Abstract
Currently, coronary artery disease (CAD) is considered a major ailment in humans with widespread prevalence. CAD also accounts for high mortality rates around the world that involves several known risk factors. Chemerin is a novel adipokinine that is associated with inflammation and adipogenesis. Furthermore, experimental and clinical data indicate that localized as well as circulating chemerin expression and activation are elevated in numerous metabolic and inflammatory diseases including psoriasis, obesity, type 2 diabetes, metabolic syndrome and cardiovascular disease. Chemerin is accepted as being a strong marker because the serum chemerin levels are increased in a CAD condition. However, the chimeric characteristics of chemerin have not been fully investigated. Although chemerin is known to be responsible for CAD development among other factors, authors still investigate it at the marker level. This review focuses on chemerin expression, processing, biological function and relevance to human diseases, and on the role of chemerin in the maintenance of a cardiovascular disease.
Keywords: cardiovascular disease, chemerin, marker
Introduction
The World Health Organization classifies cardiovascular diseases as the disorders encountered at an increased prevalence in the general population in the 21st century. Cardiovascular diseases are characterized by high mortality: morbidity levels and decreased quality of life, and are associated with serious economic problems [Heart Failure Society of America, 2006]. Predisposing factors such as diabetes mellitus, high blood pressure, high levels of cholesterol, smoking, chronic kidney disease, obesity and sedentary lifestyle, as well as factors such as age, sex, ethnic origin and genetic characteristics play important roles [Kenchaiah et al. 2002; Cole and Sperling, 2004]. The frequency of coronary artery disease (CAD) is increasing in recent years. Studies have shown that cardiovascular diseases are responsible for nearly 80% of the cardiovascular deaths occurring in countries with low and medium incomes [World Health Organization, 2010].
The role of chemerin in the development of cardiovascular diseases, and especially atherosclerosis, has been investigated. A positive correlation was shown between the chemerin secretion at the perivascular tissue and aortic and coronary atherosclerosis in autopsy studies on humans [Spiroglou et al. 2010]. In cross-sectional studies, chemerin was shown to be associated with peripheral arterial stiffness [Yoo et al. 2012] and with the number of noncalcified plaques in patients with stable chest pain [Lehrke et al. 2009]. A positive correlation was shown between patients with CAD and serum chemerin levels in case-control studies, and it was reported to play a role in determining the severity of the coronary lesions [Yan et al. 2011; Dong et al. 2011]. Echocardiography studies have detected an association between epicardial fat tissues that are responsible for a significant portion of chemerin secretion and CAD [Jeong et al. 2007]. This review highlights that chemerin may be used both as a marker and as an independent predictor of cardiovascular events.
Cardiovascular disease
Cardiovascular disease is the most frequent cause of death occurring in the USA in the last 50 years. According to the data from the USA, more than 900,000 people died and more than 12 million were newly diagnosed as having cardiovascular disease (from 1990 to 1997) [Cooper et al. 2000]. Atherosclerosis causes cardiovascular disease by atherosclerotic plaques developing on the endothelium of medium to large arteries. Atherosclerosis generally develops in vessels of all dimensions from weak to hard [Guyton, 2011]. Atherosclerosis is a chronic inflammatory response of the arterial wall to endothelial injury and the inflammation cascade [Steinberg, 2002]. The development of atherosclerotic plaques starts with the attachment of monocytes and lipids to adhesion molecules that are present on damaged or dysfunctional endothelial cells. This initial adhesion or attachment may be due to cytokines such as tumor-necrosis factor, mechanical denudation, hemodynamic forces, immune-complex deposition, irritation or chemicals. The tunica intima of the vessel wall is exposed, which is followed by monocyte diapedesis and differentiation of monocytes to macrophages by the induction of the cytokine macrophage colony-stimulating factor [Hansson, 2001]. Activated macrophages internalize the accumulated lipoproteins by becoming foam cells and creating visible fatty streaks, oxidizing them. The fatty streaks show increased amounts of polar amino acids containing elastin, which enable calcium and connective-tissue material to generate plaques [Gotleib, 1982]. Eventually, fibroblasts of the plaque-deposit-dense connective tissue cause stiffening of the arteries. Calcium salts settle with cholesterol and other lipids after stiffness and cause bony calcifications [Guyton, 2011]. Risk factors for cardiovascular diseases cause structural and functional endothelial dysfunction [Marti et al. 2001]. The endothelium is the major regulator of vascular homeostasis and maintains the balance between vasodilatation and vasoconstriction, stimulation and inhibition of smooth muscle proliferation and migration, and thrombogenesis and fibrinolysis [Lüscher and Barton, 1997; Kinlay et al. 2001]. High blood pressure, total cholesterol, high-density lipoprotein cholesterol, smoking, glucose intolerance and left ventricular hypertrophy are physiologic risk factors for cardiovascular diseases [Anderson et al. 1991]. There is also evidence that social isolation, depression, maladaptive coping methods, excessive alcohol and tobacco consumption and physiologic stress factors increase inflammation and cause endothelial dysfunction and cardiovascular diseases [Weidner, 2002].
Chemerin
Chemerin was initially identified in 1997 using differential display, as a retinoid-responsive gene present in psoriatic skin lesions [Nagpal et al. 1997]. Chemerin, also known as tazarotene-induced gene 2 (TIG2) and retinoic acid receptor responder 2 (RARRES2), is a recently discovered adipokinine that has been reported to modulate immune-system function through its binding to the chemerin receptor (ChemerinR, chemokine-like receptor 1, and G protein-coupled receptor) [Roh et al. 2007]. Chemerin signaling is tightly regulated through a number of mechanisms including expression, secretion, processing and signaling events. The precise coordination of these regulatory mechanisms is essential for establishing chemerin levels, localization and, ultimately, the activity.
Chemerin is mostly produced in visceral adipose tissue (VAT), placenta and liver, and also to a lesser extent in the lungs, heart, ovaries, kidneys and pancreas [Goralski et al. 2007; Bozaoglu et al. 2007; Issa et al. 2012; Takahashi et al. 2011]. Although chemerin levels show a diurnal rhythm similar to other adipokinines, leptin, adiponectin and omentin in mice [Parlee et al. 2010], this is believed to be minimal in humans [Tan et al. 2009]. There are studies showing high serum levels in women and the elderly [Bozaoglu et al. 2007, 2009; Lehrke et al. 2009; Stejskal et al. 2008]. Chemerin is initially synthesized as the precursor of prochemerin, preprochemerin [Wittamer et al. 2003]. Most of the circulating chemerin is in the form of inactive prochemerin and is converted to bioactive chemerin form by a proteolytic process when needed.
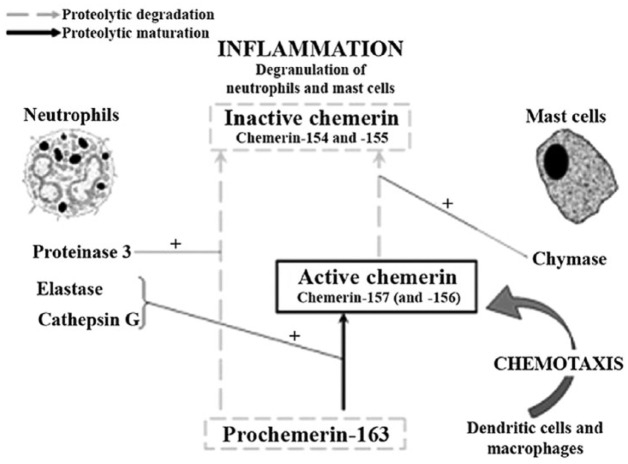
Prochemerin may be produced by various extracellular proteases of the coagulation, fibrinolytic and inflammatory cascade following secretion. These enzymes are converted into bioactive isoforms by separating the C-terminal arm of prochemerin. The type of isoform depends on the number of amino acids separated. Many different mechanisms are involved in the production of chemerin, determining local and systemic chemerin activation or inactivation directly, or by limiting present precursors, indirectly. The ratio between active and inactive isoforms is important for the determination of chemerin bioactivity. Chemerin bioactivity is related to only one function or signal pathway (and is therefore relative) according to most of the studies. Whether single chemerin isoforms have distinctive bioactivity in multiple pathways or functions is not yet clearly known [Zabel et al. 2004; Guillabert et al. 2008] (Figure 1).
Figure 1.
Proposed model for (pro)chemerin maturation and degradation. Proteinase 3 (PR3) can act as a down-regulating protease by processing prochemerin into an inactive chemerin variant.
Although data on chemerin processing and bioactivity are mostly derived from ex vivo studies, many endogenous chemerin isoforms are isolated from human samples. Different patterns of chemerin isoforms are produced in vivo in human blood (Chem-155, -157, -158), acid (Chem-157), synovial fluid (Chem-158), and cerebrospinal fluid (Chem-158). All proteases play a role in the regulation of chemerin activity via the C-terminal branch [Guillabert et al. 2008].
Currently, no information is available regarding the effect of this C-terminal processing on the tertiary or quaternary structure of chemerin, or the functional relevance of particular amino acids or amino-acid motifs within the remainder of the protein. Thus, further characterization of chemerin-isoform generation is necessary in order to fully understand the local chemerin bioactivity and the biological functions of chemerin [Guillabert et al. 2008].
Chemerin chemokine-like receptor 1 (CMKLR1) is the receptor associated with chemerin that was discovered in 1996 [Gantz et al. 1996]. Chemerin receptor 23 was found later (ChemR23) [Samson et al. 1998]. Although these were reported separately, they were in fact similar. Zabel and colleagues discovered this in 2004 [Zabel et al. 2004], and the relationship between chemR23 and chemerin was found in 2003 [Meder et al. 2003; Wittamer et al. 2003]. The most recent receptor related to chemerin is chemokine (CC motif) receptor-like 2 (CCRL2), which was discovered in 1998 [Fan et al. 1998]. Its association was also unknown until Zabel and colleagues discovered its mechanism [Zabel et al. 2008]. Chemerin and these other receptors may play multifunctional roles in the human body similar to chemokine, adipokinine and growth factors.
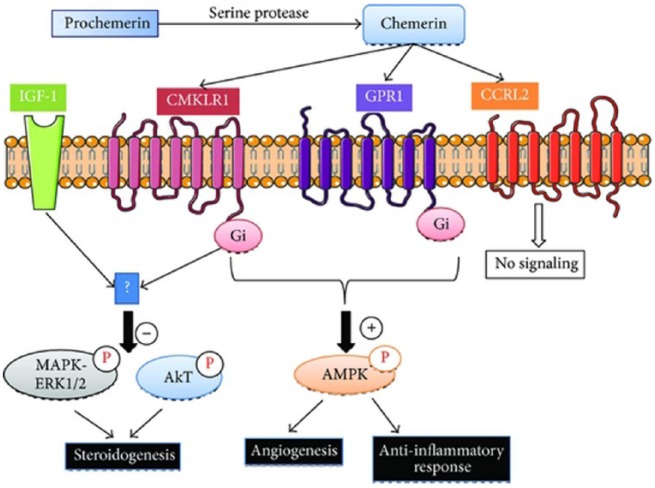
Although chemerin was shown to bind and inactivate G-protein-coupled receptor (GPR1) similarly to CMKLR1, [Barnea et al. 2008], there are no data on signal-transduction pathways in relation to GPR1. In addition to CMKLR1 and GPR1, chemerin is a ligand for a third receptor, CCRL2, that has phylogenetic homology to CC chemokine-receptor subfamilies. CCRL2-linked chemerin is not clearly known yet, and it is not believed to be a signaling receptor [Zabel et al. 2008]. On the contrary, CCRL2 is believed to focus on chemerin localization in vivo, to exert a positive effect on local chemerin concentrations, to transmit to nearby cells and thus contribute to CMKLR1, and potentially to CPR1-mediated processes [Muruganandan et al. 2010] (Figure 2).
Figure 2.
Chemerin receptors, chemerin chemokine-like receptor 1 (CMKLR1), G-protein-coupled receptor (GPR1), and chemerin chemokine-like receptor 2 (CCRL2) signaling pathways.
The three chemerin receptors have similar and dissimilar characteristics. CMKLR1 is expressed in high levels in the leukocyte populations, especially in macrophages and dendritic cells (DC), adipose tissue, bone, lungs, brain, heart and placenta [Goralski et al. 2007; Wittamer et al. 2003]. Similar to CMKLR1, GPR1 is also expressed in the adipose tissue; but GPR1 is expressed in normal levels in the central nervous system (CNS) and skeletal muscles, and in limited amounts in the leukocytes [Regard et al. 2008]. CCRL2 is present in low amounts in the adipose tissues, and in higher amounts in the lungs, heart, spleen and leukocytes [Zabel et al. 2008]. This variability in receptor localizations may contribute to common and independent signaling mechanisms of bioactive chemerin and biological functions originating from this. Little is known on signal-transduction pathways attributed to CMKLR1 and GPR1 activation. Preliminary studies have shown that CMKLR1 activation results in intracellular calcium release and decrease in AMP (cAMP) accumulation. Low-dose chemerin administration was reported to induce phosphorylation of extracellular-regulated kinase (ERK) in human adipocytes and endothelial cells by some studies [Goralski et al. 2007; Kaur et al. 2010]. This shows that inhibition or desensitization of signaling may occur in high concentration. In order to explain signaling pathways related to chemerin activity in more detail, more studies focusing on chemerin receptors are needed. Future studies should be designed to elucidate both overlapping and differential CMKLR1/GPR1 signaling pathways in a way to expose singular pathway activations of chemerin isoforms in detail.
Role of chemerin in inflammation and its relationship with systemic illness
It is now generally recognized that white adipose tissue, in addition to serving as a long-term energy store, is also an active endocrine organ that secretes a number of bioactive molecules collectively termed as adipokinine. Adipokinines are important regulators of adipose-tissue development and function that have a significant influence on glucose metabolism in various tissues, and also have an influence on the overall energy balance at the systemic level [Bluher, 2012; Pardo et al. 2012]. Chemerin, a recently discovered adipocytokine, has been shown to regulate the adipocyte differentiation and modulate the expression of adipocyte genes, such as glucose transporter-4, adiponectin, and leptin that are involved in glucose and lipid homeostasis [Goralski et al. 2007]. Consistent with this proposal, in 2007, chemerin was identified as a novel adipokinine that regulates adipogenesis and adipocyte metabolism as evidenced by experimental data showing that the loss of chemerin or CMKLR1 abro-gates adipocyte differentiation and modifies the expression of genes critical in glucose and lipid metabolism. Subsequent studies confirmed these findings and have provided experimental evidence for additional roles of chemerin in diverse biological processes including cell proliferation and differentiation, angiogenesis, renal function, and energy metabolism [Muruganandan et al. 2010]. Moreover, two additional chemerin receptors have been identified, CCRL2 and GPR1 [Zabel et al. 2008]. While chemerin is known to bind CCRL2 and GPR1, all of the biological actions currently ascribed to chemerin are elicited through the activation of CMKLR1.
The anti-inflammatory and pro-inflammatory effects of chemerin were first shown by Cash and colleagues [Cash et al. 2008]. Chemerin was shown to exert both pro-inflammatory and anti-inflammatory effects via the CMKLR1 receptor. Experimental studies on animal- and cell-based models show that chemerin and CMKLR1 have both pro-inflammatory and anti-inflammatory roles in the immune process. The data on correlations between the increase in chemerin levels in humans and inflammatory mediators support these experiments. However, it is not clear whether pro-inflammatory or anti-inflammatory activity is dominant. This may be attributed to different roles played by chemerin via various isomers in different phases of inflammation. In conclusion, although chemerin is known to be involved in immune-cell recruitment and pathological processes, presence or absence of a role played by it at the initiation, maintenance or resolution of inflammation and a protective or pathological role of increasing chemerin activity in these inflammatory disorders, are issues waiting to be resolved in ongoing research.
The first pro-inflammatory activity of chemerin was shown by its chemo-attractant characteristic for leukocytes in regions of inflammation, and expression of CMKLR1 was shown in macrophages. Also in in vitro experiments, expression of CMKLR1 was shown in the effector cells of the immune system [Zabel et al. 2004; Vermi et al. 2005; Parolini et al. 2007]. Another finding supporting the pro-inflammatory activity of chemerin is the presence of a positive correlation between pro-inflammatory cytokines such as interleukin-6 (IL-6), C-reactive protein (CRP) and tumor necrosis factor alpha (TNFα), and serum chemerin levels [Weigert et al. 2010b; Lehrke et al. 2009]. Also, increased circulating chemerin levels were found in many systemic inflammatory diseases. Main examples include Crohn’s disease, ulcerative colitis [Weigert et al. 2010b], chronic renal disease [Yamamoto et al. 2010; Pfau et al. 2010; Rutkowski et al. 2012], liver diseases [Kukla et al. 2010; Yilmaz et al. 2011], chronic pancreatitis [Adrych et al. 2012], pre-eclampsia [Stepan et al. 2011] and polycystic ovary syndrome [Tan et al. 2009]. Pfau and colleagues have shown that chemerin is an independent predictor in patients on chronic hemodialysis. Positive correlations were detected between chemerin serum levels and body mass index (BMI), fasting insulin, leptin and CRP [Pfau et al. 2010]. Sell and colleagues have found a decrease in body weight after bariatric surgery, a decrease in fat mass, improvement in insulin sensitivity, increase in inflammatory markers as well as an increase in plasma chemerin levels. They found a correlation between chemerin levels and BMI, insulin resistance, adipose tissue inflammation and liver inflammation [Sell et al. 2010]. Stejskal and colleagues have reported chemerin to be an independent marker of the metabolic syndrome, where they found chemerin to play an important role in the pathogenesis of the metabolic syndrome. [Stejskal et al. 2008]. Significant associations were found between chemerin and metabolic syndrome in animal experiments in type 2 diabetes mellitus and obesity [Bozaoglu et al. 2007] (Table 1). Chemerin was also reported to be a mediator between vascular inflammation and obesity [Landgraf et al. 2012]. It was shown that chemerin may be used as a prognostic factor in patients with non-small cell lung cancer [Zhao et al. 2011]. In light of all these data, the pro-inflammatory role played by chemerin may be attributed to its effects on both adhesion and chemotaxis of leukocytes in the inflammatory tissues via the CMKLR1 receptor.
Table 1.
The relationship of chemerin with systemic illness.
| Reported chemerin level | Disease | References | |
|---|---|---|---|
| Healthy level | Disease level | ||
| 125 | 200* | Psoriasis Vulgaris | Nakajima et al. [2010] |
| 186 | 230* | Obese | Stejskal et al. [2008] |
| 186 | 266*$ | Obese+ Met S. | Stejskal et al. [2008] |
| 94 | 144* | Tip 2 DM | Weigert et al. [2010a] |
| 190 | 230* | Tip 2 DM | Chakaroun et al. [2012] |
| 98 | 220* | Onset tip 1 DM | Verrijin Stuart et al. [2012] |
| 108 | 121* | Sleep apne | Feng et al. [2012] |
| 211 | 259* | Preeclampsia | Stepan et al. [2011] |
| 45 | 65* | Renal failure | Rutkowski et al. [2012] |
| 89 | 140 | Crohn’s | Weigert et al. [2010b] |
| 89 | 124 | Ulcerative colitis | Weigert et al. [2010b] |
| 254 | 542 | Chronic hemodialysis | Pfau et al. [2010] |
p < 0.05 versus control population.
p < 0.05 versus comparator condition.
MetS, metabolic syndrome; DM, diabetes mellitus.
Experimental evidence has shown that CMKLR1 receptors also possess anti-inflammatory activity. They exert this anti-inflammatory effect via roselvins, which act as potent inhibitors of leukocyte infiltration. Roselvins decrease the levels of interleukin-12 (IL-12) and TNFα via signals from CMKLR1 receptors [Arita et al. 2005]. Investigations on animals have shown that inhibition of endogenous chemerin activity or CMKLR1 expression increases the severity of inflammation and decreases leukocyte infiltration in some models of inflammation such as peritoneal inflammation [Wan et al. 2011], LPS-induced lipopolysaccharide (LPS) lung injury [Arita et al. 2007], and acute viral pneumonia [Cash et al. 2008]. Synthesis of pro-inflammatory mediators such as TNFα, interleukin-1b (IL-1b), IL-6, IL-12 and Regulated on Activation, Normal T Cell Expressed and Secreted (RANTES) were shown to be inhibited by chemerin in experimental studies [Cash et al. 2008]. On the other hand, this effect could not be shown clearly in independent group studies [Bondue et al. 2012]. This may be due to the triggering of monocyte adhesion by chemerin in non-leukocyte cells such as the endothelial cells.
Chemerin and cardiovascular diseases
The cardiovascular system is hard to understand due to its individual functional characteristics (such as the endothelial cells). The relationship between chemerin and the cardiovascular system could not be shown primarily, but its secondary effects were evaluated: as a chemokine, chemerin allows for chemo-attraction through the vasculature [Wittamer et al. 2003], changes endothelial adhesion levels [Yamawaki et al. 2012], and is extracellularly activated in the lumen [Wittamer et al. 2003]; as an adipokinine, chemerin adjusts lipid [Goralski et al. 2007] and glucose levels (through glucose intolerance) [Takahashi et al. 2011], possibly altering their infiltration into endothelium; and as a growth factor, it promotes micro-vessel growth to support adipocytes [Bozaoglu et al. 2010]. Changes in endothelial adhesion levels, adipokinine effect on adipose tissue [Goralski et al. 2007], and effects on glucose levels [Takahashi et al. 2011] are several of these effects. Chemo-attraction is one of the most important roles of chemerin, and by this way, macrophages interact with dendritic cells and natural killer cells and are targeted towards areas of damage [Samson et al. 1998; Zabel et al. 2004; Wittamer et al. 2003]. Similarly, intercellular adhesion molecule-1 (ICAM-1) and E-selectin interacting with the endothelium are induced by chemerin [Landgraf et al. 2012]. Chemerin increases the production of matrix myeloperoxydase (MMP). This was shown to have an effect on remodeling and growth of blood vessels in in vitro experiments [Kaur et al. 2010; Bozaoglu et al. 2010; Wang et al. 2014].
Atherosclerosis is among other conditions that have relationships with chemerin on multiple epidemiologic levels [Dessein et al. 2014; Dong et al. 2011]. This is due to receptors on macrophages [Samson et al. 1998; Wittamer et al. 2003] and the activity of chemerin in the inflammatory cascade [Zabel et al. 2004]. probably increasing the strength of macrophage activity in damaged tissues and helping immune-cell migration towards the damaged region. Presence of ChemR23s on smooth-muscle cells [Watts et al. 2013; Kostopoulos et al. 2014] may initially help healing of lesions by abnormal contractions, and then act in the cells with the occurrence of fatty streaks, thus causing progression of disease. This hypothesis partially supports that chemerin induces nitric oxide dysregulation [Neves et al. 2014], but its relationship with vascular pathologies should be proved in in vivo conditions; and replication in the human body is required in order to detect the complexity of these signals. The roles played by chemerin in lipid metabolism result in an important effect in the progression of these disorders [Goralski et al. 2007].
Studies have recently focused on the effects of chemerin on cardiovascular diseases. It may be a risk factor for CAD via the physiopathologic mechanisms mentioned above, having a close relationship with components of the metabolic syndrome that causes a high predisposition for CAD, while contributing to the inflammatory process. There are limited and conflicting conclusions on the relationship between chemerin levels and coronary atherosclerosis in present studies. In previous studies, chemerin was shown to increase the expressions of vascular pathology markers such as E-selectin and ICAM [Landgraf et al. 2012; Zakareia, 2012]. In two studies on Chinese patient populations, serum chemerin levels were significantly higher in the group with CAD, in comparison with those who did not have CAD [Xiaotao et al. 2012; Yan et al. 2011]. Also, Dong and colleagues have found higher levels of chemerin in MetS patients with CAD in comparison with MetS patients without CAD [Dong et al. 2011] (Table 2). Although significantly high serum chemerin levels were found in CAD, it is not clearly known if this increased level represents a predictor for CAD or is a result of atherosclerotic plaque morphology [Lehrke et al. 2009; Hah et al. 2011].
Table 2.
The relationship of chemerin level with cardiovascular disease.
| Reported chemerin level |
Disease | References | |
|---|---|---|---|
| Healthy level | Disease level | ||
| 95 | 133*$ | Met S+CAD | Dong et al. [2011] |
| 246 | 546* | Tip 2 DM+perivascular disease | Zakareia et al. [2012] |
| 62 | 75*$ | Tip 2 DM+HT | Yang et al. [2010] |
| 80 (One vessel) | 87* | Multiple vessel occlusion CAD | Hah et al. [2011] |
| 90 | 129* | Met S+CAD | Aksan et al. [2014] |
| 163 | 201* | Met S+CAD | Leiherer et al. [2015] |
p < 0.05 versus control population.
p < 0.05 versus comparator condition.
CAD, coronary artery disease; DM, diabetes mellitus; HT, hypertension; MetS, metabolic syndrome.
Hah and colleagues have shown a significant correlation between chemerin levels and the severity of coronary arterial stenosis in 131 patients with CAD, but they were not able to show it to be an independent risk factor for multiple-vessel disease. In this study, positive correlations were reported between serum chemerin levels and fasting glucose, triglycerides, total cholesterol, and High-sensitivity C-reactive protein (HsCRP) [Hah et al. 2011]. A correlation between circulating chemerin levels and CAD severity were found in recent case-control studies. [Yan et al. 2011; Dong et al. 2011]. Becker and colleagues were not able to show a significant increase in the area of atherosclerosis with long-term chemerin expression in their in vivo study [Becker et al. 2010], and Lehrke and colleagues did not find an association between serum chemerin levels and coronary atherosclerosis in their study evaluating coronary atherosclerosis in 303 patients with computerized tomographic angiography [Lehrke et al. 2009]. There is only one study that has evaluated circulating serum chemerin levels as a predictor of acute coronary syndrome (ACS). Aronis and colleagues have found similar ACS-group and control-group results in their case-control study. They were not able to show chemerin as a predictor of ACS in their logistic regression analysis [Aronis et al. 2014].
Bozaoglu and colleagues found higher levels of serum chemerin in MetS patients in comparison with the control group in their studies on different populations, and showed significant correlations between chemerin and BMI, blood pressure, triglyceride (TG) levels, fasting blood glucose, and high-density lipoprotein (HDL) levels [Bozaoglu et al. 2009]. Similarly, Aksan and colleagues showed higher serum chemerin levels in MetS patients in comparison with the control group, and showed a significant positive correlation between chemerin and components of metabolic syndrome such as BMI, systolic blood pressure, serum TG levels, fasting blood glucose, and a significant negative correlation with HDL-C levels [Aksan et al. 2014]. Leiherer and colleagues showed chemerin to be a significant predictive factor for cardiovascular events in patients with metabolic syndrome, independent of standard risk factors [Leiherer et al. 2015].
Chemerin was defined as an adipocytokine with pro-inflammatory actions, and positive correlations were found with arterial stiffness and coronary arterial plaques in cross-sectional studies [Spiroglou et al. 2010; Yoo et al. 2012; Lehrke et al. 2009]. Kostopoulos and colleagues evaluated the contributions of chemerin in the development of coronary atherosclerotic lesions, and detected high levels of chemerin in the periadventice adipose tissues, foam cells, vascular smooth-muscle cells in the regions of atherosclerotic lesions, and found a significant correlation between the level of chemerin released from these cells and the severity of atherosclerotic lesions [Kostopoulos et al. 2014]. Spiroglou and colleagues found a significant correlation between coronary atherosclerosis and epicardial chemerin levels [Spiroglou et al. 2010]. Hart and Greaves reported a contribution of chemerin in the progression of atherosclerosis by stimulating adhesion of macrophages to the extracellular matrix protein fibronectin and vascular cell adhesion molecule-1 (VCAM-1) [Hart and Greaves, 2010]. In addition, the chemerin molecule was shown to activate endopeptidases MMP-2 and MMP-9 belonging to matrix metalloproteinases that play a key role in plaque instability [Kaur et al. 2010], increase the expressions of adhesion molecules such as E-selectin and ICAM-1 [Landgraf et al. 2012], and was considered a new biomarker for coronary atherosclerosis.
Hyperlipidemia is considered an important risk factor in the development of cardiovascular diseases. Positive correlations between chemerin and triglycerides, low-density lipoprotein cholesterol and blood pressure levels [Bozaoglu et al. 2007; Tan et al. 2009; Chakaroun et al. 2012; Dong et al. 2011; Stejskal et al. 2008; Yoo et al. 2012; Chu et al. 2012; Xiaotao et al. 2012; Ren et al. 2012], and negative correlations with HDL cholesterol were shown in many studies [Sell et al. 2010; Chu et al. 2012; Ren et al. 2012; Alfadda et al. 2012]. There are also studies that have shown a role played by chemerin in the regulation of lipolysis via direct-signaling pathway [Bauer et al. 2011]. In in vivo studies with mice, chemerin was shown to exert its lipolytic effect via CMKLR1 receptors, not to change levels of TGs, and to cause an increase in cholesterol levels after intense expression [Becker et al. 2010]. Thus, future studies will have to concentrate on understanding the role of chemerin in lipid homeostasis better. Although the associations between chemerin and obesity, inflammation and metabolic syndrome were detected in former studies, studies of a larger scale are required to show its relationships with cardiovascular risk factors.
Although the negative effects of chemerin on vascular homeostasis and contribution to the development of coronary atherosclerosis were shown in many studies, the relationship between cardiovascular diseases and chemerin levels shown in prospective studies is not complete. Prospective chemerin studies in the detection of cardiovascular diseases in individuals in especially high-risk groups should be encouraged, and the mechanical role of chemerin in the development process of atherosclerotic plaques should be investigated. Conflicting results on the relationship between coronary atherosclerosis and serum chemerin levels may be due to study populations of different ethnic origins, use of enzyme-linked immunosorbent assay (ELISA) kits with different characteristics, and use of different atherosclerotic index evaluation methods. These studies not only support the role played by chemerin in the pathogenesis of atherosclerosis and the atherosclerotic process it contributes, but also support the autocrine and paracrine functions of chemerin in the heart.
Conclusion
Studies on chemerin as a marker seem to be more prominent among all current studies. Studies on chemerin, investigating it as a therapeutic target or as a chemerin inhibitor, as an inflammatory agent, or investigating its effects on specific diseases are considerably limited in number. There is very scarce evidence in current studies about chemerin being an independent predictor for cardiovascular diseases. More detailed and comprehensive studies are expected to provide a clearer understanding on the role played by chemerin in inflammation, and on its effects in systemic diseases, with a special emphasis on it being chosen as a target in cardiovascular diseases and bringing forth new horizons of therapeutic developments.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Sinan İnci, Departmant of Cardiology, Aksaray State Hospital, Zafer mah.Nevşehir cad. no:117, Aksaray/Merkez, Turkey.
Gökhan Aksan, Departmant of Cardiology, Şişli Etfal Education and Tracking Hospital, İstanbul, Turkey.
Pınar Doğan, Departmant of Cardiology, Aksaray State Hospital, Aksaray, Turkey.
References
- Adrych K., Stojek M., Smoczynski M., Sledzinski T., Sylwia S., Swierczynski J. (2012) Increased serum chemerin concentration in patients with chronic pancreatitis. Dig Liver Dis 44: 393–397. [DOI] [PubMed] [Google Scholar]
- Aksan G., İnci S., Nar G., Soylu K., Gedikli Ö., Yüksel S., et al. (2014) Association of serum chemerin levels with the severity of coronary artery disease in patients with metabolic syndrome. Int J Clin Exp Med 7: 5461–5468. [PMC free article] [PubMed] [Google Scholar]
- Alfadda A., Sallam R., Chishti M., Moustafa A., Fatma S., Alomaim W., et al. (2012) Differential patterns of serum concentration and adipose tissue expression of chemerin in obesity: adipose depot specificity and gender dimorphism. Mol Cell 33: 591–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson K., Odell P., Wilson P., Kannel W. (1991) Cardiovascular disease risk profiles. Am Heart J 121: 293–298. [DOI] [PubMed] [Google Scholar]
- Arita M., Bianchini F., Aliberti J., Sher A., Chiang N., Hong S., et al. (2005) Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med 201: 713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arita M., Ohira T., Sun Y., Elangovan S., Chiang N., Serhan C. (2007) Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol 178: 3912–3917. [DOI] [PubMed] [Google Scholar]
- Aronis K., Sahin-Efe A., Chamberland J., Spiro A., 3rd, Vokonas P., Mantzoros C. (2014) Chemerin levels as predictor of acute coronary events: a case-control study nested within the veterans affairs normative aging study. Metabolism 63: 760–766. [DOI] [PubMed] [Google Scholar]
- Barnea G., Strapps W., Herrada G., Berman Y., Ong J., Kloss B., et al. (2008) The genetic design of signaling cascades to record receptor activation. Proc Natl Acad Sci USA 105: 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S., Wanninger J., Schmidhofer S., Weigert J., Neumeier M., Dorn C., et al. (2011) Sterol regulatory element-binding protein 2 (SREBP2) activation after excess triglyceride storage induces chemerin in hypertrophic adipocytes. Endocrinology 152: 26–35. [DOI] [PubMed] [Google Scholar]
- Becker M., Rabe K., Lebherz C., Zugwurst J., Göke B., Parhofer K., et al. (2010) Expression of human chemerin induces insulin resistance in the skeletal muscle but does not affect weight, lipid levels, and atherosclerosis in LDL receptor knockout mice on high-fat diet. Diabetes 59: 2898–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluher M. (2012) Vaspin in obesity and diabetes: pathophysiological and clinical significance. Endocrine 41: 176–182. [DOI] [PubMed] [Google Scholar]
- Bondue B., De Henau O., Luangsay S., Devosse T., de Nadai P., Springael J., et al. (2012) The Chemerin/ChemR23 system does not affect the pro-inflammatory response of mouse and human macrophages ex vivo. PLoS ONE 7: e40043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozaoglu K., Bolton K., McMillan Zimmet P., Jowett J., Collier G., et al. (2007) Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 148: 4687–4694. [DOI] [PubMed] [Google Scholar]
- Bozaoglu K., Curran J., Stocker C., Zaibi M., Segal D., Konstantopoulos N., et al. (2010) Chemerin, a novel adipokine in the regulation of angiogenesis. J Clin Endocrinol Metab 95: 2476–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozaoglu K., Segal D., Shields K., Cummings N., Curran J., Comuzzie A., et al. (2009) Chemerin is associated with metabolic syndrome phenotypes in a Mexican-American population. J Clin Endocrinol Metab 94: 3085–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash J., Hart R., Russ A., Dixon J., Colledge W., Doran J., et al. (2008) Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J Exp Med 205: 767–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakaroun R., Raschpichler M., Kloting N., Oberbach A., Flehmig G., Kern M., et al. (2012) Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism 61: 706–714. [DOI] [PubMed] [Google Scholar]
- Cole J., Sperling L. (2004) Premature coronary artery disease: clinical risk factors and prognosis. Curr Athero Rep 6: 121–125. [DOI] [PubMed] [Google Scholar]
- Cooper R., Cutler J., Desvigne-Nickens P., Fortmann S., Friedman L., Havlik R., et al. (2000) Trends and disparities in coronary heart disease, stroke, and other cardiovascular diseases in the United States; findings of the National Conference on Cardiovascular Disease Prevention. Circulation 102: 3137–3147. [DOI] [PubMed] [Google Scholar]
- Dessein P., Tsang L., Woodiwiss A., Norton G., Solomon A. (2014) Circulating concentrations of the novel adipokine chemerin are associated with cardiovascular disease risk in rheumatoid arthritis, J Rheumatol 41: 1746–1754. [DOI] [PubMed] [Google Scholar]
- Dong B., Ji W., Zhang Y. (2011) Elevated serum chemerin levels are associated with the presence of coronary artery disease in patients with metabolic syndrome. Intern Med 50: 1093–1097. [DOI] [PubMed] [Google Scholar]
- Fan P., Kyaw H., Su K., Zeng Z., Augustus M., Carter K., et al. (1998) Cloning and characterization of a novel human chemokine receptor. Biochem Biophys Res Commun 243: 264–268. [DOI] [PubMed] [Google Scholar]
- Gantz Y., Yang Y., Miller D., Dierick H., Yamada T. (1996) Molecular cloning of a novel receptor (CMKLR1) with homology to the chemotactic factor receptors. Cytogenet Cell Genet 74: 286–290. [DOI] [PubMed] [Google Scholar]
- Goralski K., McCarthy T., Hanniman E., Zabel B., Butcher E., Parlee S., et al. (2007) Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem 282: 28175–28188. [DOI] [PubMed] [Google Scholar]
- Gotleib A. (1982) Smooth muscle and endothelial cell function in the pathogenesis of atherosclerosis. Can Med Assoc J 126: 903–908. [PMC free article] [PubMed] [Google Scholar]
- Guillabert A., Wittamer V., Bondue B., Godot V., Imbault V., Parmentier M., et al. (2008) Role of neutrophil proteinase 3 and mast cell chymase in chemerin proteolytic regulation. J Leukoc Biol 84: 1530–1538. [DOI] [PubMed] [Google Scholar]
- Guyton Hall. (2011) Textbook of Medical Physiology. Second edition. Philadelphia, PA: Saunders Publishing: 167. [Google Scholar]
- Hah Y., Kim N., Kim M., Kim H., Hur S., Yoon H., et al. (2011) Relationship between chemerin levels and cardiometabolic parameters and degree of coronary stenosis in Korean patients with coronary artery disease. Diabetes Metab J 35: 248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson G. (2001) Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol 21: 1876–1890. [DOI] [PubMed] [Google Scholar]
- Hart R., Greaves D. (2010) Chemerin contributes to inflammation by promoting macrophage adhesion to VCAM-1 and fibronectin through clustering of VLA-4 and VLA-5. J Immunol 185: 3728–3739. [DOI] [PubMed] [Google Scholar]
- Heart Failure Society of America (2006) Executive summary: HFSA 2006 Comprehensive heart failure practice guideline. J Card Fail 12: 10–38. [DOI] [PubMed] [Google Scholar]
- Issa M., Muruganandan S., Ernst M., Parlee S., Zabel B., Butcher E., et al. (2012) Chemokine-like receptor 1 regulates skeletal muscle cell myogenesis. Am J Physiol Cell Physiol 302: 1621–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong J., Jeong M., Yun K., Oh S., Park E., Kim Y., et al. (2007) Echocardiographic epicardial fat thickness and coronary artery disease. Circ J 71: 536–539. [DOI] [PubMed] [Google Scholar]
- Kaur J., Adya R., Tan B., Chen J., Randeva H. (2010) Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesis. Biochem Biophys Res Commun 391: 1762–1768. [DOI] [PubMed] [Google Scholar]
- Kenchaiah S., Evans J., Levy D., Wilson P., Benjamin E., Larson M., et al. (2002) Obesity and the risk of heart failure. N Engl J Med 347: 305–13. [DOI] [PubMed] [Google Scholar]
- Kinlay S., Libby P., Ganz P. (2001) Endothelial function and coronary artery disease. Curr Opin Lipidol. 12: 383–389. [DOI] [PubMed] [Google Scholar]
- Kostopoulos C., Spiroglou S., Varakis J., Apostolakis E., Papadaki H. (2014) Chemerin and CMKLR1 expression in human arteries and periadventitial fat: a possible role for local chemerin in atherosclerosis? BMC Cardiovasc Disord 14: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukla M., Zwirska-Korczala K., Gabriel A., Waluga M., Warakomska I., Szczygiel B., et al. (2010) Chemerin, vaspin and insulin resistance in chronic hepatitis C. J Viral Hepat 17: 661–667. [DOI] [PubMed] [Google Scholar]
- Landgraf K., Friebe D., Ullrich T., Kratzsch J., Dittrich K., Herberth G., et al. (2012) Chemerin as a mediator between obesity and vascular inflammation in children. JCEM 97: 556–564. [DOI] [PubMed] [Google Scholar]
- Lehrke M., Becker A., Greif M., Stark R., Laubender RP., von Ziegler F., et al. (2009) Chemerin is associated with markers of inflammation and components of the metabolic syndrome but does not predict coronary atherosclerosis. Eur J Endocrinol 161: 339–344. [DOI] [PubMed] [Google Scholar]
- Leiherer A., Muendlein A., Rein P., Geiger K., Fraunberger P., Drexel H., et al. (2015) Plasma chemerin is a strong and independent predictor of cardiovascular event risk. J Am Coll Cardiol 65: 10. [Google Scholar]
- Lüscher T., Barton M. (1997) Biology of the endothelium. Clin Cardiol. 20: 3–10. [PubMed] [Google Scholar]
- Marti A., Marcos A., Martinez J. (2001) Obesity and immune function relationships. Obes Rev. 2: 131–140. [DOI] [PubMed] [Google Scholar]
- Meder W., Wendland M., Busmann A., Kutzleb C., Spodsberg N., John H., et al. (2003) Characterization of human circulating TIG2 as a ligand for the orphan receptor ChemR23. FEBS Lett. 555: 495–499. [DOI] [PubMed] [Google Scholar]
- Muruganandan S., Roman A., Sinal C. (2010) Role of chemerin/CMKLR1 signaling in adipogenesis and osteoblastogenesis of bone marrow stem cells. J Bone Miner Res 25: 222–234. [DOI] [PubMed] [Google Scholar]
- Nagpal S., Patel S., Jacobe H., DiSepio D., Ghosn C., Malhotra M., et al. (1997) Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin. J Invest Dermatol 109: 91–95. [DOI] [PubMed] [Google Scholar]
- Neves K., Lobato N., Lopes R., Filgueira F., Zanotto C., Oliveira A. (2014) Chemerin reduces vascular nitric oxide/cGMP signaling in rat aorta: a link to vascular dysfunction in obesity? Clin Sci 127: 111–122. [DOI] [PubMed] [Google Scholar]
- Pardo M., Roca-Rivada A., Seoane L. (2012) Obesidomics: contribution of adipose tissue secretome analysis to obesity research. Endocrine 41: 374–383. [DOI] [PubMed] [Google Scholar]
- Parlee S., Ernst M., Muruganandan S., Sinal C., Goralski K. (2010) Serum chemerin levels vary with time of day and are modified by obesity and tumor necrosis factor-{alpha}. Endocrinology 151: 2590–2602. [DOI] [PubMed] [Google Scholar]
- Parolini S., Santoro A., Marcenaro E., Luini W., Massardi L., Facchetti F., et al. (2007) The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood 109: 3625–3632. [DOI] [PubMed] [Google Scholar]
- Pfau D., Bachmann A., Lossner U., Kratzsch J., Blüher M., Stumvoll M., et al. (2010) Serum levels of the adipokine chemerin in relation to renal function. Diabetes Care 33: 171–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regard J., Sato I., Coughlin S. (2008) Anatomical profiling of G protein-coupled receptor expression. Cell 135: 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren R., Zhang X., Xu J., Zhang H., Yu C., Cao M., et al. (2012) Chronic ethanol consumption increases the levels of chemerin in the serum and adipose tissue of humans and rats. Acta Pharmacol Sin 33: 652–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh S., Song S., Choi K., Katoh K., Wittamer V., Parmentier M., et al. (2007) Chemerin – a new adipokine that modulates adipogenesis via its own receptor. Biochem Biophys Research Comm 362: 1013–1018. [DOI] [PubMed] [Google Scholar]
- Rutkowski P., Sledzinski T., Zielinska H., Lizakowski S., Goyke E., Szrok-Wojtkiewicz S., et al. (2012) Decrease of serum chemerin concentration in patients with end stage renal disease after successful kidney transplantation. Regul Pept 173: 55–59. [DOI] [PubMed] [Google Scholar]
- Samson M., Edinger A., Stordeur P., Rucker J., Verhasselt V., Sharron M., et al. (1998) A putative chemo-attractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. Eur J Immunol 28: 1689–1700. [DOI] [PubMed] [Google Scholar]
- Sell H., Divoux A., Poitou C., Basdevant A., Bouillot J., Bedossa P., et al. (2010) Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J Clin Endocrinol Metab 95: 2892–2896. [DOI] [PubMed] [Google Scholar]
- Spiroglou S., Kostopoulos C., Varakis J., Papadaki H. (2010) Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atheroscler Thromb 17: 115–130. [DOI] [PubMed] [Google Scholar]
- Steinberg D. (2002) Atherogenesis in perspective: hypercholesterolemia and inflammation as partners in crime. Nat Med 8: 1211–1217. [DOI] [PubMed] [Google Scholar]
- Stejskal D., Karpisek M., Hanulova Z., Svestak M. (2008) Chemerin is an independent marker of the metabolic syndrome in a Caucasian population – a pilot study. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 152: 217–221. [DOI] [PubMed] [Google Scholar]
- Stepan H., Philipp A., Roth I., Kralisch S., Jank A., Schaarschmidt W., et al. (2011) Serum levels of the adipokine chemerin are increased in preeclampsia during and 6 months after pregnancy. Regul Pept 168: 69–72. [DOI] [PubMed] [Google Scholar]
- Takahashi M., Okimura Y., Iguchi G., Nishizawa H., Yamamoto M., Suda K., et al. (2011) Chemerin regulates beta-cell function in mice. Sci Rep 1: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B., Chen J., Farhatullah S., Adya R., Kaur J., Heutling D., et al. (2009) Insulin and metformin regulate circulating and adipose tissue chemerin. Diabetes 58: 1971–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermi W., Riboldi E., Wittamer V., Gentili F., Luini W., Marrelli S., et al. (2005) Role of ChemR23 in directing the migration of myeloid and plasma-cytoid dendritic cells to lymphoid organs and inflamed skin. J Exp Med 201: 509–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan M., Godson C., Guiry P., Agerberth B., Haeggstrom J. (2011) Leukotriene B4/antimicrobial peptide LL-37 proinflammatory circuits are mediated by BLT1 and FPR2/ALX and are counter-regulated by lipoxin A4 and resolvin E1. FASEB J 25: 1697–1705. [DOI] [PubMed] [Google Scholar]
- Wang C., Wu W., Liu X., To K., Chen G., Yu J., et al. (2014) Increased serum chemerin level promotes cellular invasiveness in gastric cancer: a clinical and experimental study. Peptides 51: 131–138. [DOI] [PubMed] [Google Scholar]
- Watts S., Dorrance A., Penfold M., Rourke J., Sinal C., Seitz B., et al. (2013) Chemerin connects fat to arterial contraction. Arterioscler Thromb Vasc Biol 33: 1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner G. (2002) Gender and cardiovascular health. International Encyclopedia of the social and behavioral sciences. 20: 5904–5907. [Google Scholar]
- Weigert J., Neumeier M., Wanninger J., Filarsky M., Bauer S., Wiest R., et al. (2010a) Systemic chemerin is related to inflammation rather than obesity in type 2 diabetes. Clin Endocrinol 72: 342–348. [DOI] [PubMed] [Google Scholar]
- Weigert J., Obermeier F., Neumeier M., Wanninger J., Filarsky M., Bauer S., et al. (2010b) Circulating levels of chemerin and adiponectin are higher in ulcerative colitis and chemerin is elevated in Crohn’s disease. Inflamm Bowel Dis 16: 630–637. [DOI] [PubMed] [Google Scholar]
- Wittamer V., Franssen J., Vulcano M., Mirjolet J., Le Poul E., Migeotte I., et al. (2003) Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med 198: 977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization, website: http://www.who.int.
- Xiaotao L., Xiaoxia Z., Yue X., Liye W. (2012) Serum chemerin levels are associated with the presence and extent of coronary artery disease. Coron Artery Dis 23: 412–416. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Qureshi A., Anderstam B., Heimbürger O., Barany P., Lindholm B., et al. (2010) Clinical importance of an elevated circulating chemerin level in incident dialysis patients. Nephrol Dial Transplant 25: 4017–4023. [DOI] [PubMed] [Google Scholar]
- Yamawaki H., Kameshima S., Usui T., Okada M., Hara Y. (2012) A novel adipocytokine, chemerin exerts anti-inflammatory roles in human vascular endothelial cells, Biochem Biophys Res Commun 423: 152–157. [DOI] [PubMed] [Google Scholar]
- Yan Q., Zhang Y., Hong J., Gu W., Dai M., Shi J., et al. (2011) The association of serum chemerin level with risk of coronary artery disease in Chinese adults. Endocrine 41: 281–288. [DOI] [PubMed] [Google Scholar]
- Yilmaz Y., Yonal O., Kurt R., Alahdab Y., Eren F., Ozdogan O., et al. (2011) Serum levels of omentin, chemerin and adipsin in patients with biopsy-proven nonalcoholic fatty liver disease. Scand J Gastroenterol 46: 91–97. [DOI] [PubMed] [Google Scholar]
- Yoo H., Choi H., Yang S., Kim H., Seo J., Kim S., et al. (2012) Circulating chemerin level is independently correlated with arterial stiffness. J Atheroscler Thromb 19: 59–68. [DOI] [PubMed] [Google Scholar]
- Zabel B., Nakae S., Zuniga L., Kim J., Ohyama T., Alt C., et al. (2008) Mast cell-expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis. J Exp Med 205: 2207–2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel B., Silverio A., Butcher E. (2004) Chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasma-cytoid from myeloid-dendritic cells in human blood. J Immunol 174: 244–251. [DOI] [PubMed] [Google Scholar]
- Zakareia F. (2012) Correlation of peripheral arterial blood flow with plasma chemerin and VEGF in diabetic peripheral vascular disease. Biomark Med 6: 81–87. [DOI] [PubMed] [Google Scholar]
- Zhao S., Li C., Ye Y., Peng F., Chen Q. (2011) Expression of chemerin correlates with a favorable prognosis in patients with non-small cell lung cancer. Lab Medicine 42: 553–557. [Google Scholar]