Abstract
Live poultry markets (LPMs) are crucial places for human infection of influenza A (H7N9 virus). In Yangtze River Delta, LPMs were closed after the outbreak of human infection with avian influenza A (H7N9) virus, and then reopened when no case was found. Our purpose was to quantify the effect of LPMs’ operations in this region on the transmission of influenza A (H7N9) virus. We obtained information about dates of symptom onset and locations for all human influenza A (H7N9) cases reported from Shanghai, Jiangsu and Zhejiang provinces by May 31, 2014, and acquired dates of closures and reopening of LPMs from official media. A two-phase Bayesian model was fitted by Markov Chain Monte Carlo methods to process the spatial and temporal influence of human cases. A total of 235 cases of influenza A (H7N9) were confirmed in Shanghai, Jiangsu and Zhejiang by May 31, 2014. Using these data, our analysis showed that, after LPM closures, the influenza A (H7N9) outbreak disappeared within two weeks in Shanghai, one week in Jiangsu, and one week in Zhejiang, respectively. Local authorities reopened LPMs when there was no outbreak of influenza A (H7N9), which did not lead to reemergence of human influenza A (H7N9). LPM closures were effective in controlling the H7N9 outbreak. Reopening of LPM in summer did not increase the risk of human infection with H7N9. Our findings showed that LPMs should be closed immediately in areas where the H7N9 virus is confirmed in LPM. When there is no outbreak of H7N9 virus, LPMs can be reopened to satisfy the Chinese traditional culture of buying live poultry. In the long term, local authorities should take a cautious attitude in permanent LPM closure.
Keywords: live poultry market, avian influenza A (H7N9)
Introduction
In March 2013, cases of human infection with a novel avian influenza A (H7N9) virus emerged in China[1-7]. Until May 31, 2014, two waves of human influenza A (H7N9) epidemic affected China, and caused 399 cases. The confirmed infection cases were concentrated in eastern China, including the Yangtze River Delta region and Beijing. Most H7N9 virus-infected patients developed severe pneumonia and acute respiratory distress syndrome[8] with an approximate mortality of 30%[9-10]. Chinese health authorities reported that H7N9 virus was found in domestic poultries, such as chickens, pigeons, and ducks[11-12]. Since there is no evidence of human-to-human transmission so far[13-14], the main focus of intervention measure is to reduce transmission from poultry to humans[15-17].
Live poultry was known as a potential source of human infection of H7N9 virus as 75% of H7N9 virus-infected patients reported recent exposures to poultry[6]. The most likely locations for poultry-to-human transmission of H7N9 were considered as live poultry markets (LPMs)[4-6,18-20]. In China, LPMs are common places. A survey in Guangzhou in 2006 showed that 80% of households reported visit to LPMs at least once a year[21]. Even in 2013, 47% of respondents in Guangzhou reported visiting LPMs more than one time per year[22]. Studies in Hong Kong indicated that LPMs were high risk regions of human infection with H5N1 virus[23-24]. Recently, a research on 8,943 LPMs in China has shown a strong positive association between market-level H7N9 presence and local density of LPMs[25].
In April 2013, closures of LPMs in four cities were tried as an effective measure in curtailing the spread of H7N9 virus[20]. However, closures of LPMs were only temporary, and LPMs were reopened in June 2013. The influence of reopening LPMs on H7N9 virus transmission is still lack of analysis. Since H7N9 virus was initially detected in the Yangtze River Delta, we aimed to assess the effects of closing and reopening LPMs around this area. In this study, we proposed a two-phase Bayesian network, which may provide scientific evidence for authorities to employ more comprehensive measures to LPMs in those regions affected by H7N9 virus.
Materials and methods
Data source
Shanghai and two adjacent provinces, Jiangsu and Zhejiang, were selected as the region for the current study. As of 31 May 2014, a total of 235 laboratory-confirmed human cases of H7N9 had occurred in the region. For each case, we collected information about the dates of symptom onset and locations from H7N9 epidemic report released by the National Health and Family Planning Commission of the People’s Republic of China (NHFPC). By the same way, we obtained the dates of closing and reopening of LPMs in the region.
Spatial and temporal analysis
To assess the effect of LPMs’ operations on the epidemic of H7N9, we proposed a two-phase Bayesian model. We segmented the human cases of each province into time series. For convenience, we treated the first week in 2013 as the first week in our study. Let Xi,t denote the number of cases at the tth week in province i, and Zi,t denote the phase of epidemic in city or province i, where Zi,t = 0,1 corresponds to the non-outbreak phase and outbreak phase, respectively. Index i = 1, 2, 3 denotes Shanghai, Jiangsu, and Zhejiang, respectively.
To measure the effect of LPM closure and reopening in each specified city or province, we firstly assumed that the weekly numbers of human cases obey the conditional Poisson distribution under the given phase Z for each specified city or province. Then, we introduced the spatial and temporal dependence on the phases of all provinces.
For Shanghai, we supposed that the observation X1,t obeys a Poisson distribution under the given phase Z1,t. Given Z1,t = 0, which denotes that there is no outbreak of influenza A (H7N9):
where λ1,t denotes the mean number of H7N9 cases when there is no outbreak of H7N9, where the mean number refers to the mean number of H7N9 cases every week. Since Shanghai closed LPMs in the 14th and 53th week, and reopened in the 25th and 70th week, respectively, we segmented λ1,t into five different time periods as below:
 |
When Z1,t =1,which means the presence of outbreak of H7N9, we assumed:
where ρ1,t denotes the infection force of H7N9 transmission. Similarly, we segmented ρ1,t like λ1,t:
 |
In the same way as (1)-(4), we constructed the conditional Poisson distribution of X2,t and X3,t for Jiangsu and Zhejiang, respectively.
To investigate the patterns of H7N9 outbreak, we adopted the spatial and temporal dependence into the model, where the phase Z1,t for Shanghai at week t was not only dependent on its previous phase Z1,t-1, but also on its adjacent provinces. This pattern can be described by a generalized linear model[26]. For province i at week t, the probability of Zi,t is defined as follows:
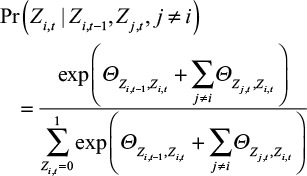 |
where Θ and Φ were parameter matrices that denoted the temporal and spatial influences. Since phase Z takes only two possible values, the Θ is a 2 × 2 matrix. Θm,n measures the possibility that phase Zi,t takes value n given the previous phase Zi,t-1 equals to m. The matrix Φ is also a 2 × 2 matrix, in which Φl,k denotes the possibility that phase Zi,t takes value k given that the phase of the adjacent location Zj,t equals to l. Each element in Θ and Φ obeys a zero-mean Gaussian prior distribution.
The sequence of state Z for a specific city reflects the local effect of LPM closure or reopening. This Bayesian model is similar to models previously used to detect the influenza from Twitter[26]. We compute all the parameters by Markov Chain Monte Carlo methods in R software (Version 3.2.0).
Results
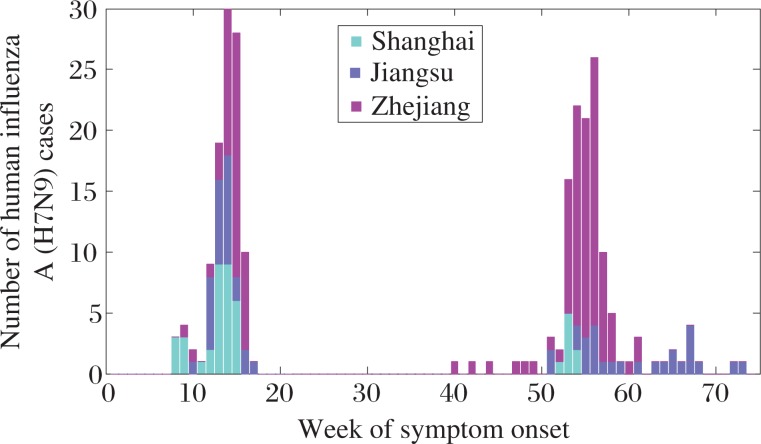
As of 31 May 2014, there were 235 laboratory-confirmed human cases of H7N9 reported in the study areas, 41 cases in Shanghai, 55 cases in Jiangsu, and 139 cases in Zhejiang, respectively (Fig. 1). The first epidemic wave occurred between19 February 2013 and 5 April 2013 and led to 107 cases, and the second wave caused 128 cases between 7 October 2013 and 22 May 2014.
Fig. 1. Weekly distribution of human influenza A (H7N9) cases.
The weekly number of H7N9 in Shanghai, Jiangsu, and Zhejiang are plotted, where we treat the first week in 2013 as the 1st week of our model.
For prevention of the first epidemic wave in 2013, local authorities closed LPMs in Shanghai on April 6, in Jiangsu on April 16, and in Zhejiang on April 22, respectively. When this wave ended, LPMs reopened in Shanghai on June 20, Jiangsu on June 1, and Zhejiang on May 27, respectively. For the second epidemic wave in 2014, the three provinces have taken different strategies on LPMs based on their epidemic situations. Shanghai closed and reopened LPMs on January 3 and April 30 in 2014, respectively. Jiangsu did not close LPMs during the second epidemic wave. Zhejiang closed all LPMs on February 8 in 2014, which never reopened. The details of dates of closing and opening of LPMs are shown in Table 1.
Table 1. The dates of closing and reopening of live poultry markets (LPMs) in Shanghai, Jiangsu and Zhejiang provinces.
| Location | Date of first closure | Date of first opening | Date of second closure | Date of second opening |
|---|---|---|---|---|
| Shanghai | April 6, 2013 (the 14th week)* | June 20, 2013 (the 25th week)* | January 3, 2014 (the 53th week)* | April 30, 2014 (the 70th week)* |
| Jiangsu | April 16, 2013 (the 15th week)* | June 1, 2013 (the 22th week)* | -** | -** |
| Zhejiang | April 22, 2013 (the 16th week)* | May 27, 2013 (the 21th week)* | February 8, 2014 (the 58th week)* | -** |
Data in parentheses are corresponding week of LPM closure (opening), where we treated the first week in 2013 as the 1st week of our model.
There is no closure or reopening of LPMs.
The analysis of phase and estimated number of H7N9 cases
Based on the two-phase Bayesian model, we estimated the phases for each province. The Kruskal-Wallis test with chi-squared = 88.728, df = 1, P-value<2.2e-16 showed a significant difference between the outbreak-phase and the non-outbreak phase.
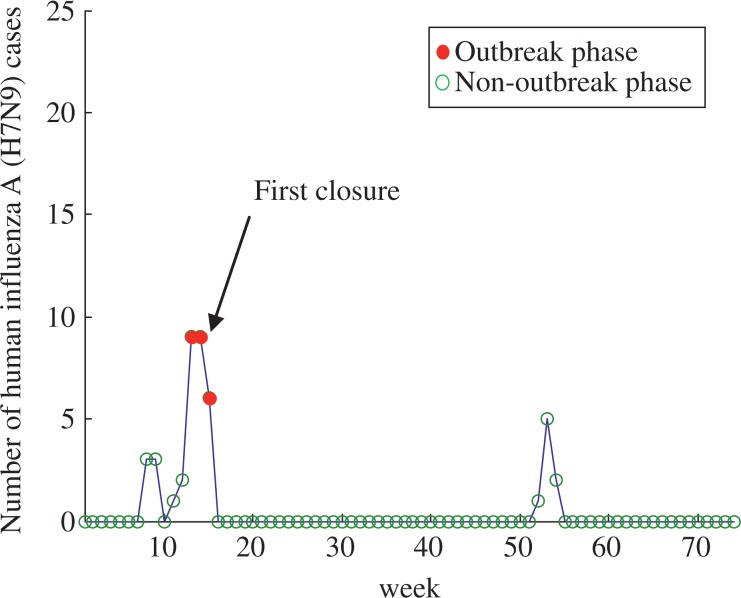
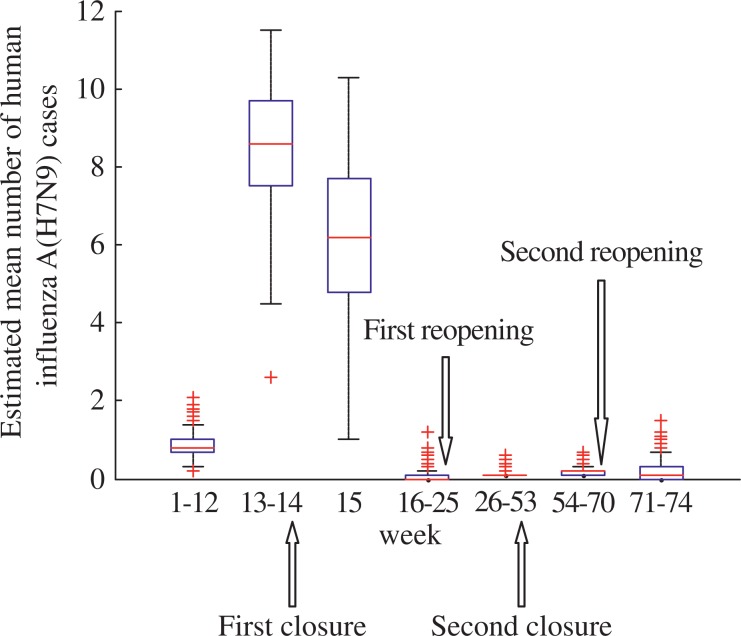
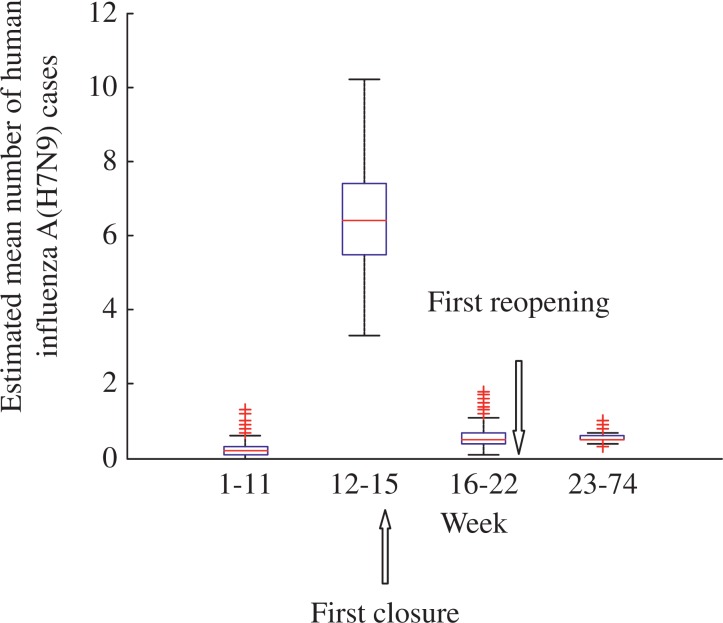
The number of cases and phases of H7N9 epidemic for Shanghai are shown in Fig. 2. Outbreak phases in Shanghai only occurred among the 13th and 15th week. The date of first closure of LPMs in Shanghai was the 14th week. For the first closure of LPMs, the number of cases decreased from 8.5 at the 14th week to 6.2 at the 15th week. From the 16th week to 25th week, the number of H7N9 cases was estimated at 0.07. The estimated mean number of H7N9 cases before and after the first reopening of LPMs was 0.07 and 0.14, respectively. The estimated mean number of H7N9 cases before and after the second reopening of LPMs was 0.18 and 0.19, respectively. The box-plot analysis and details of the estimated mean number of H7N9 cases are shown in Fig. 3 and Table 2, respectively.
Fig. 2. Number of human influenza A (H7N9) cases and phases of epidemic in Shanghai.
The blue line denotes the number of human cases, and the red circles and green circles denotes the outbreak phase and non-outbreak phase of Shanghai, respectively. In our model, Shanghai only occurred one wave of H7N9 epidemic from the 13th week to the 15th week.
Fig. 3. The box-plot of mean number of human influenza A (H7N9) cases in Shanghai.
The mean numbers of H7N9 cases before and after live poultry markets were open or closed in Shanghai were analyzed.
Table 2. Estimated mean number of human influenza A (H7N9) cases in different time period segmented by the closures and reopening LPMs of Shanghai, Jiangsu and Zhejiang.
| Location | Exposure time | Estimation of mean number of H7N9 cases under the non-outbreak phase (95% CI) | Estimation of mean number of H7N9 cases under the outbreak phase (95% CI) |
|---|---|---|---|
| Shanghai | Before the first closure of LPMs | 0.8448 (0.8277-0.8618) | 8.5227 (8.414-8.6313) |
| After the first closure of LPMs | 0.0655 (0.0576-0.0735) | 6.2444 (6.115-6.3739) | |
| After the first reopening of LPMs | 0.1307 (0.1263-0.1351) | No outbreak phases were found after the first reopening | |
| After the second closure of LPMs | 0.1804 (0.1745-0.1864) | No outbreak phase were found after the second closure | |
| After the second reopening of LPMs | 0.1892 (0.1748-0.2035) | No outbreak phase were found after the second reopening | |
| Jiangsu | Before the first closure of LPMs | 0.2410 (0.2295-0.2525) | 6.4883 (6.3945-6.5821) |
| After the first closure of LPMs | 0.5684 (0.5502-0.5866) | No outbreak phases were found after the first closure | |
| After the first reopening of LPMs | 0.5406 (0.5343-0.5469) | No outbreak phases were found after the first reopening | |
| Zhejiang | Before the first closure of LPMs | 0.5854 (0.5722-0.5986) | 9.8736 (9.8189-9.9283) |
| After the first closure of LPMs | 0.1433 (0.1324-0.1543) | No outbreak phases were found after the first closure | |
| After the first reopening of LPMs | 0.4276 (0.4185-0.4367) | 10.1064 (10.0852-10.1367) | |
| After the second closure of LPMs | 0.2505 (0.2427-0.2582) | No outbreak phases were found after the second closure |
CI: confidence interval.
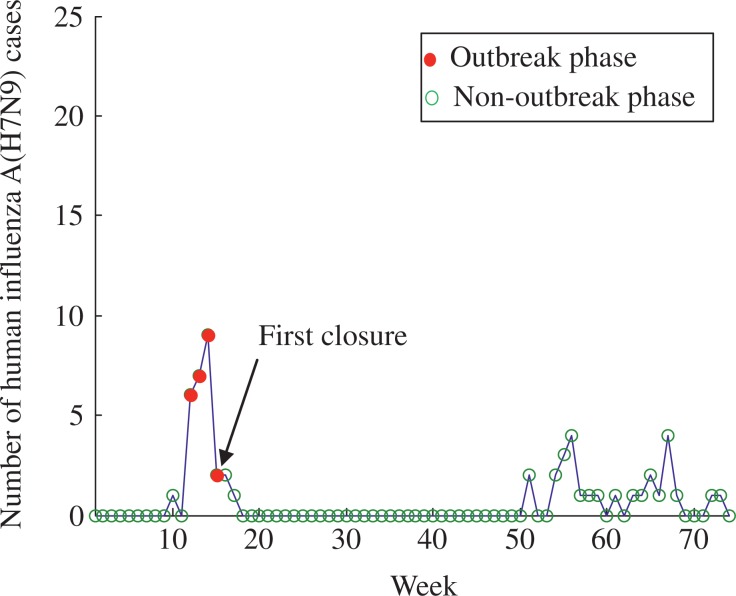
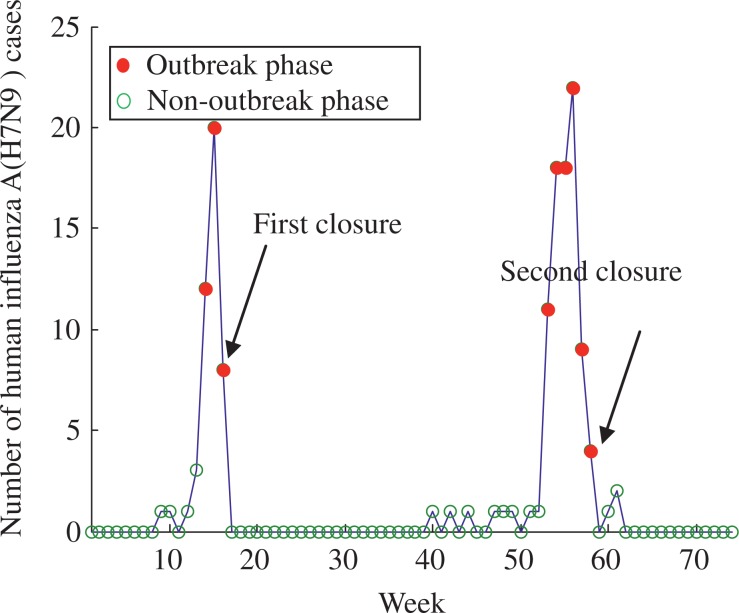
The numbers of cases and phases of H7N9 epidemic for Jiangsu are shown in Fig. 4, where the outbreak phases only occurred between the 12th and 15th week. The da e of he firs closure of LPMs in Jiangsu was the 15th week. The number of H7N9 cases before and after the first closure was 6.5 and 0.57, respectively. The estimated mean number of H7N9 cases before and after the first reopening was 0.57 and 0.54, respectively. The box-plot analysis and details of the estimated mean number of H7N9 cases are shown in Fig. 5 and Table 2, respectively.
Fig. 4. Number of human influenza A (H7N9) cases and phases of epidemic in Jiangsu.
The blue line denotes the number of human cases, and the red circles and green circles denotes the outbreak phase and no-outbreak phase of Jiangsu, respectively. In our model, Jiangsu only occurred one wave of H7N9 epidemic from the 12th week to the 15th week.
Fig. 5. The box-plot of mean number of human influenza A (H7N9) cases in Jiangsu.
The mean numbers of H7N9 cases before and after LPMs were closed and opened in Jiangsu were analyzed.
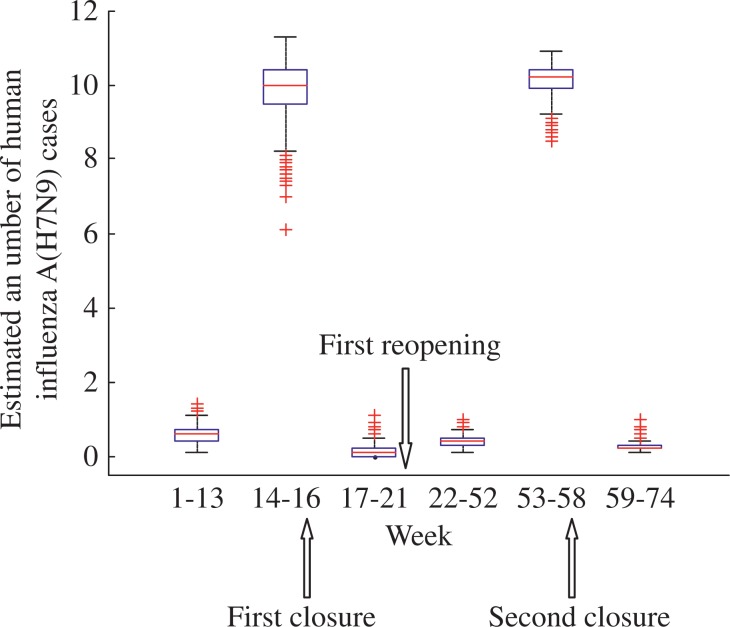
The number of cases and phases of H7N9 cases for Zhejiang are shown in Fig. 6. The outbreak phases in Zhejiang occurred among the 14th-16th week, and the 53th-58th week. The dates of the first and second closure of LPMs were the 16th and 58th week, respectively. For the first closure, the estimated mean number of H7N9 cases decreased from 9.9 to 0.14. The estimated mean number of H7N9 cases before and after the second closure was 10.1 and 0.25, respectively. The estimated mean number of H7N9 cases before and after the first reopening was 0.14 and 0.43, respectively. The box-plot analysis and details of the estimated mean number of H7N9 cases are shown in Fig. 7 and Table 2, respectively.
Fig. 6. Number of human influenza A (H7N9) cases and phases of epidemic in Zhejiang.
The blue line denotes the number of human cases, and the red circles and green circles denotes the outbreak phase and no-outbreak phase of Zhejiang, respectively. In our model, Zhejiang had two waves of H7N9 epidemic. The first wave happened from the 14th week to 16th week, and the second wave from the 53th week to the 58th week.
Fig. 7. The box-plot of mean number of human influenza A (H7N9) cases in Zhejiang.
The mean numbers of H7N9 cases before and after the LPMs’ operations in Zhejiang were analyzed.
Relationships between temperature and H7N9 cases
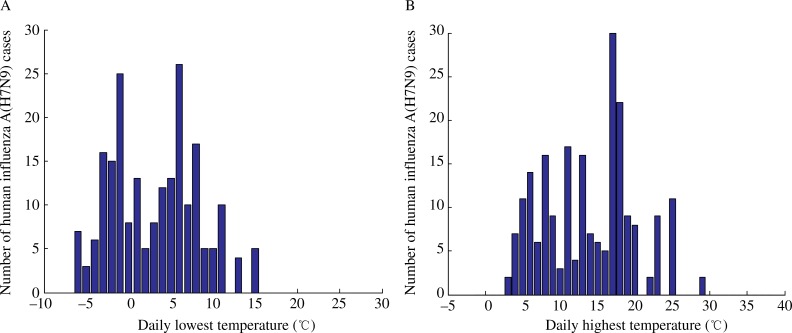
The relationships between the number of H7N9 cases and temperature are shown in Fig. 8, which plots the daily lowest and highest temperatures as well as the number of cases in Shanghai, Jiangsu, and Zhejiang between February 2013 and May 2014. The highest temperature ranged from 3°C to 29°C, and the lowest temperature ranged from -4°C to 15°C.
Fig. 8. The relationship between the number of human influenza A (H7N9) cases and the daily lowest temperature in (A), and daily highest temperature in (B).
Spatial and temporal dependence
Our model analyzed the spatial and temporal dependence for the cases of H7N9 in Shanghai, Jiangsu, and Zhejiang provinces. We estimated the temporal dependence of phase Z. When there was no outbreak of H7N9 at the (t-1)th week, the probability of no outbreak of H7N9 at the tth week was 0.97 (95% CI: 0.9671-0.9686). When there happened an outbreak of H7N9 at the (t-1)th week, the probability of the outbreak of H7N9 at the tth week was 0.72 (95% CI: 0.7143-0.7279). The spatial dependence of phase Z was also considered. The province had a high probability of 0.96 (95% CI: 0.9607-0.9617) of the no-outbreak phase when there was no outbreak of H7N9 at adjacent provinces. The probability of outbreak phase was 0.21 (95% CI: 0.2049-0.2099) when adjacent provinces had outbreak of H7N9.
Discussion
Our model systematically evaluated the effects of intervention measures to the LPMs on the epidemic of H7N9 in the study areas. According to our findings, Shanghai and Jiangsu had one wave of H7N9 epidemic, while Zhejiang had two waves of epidemic. The H7N9 epidemic in Shanghai started at the 13th week, and LPMs were shut down on the next week. Then, the H7N9 epidemic faded after the 15th week. Our model showed that the closure of LPMs reduced the mean number of H7N9 cases from 8.5277 at the 13th-14th week to 0.07 at the 16th-25th week. Furthermore, the effectiveness of closures of LPMs in Jiangsu and Zhejiang can also be found by our model in a similar way. This result supported the idea that LPMs have a major role in poultry-to-human transmission of H7N9 virus[20,27]. From the public health standpoint, once signs of outbreak of human infection with H7N9 virus are detected by routine monitoring, especially in winter and spring, local authorities should decisively close LPMs as soon as possible to block the epidemic.
Conversely, we found that reopening LPMs in the non-outbreak phase did not increase the risk of human infections with H7N9 virus. As mentioned before, Shanghai reopened the LPMs twice, while Jiangsu and Zhejiang reopened LPMs once. The date of the first reopening of LPMs in Shanghai was June 20, 2013. In our model, Shanghai remained in the non-outbreak phases of H7N9 epidemic after reopening. The mean number of H7N9 cases before and after the reopening was estimated at 0.07 and 0.14, respectively. Similarly, the second reopening of LPMs in Shanghai, and reopening of LPMs in Jiangsu and Zhejiang did not lead to outbreak of H7N9 epidemic. Climate conditions in the hot summer may not be suitable for H7N9 virus to survive. Many experimental studies on H5N1 have revealed that temperature can affect the survival time of the H5N1 virus[28-29]. It is also reported that H7N9 virus is inactivated in high temperature[30]. Analysis of H7N9 cases and their corresponding temperature showed that there were no cases when the lowest daily temperature were less than 15°C or the highest daily temperature exceeded over 29°C. In this case, closing LPMs would not be a wise decision to the local authorities, taking into account the heavy cost.
The spatial and temporal dependence of the outbreak of H7N9 epidemic was discussed in our model. Analysis of temporal dependence showed that, in the same province, the phases of the adjacent weeks showed strong correlations. That is, if the outbreak phase of H7N9 epidemic was found at the present, the probability of occurring of H7N9 at the next week was as high as 0.97. This result suggested that, when the H7N9 epidemic was in the outbreak phase, LPMs should be rapidly closed to protect against poultry-to-human transmission. Spatial analysis revealed that, in the same week, the phases of the adjacent provinces showed mild correlations. This result was from the analysis of all cases between 2013 and 2014. In 2013, the first wave of H7N9 occurred almost on the same week in Shanghai, Jiangsu and Zhejiang. This phenomenon in 2013 reflected a strong spatial correlation on the epidemic outbreak among adjacent provinces. But after effective precautionary measures were taken in 2014, only Zhejiang detected the outbreak of H7N9 cases in the 53rd-58th week. No outbreak of H7N9 occurred in Shanghai and Jiangsu. This phenomenon in 2014 showed that the spatial correlation in epidemic outbreak among adjacent provinces was weak. Combining all outbreak phases in 2013 and 2014, the spatial analysis of our model showed a mild spatial correlation.
Currently, the considerations of permanent LPM closure and central slaughtering for the poultry supply chain are fiercely discussed. Although the evidence has shown that LPM closures can dramatically cut down H7N9 cases, the policymakers should also consider the social and economic impacts of LPM closures. At the end of June 2013, the first wave of epidemic caused 60 billion RMB loss in China's poultry industry, and more than 50 million enterprises and farmers suffered losses[10]. Based on our findings, we propose that local authorities should be more reasonable in using the strategy of closing LPMs.
Limitations of our study have to be addressed here. Firstly, we made a simple assumption that LPMs of each province were closed on the same week. In fact, even in the same province, the dates of LPM closures in different cities may have one week's bias. In Jiangsu, the provincial government started Level 4 Emergency Response to avian influenza on April 3, 2013[31]. Based on this response, LPMs were shut down on April 8 (the 14th week) in Nanjing and Suzhou, on April 9 (the 14th week) in Zhenjiang, on April 16 (the 15th week) in Yancheng and Xuzhou. Our model assumed 15th week as the date of LPM closures for Jiangsu. This assumption may have certain impact on effective analysis of LPM closures. To deal with this situation, a more complex model needs to be considered. Secondly, we targeted our surveys to three provinces in the Yangtze River delta region, where most H7N9 cases occurred. We were unable to examine the effect of LPM closure and reopening in other Chinese provinces which had small numbers of H7N9 cases.
Despite these limitations, it is noteworthy that we have analyzed the effectiveness of closing and reopening of LPMs by using the H7N9 epidemic phases which were built through a two-phase Bayesian model. The result can provide a basis for local authorities for taking proper control strategies on LPMs.
References
- [01].WHO/GAR (2013). Human infection with influenza A (H7N9) virus in China[R/OL] Available:http://www.who.int/csr/don/2013_04_01/en. Accessed 1 April 2013 [Google Scholar]
- [02].Yang F, Wang J, Jiang L et al. A fatal case caused by novel H7N9 avian influenza A virus in China[J/OL] Emerg Microbes Infect. 2013;2(4):el9. doi: 10.1038/emi.2013.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [03].Gao R, Cao B, Hu Y et al. Human infection with a novel avian-origin influenza A (H7N9) virus[J] N Engl J Med. 2013;368(20):1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- [04].Li Q, Zhou L, Zhou Met al. Epidemiology of human infections with avian influenza A (H7N9) virus in China[J] N Engl J Med 2014370:520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [05].Chen Y, Liang W, Yang Set al. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome[J] Lancet ,201338198811916–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [06].Cowling BJ, Jin L, Lau EHYet al. Comparative epidemiology of human infections with avian influenza A H7N9 and H5N1 viruses in China: a population-based study of laboratory-confirmed cases[J] Lancet ,20133829887129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [07].Zhuang Q, Wang S, Wu Met al. Epidemiological and risk analysis of the H7N9 subtype influenza outbreak in China at its early stage[J] Chin Sci Bull ,201358:1403–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [08].Gao H, Lu H, Cao B, Du B, Shang H et al. Clinical findings in 111 cases of influenza A (H7N9) virus infection[J] N Engl J Med. 2013;368(24):2277–2285. doi: 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- [09].Li Q, Zhou L, Zhou M, Chen Zet al. Preliminary Report: Epidemiology of the Avian Influenza A (H7N9) Outbreak in China[J] N Engl J Med 2013370:520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. China’s Ministry of Agriculture (2014). National plan for Eradication of avian influenza A (H7N9)[R/OL]. Available:http://www.chinairn.com/news/20140221/13542832.html [Google Scholar]
- [11].Kahn RE, Richt JA. The Novel H7N9 Influenza A Virus: Its Present Impact and Indeterminate Future[J] Vector-Borne Zoonot. 2013;13(6):347–348. doi: 10.1089/vbz.2013.999.ceezad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Shi J, Deng G, Liu Pet al. Isolation and characterization of H7N9 viruses from live poultry markets-Implication of the source of current H7N9 infection in humans[J] Chin Sci Bull 201358:1857–1863. [Google Scholar]
- [13].Li Q, Zhou L, Zhou Met al. Preliminary report: Epidemiology of the avian influenza A (H7N9) outbreak in China[J] N Engl J Med 2014370:520–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Zhu H, Wang D, Kelvin DJet al. Infectivity, transmission, and pathology of human-isolated H7N9 influenza virus in ferrets and pigs[J] Science 2013341:183–186. [DOI] [PubMed] [Google Scholar]
- [15].Horby P. H7N9 is a virus worth worrying about[J] Nature. 2013;496(7446):399. doi: 10.1038/496399a. [DOI] [PubMed] [Google Scholar]
- [16].Uyeki TM, Cox NJ. Global concerns regarding novel influenza A (H7N9) virus infections[J] N Engl J Med. 2013;368(20):1862–1864. doi: 10.1056/NEJMp1304661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Han J, Jin M, Zhang P et al. Epidemiological link between exposure to poultry and all influenza A (H7N9) confirmed cases in Huzhou city, China, March to May 2013[J] Euro Surveill, 2013;18(20):20481. [PubMed] [Google Scholar]
- [18].He Y, Liu P, Tang S et al. Live poultry market closure and control of avian influenza A (H7N9), Shanghai, China[J] Emerg Infect Dis. 2014;20(9):1565–1566. doi: 10.3201/eid2009.131243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Bao C, Cui L, Zhou M et al. Live-animal markets and influenza A (H7N9) virus infection[J] N Engl J Med. 2013;368(24):2337–2339. doi: 10.1056/NEJMc1306100. [DOI] [PubMed] [Google Scholar]
- [20].Yu H, Wu J, Cowling BJ et al. Effect of closure of live poultry markets on poultry-to-person transmission of avian influenza A H7N9 virus: an ecological study[J] Lancet. 2014;383(9916):541–548. doi: 10.1016/S0140-6736(13)61904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liao Q, Lam WT, Leung GM et al. Live poultry exposure, Guangzhou, China, 2006[J] Epidemics. 2009;1(4):207–212. doi: 10.1016/j.epidem.2009.09.002. [DOI] [PubMed] [Google Scholar]
- [22].Wang L, Cowling BJ, Wu P et al. Human exposure to live poultry and psychological and behavioral responses to influenza A(H7N9), China[J] Emerg Infect Dis. 2014;20(8):1296–1305. doi: 10.3201/eid2008.131821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shortridge KF, Gao P, Guan Y et al. Interspecies transmission of influenza viruses: H5N1 virus and a Hong Kong SAR perspective[J] Vet Microbiol. 2000;74(1–2):141–147. doi: 10.1016/s0378-1135(00)00174-7. [DOI] [PubMed] [Google Scholar]
- [24].Mounts AW, Kwong H, Izurieta HS et al. Case-control study of risk factors for avian influenza A(H5N1) disease, Hong Kong, 1997[J] J Infect Dis. 1999;180(2):505–508. doi: 10.1086/314903. [DOI] [PubMed] [Google Scholar]
- [25].Gilbert M, Golding N, Zhou H et al. Predicting the risk of avian influenza A H7N9 infection in live-poultry markets across Asia[J] Nat Commun. 2014;5:4116. doi: 10.1038/ncomms5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li J, Cardie C.Early stage influenza detection from twitter. arXiv:1309.7340[cs.SI]. Available:http://arxiv.org/abs/1309.7340 [Google Scholar]
- [27].Gao G. Influenza and the Live Poultry Trade[J] Science. 2014;344(6181):235. doi: 10.1126/science.1254664. [DOI] [PubMed] [Google Scholar]
- [28].Paek MR, Lee Y J, Yoon H et al. Survival rate of H5N1 highly pathogenic avian influenza viruses at different temperatures[J] Poult Sci. 2010;89(8):1647–1650. doi: 10.3382/ps.2010-00800. [DOI] [PubMed] [Google Scholar]
- [29].Tiensin T, Nielen M, Songserm Tet al. Geographic and temporal distribution of highly pathogenic avian influenza A virus (H5N1) in Thailand, 2004–2005: an overview[J] Avian Dis 2007511 Suppl):182–188. [DOI] [PubMed] [Google Scholar]
- [30].Zhang Z, Xia Y, Lu Y et al. Prediction of H7N9 epidemic in China[J] Chin Med J. 2014;127(2):254–260. [PubMed] [Google Scholar]
- [31].Jiangsu Province (2013). Jiangsu starts the 4th level Emergency Response to avian influenza[EB/OL]. Available:http://www.chinanews.com/gn/2013/04-03/4703079.shtml [Google Scholar]