Abstract
HIV-associated neurocognitive disorder (HAND) consists of motor and cognitive dysfunction in a relatively large percentage of patients with AIDS. Prior work has suggested that at least part of the neuronal and synaptic damage observed in HAND may occur due to excessive stimulation of NMDA-type glutamate receptors (NMDARs). Here, we compared pharmacological and genetic manipulation of NMDAR activity using an improved derivative of the NMDAR antagonist memantine, termed NitroMemantine, and the modulatory NMDAR subunit GluN3A in the HIV/gp120 transgenic (tg) mouse model of HAND. Interestingly, we found that while both NitroMemantine and GluN3A have been shown to inhibit NMDAR activity, NitroMemantine protected synapses in gp120 tg mice, but overexpression of GluN3A augmented the damage. Given recent findings in the field, one explanation for this apparently paradoxical result is the location of the NMDARs primarily affected, with NitroMemantine inhibiting predominantly extrasynaptic pathologically-activated NMDARs, but GluN3A disrupting normal NMDAR-mediated neuroprotective activity via inhibition of synaptic NMDARs.
Keywords: HIV-associated neurocognitive disorder (HAND), AIDS, NMDA-type glutamate receptors, GluN3A, gp120, memantine, NitroMemantine
Introduction
HIV/AIDS can affect the brain to cause motor and cognitive dysfunction, termed HIV-associated neurocognitive disorder (HAND). Despite the fact that both morbidity and survival from HIV/AIDS have improved with combination Antiretroviral Therapy (cART), the prevalence of HAND continues to increase as people live longer with the disease (Kolson 2002; McArthur et al. 2004; Mocchetti et al. 2008). This finding indicates that cART does not completely protect from neurological complications and mandates the future development of neuroprotective therapy for HAND. There are currently a number of rodent models for HAND, and, among these, the HIV/gp120 envelope protein-expressing transgenic (tg) mouse displays several neuropathologic features of HAND, including dendritic damage, synaptic loss, and neuronal dropout (Toggas et al. 1994; Garden et al. 2002; Portula et al. 2005; Gorantla et al. 2007). Improving these parameters in gp120-tg mice can be predictive of effects in subsequent human clinical trials (Toggas et al. 1996; Schifitto et al. 2007), indicating the potential usefulness of the animal model in therapeutic evaluations.
Prior reports have shown that gp120-induced neuronal and synaptic damage is mediated, at least in part, by overstimulation of NMDA-type glutamate receptors (NMDARs) (for reviews see Lipton and Gendelman 1995; Kaul et al. 2001). Along these lines, hyperactivation of NMDARs is well known to result in excitotoxic neurodegeneration (Lipton and Rosenberg 1994; Dingledine et al. 1999; Cull-Candy et al. 2001). Memantine, an uncompetitive NMDAR antagonist, is an FDA- and European Medicines Agency (EMA)-approved drug for moderate-to-severe Alzheimer’s disease (AD; Lipton 2006). Memantine acts as an open channel-blocker of NMDAR-associated channels (Chen et al. 1992; Chen and Lipton 1997), thus inhibiting excessive NMDAR activation. Memantine was shown to exhibit a neuroprotective effect in gp120-tg mice (Toggas et al., 1996). In addition, although the results were not definitive, memantine was shown in a human phase 2 clinical trial to potentially offer improvement in HIV-associated dementia (a severe form of HAND) (Schifitto et al. 2007). Accordingly, our group recently developed a series of improved derivatives of memantine, termed NitroMemantines (Lipton 2006; Wang et al. 2006; Talantova et al. 2013). NitroMemantines act not only as channel blockers via their memantine moiety, but also allosterically inhibit NMDAR activity via an adducted nitro group that reacts with redox modulatory sites on the receptor (Lipton 2006; Talantova et al. 2013). Importantly, both memantine and NitroMemantine preferentially inhibit extrasynaptic NMDARs, while relatively sparing synaptic NMDAR activity (Xia et al. 2010; Talantova et al. 2013).
Conventional NMDARs require two distinct subunits, GluN1 (formerly known as NR1) plus GluN2A-D (NR2A-D), to form functional channels (Hollmann and Heinemann 1994). We and our colleagues identified a third family of NMDAR subunits, designated GluN3A/B (formerly designated NR3A/B) (Ciabarra et al. 1995; Sucher et al. 1995; Das et al. 1998). The level of GluN3A mRNA in the cerebrocortex peaks at postnatal day (P)7-10 and then rapidly decreases. In adult cortex and hippocampus, GluN3A expression is low (Wong et al. 2002). In heterologous expression systems, the addition of GluN3A decreases unitary channel conductance and Ca2+ permeability of GluN1/GluN2 channels (Das et al. 1998; Pérez-Otaño et al. 2001; Sasaki et al. 2002). In our previous studies, transgenic mice expressing exogenous GluN3A subunits in the adult brain manifest reduced Ca2+ permeability of NMDA-evoked currents (Tong et al. 2008), and, accordingly, diminished neuronal injury caused by various acute excitotoxic insults such as focal cerebral ischemia (Nakanishi et al. 2009). Other investigators have shown that GluN3A overexpression protected against striatal lesions caused by acute injection of 3-nitroproprionic acid (Martinez-Turrilas et al., 2012).
However, a recent study suggests that GluN3A protein levels are abnormally increased in the brains of Huntington’s disease (HD) patients and HD mouse models. Furthermore, genetic ablation of GluN3A in the HD mouse lines rescued the chronic pathological phenotypes of these mice, indicating that the abnormally expressed GluN3A in the brain may be pathogenic (Marco et al. 2013). Moreover, expression of GluN3A causes spine destabilization and hence synaptic loss (Das et al. 1998; Kehoe et al. 2014).
In the current study, we attempt to evaluate the role of NMDAR in the pathogenesis of gp120-induced neuronal and synaptic injury using both pharmacologic and genetic manipulations. Specifically, we examined the effects of the drug NitroMemantine versus chronic overexpression of the GluN3A subunit on gp120-tg mouse brain. Our results indicate that, while inhibition of NMDARs by NitroMemantine protects from gp120, chronic overexpression of GluN3A leads to neuronal and synaptic injury in the mouse hippocampus, augmenting gp120-associated damage.
Materials and Methods
Animals
Transgenic mice expressing HIV/gp120 under the control of a modified murine glial fibrillary acidic protein (GFAP) promoter were obtained from Dr. Lennart Mucke (Gladstone Institute, University of California, San Francisco, San Francisco, CA). Characteristics of these mice have been previously presented (Toggas et al. 1994; Garden et al. 2002; Kang et al. 2010).
GluN3A overexpressing transgenic (GluN3A-tg) mice have previously been generated and characterized by our group (Tong et al. 2008; Nakanishi et al. 2009). Briefly, we have generated transgenic mice carrying GluN3A transgene controlled by the tetracycline transactivator (tTA) responsive promoter. We then genetically crossed these mice with the tTA transgenic mice with the α-calmodulin dependent kinase II (CaMKII) promoter (Mayford et al. 1996). This Tet-off system produces the overexpression of GluN3A in the broad area of the forebrain in the mice carrying both tTA and GluN3A transgenes (Tong et al. 2008). We then crossed gp120- and GluN3A-tg mice in order to produce double transgenic lines with the gp120 and GluN3A transgenes. The genetic backgrounds of the gp120- and GluN3A-tg mice are C57BL/6 × SLG and C57BL/6, respectively. All experiments were performed in accordance with Institutional guidelines concerning care and treatment of vertebrate animals.
NitroMemantine (YQW-036) Drug Delivery
Saline solutions containing the lead NitroMemantine candidate, YQW-036, were prepared fresh just prior to administration; 4.6 µmol/kg of YQW-036 was injected intraperitoneally (i.p.) every 12 hours for a duration of three months starting at 6 months of age, as we have described previously (Talantova et al. 2013).
Quantitative Neuropathological Analysis
For immunostaining, three serial sections of corresponding mouse brain regions were analyzed. For assessment of neuronal and synaptic changes, sections were immunolabeled with antibodies against microtubule associated protein-2 (MAP2) (Chemicon, Temecula, CA, USA) to label neuronal cell bodies and dendrites, NeuN (Chemicon) to label neuronal nuclei and cell bodies, and synaptophysin (DAKO, Carpinteria, CA, USA) to label presynaptic vesicles of neurons. Primary antibody staining was identified with fluorescently-tagged secondary antibodies. Immunolabeled, blind-coded sections were imaged serially using a laser-scanning confocal microscopy or deconvolution microscopy (Garden et al. 2001; Kang et al. 2010). Digitized images of three optical sections (40 µm in thickness) were analyzed with Slidebook 5.0 software (Intelligent Imaging Innovations, Denver, CO). The hippocampus, including the molecular layer of the dentate gyrus and pyramidal layer of the CA1 region, was analyzed quantitatively for each antibody label.
Statistical Analysis
Data are expressed as mean + SEM in each experiment. Statistical comparisons were made by a Student’s t test for pairwise comparisons, and by an analysis of variance (ANOVA) with post hoc Newman-Keuls test for multiple comparisons. Statistical significance was taken at a level of P < 0.05.
Results
NitroMemantine Treatment of gp120-Transgenic Mice Increases MAP2 and Synaptophysin Immunoreactivity
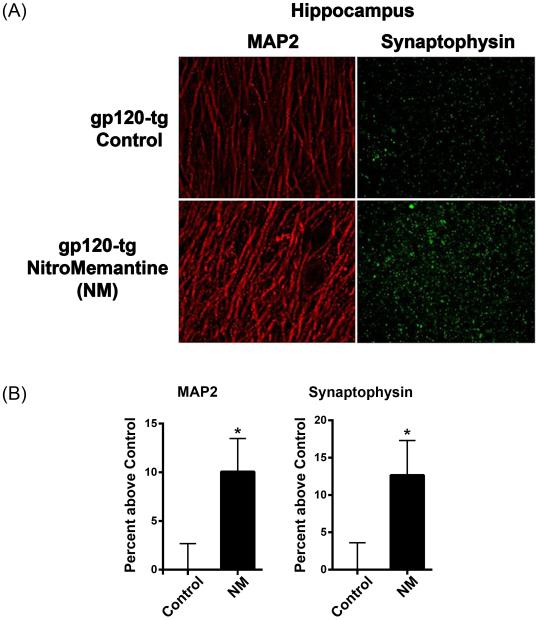
Initially, we evaluated the effect of NitroMemantine (NM) treatment on neuronal integrity in HIV/gp120 transgenic (tg) mice. Our prior studies assessed dendritic and presynaptic damage in gp120-tg mice by quantitative immunohistochemistry using the markers MAP2 and synaptophysin, respectively. We found that these mice exhibit significant reductions in dendritic complexity and synapses compared to wild-type mice by 1.5-2.5 months of age (Toggas et al., 1994; Toggas et al., 1996; Garden et al., 2001; Kang et al., 2010). In the current study, we treated 6-month-old gp120-tg mice with NitroMemantine at a standard dose of 4.6 µmol/kg body weight administered intraperitoneally (i.p.) twice a day (Talantova et al. 2013) and compared their outcome with PBS-treated or untreated controls. After three months of treatment, the mice were sacrificed, brain sections prepared and immunostaining performed using antibodies against the neuronal dendritic marker MAP2 and presynaptic protein synaptophysin. Confocal immunocytochemical images of the hippocampus revealed an increase in the expression levels of both MAP2 and synaptophysin in gp120-tg mice treated with NitroMemantine relative to the control mice (Fig. 1a). Quantifying the expression levels of MAP2 and synaptophysin by measuring the percent area of the dendritic neuropil occupied by MAP2 or terminals labeled by synaptophysin showed that both dendrites and terminals were significantly increased in the hippocampus of NitroMemantine-treated gp120-tg mice (Fig. 1b). These data suggest that treatment with NitroMemantine protects both dendritic and synaptic structures in gp120-tg mouse brain.
Fig. 1.
Effects of NitroMemantine (NM) treatment on HIV/gp120 transgenic mice. a Representative histological images of brain slices from six-month-old gp120 transgenic mice treated with NM. Staining for MAP2 positive neuronal dendrites (red) and synaptophysin (green) are shown in the hippocampus. b Quantification of immunoreactivity of MAP2 and synaptophysin in gp120 transgenic mice with NM treatment. In order to evaluate synaptic integrity, we measured the percent area of the neuropil occupied by immunoreactive terminals and dendrites. In the hippocampus of NM-treated gp120-tg mice, both MAP2+ dendrites and synaptophysin+ terminals were significantly increased compared to controls. Values presented as mean percentage difference from control + SEM, n = 6 mice per treatment group. *P < 0.04 for MAP2, *P < 0.02 for synaptophysin.
Chronic Overexpression of GluN3A in Wild-Type (WT) and gp120-tg Mice Decreases NeuN Expression in the Dentate Gyrus
Next, we sought to evaluate the effects of chronic overexpression of the GluN3A subunit in WT or gp120-tg mice. Overexpression of GluN3A has been shown to be both protective and detrimental in various models of neurodegenerative disorders, and the observed action may depend on the site of GluN3A (Nakanishi et al. 2009; Marco et al., 2013). To study the effect of GluN3A on gp120-tg mice, we generated GluN3A-overexpressing transgenic mice (GluN3A-tg) using the Tet-Off system. These mice carry two transgenes – the tetracycline transactivator (tTA) transgene controlled by the α-calmodulin dependent kinase II (CaMKII) promoter and the GluN3A transgene controlled by the tTA-responsive promoter. We previously showed that these GluN3A-tg mice express GluN3A in the broad area of the forebrain (Tong et al. 2008). For the current experiment, we genetically crossed GluN3A-tg mice with gp120-tg mice and evaluated the effects of chronic GluN3A overexpression on neuronal and synaptic marker expression by immunohistochemistry.
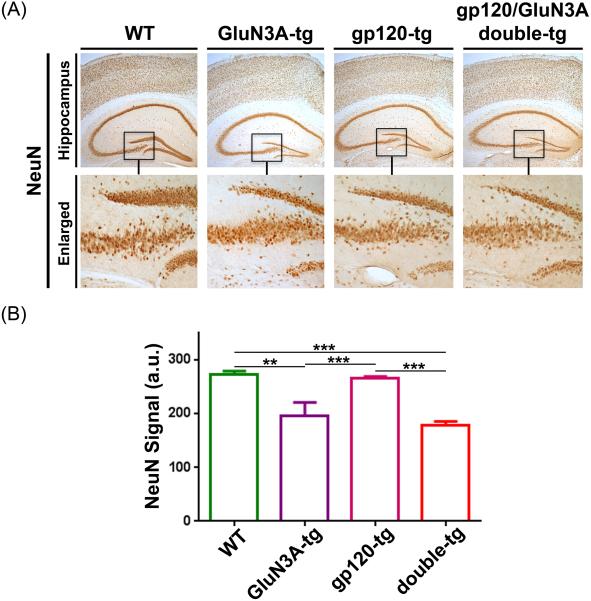
We examined the levels of the neuronal marker NeuN in the hippocampus of WT, GluN3A-tg, gp120-tg and gp120/GluN3A (double)-tg mice at 9 mos of age. Immunohistochemistry revealed that expression of GluN3A reduced NeuN in the dentate gyrus of the hippocampus (Fig. 2a). Quantification of NeuN signal revealed that chronic GluN3A overexpression significantly reduced NeuN in the dentate gyrus in gp120-tg mice somewhat greater than in WT mice (Fig. 2b). A significant (P < 0.05) but more subtle effect on NeuN dropout was observed in the CA1 region of the hippocampus in the same sections. Therefore, GluN3A overexpression in these models appears to lead to neurodegeneration in this area of the brain.
Fig. 2.
Effects of chronic overexpression of GluN3A in wild-type and gp120-trangenic (tg) mice. a Immunostaining with NeuN antibody on the cerebral cortex and hippocampal brain sections of WT, GluN3A-tg, gp120-tg and double (gp120/GluN3A)-tg mice. b Quantification of NeuN signals (in arbitrary units) in the dentate gyrus (DG) area of hippocampus and cerebral cortex in WT (n = 4), GluN3A tg (n = 5), gp120 tg (n = 7), and double tg mice (n = 7). Chronic overexpression of GluN3A results in significant decrease in NeuN immunoreactivity both in the DG area of both WT and gp120-tg mice. Values presented as mean percentage difference from control + SEM. **P < 0.01, ***P < 0.001.
Chronic Overexpression of GluN3A in WT and gp120-tg Mice Decreases MAP2 and Synaptophysin Immunoreactivity in the Hippocampus
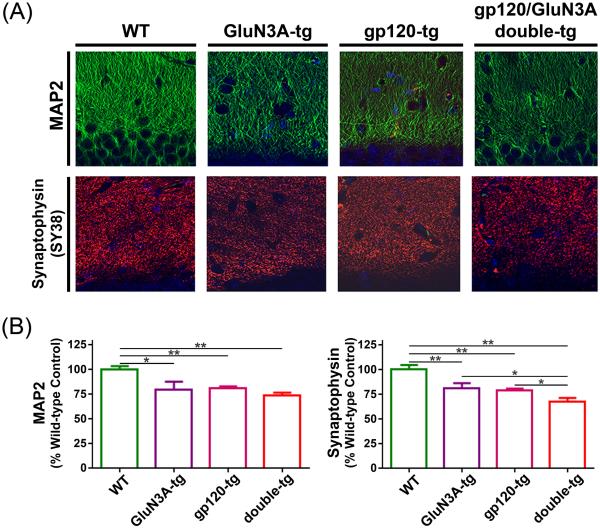
The degree of cognitive decline is most closely associated with dendritic and synaptic damage in HAND as well as in other dementias (Terry et al. 1991; Masliah et al. 1994; Everall et al. 1999). Hence, we next performed immunohistochemistry using MAP2 and synaptophysin antibodies on hippocampal sections. Representative images of MAP2 and synaptophysin staining show decrements in the hippocampus (Fig. 3a). Fig. 3b shows quantification in the hippocampus of MAP2+ dendrites and synaptophysin+ presynaptic terminals. MAP2 staining was decreased in GluN3A-tg and gp120-tg mice relative to WT mice. Strikingly, synaptophysin staining was reduced in both GluN3A-tg and gp120-tg mice compared to WT, but to an even more significant degree in the double transgenic (GluN3A/gp120) mouse compared to the single transgenics. This finding is consistent with the greatest degree of synaptic loss occurring in the GluN3A/gp120 double transgenic mouse hippocampus. It thus appears that GluN3A overexpression contributes to synaptic damage in these mice.
Fig. 3.
Effects of chronic overexpression of GluN3A in WT and gp120-trangenic (tg) mice. a Immunostaining with MAP2 and synaptophysin (SY38) antibodies on hippocampal brain sections of WT, GluN3A-tg, gp120-tg and double-tg (gp120/GluN3A) mice. b Quantification of MAP2 and synaptophysin signals in the dentate gyrus (DG) of hippocampus as percentage of WT control mice (n = 4) for GluN3A tg (n = 5), gp120 tg (n = 7), and double tg mice (n = 7). Chronic overexpression of GluN3A resulted in significant decrease in MAP2 labeling of dendrites and synaptophysin staining of terminals in the hippocampus. Double transgenic mice were the most significantly affected. *P < 0.05, **P < 0.01.
Discussion
In the current study, we sought to evaluate the potential role of NMDARs in neuronal and synaptic damage in HIV/gp120-tg mice using in vivo administration of the drug NitroMemantine or genetic overexpression of the modulatory NMDAR subunit GluN3A. Our results indicate that NitroMemantine is protective against gp120-induced neuronal damage and synaptic loss in the hippocampus. In contrast, chronic overexpression of GluN3A enhanced synaptic injury in both gp120-tg and WT mice.
Given that NitroMemantine is an NMDAR antagonist and that GluN3A acts as an inhibitory subunit of the NMDAR, how can we reconcile these two apparently contradictory outcomes? First, several differences in methodology between the two treatment groups can be highlighted. HIV/gp120 tg mice were treated with NitroMemantine between ages 6 and 9 months of age for three months, while GluN3A transgene expression, under control of the CaMKII promoter, was chronically overexpressed in the brain for the duration of the mouse’s life. In addition, while NitroMemantine was systemically administered, GluN3A overexpression was spatially restricted to the forebrain due to the nature of the genetic manipulation. These temporal and spatial differences in NMDAR inhibition might have led to differences in their effects on neuronal and synaptic injury.
Second, and probably more important, are the differential effects of this pharmacological intervention and the genetic approach. NitroMemantine has been shown to preferentially inhibit extrasynaptic compared to synaptic NMDARs due, at least in part, to the drug’s mechanism of action as an uncompetitive antagonist with a 2013). In contrast, genetically-overexpressed GluN3A subunits inhibit miniature excitatory postsynaptic currents mediated by NMDARs located synaptically as well as extrasynaptically-evoked currents (Tong et al. 2008). Consistent with this notion, Martinez-Turrilas et al. (2012) reported that overexpressed GluN3A subunits can be found at both synaptic and extrasynaptic sites by biochemical fractionation. It has been established that extrasynaptic versus synaptic NMDAR activation trigger distinct signal pathways, leading to differential outcomes. Namely, extrasynaptic NMDAR activation contributes to excitotoxic signaling pathways, while synaptic NMDAR activation protects against excitotoxicity (Hardingham and Bading 2010). If both extrasynaptic and synaptic receptors are blocked chronically, then the ill effects of synaptic receptors predominate, resulting in synaptic damage and neurotoxicity (Okamoto et al. 2009). Therefore, it is likely that the pathological effects of synaptic GluN3A overexpression outweigh the beneficial effects on extrasynaptic GluN3A. This same phenomenon has also been observed in a Huntington’s disease mouse model (Marco et al. 2013). In contrast, NitroMemantine’s preferential inhibition of extrasynaptic NMDARs renders this drug beneficial against excitotoxic insults. Mechanism notwithstanding, our studies indicate that NitroMemantine has potential as a neurotherapeutic against HAND.
Acknowledgments
We thank James Parker for graphic assistance. This work was supported in part by NIH grants P01 HD29587, P30 NS076411, R21 NS083415, and R01 EY09024 to S.A.L. Behavior Research Foundation to S.A.L.
Footnotes
Compliance with Ethical Standards
Competing interests: S.A.L. is the named inventor on worldwide patents for memantine (Namenda®) and NitroMemantine for the treatment of neurodegenerative diseases. Following Harvard University guidelines, he participates in a royalty sharing agreement with his former institution Harvard Medical School/Boston Children’s Hospital for the licensing of the memantine patents to Forest Laboratories/Actavis. He is also Scientific Co-Founder of Adamas Pharmaceuticals, Inc., which has agreements with Forest Laboratories/Actavis for co-developing long-lasting formulations of memantine. The authors declare that they have no other competing interests.
References
- Das S, Sasaki YF, Rothe T, et al. Increased NMDA current and spine density in mice lacking the NMDA receptor subunit NR3A. Nature. 1998;393:377–381. doi: 10.1038/30748. [DOI] [PubMed] [Google Scholar]
- Everall IP, Heaton RK, Marcotte TD, et al. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. Brain Pathol. 1999;9:209–217. doi: 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garden GA, Budd SL, Tsai E, et al. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J Neurosci. 2002;22:4015–4024. doi: 10.1523/JNEUROSCI.22-10-04015.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorantla S, Liu J, Sneller H, et al. Copolymer-1 induces adaptive immune anti-inflammatory glial and neuroprotective responses in a murine model of HIV-1 encephalitis. J Immunol. 2007;179:4345–4356. doi: 10.4049/jimmunol.179.7.4345. [DOI] [PubMed] [Google Scholar]
- Hardingham GE, Bading H. Synaptic versus extrasynaptic NMDA receptor signalling: implications for neurodegenerative disorders. Nat Rev Neurosci. 2010;11(10):682–696. doi: 10.1038/nrn2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YJ, Digicaylioglu M, Russo R, et al. Erythropoietin plus insulin-like growth factor-I protects against neuronal damage in a murine model of human immunodeficiency virus-associated neurocognitive disorders. Ann Neurol. 2010;68:342–352. doi: 10.1002/ana.22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kehoe LA, Bellone C, De Roo M, et al. GluN3A promotes dendritic spine pruning and destabilization during postnatal development. J Neurosci. 2014;34(28):9213–9221. doi: 10.1523/JNEUROSCI.5183-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolson DL. Neuropathogenesis of central nervous system HIV-1 infection. Clin Lab Med. 2002;22:703–717. doi: 10.1016/s0272-2712(02)00009-4. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Rosenberg PA. Mechanisms of disease: Excitatory amino acids as a final common pathway in neurologic disorders. N Engl J Med. 1994;330:613–622. doi: 10.1056/NEJM199403033300907. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Gendelman HE. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: Memantine and beyond. Nature Rev Drug Disc. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- Marco S, Giralt A, Petrovic MM, et al. Suppressing aberrant GluN3A expression rescues synaptic and behavioral impairments in Huntington's disease models. Nat Med. 2013;19(8):1030–1038. doi: 10.1038/nm.3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Turrillas R, Puerta E, Chowdhury D, Marco S, Watanabe M, Aguirre N, Pérez-Otaño I. The NMDA receptor subunit GluN3A protects against 3-nitroproprionic-induced striatal lesions via inhibition of calpain activation. Neurobiol Dis. 2012;48:290–298. doi: 10.1016/j.nbd.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Masliah E, Mallory M, Hansen L, DeTeresa R, Alford M, Terry R. Synaptic and neuritic alterations during the progression of Alzheimer's disease. Neurosci Lett. 1994;174:67–72. doi: 10.1016/0304-3940(94)90121-x. [DOI] [PubMed] [Google Scholar]
- McArthur JC. HIV dementia: an evolving disease. J Neuroimmunol. 2004;157:3–10. doi: 10.1016/j.jneuroim.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Mocchetti I, Bachis A, Masliah E. Chemokine receptors and neurotrophic factors: potential therapy against aids dementia? J Neurosci Res. 2008;86:243–255. doi: 10.1002/jnr.21492. [DOI] [PubMed] [Google Scholar]
- Nakanishi N, Tu S, Shin Y, et al. Neuroprotection by the NR3A subunit of the NMDA receptor. J Neurosci. 2009;29:5260–5265. doi: 10.1523/JNEUROSCI.1067-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S-i, Pouladi M, Talantova M, et al. Balance between synaptic versus extrasynaptic NMDA receptor activity influences inclusions and neurotoxicity of mutant huntingtin. Nature Med. 2009;15:1407–1413. doi: 10.1038/nm.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potula R, Poluektova L, Knipe B, et al. enhances elimination of virus-infected macrophages in an animal model of HIV-1 encephalitis. Blood. 106:2382–2390. doi: 10.1182/blood-2005-04-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki YF, Rothe T, Premkumar LS, et al. Characterization and comparison of the NR3A subunit of the NMDA receptor in recombinant systems and primary cerebrocortical neurons. J Neurophysiol. 2002;87:2052–2063. doi: 10.1152/jn.00531.2001. [DOI] [PubMed] [Google Scholar]
- Schifitto G, Navia GA, Yiannoustsos CT, et al. Memantine and HIV-associated cognitive impairment: a neuropsychological and proton magnetic resonance spectroscopy study. AIDS. 2007;21:1877–1886. doi: 10.1097/QAD.0b013e32813384e8. [DOI] [PubMed] [Google Scholar]
- Talantova M, Sanz-Blasco S, Zhang X, et al. Aβ induces astrocytic glutamate release, extrasynaptic NMDA receptor activation, and synaptic loss. Proc Natl Acad Sci U S A. 2013;110:E2518–2527. doi: 10.1073/pnas.1306832110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry RD, Masliah E, Salmon DP, et al. Physical basis of cognitive alterations in Alzheimer's disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;40:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Rockenstein EM, et al. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- Toggas SM, Masliah E, Mucke L. Prevention of HIV-1 gp120-induced neuronal damage in the central nervous system of transgenic mice by the NMDA receptor antagonist memantine. Brain Res. 1996;706:303–307. doi: 10.1016/0006-8993(95)01197-8. [DOI] [PubMed] [Google Scholar]
- Tong G, Takahashi H, Tu S, et al. Modulation of NMDA receptor properties and synaptic transmission by the NR3A subunit in mouse hippocampal and cerebrocortical neurons. J Neurophysiol. 2008;99:122–132. doi: 10.1152/jn.01044.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H-K, Liu XB, Matos MF, et al. Temporal and anatomical expression of NMDA receptor subunit NR3A in the mammalian brain. J Comp Neurol. 2002;450:303–317. doi: 10.1002/cne.10314. [DOI] [PubMed] [Google Scholar]
- Xia P, Chen H-SV, Zhang D, Lipton SA. Memantine preferentially blocks extrasynaptic over synaptic NMDA receptor currents in hippocampal autapses. J Neurosci. 2010;30:11246–11250. doi: 10.1523/JNEUROSCI.2488-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]