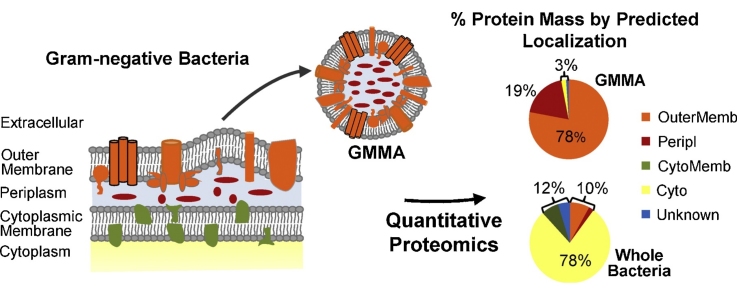
Graphical abstract
Abbreviations: −p, absence of virulence plasmid (either pSS or pINV); +p, presence of virulence plasmid (either pSS or pINV); Cyto, cytoplasm; CytoM, cytoplasmic membrane; DOMV, detergent-derived outer membrane vesicles; E. coli, Escherichia coli; GMMA, Generalized Modules for Membrane Antigens; NOMV, native outer membrane vesicles; OM, outer membrane; OM lipo, outer membrane lipoprotein; OMV, outer membrane vesicles; OAg, O antigen; PP, periplasm; SD, standard deviation; Sf2a, hyperblebbing plasmid-cured OAg-deficient Shigella flexneri 2a 2457T; SHIFL, Shigella flexneri protein database, mixed serotypes; SHIF8, Shigella flexneri serotype 5b protein database; SHISO, Shigella sonnei 53G protein database; SHISS, Shigella sonnei 046 protein database; SOMV, spontaneous outer membrane vesicles; Ss, hyperblebbing plasmid-cured OAg-deficient Shigella sonnei 53G; vs, versus
Keywords: Shigella sonnei, Shigella flexneri 2a, Proteomics, GMMA, Vaccine, iBAQ
Abstract
Outer membrane blebs are naturally shed by Gram-negative bacteria and are candidates of interest for vaccines development. Genetic modification of bacteria to induce hyperblebbing greatly increases the yield of blebs, called Generalized Modules for Membrane Antigens (GMMA). The composition of the GMMA from hyperblebbing mutants of Shigella flexneri 2a and Shigella sonnei were quantitatively analyzed using high-sensitivity mass spectrometry with the label-free iBAQ procedure and compared to the composition of the solubilized cells of the GMMA-producing strains. There were 2306 proteins identified, 659 in GMMA and 2239 in bacteria, of which 290 (GMMA) and 1696 (bacteria) were common to both S. flexneri 2a and S. sonnei. Predicted outer membrane and periplasmic proteins constituted 95.7% and 98.7% of the protein mass of S. flexneri 2a and S. sonnei GMMA, respectively. Among the remaining proteins, small quantities of ribosomal proteins collectively accounted for more than half of the predicted cytoplasmic protein impurities in the GMMA. In GMMA, the outer membrane and periplasmic proteins were enriched 13.3-fold (S. flexneri 2a) and 8.3-fold (S. sonnei) compared to their abundance in the parent bacteria. Both periplasmic and outer membrane proteins were enriched similarly, suggesting that GMMA have a similar surface to volume ratio as the surface to periplasmic volume ratio in these mutant bacteria. Results in S. flexneri 2a and S. sonnei showed high reproducibility indicating a robust GMMA-producing process and the low contamination by cytoplasmic proteins support the use of GMMA for vaccines. Data are available via ProteomeXchange with identifier PXD002517.
1. Introduction
Shigellae are Gram-negative enterobacteria classified into fifty different serotypes based on the carbohydrate composition of the outer polysaccharide antigen (O antigen, OAg) of the lipopolysaccharide (LPS) (Levine et al., 2007). The worldwide dominant serotypes are S. sonnei and S. flexneri 2a (Levine et al., 2007, Chang et al., 2012, Livio et al., 2014). They are the etiological agent of shigellosis, one of the most frequent causes of medium and severe diarrhea in developing countries, especially in children under five years of age (Kotloff et al., 2013). Currently, no Shigella vaccine is widely available.
Gram-negative bacteria naturally shed outer membranes in a process of blebbing. These particles are known as native outer membrane vesicles (NOMV), spontaneous outer membrane vesicles (SOMV), or sometimes just OMV. They are quite different from the outer membrane vesicles derived by detergent extraction of whole bacteria (also usually called OMV or sometimes DOMV) and used in meningitis vaccines (Holst et al., 2013), due to the depletion of lipoproteins and lipooligosaccharides by the detergent from DOMV (Ferrari et al., 2006, van de Waterbeemd et al., 2013). The composition of NOMV reflects the composition of the outer membrane of the donor bacteria, an important interface between the bacterial cell and its environment, and thus outer membrane blebs represent ideal candidates for vaccine development (Ellis and Kuehn, 2010). Blebs present these antigens in the natural membrane context and in the presence of powerful stimulators of the innate immune system (Ferrari et al., 2006, Park et al., 2011, Ellis et al., 2010) and elicit protection in animal models against bacterial infections (Alaniz et al., 2007, Schild et al., 2008, Park et al., 2011), including Shigella (Camacho et al., 2011, Mitra et al., 2013). Therefore NOMV have been proposed for use as vaccines (Ellis and Kuehn, 2010). However, NOMV are generally present at low concentrations in bacterial cultures. Using genetic modification, it is possible to induce high level shedding of blebs (“gemma” in Italian), called Generalized Modules for Membrane Antigens (GMMA) (Berlanda Scorza et al., 2012), that together with high-yield, industrial processes form the bases for a practical vaccine platform (Berlanda Scorza et al., 2012, Gerke et al., 2015).
Several studies have identified proteins in the proteome of outer membrane blebs, e.g. from Escherichia coli (Berlanda Scorza et al., 2008), Helicobacter pylori (Mullaney et al., 2009), Edwardsiella tarda (Park et al., 2011), Francisella novicida (Pierson et al., 2011), Neisseria meningitidis (van de Waterbeemd et al., 2013) and Haemophilus influenza (Roier et al., 2015). These studies, including our previous analysis of Shigella sonnei GMMA (Berlanda Scorza et al., 2012), conducted qualitative analyses of the proteome to document the proteins detectable. When the results were expressed by number of proteins identified, cytoplasmic proteins that are not predicted to be included in outer membrane blebs comprised a substantial proportion of the total (Berlanda Scorza et al., 2008, Berlanda Scorza et al., 2012, Choi et al., 2011). The actual proportion appears to depend on the sensitivity of the method (2D gel-based proteomics (Berlanda Scorza et al., 2008, Berlanda Scorza et al., 2012) versus LC–MS/MS (Choi et al., 2011)) and the sensitivity of the detection. The higher the sensitivity, the more low abundant cytoplasmic proteins are detected and thus the smaller the detected proportion of predicted periplasmic and outer membrane proteins.
Two studies used labeling techniques to quantitatively compare the ratio of the quantity of each of the detectible proteins in two or more related membrane samples from N. meningitidis. Chemical isotopic labeling using heavy (CD2O) or light (CH2O) formaldehyde has been used to measure the ratio of individual proteins in spontaneously released (SOMV), chelate induced (NOMV) and detergent extracted (DOMV) vesicles from an unencapsulated recombinant B group Neisseria meningitis carrying deletions of galE to truncate the LOS, lplX to give a penta-acyl Lipid A structure and rmpM to increase spontaneous OMV production (van de Waterbeemd et al., 2013). Metabolic labeling with 15N was used in another study of unencapsulated N. meningitidis to quantitatively measure the relative abundance of proteins in SOMV with outer membrane preparations (Lappann et al., 2013). Neither of these methods provides quantitative information on the abundance of each protein present within a single sample. For developing outer membrane vaccines, we need to know the relative mass of each protein present in a single vaccine preparation, especially for the outer membrane and periplasmic proteins.
One study, the proteomic analysis of detergent extracted and spontaneously released OMV of H. influenza (Roier et al., 2015), used a label free technique to rank proteins within each sample on the basis of their Mascot ion scores. While this a measure of the probability that the protein derived peptides are correctly identified it is not a direct measure of the relative abundance.
Two studies have used label free quantification of proteins present in OMV to estimate the relative amount of all the proteins detected:
-
•
The Lappman et al. study (Lappann et al., 2013) quoted above, in addition to using metabolic labeling to determine ratio in different preparations, also used a non-labeled technique, normalized spectral abundance factors (Zybailov et al., 2006) to estimate the relative amounts of each protein within each sample.
-
•
The abundance of proteins in spontaneously released OMV from Pseudomonas aeruginosa (Choi et al., 2011), was measured using the APEX tool (Lu et al., 2007).
As part of a program of developing a GMMA-based Shigella vaccine (Gerke et al., 2015), we used the iBAQ index (Schwanhäusser et al., 2011) for the non-labeled, quantitative proteomic analysis of GMMA from hyperblebbing S. sonnei 53G (Ss) and Shigella flexneri 2a 2457T (Sf2a) strains and show that both preparations contain low levels of cytoplasmic proteins, supporting the use of GMMA as vaccines for delivering outer membrane and periplasmic antigens.
2. Materials and methods
2.1. Shigella strains and bacterial growth condition
In this study we used previously described hyperblebbing (ΔtolR) O antigen-deficient strains of S. sonnei 53G and S. flexneri 2a 2457T that were cured of the virulence plasmid: S. sonnei −p ΔtolR (Berlanda Scorza et al., 2012) (in this study abbreviated as Ss) and S. flexneri 2a −p ΔtolR ΔrfbG (Rossi et al., 2014) (in this study abbreviated as Sf2a).
The defined medium for S. sonnei was described previously (Berlanda Scorza et al., 2012) and had the following composition: 30 g/L glycerol, 2.5 g/L aspartic acid, 13.3 g/L KH2PO4, 1.2 g/L (NH4)2HPO4 4 g/L, MgSO4*7H2O, 1.7 g/L citric acid, 2.5 mg/L CoCL2*6H2O, 15 mg/L MnCl2*4H2O, 1.5 mg/L CuCl2*2H2O, 3 mg/L H3BO3, 2.5 mg/L Na2MoO4*2H2O, 13 mg/L Zn(CH3COO)2*2H2O, 2 μM ferric citrate, 50 mg/L thiamine, 10 mg/L nicotinic acid. For Sf2a, 4 g/L of glucose was also added. Both strains were grown at 37 °C.
2.2. Preparation of GMMA and solubilized bacteria
To produce GMMA, the strains were grown in medium supplemented with antibiotics (30 μg/mL kanamycin for Ss; 30 μg/mL kanamycin and 100 μg/mL erythromycin for Sf2a) to OD600nm = 0.3. The cultures were then diluted into 1 L of fresh medium (without antibiotics) to a starting OD600nm of 0.05 and incubated at 37 °C. After growing to OD600nm of 0.4, in case of Ss, and of 0.3, in case of Sf2a, cultures were centrifuged for 15 min at 5000 × g and the supernatants removed for GMMA preparation.
The bacterial pellets were solubilized after two washes with cold phosphate buffered saline (PBS) by resuspending in lysis buffer (0.8 M urea, 0.4% SDS, 1 mM DTT and 20 mM Tris, pH 7.4) to prepare lysates.
The culture supernatants were filtered through a 0.22 μm filter unit (Millipore). The filtered samples were concentrated in a Stirred Cell Model 8400 (Millipore) through a 100,000 Da regenerated cellulose membrane (Millipore). GMMA were then separated from soluble proteins by ultracentrifugation at 186,000 × g for 2 h at 4 °C (Optima™ L-series, 45Ti rotor, Beckman Instruments). The GMMA pellets were washed once with cold PBS, resuspended in 20 mM Tris, pH 7.4, then filtered through a 0.22 μm filter.
2.3. Total protein quantification
The protein concentration in GMMA and solubilized bacteria was determined using Pierce Microplate BCA Protein Assay Kit-Reducing Agent Compatible (Thermo Scientific). Prior to the determination GMMA were diluted into the buffer used to prepare the solubilized bacteria.
2.4. SDS-PAGE
GMMA (13 μg) and cell lysates (26 μg) were mixed with NuPAGE SDS sample buffer (Invitrogen) containing 5 mM Tris(2-carboxyethyl)phosphine hydrochloride solution (TCEP, Sigma), boiled for 10 min, and iodoacetamide (IAA, Sigma) was then added to a final concentration of 10 mM. Following 30 min incubation in the dark at room temperature, the samples were loaded into NuPAGER 12% Bis-Tris gels using SeeBlue Plus 2 Pre-Stained Standard (Invitrogen) for molecular weight calibration and MOPS as running buffer (Invitrogen). Following electrophoresis, the gel was then fixed with 40% methanol (MeOH), 2% acetic acid for 30 min stained overnight with Brilliant Blue G-colloidal solution (Sigma), and destained with 30% MeOH. Gels were scanned with an Epson Expression 1640XL scanner.
2.5. In-gel protein digestion
Each lane of the gel was excised in 15–16 slices; bands and blank regions were excised separately. Each slice was then cut into small pieces and transferred into a 96-well pierced plate (Proxeon) stacked on a normal plate. The gel pieces were destained with 50% 50 mM triethylammonium bicarbonate buffer (TEAB) pH 8.0, 50% acetonitrile for 30 min at 37 °C with shaking. The liquid was removed by centrifugation of the Proxeon plate. The destaining step was repeated until the gel pieces were completely white. The samples were incubated with acetonitrile, the liquid was removed, and the plate was air-dried. The gel pieces were digested with 0.15 μg/well of trypsin. Prior to digestion 50 fmol/well of enolase were added as internal standard for the label-free quantification. After 2 h of digestion at 37 °C, MeOH was added to give a final concentration of 33% and another 0.15 μg/well of trypsin was added to each well and the digestion was performed for additional 3 h at 37 °C, followed by 25 °C overnight. Peptides were extracted at 37 °C using one change of 50% acetonitrile (20 min), 2 changes of 50% acetonitrile/0.25% formic acid (30 min each), and one change of pure acetonitrile (30 min), dried, and stored frozen at −20 °C until analysis.
2.6. LC/MS–MS analysis
The samples were analyzed with on-line nano LC–MS/MS on an Ultimate 3000 RSLCnano System (Dionex) coupled to a LTQ Orbitrap Velos (Thermo Fisher) hybrid mass spectrometer equipped with a nanospray source. Peptides were separated on a 75 μm id × 50 cm PepMap RSLC column (2 μm, Dionex) over a 120 min linear gradient of 4–32% CH3CN/0.1% FA. The LTQ Orbitrap Velos mass spectrometer was operated in the “top 10” data-dependent acquisition mode. The 10 most abundant multiply-charged precursor ions in the MS survey scan in the Orbitrap (m/z 400–1500, with the lock mass at 445.120025), with a minimal signal above 2000 counts, were dynamically selected for Collision induced Dissociation (MS/MS) in the LTQ Velos ion trap. The preview mode of FT master scan was disabled. The Orbitrap resolution was set to 60,000 at m/z 400. The dynamic exclusion was set to a mass width of ±20 ppm and a duration of 45 s. To achieve high mass accuracy, the AGC (Automatic Gain Control) was set to 1 × 106 for the full MS survey in the Orbitrap, and to 5000 for the MS/MS in the LTQ Velos, both with a maximum injection time of 200 ms.
The raw data were analyzed by MaxQuant software, version 1.2.2.5 for both protein identification and protein quantification. The Andromeda search engine was used to search the MS/MS spectra using the following parameters: trypsin/P with maximum 3 missed cleavage sites; peptide mass tolerance at first search was set at 20 ppm; MS/MS fragment mass tolerance at 0.50 Da, top 6 MS/MS peaks per 100 Da, and a minimum peptide length of 6 amino acids. Fixed modification for carbamidomethylation of cysteines and variable modifications for acetylation of protein N-termini, deamidation of asparagines and glutamines and oxidation of methionines were used. Identified peptides were searched against the available protein sequences from S. flexneri (SHIFL and SHIF8 protein databases) and S. sonnei (SHISS and SHISO databases) downloaded from UniProt (www.uniprot.org). One identified and quantified peptide was considered sufficient to call a protein present based on the fact that several predicted small proteins contained only 1 observable peptide. However most (2181 of 2306 proteins) were identified on the basis of multiple peptides (Table S1). False discovery rates were estimated based on the target-decoy approach (Balgley et al., 2007) using matches to reversed sequences in a concatenated database. The false discovery rate was set to 1% for proteins and peptides. Protein groups with a posterior error probability value over 0.01 or groups containing entries from the decoy database or contaminants were discarded. Since multiple databases were used, the software occasionally assigned homologs into different protein groups, e.g. where one detected peptide spanned a polymorphic region. To group genuine homologs and identify the protein group duplications, peptide IDs for each protein were matched with the peptide ID for all other proteins. The protein sequences of the members of the matching protein groups were then aligned to ensure that these were indeed genuine homologs and all members combined in a single protein group.
2.7. iBAQ individual protein quantification
The quantity of each protein was assessed as the Intensity-Based Absolute Quantification Index (iBAQ) (Schwanhäusser et al., 2011). The iBAQ of a protein/protein group is the sum of peak intensities of all peptides divided by the number of theoretically observable peptides. iBAQ values are approximately proportionate to the number of moles of protein present and thus iBAQi/ΣiBAQj is the relative molar amount of protein i. In this paper we present results as the relative mass of protein present (mi), calculated for each protein i as: mi = iBAQi·Mi/Σ(iBAQj·Mj), where Mi is the theoretical molecular weight of the protein i (Ishihama et al., 2005, Schwanhäusser et al., 2011).
2.8. Bioinformatics
The sequences of the identified proteins were analyzed by different bioinformatics tools. The prediction of the sub-cellular protein localization was carried out using LIPO (Berven et al., 2006) to look for inner and outer membrane lipoproteins. Proteins not identified as lipoproteins or scored as low probability lipoproteins were analyzed with PSORTb v3.0 (Yu et al., 2010). Finally, proteins still not assigned a definitive location were analyzed by SignalP 4.1 (Petersen et al., 2011) using a combination of artificial neural networks trained on proteins with signal peptides from Gram-negative bacteria. Proteins found to have a signal peptide were assumed to be periplasmic. 21 cases were manually checked, where peptides identified by mass spectrometry matched pairs of orthologs in the databases that had different predicted locations. For several of these, the different databases had assumed a different gene structure with different start codons. Where this occurred both members of the pair were assigned the same location based on the following: if one of the pair had a well-defined lipobox or signal peptide both members of the pair were assigned to a lipoprotein or periplasmic location, respectively. For proteins still unresolved, the pair was assigned the location of the member with the highest PSORTb prediction score.
For proteins that were identified only in S. sonnei 53G or only in S. flexneri 2a 2457T GMMA or Lysates the theoretical presence in the predicted proteome of the other strain was first assessed using protein BLAST (National library of Medicine, http://blast.st-va.ncbi.nlm.nih.gov/Blast.cgi). If no homologous protein was identified in the strain of interest, nucleotide BLAST of the corresponding gene was used. In silico translation (ExPASy Biofinformatics Resources Portal, http://web.expasy.org/translate/) was used to assess if a nucleotide BLAST hit is likely to be a functional protein coding gene (e.g. absence of frame shifts). For positive hits, a cut-off of 90% coverage and 95% identity of the protein sequence were used to call the protein theoretically present. The cut-off was based on the observed variation of proteins within selected protein groups.
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (Vizcaino et al., 2014) via the PRIDE partner repository with the dataset identifier PXD002517.
3. Results
3.1. Composition of predicted outer membrane and periplasmic proteins in GMMA
Analysis of solubilized whole cells and GMMA from the O antigen- and plasmid-negative S. flexneri 2a 2457T −p ΔtolR ΔrfbG (Sf2a Lysate and Sf2a GMMA respectively), and solubilized whole cell and GMMA from the O antigen and plasmid-negative S. sonnei 53G −p ΔtolR (Ss Lysate and Ss GMMA respectively) identified 2306 unique protein groups (in the following referred to as proteins) with an estimated abundance based on iBAQ quantification (Table S1, listed by the protein with the highest abundance in the protein group). Of these proteins, 2239 were found in at least one of the lysates, 659 of the proteins were identified in at least one of the GMMA samples. The protein abundance in GMMA closely followed the subcellular prediction made by a combination of LIPO (Berven et al., 2006), PSORTb (Yu et al., 2010), and SignalP (Petersen et al., 2011) with 95.7% and 98.7% of the protein mass found in the Sf2a and Ss GMMA, respectively, coming from predicted outer membrane (including outer membrane lipoproteins, OM lipo) or periplasmic proteins (Table 1). Of the 659 proteins found in GMMA, the 40 most abundant proteins account for 87.3% and 82.8% of the protein mass in Sf2a and Ss GMMA (Table 2). Compared to their abundance in the corresponding lysates, most of the outer membrane and periplasmic proteins are approximately 10-fold enriched in GMMA as a proportion of total protein (Fig. 1A and B). In accordance with the enrichment in GMMA, they comprise only 8.3% of the protein mass in Sf2a Lysates and 11.4% in Ss Lysates, respectively.
Table 1.
Predicted location of proteins found in Sf2a and Ss GMMA and Lysates.
| Predicted location | GMMA |
Lysates |
||||||
|---|---|---|---|---|---|---|---|---|
| % mass |
Number of proteins |
% mass |
Number of proteins |
|||||
| Sf2a | Ss | Sf2a | Ss | Sf2a | Ss | Sf2a | Ss | |
| Outer membrane | ||||||||
| Outer membrane integral proteins (OM) | 47.85 | 48.12 | 33 | 39 | 3.37 | 5.04 | 22 | 33 |
| Outer membrane lipoprotein (OM lipo) | 28.00 | 32.34 | 66 | 75 | 2.72 | 4.05 | 52 | 59 |
| Periplasmic | 19.86 | 18.25 | 105 | 133 | 2.2 | 2.28 | 90 | 127 |
| Total predicted outer membrane/periplasmic | 95.70 | 98.71 | 204 | 247 | 8.29 | 11.37 | 164 | 219 |
| Cytoplasmic | ||||||||
| Ribosomal | 0.86 | 0.28 | 43 | 35 | 15.03 | 15.08 | 53 | 53 |
| Other cytoplasmic | 0.63 | 0.20 | 177 | 93 | 65.08 | 61.30 | 1137 | 1198 |
| Cytoplasmic membrane | ||||||||
| Tail-specific protease | 1.15 | 0.21 | 1 | 1 | 0.03 | 0.02 | 1 | 1 |
| Other cytoplasmic membrane | 0.24 | 0.13 | 53 | 29 | 7.00 | 6.44 | 291 | 346 |
| Extracellular | 0.00 | – | 1 | 0 | 0.02 | 0.01 | 5 | 4 |
| Unknown | ||||||||
| YajG | 1.28 | 0.34 | 1 | 1 | 0.04 | 0.05 | 1 | 1 |
| Other unknown | 0.13 | 0.13 | 35 | 28 | 4.51 | 5.72 | 216 | 245 |
| Total predicted non-OM/periplasmic | 4.30 | 1.29 | 311 | 187 | 91.71 | 88.63 | 1704 | 1848 |
Table 2.
40 most abundant proteins found in GMMA (by average % mass in Sf2a and Ss GMMA).
| Gene name | Location | Protein name | Uniprot name | Average % mass in GMMA | % mass in Sf2a GMMA | % mass in Ss GMMA |
|---|---|---|---|---|---|---|
| SF2457T_0298 | OM | Outer membrane protein C | E3XX60_SHIFL | 13.411 | 14.619 | 12.203 |
| SF2457T_0799 | OM | Outer membrane protein A | E3XYK2_SHIFL | 11.943 | 10.615 | 13.271 |
| SS53G_3941 | OM | Outer membrane protein X | E9UMG3_SHISO | 8.506 | 11.829 | 5.183 |
| SS53G_0415 | OM lipo | Repeated sequence found in lipoLPP family prot. | E7JU63_SHISO | 7.261 | 9.955 | 4.566 |
| SF2457T_4977 | PP | Protease Do (HtrA) | E3YA49_SHIFL | 4.604 | 7.366 | 1.842 |
| SS53G_2965 | OM lipo | Entericidin B | E7K193_SHISO | 4.257 | 0.101 | 8.413 |
| SS53G_0370 | OM lipo | Outer membrane lipoprotein pcp | E7JU20_SHISO | 3.528 | 4.570 | 2.485 |
| tolB | PP | Protein tolB | TOLB_SHISS | 2.859 | 2.637 | 3.080 |
| SS53G_0760 | OM | Outer membrane porin protein LC | E7JV54_SHISO | 2.419 | 0.479 | 4.358 |
| SF2457T_3748 | OM | Outer membrane protein | E3Y6Q5_SHIFL | 2.145 | 2.654 | 1.636 |
| yaeT | OM | Outer membrane protein assembly factor yaeT | YAET_SHISS | 1.646 | 2.258 | 1.035 |
| lpoA | OM lipo | Penicillin-binding protein activator LpoA | LPOA_SHIF8 | 1.595 | 1.679 | 1.510 |
| SF2457T_0832 | OM | Outer membrane protein F | E3XYN5_SHIFL | 1.471 | 1.036 | 1.905 |
| SGF_01778 | OM lipo | Glycoprotein-polysaccharide metabolism | E7TB57_SHIFL | 1.465 | 0.280 | 2.650 |
| SS53G_1454 | OM lipo | Osmotically inducible lipoprotein B | E7JX36_SHISO | 1.256 | 1.692 | 0.820 |
| lamB | OM | Maltoporin | LAMB_SHISS | 1.156 | 0 | 2.312 |
| slp | OM lipo | OM protein induced after carbon starvation | Q3YWI0_SHISS | 1.151 | 0.014 | 2.288 |
| SGF_00427 | OM | Aerobactin siderophore receptor IutA | E7T7F9_SHIFL | 0.977 | 1.486 | 0.468 |
| SF2457T_4357 | PP | Peptidyl-prolyl cis-trans isomerase | E3Y8E7_SHIFL | 0.932 | 0.968 | 0.896 |
| pal | OM lipo | Peptidoglycan-associated lipoprotein | E7K8X6_SHISO | 0.873 | 0.930 | 0.816 |
| SS53G_1748 | PP | Putative uncharacterized protein | E7JXX8_SHISO | 0.869 | 1.080 | 0.658 |
| SS53G_0544 | OM lipo | SmpA/OmlA family protein | E7JUJ2_SHISO | 0.829 | 0.170 | 1.489 |
| yajG | Unknown | Putative polymerase/proteinase | Q3Z4W9_SHISS | 0.808 | 1.276 | 0.340 |
| SF2457T_2094 | PP | Periplasmic oligopeptide-binding protein | E3Y260_SHIFL | 0.769 | 1.065 | 0.473 |
| SF2457T_2279 | CytoM | Tail-specific protease | E3Y2N8_SHIFL | 0.679 | 1.152 | 0.206 |
| surA | PP | Chaperone surA | SURA_SHISS | 0.623 | 0.787 | 0.458 |
| SF2457T_0435 | OM | Long-chain fatty acid outer membrane transporter | E3XXF4_SHIFL | 0.622 | 0.062 | 1.181 |
| SS53G_1935 | OM lipo | SmpA/OmlA family protein | E7JYG3_SHISO | 0.611 | 0.858 | 0.364 |
| degQ | PP | Serine endoprotease | Q3YX14_SHISS | 0.592 | 0.053 | 1.132 |
| SS53G_3947 | PP | Putative uncharacterized protein | E9UMG9_SHISO | 0.540 | 0.816 | 0.265 |
| SF2457T_3200 | OM lipo | Pectinesterase B | E3Y553_SHIFL | 0.532 | 0.651 | 0.414 |
| SF2457T_3582 | OM lipo | Peptidase family M48 family protein | E3Y5Y2_SHIFL | 0.530 | 0.555 | 0.504 |
| livJ | PP | High-affinity amino acid transport system | Q3YW68_SHISS | 0.521 | 0.264 | 0.777 |
| yeaF | OM | Putative uncharacterized protein yeaF | Q3Z2C1_SHISS | 0.507 | 0.479 | 0.535 |
| SS53G_4569 | OM lipo | Putative phospholipid-binding domain protein | E7K576_SHISO | 0.468 | 0.535 | 0.401 |
| SF2457T_4988 | OM | Ferrichrome-iron receptor | E3YA60_SHIFL | 0.435 | 0.816 | 0.053 |
| SF2457T_4129 | PP | PP dipeptide transport protein | E3Y7S0_SHIFL | 0.434 | 0.401 | 0.467 |
| ybjP | OM lipo | Putative enzyme | Q0T8Q3_SHIF8 | 0.414 | 0.429 | 0.400 |
| SF2457T_3629 | OM | Serine protease eatA | E3Y628_SHIFL | 0.410 | 0.115 | 0.705 |
| yfgL | OM-Lipo | Outer membrane assembly lipoprotein YfgL | E7JUN3_SHISO | 0.409 | 0.571 | 0.246 |
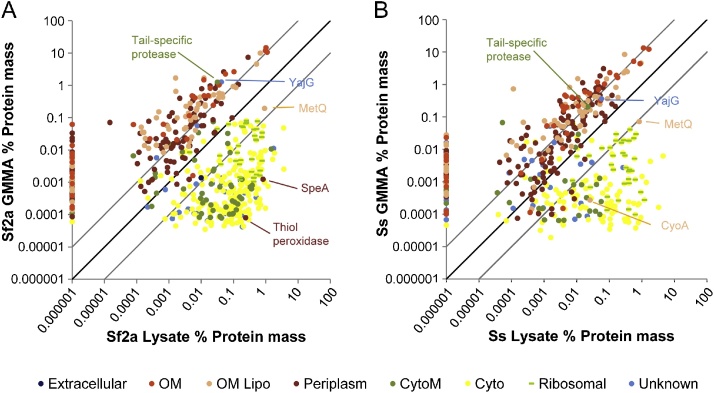
Fig. 1.
The relative abundance of proteins identified in GMMA compared to their abundance in whole cell lysates. (A) S. flexneri 2a and (B) S. sonnei. Proteins are plotted according to the percent of the estimated protein mass in the two samples and color coded according to the predicted location as follows: light orange, outer membrane lipoprotein; dark orange, integral outer membrane proteins; dark red, periplasmic protein; dark blue, extracellular; olive green, cytoplasmic membrane; yellow, cytoplasmic. Ribosomal proteins are distinguished from other cytoplasmic proteins by the green bar. Only proteins identified in GMMA are plotted. Proteins detected in GMMA but not in lysates were assigned 0.000001% mass and are plotted on the vertical axis. Proteins equally abundant in the GMMA and Lysate would be expected to lie on the dark diagonal line. The light diagonal lines above and below the identity line are guidance lines marking the theoretical positions of proteins enriched 10-fold in GMMA or 10-fold in the lysate, respectively. (For interpretation of the references to color in the figure legend, the reader is referred to the web version of this article.)
The non-outer membrane and non-periplasmic protein category was dominated by similar proteins for both Sf2a and Ss GMMA. Small quantities of ribosomal proteins collectively comprise more than half of the mass of the cytoplasmic proteins in GMMA (Table 1 and Table S2). In addition, Tail-specific protease (E3Y2N8_SHIFL) predicted to be located at the cytoplasmic membrane and YajG, a putative polymerase/proteinase (Q3Z4W9_SHISS) with no definitive subcellular localization, are the major contributors to the non-outer membrane/periplasmic components (Table 1).
There were a few proteins that show an anomalous distribution, expected to be GMMA components from the predicted locations but were preferentially found in the lysate (Fig. 1 and Table S1). Predicted outer membrane lipoproteins MetQ (d-methionine-binding lipoprotein MetQ, E7K3R2_SHISO) and CyoA (Ubiquinol oxidase, subunit II, E7K828_SHISO), were more abundant in lysates than in GMMA (MetQ: 0.91% and 0.82% in Sf2a and Ss Lysates vs 0.19% and 0.07% in Sf2a and Ss GMMA, respectively; CyoA: 0.0255% in Ss lysate vs 0.0003% in Ss GMMA). In contrast, outer membrane predicted thiol peroxidase (E3Y2F2_SHIFL) and SpeA (Biosynthetic arginine decarboxylase, Q3YXT3_SHISS) were only detected in Sf2a GMMA although they were prominent in both lysates. In addition, ten predicted periplasmic proteins were found in both the Sf2a and Ss Lysates, but not in either of the GMMA samples (Table S1).
Proteins of specific interest in GMMA were iron-regulated outer membrane proteins as they elicited protection in animal models against E. coli (Fox et al., 2009), Salmonella (Kaneshige et al., 2009), and Bordetella pertussis (Alvarez Hayes et al., 2013). Approximately 2.4% of the protein mass were comprised by iron-uptake proteins in both Ss and Sf2a GMMA. Aerobactin siderophore receptor IutA (E7T7F9_SHIFL), Ferrichrome-receptor FepA (E3YA60_SHIFL) and Ferrichrome-iron receptor FhuA (E7K3K6_SHISO) were present in both strains. CirA and iron (III) dicitrate transport protein FecA (E7K2N5_SHISO) were additionally detected in Ss GMMA (Table S1).
In order to measure the enrichment of proteins in the GMMA compared to the whole cell lysate, we did non-weighted linear regressions of the log-transformed percentages of each predicted outer membrane and periplasmic protein in GMMA versus lysate for proteins with a mass abundance of greater than 0.01%. This gave slopes of 0.83 ± 0.12 and 0.85 ± 0.09 for the Sf2a and Ss preparations, respectively. At the mid-points of the regression, there was a predicted 13.3-fold enrichment (95% confidence interval 9.1–19.5) in Sf2a GMMA and 8.3-fold (6.5–10.7) in Ss GMMA. Neither slope was significantly different from 1, consistent with a similar enrichment of all (across the abundance range) predicted periplasm and outer membrane proteins in GMMA (Fig. 1). There was no statistically significant difference in the enrichment of membrane proteins compared to periplasmic proteins in either the Sf2a or Ss preparations (p = 0.94 and 0.85 by t test, for Sf2a and Ss, respectively).
3.2. Comparative analysis of Sf2a and Ss GMMA and lysates
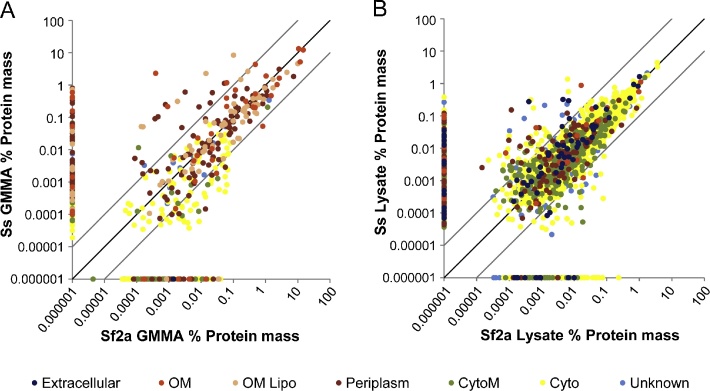
By comparing the 4 samples, we assessed the consistency of the samples. Of the 659 proteins identified in at least one of the GMMA samples, 290 (44%) were common to both and these accounted for 99.6% and 94.9%, of the protein mass in Sf2a GMMA and Ss GMMA, respectively. Similarly of the 2239 proteins identified in at least one of the lysates, 1696 were common (76%) and these accounted for 99.0% and 96.4% of the total protein mass in the Sf2a lysate and Ss lysate, respectively. Significantly, the abundance of the common proteins was highly correlated in each of the pairs of samples. Direct correlation plots (Fig. 2) show that the majority of proteins lie close to the identity line and the Pearson correlation coefficients were 0.80 and 0.91 for GMMA and lysates, respectively (p < 0.0001 for both).
Fig. 2.
The relative abundance of proteins identified in Ss GMMA compared to Sf2a GMMA (A) and in Ss Lysate compared to Sf2a Lysates (B). The color codes are as described in the legend for Fig. 1, except ribosomal proteins are not explicitly marked but are part of the cytoplasmic proteins. Proteins detected in GMMA or Lysates from only 1 strain were assigned 0.000001% mass in the other strain and plotted on the axes. The light diagonal lines above and below the identity line are guidance lines marking the theoretical positions of proteins enriched 10-fold in Ss or 10-fold in Sf2a samples, respectively. (For interpretation of the references to color in the figure legend, the reader is referred to the web version of this article.)
There were a small number of proteins that were relatively more abundant in the Ss GMMA than in the Sf2a GMMA, including some proteins of moderate abundance that were only seen in the Ss GMMA (Table 3). Approximately half of these proteins were confirmed to be absent in Sf2a by genome analysis (Table 3). In contrast, there were only 4 moderately abundant proteins with predicted outer membrane or periplasmic localization in Sf2a GMMA that were not found in Ss GMMA of which by genome analysis only 1 protein would be expected to be present in Sf2a (Table 4).
Table 3.
Proteins preferentially found in Ss GMMA.
| Gene name | Location | Protein name | Uniprot name | % mass in Ss GMMA | Ratio | Theoretically present in Sf2a* |
|---|---|---|---|---|---|---|
| Predicted outer membrane and periplasmic localized proteins with at least 0.01% mass in Ss GMMA not found in Sf2a GMMA | ||||||
| SS53G_2461 | PP | Maltose-binding periplasmic protein | E7JZV4_SHISO | 0.799 | −** | + |
| SS53G_0290 | OM lipo | TonB-dependent Receptor Plug domain protein | E7JTU0_SHISO | 0.732 | − | − |
| SS53G_3463 | OM | Iron(III) dicitrate transport protein fecA | E7K2N5_SHISO | 0.589 | − | − |
| cirA | OM | OM receptor for iron-regulated colicin I receptor | Q3Z051_SHISS | 0.430 | − | − |
| exc | OM lipo | Entry exclusion protein 2 | A4SH43_SHISS | 0.423 | − | − |
| SS53G_3761 | OM | Ferrichrome-iron receptor | E7K3K6_SHISO | 0.396 | − | − |
| SS53G_2464 | PP | Maltose operon periplasmic protein | E7JZV7_SHISO | 0.337 | − | + |
| ycdO | PP | UPF0409 protein ycdO | YCDO_SHISS | 0.276 | − | + |
| SS53G_0127 | OM | Putative tonB-dependent receptor yncD | E7JTC8_SHISO | 0.159 | − | − |
| hdeA | PP | Putative uncharacterized protein hdeA | Q3YWI4_SHISS | 0.140 | − | + |
| mdoG | PP | Glucans biosynthesis protein G | OPGG_SHISS | 0.106 | − | + |
| SS53G_2146 | PP | Putative uncharacterized protein | E7JZ10_SHISO | 0.064 | − | − |
| SS53G_0128 | PP | PQQ enzyme repeat family protein | E7JTC9_SHISO | 0.058 | − | − |
| SS53G_5268 | OM | TonB-dependent Receptor Plug domain protein | E7K7A5_SHISO | 0.053 | − | + |
| SS53G_2796 | OM lipo | Inner membrane lipoprotein yiaD | E7K0S5_SHISO | 0.051 | − | + |
| SSON_1625 | PP | Putative sulfatase | Q3Z1P2_SHISS | 0.047 | − | − |
| SS53G_0368 | OM lipo | Putative uncharacterized protein | E7JU18_SHISO | 0.036 | − | − |
| SS53G_4157 | PP | Putative uncharacterized protein | E7K461_SHISO | 0.032 | − | + |
| yehZ | PP | Putative transport system permease protein | Q3Z073_SHISS | 0.026 | − | + |
| SS53G_4973 | PP | Acid-resistance protein | E7K6I7_SHISO | 0.025 | − | + |
| SS53G_0106 | PP | Bacterial extracellular solute-binding family protein | E7JTA7_SHISO | 0.024 | − | − |
| SSON_1637 | PP | Putative hemin-binding lipoprotein | Q3Z1N0_SHISS | 0.022 | − | − |
| tauA | PP | Taurine transport system periplasmic protein | Q3Z543_SHISS | 0.019 | − | − |
| SS53G_3203 | OM lipo | Putative uncharacterized protein | E7K1X5_SHISO | 0.015 | − | + |
| Predicted outer membrane and periplasmic localized proteins highly enriched in Ss GMMA compared to Sf2a GMMA*** | ||||||
| lamB | OM | Maltoporin | LAMB_SHISS | 2.312 | 6017 | |
| slp | OM lipo | Outer membrane protein induced after carbon starvation | Q3YWI0_SHISS | 2.288 | 163 | |
| glpQ | PP | Glycerophosphodiester phosphodiesterase, periplasmic | Q3YZW7_SHISS | 0.609 | 193 | |
| SS53G_4155 | OM lipo | Putative polysaccharide export protein gfcE | E7K459_SHISO | 0.313 | 170 | |
| SS53G_3546 | PP | Putative phospholipid-binding domain protein | E7K2W6_SHISO | 0.249 | 321 | |
| SS53G_4156 | OM | Putative uncharacterized protein | E7K460_SHISO | 0.216 | 239 | |
| SS53G_5756 | PP | Glutamate/aspartate periplasmic-binding protein | E7K8P1_SHISO | 0.105 | 230 | |
| ycdB | PP | Peroxidase ycdB (Deferrochelatase/peroxidase EfeB) | YCDB_SHISS | 0.091 | 246 | |
| ymcC | OM lipo | Putative regulator | Q3Z3D5_SHISS | 0.052 | 376 | |
| blc | OM lipo | Outer membrane lipoprotein | Q3YUI9_SHISS | 0.051 | 329 | |
Present if protein sequence with ≥90% coverage and ≥95% identity was identified in Sf2a proteome or translated genome.
Protein not detected in Sf2a GMMA.
Proteins >0.01% total protein mass and with at least 100× higher % mass in Ss GMMA than Sf2a GMMA.
Table 4.
Proteins preferentially found in Sf2a GMMA.
| Gene name | Location | Protein name | Uniprot name | % mass in Sf2a GMMA | Theoretically present in Ss* |
|---|---|---|---|---|---|
| Predicted outer membrane and periplasmic localized proteins with at least 0.01% mass in Sf2a GMMA not found in Ss GMMA | |||||
| SF2457T_1008 | OM lipo | Uncharacterized lipoprotein ydcL | E3XZ64_SHIFL | 0.035 | − |
| SF2457T_0155 | OM lipo | Lipoprotein | E3XWR9_SHIFL | 0.035 | − |
| sitA | PP | Iron transport protein | Q3Z1C5_SHISS | 0.024 | + |
| SF2457T_3658 | OM | Antigen 43 | E3Y656_SHIFL | 0.014 | − |
Present if protein sequence with ≥90% coverage and ≥95% identity was identified in Ss proteome or translated genome.
For proteins detected both in Sf2a and Ss we assessed the reproducibility of protein abundance in the Sf2a and Ss Lysate based on the log ratio of iBAQ. Considering the common 1696 proteins, the distribution of the log ratios closely followed a normal distribution with a best fit (minimized sum of errors squared) mean of 0.0054 with SD of 0.42 (Fig. S1A). Thus, for the 1696 proteins the average correlation of the % mass was 1.01 (antilog of 0.0054). The impact of the magnitude of the iBAQ on the mean and SD of the log ratios was tested by dividing the protein population into 10 groups of increasing iBAQ. No variation in the log ratio or SD was seen in groups with geometric mean iBAQ ranging from 10,000 to 10,000,000 (Fig. S1B). This is consistent with the individual abundance plots in Fig. 2B and previous studies using iBAQ (Schwanhäusser et al., 2011). There was a small deviation with approximately 4.5% of Ss proteins with an average of 10× greater abundance that the corresponding Sf2a proteins (Fig. S1A and Table S1), similar to the findings in GMMA (Table 3).
We investigated the technical reproducibility by measuring the log ratio of iBAQ and corresponding SD for pairs of ribosomal proteins in the two lysate samples. We assume that all ribosomes have stoichiometric amounts of each protein and also that there is negligible amounts of ribosomal protein not in ribosomes. Thus variation in the ratio of the iBAQ for the 53 pairs of ribosomal proteins in the two lysates is a measure of the technical reproducibility of the methods (Table S2). The ratio of molar abundance of ribosomal proteins in the 2 samples had on average, a ratio of 1.11. Thus the ratio of ribosomal proteins in the two samples was similar to the ratios of all proteins (1.11 vs 1.01), but the standard deviation of the log ratio was lower for the ribosomal proteins compared to all proteins (0.22 vs 0.42).
The accuracy of using iBAQ to estimate the molar concentration of proteins was assessed by comparing the iBAQ values of the individual ribosomal proteins within each lysate (Table S2), again assuming a negligible amount of free ribosomal proteins, as ribosomal proteins should be present in the same molar quantity regardless of the size variation. Similar to other studies (Arike et al., 2012), there was appreciable variation in the molar estimates with the 95% confidence interval calculated from the log transformed iBAQ covering a 10.7- and 8.5-fold range for the Sf2a and Ss lysates, respectively.
Since we were able to show that most proteins are present in approximately equal relative quantities in the Ss and Sf2a Lysates, we used the frequency at which the orthologs were detected in both the Ss and Sf2a Lysates to estimate detection limit of this assay. First, for each protein that was detected only in either the Ss or the Sf2a Lysates, we checked if the gene was present in both genomes and was likely to be functional (i.e. absence of frame shift mutations). This resulted in 2017 proteins where at least one was detected and both Ss and Sf2a potentially had functional genes. We ranked the proteins in order of increasing individual iBAQ or average iBAQ (where both where present), and then divided the set into 40 groups of increasing iBAQ. We counted the percentage of cases where proteins were detected in both samples (conditional on detecting at least one) and plotted this against the mean iBAQ of the group (Fig. S2). We assumed that the probability (p) of detecting a protein in a single sample was a simple hyperbolic function of iBAQ (p = iBAQ/(iBAQ + iBAQ0.5), where iBAQ0.5 is the iBAQ with a 50% probability of detection. We then did an unweighted fit of the percentage of cases where proteins were detected in both samples (% two proteins detected = 100p2/(2(1 − p)p + p2) to iBAQ, minimizing the sum of error squared. This gave a good fit (r2 = 0.754) with an iBAQ0.5 of 6134.
4. Discussion
In previous studies that identified proteins in outer membrane blebs (SOMV, NOMV and GMMA), proteins with predicted outer membrane or periplasmic localization accounted for half or fewer of all identified proteins (Berlanda Scorza et al., 2008, Berlanda Scorza et al., 2012, Choi et al., 2011, Lappann et al., 2013, Mullaney et al., 2009, Park et al., 2011, Pierson et al., 2011, Roier et al., 2015, van de Waterbeemd et al., 2013, Zybailov et al., 2006). In the study presented here, we also found less than half of all proteins to have predicted outer membrane or periplasmic localization, with 265 of 659 proteins identified in either Sf2a or Ss GMMA. Analyzing the protein content quantitatively gives a different view. As measured by iBAQ, nearly all of the protein content in the GMMA is predicted to be derived from outer membrane or periplasmic localized proteins: in this study 95.7% and 98.7% of the protein content in Sf2a and Ss GMMA, respectively.
The most abundant outer membrane proteins in the GMMA were identified as OmpA, OmpC, and OmpX consistent with previous observations with Ss GMMA (Berlanda Scorza et al., 2012) and with the preparation of GMMA from cultures in the early exponential phase (Zhu et al., 2007). Surprisingly, Entericidin B and not Lpp (Braun, 1975), the most abundant lipoprotein in E. coli, was the most abundant lipoprotein in Ss GMMA (8.4%) while it comprised only 0.1% mass in Sf2a GMMA. This difference could be related to the difference in the media composition.
A total of 60 samples (15 gel slices of each of the 4 preparations, Fig. S3) were used in this study and the large number of samples made technical or biological replicates problematic. Therefore, the estimates of the reliability of the study relies on the consistency between the Sf2a and Ss GMMA samples, between the Sf2a and Ss Lysate samples and between the corresponding GMMA and Lysate samples. Several measures of the reproducibility and precision were included in the analysis.
Estimates of the relative mass of the proteins depends on the assumption that iBAQ is a useful measure of the relative molar quantities of the proteins present. Analysis of the iBAQ values of ribosomal proteins within a sample provides an estimate of the accuracy in this study: the calculated molar ratios varied with a 95% confidence interval of approximately 9.6-fold (Table S2) in line with other studies (Arike et al., 2012, Maier et al., 2011, Ishihama et al., 2008). Although the uncertainty of individual protein abundances is relatively large, when averaged over large numbers of proteins the error in the estimate, e.g. of cytoplasmic contamination in GMMA, will be small. Comparison of the ribosomal data between the Sf2a and Ss samples showed that the reproducibility between samples is good. The variance of the log ratios of the ribosomal proteins was 0.049 (i.e. 95% confidence interval of 2.7-fold) but the variance of the 1696 common proteins in the lysate was 0.17. This suggests that approximately a quarter of the variation between the samples from Sf2a and Ss results from variation within the method, e.g. sample preparation or the mass spectrometry, whereas three quarters of the variance is a true difference between the Sf2a and Ss Lysates, reflecting differential abundance of specific proteins.
There are two groups of proteins where the reproducibility between samples was much less than expected: 4.5% of proteins were approximately 10× overexpressed in Ss compared to Sf2a. In addition, 11 of the 191 protein present in the Ss GMMA at an abundance of >0.01% were not detected in Sf2a despite the corresponding gene being present in Sf2a and 10 of the 191 proteins were >100 times more abundant than in Sf2a GMMA. In contrast, only 1 protein present in Sf2a GMMA at >0.01% of total protein was not detected in Ss GMMA though the gene is present (Table 3, Table 4). The iBAQ score in the Ss or Sf2a GMMA for the proteins absent in GMMA from the other strain ranged from 326,930 to 10,197,000. If the detection limit for the GMMA samples was similar to that assessed on the Lysate, the corresponding probability of detecting these proteins ranged from 0.965 to 0.999. Therefore, it is unlikely that these were present at a similar abundance and missed by chance. Of the proteins that were highly enriched in Ss GMMA compared to Sf2a GMMA, 3 are related to maltose metabolism: maltose-binding periplasmic protein (E7JZV4_SHISO), a homologue of MalE (Chapon, 1982), maltose operon periplasmic protein (E7JZV7_SHISO), a homologue of MalM (Gilson et al., 1986), and maltoporin LamB (LAMB_SHISS). The corresponding genes are part of the malT operon and subject to catabolite repression (Chapon, 1982) and thus the addition of glucose to the growth medium for Sf2a but not for Ss is a possible reason for the low or absent expression of these three genes. For the other proteins, the reason for the relative abundance in Ss is unclear.
Since the lysates are solubilized whole cells, all the proteins found in GMMA should also be in the lysates, albeit at lower relative abundance. Comparison of the Sf2a GMMA with the Sf2a Lysate and Ss GMMA with the Ss Lysate (Fig. 1) confirmed this assumption and demonstrated that most of the proteins detected in the GMMA samples were also present in the lysates at approximately the same abundance ranks. Moreover, comparison of Sf2a Lysate with Ss Lysate showed that most of the proteins were conserved in the samples from the two strains and that on average were present at the same abundance (Fig. 2B). Similarly, the common proteins in Sf2a GMMA with Ss GMMA accounting for 99.6% and 94.9% of the protein mass, respectively, were present in close to the same mass rank order.
In both the Sf2a and Ss GMMA, approximately 20% of the protein was predicted to be periplasmic. This was twice the value for outer membrane blebs from P. aeruginosa (10% (Choi et al., 2011)) and three times that for N. meningitidis (6.2% (Lappann et al., 2013)) suggesting that the Pseudomonas NOMV and Neisseria SOMV had a larger surface to volume ratio than the Shigella GMMA in this study. A surprising observation in the current study was the approximately 10-fold enrichment of both periplasmic and outer membrane proteins in GMMA compared to total cell proteins (Fig. 1A and B). This suggests that the GMMA co-incidentally have a similar surface to volume ratio as the surface to periplasmic volume ratio of the bacteria. Electron microscopy of hyperblebbing bacteria with the tolR mutation showed that the separation between inner and outer membrane is substantially greater than in the wild-type bacteria (Meloni E, unpublished data). Thus, the genetic enhancement bleb formation may be linked to the enrichment of periplasmic proteins in Sf2a and Ss GMMA (approximately 20% in both) compared to spontaneously released vesicles from P. aeruginosa (10% (Choi et al., 2011)) and N. meningitidis (6.2% (Lappann et al., 2013)).
The “purity” of the GMMA with respect to outer membrane and periplasmic proteins is likely to be even higher than the initial analysis suggested: Two proteins, Tail-specific protease and YajG, highly enriched in GMMA but not predicted to be outer membrane or periplasmic proteins (Fig. 1) appear to be misclassified using the sequences from the Shigella database. Tail-specific protease is known to be a soluble periplasmic protease in E. coli (Keiler and Sauer, 1996) and the YajG homologue in E. coli is a lipoprotein with a classic LIPO box (Boudet et al., 2007). Taking this into account, 98.1% and 99.3% of Sf2a and Ss GMMA, respectively, would be periplasmic or outer membrane localized proteins. Much of the remaining protein content is ribosomal proteins. The GMMA used in this study were purified by ultracentrifugation under conditions that contaminating ribosomes, if present in the medium as a result of low-level bacterial lysis, are likely to have been co-sedimented with the GMMA. It will be interesting to see if GMMA purified using filtration, for use in vaccines are also contaminated with ribosomes.
These results are different to the data from naturally shed outer membrane vesicles from N. meningitidis where 85.8% of the protein mass in the SOMV was predicted to be periplasmic (6.2%) or outer membrane (79.6%) (Lappann et al., 2013). For P. aeruginosa NOMV the prediction was even lower with approximately 47% of the protein in the vesicles predicted to be periplasmic or outer membrane (Choi et al., 2011). Importantly, the cytoplasmic and inner membrane predicted proteins that would be considered impurities in an outer membrane bleb-based vaccine accounted for 13.9% in Neisseria SOMV (Lappann et al., 2013) or 38% in Pseudomonas NOVM (Choi et al., 2011) in contrast to 4.4% in Sf2a GMMA and only 1.3% in Ss GMMA (or only 1.9% and 0.7%, respectively, assuming misclassification of Tail-specific protease and YajG).
It is unlikely that these differences relate to differences in the surface polysaccharide content or LPS/LOS structure: The Pseudomonas was presumably encapsulated (not specified in the paper), but the Neisseria had the capsule deleted and neither Shigella lines were encapsulated (Caboni et al., 2015). By deleting the O antigen, the LPS of the Shigella lines had a similar size and structure to the LOS present in the Neisseria. The Pseudomonas and the Shigella had a wild type Lipid A component of their LPS, the Neisseria contained the lpxL mutation that results in penta-acylated lipid A. It is possible that the differences observed in these studies relates to the use of the tolR mutation in Shigella that result in a very high levels of blebbing. By substantially increasing the production of GMMA, the level of impurities in the resulting GMMA preparation is likely to be substantially reduced. This is a critical point for vaccine production: it suggests that cytoplasmic proteins are not intrinsically bound to GMMA and therefore their presence needs to be carefully controlled.
In conclusion, using quantitative proteomics, we show that we can produce GMMA from S. sonnei and S. flexneri 2a that contain the expected outer membrane and periplasmic proteins and have the low levels of cytoplasmic protein contamination. This makes their composition and purity suitable for vaccines.
Acknowledgements
We thank Thomas Connor (WTSI) for discussion and help with genomic analyses and Calman MacLennan (NVGH) for discussion and critically reading the manuscript. The research received funding from the Wellcome Trust, grant WT098051, the European Union Seventh Framework Programme [FP7/2007-2013] under Grant Agreement 261472 ‘STOPENTERICS’. This work was also partially funded by a grant from Novartis to the Novartis Vaccines Institute of Global Health, prior to its sale to GSK. The funders had no role in the design of the experiments, the interpretation of the data, writing this paper or the decision to publish.
Footnotes
Supplementary material related to this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ijmm.2015.12.003.
Appendix A. Supplementary data
The following are Supplementary data to this article:
The table lists the 2306 proteins identified in the 4 samples tested: S. flexneri 2a GMMA (Sf2a GMMA), S. flexneri 2a lysate (Sf2a Lysate), S. sonnei GMMA (Ss GMMA) and S. sonnei lysate (Ss Lysate), by gene name, UniProt name, predicted location, protein name, molecular weight, sequence length, peptides detected and with the calculated relative abundance expressed as a % moles and as % mass.
References
- Alaniz R.C., Deatherage B.L., Lara J.C., Cookson B.T. Membrane vesicles are immunogenic facsimiles of Salmonella typhimurium that potently activate dendritic cells, prime B and T cell responses, and stimulate protective immunity in vivo. J. Immunol. 2007;179:7692–7701. doi: 10.4049/jimmunol.179.11.7692. [DOI] [PubMed] [Google Scholar]
- Alvarez Hayes J., Erben E., Lamberti Y., Principi G., Maschi F., Ayala M., Rodriguez M.E. Bordetella pertussis iron regulated proteins as potential vaccine components. Vaccine. 2013;31:3543–3548. doi: 10.1016/j.vaccine.2013.05.072. [DOI] [PubMed] [Google Scholar]
- Arike L., Valgepea K., Peil L., Nahku R., Adamberg K., Vilu R. Comparison and applications of label-free absolute proteome quantification methods on Escherichia coli. J. Proteomics. 2012;75:5437–5448. doi: 10.1016/j.jprot.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Balgley B.M., Laudeman T., Yang L., Song T., Lee C.S. Comparative evaluation of tandem MS search algorithms using a target-decoy search strategy. Mol. Cell Proteomics. 2007;6:1599–1608. doi: 10.1074/mcp.M600469-MCP200. [DOI] [PubMed] [Google Scholar]
- Berlanda Scorza F., Colucci A.M., Maggiore L., Sanzone S., Rossi O., Ferlenghi I., Pesce I., Caboni M., Norais N., Di Cioccio V., Saul A., Gerke C. High yield production process for Shigella outer membrane particles. PLOS ONE. 2012;7:e35616. doi: 10.1371/journal.pone.0035616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlanda Scorza F., Doro F., Rodriguez-Ortega M.J., Stella M., Liberatori S., Taddei A.R., Serino L., Gomes D.M., Nesta B., Fontana M.R., Spagnuolo A., Pizza M., Norais N., Grandi G. Proteomics characterization of outer membrane vesicles from the extraintestinal pathogenic Escherichia coli DtolR IHE3034 mutant. Mol. Cell Proteomics. 2008;7:473–485. doi: 10.1074/mcp.M700295-MCP200. [DOI] [PubMed] [Google Scholar]
- Berven F.S., Karlsen O.A., Straume A.H., Flikka K., Murrell J.C., Fjellbirkeland A., Lillehaug J.R., Eidhammer I., Jensen H.B. Analysing the outer membrane subproteome of Methylococcus capsulatus (Bath) using proteomics and novel biocomputing tools. Arch. Microbiol. 2006;184:362–377. doi: 10.1007/s00203-005-0055-7. [DOI] [PubMed] [Google Scholar]
- Boudet J., Chouquet A., Chahboune A., Giustini C., Joris B., Simorre J.P., Bougault C. 1H, 13C and 15N resonance assignments of YajG, an Escherichia coli protein of unknown structure and function. Biomol. NMR Assign. 2007;1:89–91. doi: 10.1007/s12104-007-9025-0. [DOI] [PubMed] [Google Scholar]
- Braun V. Covalent lipoprotein from the outer membrane of Escherichia coli. Biochim. Biophys. Acta. 1975;415:335–377. doi: 10.1016/0304-4157(75)90013-1. [DOI] [PubMed] [Google Scholar]
- Caboni M., Pedron T., Rossi O., Goulding D., Pickard D., Citiulo F., MacLennan C.A., Dougan G., Thomson N.R., Saul A., Sansonetti P.J., Gerke C. An O antigen capsule modulates bacterial pathogenesis in Shigella sonnei. PLoS Pathog. 2015;11:e1004749. doi: 10.1371/journal.ppat.1004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho A.I., de Souza J., Sanchez-Gomez S., Pardo-Ros M., Irache J.M., Gamazo C. Mucosal immunization with Shigella flexneri outer membrane vesicles induced protection in mice. Vaccine. 2011;29:8222–8229. doi: 10.1016/j.vaccine.2011.08.121. [DOI] [PubMed] [Google Scholar]
- Chang Z., Lu S., Chen L., Jin Q., Yang J. Causative species and serotypes of shigellosis in mainland China: systematic review and meta-analysis. PLOS ONE. 2012;7:e52515. doi: 10.1371/journal.pone.0052515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapon C. Role of the catabolite activator protein in the maltose regulon of Escherichia coli. J. Bacteriol. 1982;150:722–729. doi: 10.1128/jb.150.2.722-729.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D.-S., Kim D.-K., Choi S.J., Lee J., Choi J.-P., Rho S., Park S.-H., Kim Y.-K., Hwang D., Gho Y.S. Proteomic analysis of outer membrane vesicles derived from Pseudomonas aeruginosa. Proteomics. 2011;11:3424–3429. doi: 10.1002/pmic.201000212. [DOI] [PubMed] [Google Scholar]
- Ellis T.N., Kuehn M.J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 2010;74:81–94. doi: 10.1128/MMBR.00031-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis T.N., Leiman S.A., Kuehn M.J. Naturally produced outer membrane vesicles from Pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect. Immun. 2010;78:3822–3831. doi: 10.1128/IAI.00433-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari G., Garaguso I., Adu-Bobie J., Doro F., Taddei A.R., Biolchi A., Brunelli B., Giuliani M.M., Pizza M., Norais N., Grandi G. Outer membrane vesicles from group B Neisseria meningitidis Dgna33 mutant: proteomic and immunological comparison with detergent-derived outer membrane vesicles. Proteomics. 2006;6:1856–1866. doi: 10.1002/pmic.200500164. [DOI] [PubMed] [Google Scholar]
- Fox J.T., Thomson D.U., Drouillard J.S., Thornton A.B., Burkhardt D.T., Emery D.A., Nagaraja T.G. Efficacy of Escherichia coli O157:H7 siderophore receptor/porin proteins-based vaccine in feedlot cattle naturally shedding E. coli O157. Foodborne Pathog. Dis. 2009;6:893–899. doi: 10.1089/fpd.2009.0336. [DOI] [PubMed] [Google Scholar]
- Gerke C., Colucci A.M., Giannelli C., Vitali C.G., Auerbach J., Sollai L., Rossi O., Sanzone S., Di Cioccio V., Saul A. Shigella sonnei GMMA vaccine, 1790GAHB. PLOS ONE. 2015;10:e0134478. doi: 10.1371/journal.pone.0134478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson E., Rousset J.P., Charbit A., Perrin D., Hofnung M. malM, a new gene of the maltose regulon in Escherichia coli K12. I. malM is the last gene of the malK-lamB operon and encodes a periplasmic protein. J. Mol. Biol. 1986;191:303–311. doi: 10.1016/0022-2836(86)90127-0. [DOI] [PubMed] [Google Scholar]
- Holst J., Oster P., Arnold R., Tatley M.V., Næss L.M., Aaberge I.S., Galloway Y., McNicholas A., O’Hallahan J., Rosenqvist E., Black S. Vaccines against meningococcal serogroup B disease containing outer membrane vesicles (OMV): lessons from past programs and implications for the future. Hum. Vaccin. Immunother. 2013;9:1241–1253. doi: 10.4161/hv.24129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihama Y., Oda Y., Tabata T., Sato T., Nagasu T., Rappsilber J., Mann M. Exponentially modified protein abundance index (emPAI) for estimation of absolute protein amount in proteomics by the number of sequenced peptides per protein. Mol. Cell Proteomics. 2005;4:1265–1272. doi: 10.1074/mcp.M500061-MCP200. [DOI] [PubMed] [Google Scholar]
- Ishihama Y., Schmidt T., Rappsilber J., Mann M., Hartl F.U., Kerner M.J., Frishman D. Protein abundance profiling of the Escherichia coli cytosol. BMC Genomics. 2008;9:102. doi: 10.1186/1471-2164-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshige T., Yaguchi K., Ohgitani T. Siderophore receptor IroN is an important protective antigen against Salmonella infection in chickens. Avian Dis. 2009;53:563–567. doi: 10.1637/8925-051309-Reg.1. [DOI] [PubMed] [Google Scholar]
- Keiler K.C., Sauer R.T. Sequence determinants of C-terminal substrate recognition by the Tsp protease. J. Biol. Chem. 1996;271:2589–2593. doi: 10.1074/jbc.271.5.2589. [DOI] [PubMed] [Google Scholar]
- Kotloff K.L., Nataro J.P., Blackwelder W.C., Nasrin D., Farag T.H., Panchalingam S., Wu Y., Sow S.O., Sur D., Breiman R.F., Faruque A.S., Zaidi A.K., Saha D., Alonso P.L., Tamboura B., Sanogo D., Onwuchekwa U., Manna B., Ramamurthy T., Kanungo S., Ochieng J.B., Omore R., Oundo J.O., Hossain A., Das S.K., Ahmed S., Qureshi S., Quadri F., Adegbola R.A., Antonio M., Hossain M.J., Akinsola A., Mandomando I., Nhampossa T., Acácio S., Biswas K., O’Reilly C.E., Mintz E.D., Berkeley L.Y., Muhsen K., Sommerfelt H., Robins-Browne R.M., Levine M.M. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case–control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- Lappann M., Otto A., Becher D., Vogel U. Comparative proteome analysis of spontaneous outer membrane vesicles and purified outer membranes of Neisseria meningitidis. J. Bacteriol. 2013;195:4425–4435. doi: 10.1128/JB.00625-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M.M., Kotloff K.L., Barry E.M., Pasetti M.F., Sztein M.B. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat. Rev. Microbiol. 2007;5:540–553. doi: 10.1038/nrmicro1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livio S., Strockbine N.A., Panchalingam S., Tennant S.M., Barry E.M., Marohn M.E., Antonio M., Hossain A., Mandomando I., Ochieng J.B., Oundo J.O., Qureshi S., Ramamurthy T., Tamboura B., Adegbola R.A., Hossain M.J., Saha D., Sen S., Faruque A.S., Alonso P.L., Breiman R.F., Zaidi A.K., Sur D., Sow S.O., Berkeley L.Y., O’Reilly C.E., Mintz E.D., Biswas K., Cohen D., Farag T.H., Nasrin D., Wu Y., Blackwelder W.C., Kotloff K.L., Nataro J.P., Levine M.M. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin. Infect. Dis. 2014;59:933–941. doi: 10.1093/cid/ciu468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu P., Vogel C., Wang R., Yao X., Marcotte E.M. Absolute protein expression profiling estimates the relative contributions of transcriptional and translational regulation. Nat. Biotechnol. 2007;25:117–124. doi: 10.1038/nbt1270. [DOI] [PubMed] [Google Scholar]
- Maier T., Schmidt A., Guell M., Kuhner S., Gavin A.-C., Aebersold R., Serrano L. Quantification of mRNA and protein and integration with protein turnover in a bacterium. Mol. Syst. Biol. 2011;7:511. doi: 10.1038/msb.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S., Chakrabarti M.K., Koley H. Multi-serotype outer membrane vesicles of Shigellae confer passive protection to the neonatal mice against shigellosis. Vaccine. 2013;31:3163–3173. doi: 10.1016/j.vaccine.2013.05.001. [DOI] [PubMed] [Google Scholar]
- Mullaney E., Brown P.A., Smith S.M., Botting C.H., Yamaoka Y.Y., Terres A.M., Kelleher D.P., Windle H.J. Proteomic and functional characterization of the outer membrane vesicles from the gastric pathogen Helicobacter pylori. Proteomics Clin. Appl. 2009;3:785–796. doi: 10.1002/prca.200800192. [DOI] [PubMed] [Google Scholar]
- Park S.B., Jang H.B., Nho S.W., Cha I.S., Hikima J., Ohtani M., Aoki T., Jung T.S. Outer membrane vesicles as a candidate vaccine against edwardsiellosis. PLoS ONE. 2011;6:e17629. doi: 10.1371/journal.pone.0017629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen T.N., Brunak S., von Heijne G., Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat. Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Pierson T., Matrakas D., Taylor Y.U., Manyam G., Morozov V.N., Zhou W., van Hoek M.L. Proteomic characterization and functional analysis of outer membrane vesicles of Francisella novicida suggests possible role in virulence and use as a vaccine. J. Proteome Res. 2011;10:954–967. doi: 10.1021/pr1009756. [DOI] [PubMed] [Google Scholar]
- Roier S., Blume T., Klug L., Wagner G.E., Elhenawy W., Zangger K., Prassl R., Reidl J., Daum G., Feldman M.F., Schild S. A basis for vaccine development: comparative characterization of Haemophilus influenzae outer membrane vesicles. Int. J. Med. Microbiol. 2015;305:298–309. doi: 10.1016/j.ijmm.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Rossi O., Pesce I., Giannelli C., Aprea S., Caboni M., Citiulo F., Valentini S., Ferlenghi I., MacLennan C.A., D’Oro U., Saul A., Gerke C. Modulation of endotoxicity of Shigella generalized modules for membrane antigens (GMMA) by genetic lipid a modifications: relative activation of TLR4 and TLR2 pathways in different mutants. J. Biol. Chem. 2014;289:24922–24935. doi: 10.1074/jbc.M114.566570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schild S., Nelson E.J., Camilli A. Immunization with Vibrio cholerae outer membrane vesicles induces protective immunity in mice. Infect. Immun. 2008;76:4554–4563. doi: 10.1128/IAI.00532-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwanhäusser B., Busse D., Li N., Dittmar G., Schuchhardt J., Wolf J., Chen W., Selbach M. Global quantification of mammalian gene expression control. Nature. 2011;473:337–342. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- van de Waterbeemd B., Mommen G.P.M., Pennings J.L.A., Eppink M.H., Wijffels R.H., van der Pol L.A., de Jong P.J.M. Quantitative proteomics reveals distinct differences in the protein content of outer membrane vesicle vaccines. J. Proteome Res. 2013;12:1898–1908. doi: 10.1021/pr301208g. [DOI] [PubMed] [Google Scholar]
- Vizcaino J.A., Deutsch E.W., Wang R., Csordas A., Reisinger F., Rios D., Dianes J.A., Sun Z., Farrah T., Bandeira N., Binz P.A., Xenarios I., Eisenacher M., Mayer G., Gatto L., Campos A., Chalkley R.J., Kraus H.J., Albar J.P., Martinez-Bartolome S., Apweiler R., Omenn G.S., Martens L., Jones A.R., Hermjakob H. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 2014;32:223–226. doi: 10.1038/nbt.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N.Y., Wagner J.R., Laird M.R., Melli G., Rey S., Lo R., Dao P., Sahinalp S.C., Ester M., Foster L.J., Brinkman F.S. PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics. 2010;26:1608–1615. doi: 10.1093/bioinformatics/btq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L., Liu X.K., Zhao G., Zhi Y.D., Bu X., Ying T.Y., Feng E.L., Wang J., Zhang X.M., Huang P.T., Wang H.L. Dynamic proteome changes of Shigella flexneri 2a during transition from exponential growth to stationary phase. Genomics Proteomics Bioinform. 2007;5:111–120. doi: 10.1016/S1672-0229(07)60021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zybailov B., Mosley A.L., Sardiu M.E., Colema M.K., Florens L., Washburn M.P. Statistical analysis of membrane proteome expression changes in Saccharomyces cerevisiae. J. Proteome Res. 2006;5:2339–2347. doi: 10.1021/pr060161n. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The table lists the 2306 proteins identified in the 4 samples tested: S. flexneri 2a GMMA (Sf2a GMMA), S. flexneri 2a lysate (Sf2a Lysate), S. sonnei GMMA (Ss GMMA) and S. sonnei lysate (Ss Lysate), by gene name, UniProt name, predicted location, protein name, molecular weight, sequence length, peptides detected and with the calculated relative abundance expressed as a % moles and as % mass.