Abstract
In human lung fibrotic lesions, fibroblasts were shown to be closely associated with immature dendritic cell (DC) accumulation. The aim of the present pilot study was to characterize the role of pulmonary fibroblasts on DC phenotype and function, using co-culture of lung fibroblasts from patients with idiopathic pulmonary fibrosis (IPF) and from control patients, with a DC cell line MUTZ-3. We observed that co-culture of lung control and IPF fibroblasts with DCs reduced the expression of specific DC markers and down-regulated their T-cell stimulatory activity. This suggests that pulmonary fibroblasts might sustain chronic inflammation in the fibrotic lung by maintaining in situ a pool of immature DCs.
Keywords: Lung, Fibroblasts, Dendritic cells, Fibrosis
Findings
Background
We previously reported that in human fibrotic interstitial lung disease, immature DC accumulate in large numbers in areas of established fibrosis, in the close vicinity of fibroblasts expressing the main chemokines involved in the recruitment of immune cells [1–3]. Accumulating evidence indicates that the stromal microenvironment plays an important role in the regulation of the trafficking and phenotype and function regulation of immune cells, including DC [4, 5].
Fibroblasts and other stromal cells from various tissues have been shown to modulate DC differentiation and activation [6–8] and may induce the development/differentiation of DCs from blood monocytes or CD34+ hematopoietic precursors [9, 10]. Kitamura et al [11] reported that fibroblasts have a crucial role in regulating both fibrotic and immune responses in the lung by chemokine secretion, favoring dendritic cell trafficking, through TGF-β αvβ8-mediated activation. Dermal fibroblasts were also reported to act as potent immunoregulatory cells able to modulate the maturation of DCs [12–14].
Herein, we aimed to characterize in vitro the influence of pulmonary fibroblasts on DC phenotype and function, using co-culture of human pulmonary fibroblasts obtained from patients with idiopathic pulmonary fibrosis (IPF) and from control patients, with immature DCs developed from the precursor cell line MUTZ-3 [15].
Materials
Primary fibroblasts
Primary fibroblasts were derived in Dulbecco’s modified Eagle’s medium (DMEM, Life Technologies-Invitrogen, Courtaboeuf, France) supplemented with 10 % FCS (Life Technologies-Invitrogen) from lung tissue biopsies obtained from 6 patients with IPF (age = 65.4 ± 10.6, 2 F), and from 6 patients with removal of a primary lung tumor without any fibrosis (control patients, age = 53 ± 14, 3 F), according to previous detailed procedures [16]. Fibroblasts were used at passage 5. No significant morphological and phenotypic differences were observed between IPF and control fibroblasts. Informed consent was obtained before lung sampling. The protocol was approved by the ethical committee of Paris-Ile de France 1.
MUTZ-3 dendritic cells
The CD34+ human MUTZ-3 progenitor cell line (obtained from DSMZ, Braunschweig, Germany) maintained in α-MEM (Life Technologies) supplemented with 20 % FCS and 0.25 ng/ml rhGM-CSF (Gentaur, Bruxelles, Belgium) express CD34 for 80 % and HLA-DR+ for 70 %, as previously reported [17]. Generation of dendritic-like cells MUTZ-3 DCs was obtained by culturing MUTZ-3 progenitor (4×105cells/ml) for 7 days in α-MEM with 20 % FCS, 10 ng/ml rhGM-CSF, 10 ng/ml rhIL-4 (Gentaur), and 0.5 ng/ml TNF-α (R&D Systems Europe, Lille, France) (MUTZ − 3 DC medium) [17].
Co-cultures
MUTZ-3 DCs (1×106) were co-cultured with fibroblast monolayers (80–90 % confluence) for 48 h in MUTZ-3 DC medium either in 6-well tissue culture plates for direct contact or in Transwell® support. As controls, MUTZ-3 DCs and fibroblasts alone were cultured for 48 h under the same conditions.
Mixed Leukocyte Reaction
For mixed leukocyte reaction (MLR), human peripheral blood mononuclear cells were isolated from one leucocyte-enriched buffycoat, obtained from Etablissement Français du Sang (Paris, France), by Lymphocyte Separation MediumM (GE Healthcare, PAA, Austria) density gradient centrifugation, cultured in RPMI 1640 (Gibco, Life Technologies) with 10 % heat inactivated human AB serum (RPMI complete medium) for 2 h in order to eliminate adherent mononuclear cells and resuspended at 1×106 cells/ml RPMI complete medium. MUTZ-3 DCs cultured alone or in the presence of fibroblasts as indicated above were collected, washed once and resuspended in RPMI complete medium also. 2×104 DC were cultured with 1×105 allogeneic T cells in 96-well round-bottom plates, in a final volume of 200 μl for each well. All determinations were performed in sixplicate. Cell proliferation was assessed for 6 days, with during the last 18 h of the culture period, addition of 1 μCi of 3H-thymidine (Perkin-Elmer Wallac, Courtabeuf, France) to each well. The cells were then harvested onto FilterMat filters (Wallac) by using the Harvester 96 cell Tomtec harvester, and incorporated radioactivity was determined using a MicroBeta plate reader (Wallac Trilux 1450 MicroBeta Liquid Scintillation Counter & Luminometer, WS-Trilux 1450).
Flow Cytometry
Cell surface staining was performed in phosphate-buffered saline (PBS, Life Technologies) (without calcium and magnesium) containing 1 % bovine serum albumin (BSA) (Sigma-Aldrich) using fluorochrome-conjugated monoclonal antibodies recognizing MHC class II and DC-specific molecules: HLA-DR-PE (Pharmingen-BD, L’Isle d’Abeau, France), CD1a-PE, CD34-FITC, CD83-PE, CD86-PE, and CD209-PE (Immunotech-Beckman Coulter, Marseille, France) for 20 min at 4 °C. After two washes in PBS/BSA, the cells were fixed in formaldehyde 1 % and subsequently analyzed using an Epics cytometer (Beckman Coulter). Appropriate gating was used to exclude cell debris. Gating on isotype control staining was used to identify the percentage of cells expressing DC-specific markers. Data were analyzed with FlowJo software (Tree Star, Inc., San Carlos, CA). Analysis of each epitope staining was assessed using both the percentage of positive cells (%) and the mean fluorescence intensity (MFI) of DCs.
Cytokine Production
Supernatants from MUTZ-3 DC and fibroblasts either cultured alone or in co-culture were centrifuged (1600 rpm, 10 min, 4 °C) and frozen at −70 °C until assayed using specific enzyme-linked immunosorbent assays (ELISAs) from R&D Systems (Quantikine, R&D Systems, Abingdon, UK).
Statistical analysis
Data are presented as means (± SEM), or as individual values and median. Comparisons were performed with Wilcoxon paired nonparametric test for group comparisons or Mann–Whitney U test. P values < 0.05 were considered significant. Statistical analysis was performed with Prism 5 (Graphpad Software Inc., La Jolla, CA USA).
Results
MUTZ-3 DCs obtained after a 7 day-differentiation culture (n = 12) were characterized by a low CD34 expression (<15 %), and the expression of CD209 (33.7 ± 1.6 %, mean ± SD) (marker of immaturity), HLA-DR (66.7 ± 2.8 %) and CD86 (59.5 ± 1 %). Few cells, if any, expressed the maturation marker CD83 (0.68 ± 0.23 %). These immature cells can undergo significant maturation by stimulation with 25 ng/ml TNF-α during 48 h as detected by an increased expression of HLA-DR (88.6 ± 1.3 % p ≤ 0.001) and the induction of the maturation marker CD83 (47.2 ± 2.1 %).
Co-culture of lung fibroblasts with immature MUTZ3-DCs for 48 h induced a 20 % decrease of HLA-DR expression and a 40 % decrease in CD83 and CD86 expression by DCs (Fig. 1). CD209 expression by DC was not significantly altered by co-culture (data not shown). It is noteworthy that IPF and control fibroblasts had a similar inhibitory effect on DCs.
Fig. 1.
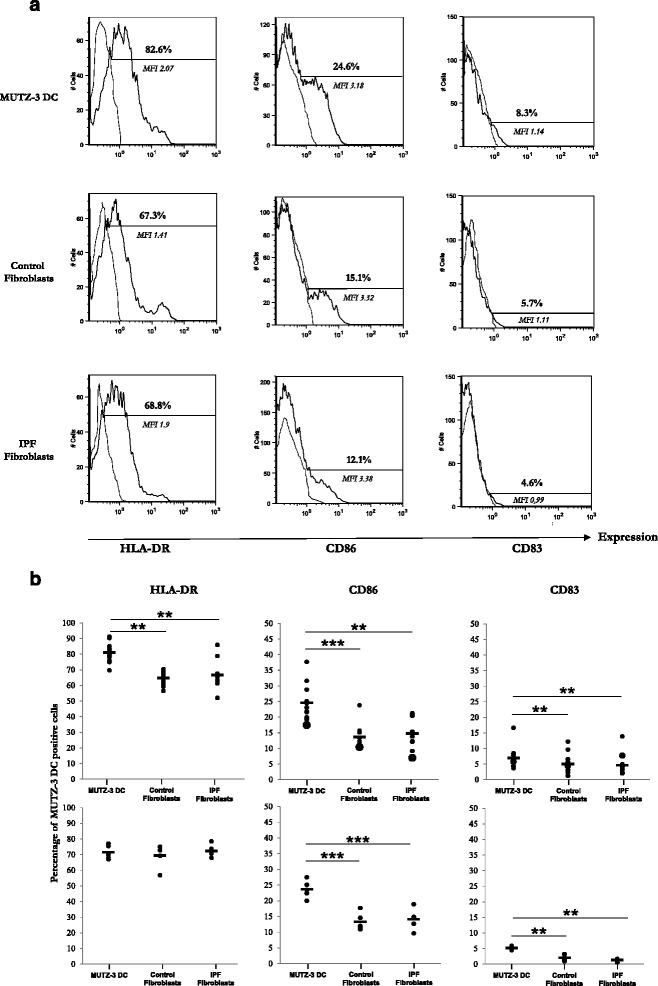
Phenotype of MUTZ-3 DCs alone or cultured with control or IPF fibroblasts for 48 h. Immature MUTZ-3 DCs (1×106) were directly added to fibroblasts monolayers grown to confluence in 6-well tissue culture plates (direct contact) or fibroblasts (low compartment) and MUTZ-3 DCs (high compartment) were separated using modified Boyden chambers (Transwell® permeable support-0.4 μm) to prevent direct cell-cell contact, for 48 h in MUTZ-3 DC medium (Transwell condition). Controls were MUTZ-3 DCs cultured alone for 48 h in the same condition. Flow Cytometry analysis was assessed on DCs stained with monoclonal antibodies HLA-DR-PE (Pharmingen-BD), CD34-FITC, CD83-PE, CD86-PE, and CD209-PE (Immunotech-Beckman Coulter), and acquired with an Epics cytometer (Beckman Coulter). a One representative experiment or (b) percentage of positive cells for HLA-DR, CD86, and CD83 expression, represented as mean (dark horizontal bars) and individual values (n = 10) for direct contact co-cultures (upper panels) and Transwell® co-cultures (lower panels). MFI: Mean fluorescence intensity. **p < 0.01, ***p < 0.001. Statistical comparisons were performed with Wilcoxon paired nonparametric test for group comparisons or Kruskal-Wallis for unpaired nonparametric test, using Prism 5 (Graphpad Software Inc.)
In accordance with the decreased expression of activation/maturation markers, co-culture of lung fibroblasts from either control or IPF patients and MUTZ-3 DCs induced a significant 30 % reduction of lymphocyte allostimulatory activity as compared to that observed for MUTZ-3 DCs cultured alone (Table 1).
Table 1.
Mixed leukocyte reaction (MLR)
| Conditions | MUTZ-3 DC medium | Contact Co-culture | |
| medium | Control Fibro | IPF Fibro | |
| Lymphocyte Proliferation (mean cpm ± SD, n = 3) | 56,870 ± 6110 | 40,236 ± 6047 | 34,322 ± 2843 |
| p value (versus MUTZ-3 DC) | 0.02 | 0.01 | |
| Conditions | MUTZ-3 DC medium | Transwell Co-culture | |
| medium | Control Fibro | IPF Fibro | |
| Lymphocyte Proliferation (mean cpm ± SD, n = 3) | 36,967 ± 4942 | 29,328 ± 3215 | 26,890 ± 6564 |
| p value (versus MUTZ-3 DC) | 0.04 | 0.05 | |
MLR was performed by culturing with 100 000 lymphocytes (depleted of adherent mononuclear cells by a 2 h-lasting culture) and 20 000 MUTZ-3 DCs previously cultured alone (medium) or co-cultured with control or IPF fibroblasts for 48 h (as in Fig. 1) in 200 μl RPMI complete medium (n = 6 for each condition) for 6 days During the last 18 h, 1 μCi of 3H-thymidine (Perkin-Elmer Wallac) was added per well. Incorporated radioactivity was determined using a MicroBeta plate reader (Wallac WS-Trilux 1450
Results are presented as mean (±SD) lymphocyte proliferation induced by MUTZ-3 DCs previously alone (medium) or co-cultured with control or IPF fibroblasts either in direct contact or in transwell conditions during 48 h before MLR
3H-thymidine incorporation of 105 lymphocytes alone was 605 ± 436 cpm and 3H-thymidine incorporation of 20 000 MUTZ3 DC alone was 138 ± 59 cpm, (mean ± SD, n = 6)
Lung fibroblasts might alter MUTZ-3 DC phenotype by either direct cell-cell contact or soluble factors. Of interest, MUTZ-3 DCs co-cultured with fibroblasts using modified Boyden chambers (Transwell® support) to prevent direct cell-cell contact did also show significant decrease in DC CD86 and CD83 markers (Fig. 1blower panels), decreases that were not significantly different from those observed in cell-cell contact conditions (upper panels).
Numerous cytokines such as IL-6, G-CSF, M-CSF, VEGF, and TGF-β [18, 19] are produced by fibroblasts in the microenvironment and may inhibit MUTZ-3 DC maturation and function. Accordingly, we detected measurable levels of these cytokines in the culture supernatants of control and IPF fibroblasts, either alone or in co-culture (Fig. 2). Although IL6 production was lower in control fibroblasts alone compared to IPF ones, no differences were observed between the two fibroblast types when co-cultured with MUTZ3-DC, either in contact or not. Noteworthy, a significant decrease in MCSF production was detected for both populations when cultured in contact with MUTZ-3 cells.
Fig. 2.
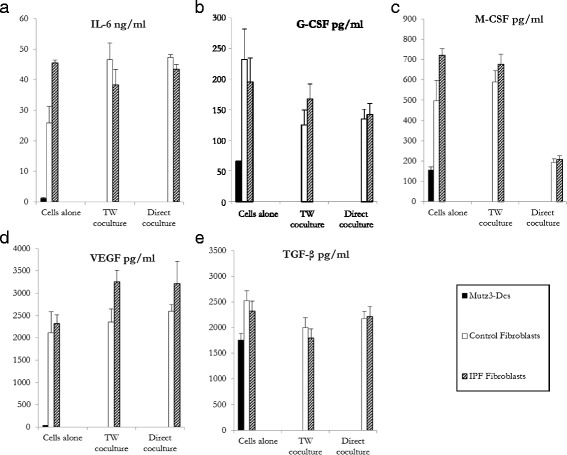
Production of the cytokines IL-6, G-CSF, M-CSF, VEGF and TGF-β by MUTZ-3 DC (dark), control fibroblasts (white) or IPF (dashed) fibroblasts, cultured either alone or in direct contact or using Transwell (TW) support. Mean levels (± SEM, n = 6) of (a) IL-6, (b) G-CSF, (c) M-CSF, (d) VEGF, and (e) TGF-β, assessed by ELISA after 48 h of both culture types. Cytokine levels were analysed using the normality test. The Mann-Whitney U and Student t test were used to compare the mean values of cytokines between patients and control subjects. TW: transwell. *p < 0.05
Discussion
The present study demonstrates that lung fibroblasts co-cultured with DCs, differentiated from CD34+ MUTZ-3 precursors and closely resembling monocyte-derived DCs in terms of morphology, phenotype and function [15, 17, 20–22], induced a reduced MHC classII, CD86, and CD83 expression by DCs, with a decrease in their T-cell stimulatory activity. These findings support the notion that lung fibroblasts are effective in maintaining DCs in an immature state within fibrotic lesions. Our results are in line with recent data showing that human dermal fibroblasts are potent immunoregulatory cells inhibiting allogeneic T-cell activation by DCs [11]. Moreover, dermal fibroblasts inhibited T-cell proliferation in response to DCs with similar potency to bone marrow-derived mesenchymal stem cells (MSC), suggesting that immunosuppression is a general property of stromal cells [11, 23].
The absence of difference between IPF and control fibroblasts in our study suggests that the inhibitory effect of lung fibroblasts on DC differentiation and function is an intrinsic property of this cell type, independently of the physiological context. This possibility, which fits well with the recently documented immunoregulatory function of MSC [24], needs to be further assessed with fibroblasts isolated from various other tissues.
Conclusion
In conclusion, our findings suggest that lung fibroblasts by their ability to modulate DC differentiation and function, are able to participate in pulmonary inflammatory and immune responses. Whereas their role in production of extracellular matrix proteins is a key factor in healing and remodeling, their likely interaction with lung DCs might be also considered as critical in inflammatory lung disorders.
Acknowledgements
This work was supported by the European Commission (FP7 project #202224, European IPF Network), by the Agence Nationale de la Recherche (ANR Physio 2006, FIBROPNEUMO) and by the Chancellerie des Universités de Paris (Legs Poix). Paul Soler was the recipient of a Contrat d’Interface Inserm - Assistance Publique/Hôpitaux de Paris. Olivia Freynet was supported by a grant from the Société de Pneumologie de Langue Française (SPLF).
Abbreviations
- DC
Dendritic cells
- IPF
Idiopathic pulmonary fibrosis
- MSC
Mesenchymal stem cells
Footnotes
Bruno Crestani, Paul Soler and Laurence Michel are co-seniors.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
OF conceived and performed the experiments, revised the manuscript. JM-S conceived and performed the experiments, analyzed the results, revised the manuscript. FJ-L performed the experiments. AM, BC, PS and LM conceived the experiments, analyzed the results, revised the manuscript. All authors read and approved the final manuscript.
Contributor Information
Bruno Crestani, Phone: 33 1 40256800, Email: bruno.crestani@bch.aphp.fr.
Laurence Michel, Email: laurence.michel@inserm.fr.
References
- 1.Lipscomb MF, Masten BJ. Dendritic cells: immune regulators in health and disease. Physiol Rev. 2002;82:97–130. doi: 10.1152/physrev.00023.2001. [DOI] [PubMed] [Google Scholar]
- 2.Marchal-Somme J, Uzunhan Y, Marchand-Adam S, Kambouchner M, Valeyre D, et al. Dendritic cells accumulate in human fibrotic interstitial lung disease. Am J Respir Crit Care Med. 2007;176:1007–1014. doi: 10.1164/rccm.200609-1347OC. [DOI] [PubMed] [Google Scholar]
- 3.Marchal-Somme J, Uzunhan Y, Marchand-Adam S, Valeyre D, Soumelis V, et al. Cutting edge: nonproliferating mature immune cells form a novel type of organized lymphoid structure in idiopathic pulmonary fibrosis. J Immunol. 2006;176:5735–5739. doi: 10.4049/jimmunol.176.10.5735. [DOI] [PubMed] [Google Scholar]
- 4.Parsonage G, Filer AD, Haworth O, Nash GB, Rainger GE, et al. A stromal address code defined by fibroblasts. Trends Immunol. 2005;26:150–156. doi: 10.1016/j.it.2004.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svensson M, Kaye PM. Stromal-cell regulation of dendritic-cell differentiation and function. Trends Immunol. 2006;27:580–587. doi: 10.1016/j.it.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 6.Berthier R, Rizzitelli A, Martinon-Ego C, Laharie AM, Collin V, et al. Fibroblasts inhibit the production of interleukin-12p70 by murine dendritic cells. Immunology. 2003;108:391–400. doi: 10.1046/j.1365-2567.2003.01573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butler M, Ng CY, van Heel DA, Lombardi G, Lechler R, et al. Modulation of dendritic cell phenotype and function in an in vitro model of the intestinal epithelium. Eur J Immunol. 2006;36:864–874. doi: 10.1002/eji.200535497. [DOI] [PubMed] [Google Scholar]
- 8.Zhang M, Tang H, Guo Z, An H, Zhu X, et al. Splenic stroma drives mature dendritic cells to differentiate into regulatory dendritic cells. Nat Immunol. 2004;5:1124–1133. doi: 10.1038/ni1130. [DOI] [PubMed] [Google Scholar]
- 9.Guironnet G, Dezutter-Dambuyant C, Gaudillere A, Marechal S, Schmitt D, et al. Phenotypic and functional outcome of human monocytes or monocyte-derived dendritic cells in a dermal equivalent. J Invest Dermatol. 2001;116:933–939. doi: 10.1046/j.1523-1747.2001.01355.x. [DOI] [PubMed] [Google Scholar]
- 10.Mollah ZU, Aiba S, Manome H, Yoshino Y, Tagami H. Cord blood CD34+ cells differentiate into dermal dendritic cells in co-culture with cutaneous fibroblasts or stromal cells. J Invest Dermatol. 2002;118:450–460. doi: 10.1046/j.0022-202x.2001.01692.x. [DOI] [PubMed] [Google Scholar]
- 11.Kitamura H, Cambier S, Somanath S, Barker T, Minagawa S, et al. Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin αvβ8-mediated activation of TGF-β. J Clin Invest. 2011;121:2863–75. doi: 10.1172/JCI45589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, et al. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol. 2007;179:1595–1604. doi: 10.4049/jimmunol.179.3.1595. [DOI] [PubMed] [Google Scholar]
- 13.Saalbach A, Klein C, Sleeman J, Sack U, Kauer F, et al. Dermal fibroblasts induce maturation of dendritic cells. J Immunol. 2007;178:4966–4974. doi: 10.4049/jimmunol.178.8.4966. [DOI] [PubMed] [Google Scholar]
- 14.Saalbach A, Klein C, Schirmer C, Briest W, Anderegg U, et al. Dermal fibroblasts promote the migration of dendritic cells. J Invest Dermatol. 2010;130:444–454. doi: 10.1038/jid.2009.253. [DOI] [PubMed] [Google Scholar]
- 15.van Helden SF, van Leeuwen FN, Figdor CG. Human and murine model cell lines for dendritic cell biology evaluated. Immunol Lett. 2008;117:191–197. doi: 10.1016/j.imlet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Marchand-Adam S, Fabre A, Mailleux A, Marchal J, Quesnel C, et al. Defect of pro-hepatocyte growth factor activation by fibroblasts in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:58–66. doi: 10.1164/rccm.200507-1074OC. [DOI] [PubMed] [Google Scholar]
- 17.Larsson K, Lindstedt M, Borrebaeck CA. Functional and transcriptional profiling of MUTZ-3, a myeloid cell line acting as a model for dendritic cells. Immunology. 2006;117:156–166. doi: 10.1111/j.1365-2567.2005.02274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 19.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 20.Masterson AJ, Sombroek CC, De Gruijl TD, Graus YM, van der Vliet HJ, et al. MUTZ-3, a human cell line model for the cytokine-induced differentiation of dendritic cells from CD34+ precursors. Blood. 2002;100:701–703.21. doi: 10.1182/blood.V100.2.701. [DOI] [PubMed] [Google Scholar]
- 21.Santegoets SJ, Schreurs MW, Masterson AJ, Liu YP, Goletz S, et al. In vitro priming of tumor-specific cytotoxic T lymphocytes using allogeneic dendritic cells derived from the human MUTZ-3 cell line. Cancer Immunol Immunother. 2006;2006(55):1480–1490. doi: 10.1007/s00262-006-0142-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundberg K, Albrekt AS, Nelissen I, Santegoets S, de Gruijl TD, et al. Transcriptional profiling of human dendritic cell populations and models--unique profiles of in vitro dendritic cells and implications on functionality and applicability. PLoS One. 2013;8(1):e52875. doi: 10.1371/journal.pone.0052875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Q, Guo Z, Xu X, Xia S, Cao X. Pulmonary stromal cells induce the generation of regulatory DC attenuating T-cell-mediated lung inflammation. Eur J Immunol. 2008;38:2751–2761. doi: 10.1002/eji.200838542. [DOI] [PubMed] [Google Scholar]
- 24.Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566–2573. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]


