Abstract
Background
Prostate cancer persisting in the primary site after systemic therapy may contribute to emergence of resistance and progression. We previously demonstrated molecular characteristics of lethal cancer in the prostatectomy specimens of patients presenting with lymph node metastasis after chemohormonal treatment. Here we report the post-treatment outcomes of these patients and assess whether a link exists between surgery and treatment-free/cancer-free survival.
Methods
Patients with either clinically detected lymph node metastasis or primaries at high risk for nodal dissemination were treated with androgen ablation (ADT) and docetaxel. Those responding with prostate-specific antigen (PSA) concentration <1 ng/mL were recommended surgery 1 year from enrollment. ADT was withheld postoperatively. The rate of survival without biochemical progression 1 year after surgery was measured to screen for efficacy.
Results
Forty patients were enrolled and 39 were evaluable. Three patients (7.7%) declined surgery. Of the remaining 36, 4 patients experienced disease progression during treatment and 4 more did not reach PSA <1. Twenty-six patients (67%) completed surgery, and 13 (33%) were also progression-free 1 year postoperatively (8 with undetectable PSA). With a median follow-up of 61 months, time to treatment failure was 27 months in the patients undergoing surgery. The most frequent patterns of first disease recurrence were biochemical (10 patients) and systemic (5).
Conclusions
Half of the patients undergoing surgery were off treatment and progression-free 1 year following completion of all therapy. These results suggest that integration of surgery is feasible and may be superior to systemic therapy alone for selected prostate cancer patients presenting with nodal metastasis.
Keywords: lymphatic metastasis, prostate cancer, hormone therapy, docetaxel, prostatectomy
Introduction
In everyday practice, surgery or radiation are frequently considered futile in the treatment of prostate cancer patients presenting with clinically detected metastases in lymph nodes (LN).1 Although the prognosis of these patients is generally poorer than that of those whose disease has not disseminated, specific features of the primary tumor remain of critical importance.2,3 Gleason score (tumor grade), primary tumor bulk (T-stage), and extent of LN involvement (including number of positive nodes, tumor burden within nodes, and extrapelvic metastasis) have all been noted to affect outcomes.4,5 Poorly differentiated primary tumors and ≥3 involved nodes confer the highest risk of systemic progression and cancer–specific death.1,2,6,7 The optimal integration of systemic and local therapies for these patients remains a dilemma.
Men presenting with nodal metastases are commonly treated with long-term androgen-deprivation therapy (ADT) alone or with radiation. The use of single modality ADT is associated with a high probability of eventual local disease progression (up to 60%), which may result in urinary retention, hematuria, and pain, among other symptoms.8 Results from studies integrating ADT and curative-intent surgery/radiation in men at risk for or with regional cancer spread have led to an increasing recognition that local therapy is an essential part of the optimal treatment for these patients (see Verhagen et al.9 for a recent review).3,6,7,10–18
We hypothesized that in patients clinically presenting with nodal metastasis or with primaries unlikely to be completely resected upfront, prostate cancer progression might be prevented or delayed using surgical consolidation of systemic therapy. A trial was designed that included 1 year of presurgical treatment followed in responding patients by prostatectomy. We previously reported on the molecular characterization of the residual cancers, and demonstrated that potentially lethal cancer foci persist in the removed prostate.19 Here we present the post-treatment outcomes of these selected patients, with the primary goal to determine the rate of prostate-specific antigen (PSA) <1 ng/mL at 1 year following completion of therapy and treatment-free survival, and secondary goal to link outcomes to morphologic and clinical characteristics.
Materials and Methods
Patients and treatment
In this single-arm phase II clinical trial (NCT01076335), patients were required to have histologically confirmed prostate adenocarcinoma and no surgical contraindications. Enrollment took place from April 21, 2005 through March 31, 2008. At least one of the following inclusion criteria was needed: biopsy/pretreatment dissection confirming LN metastasis; pelvic and/or abdominal lymphadenopathy ≥2 cm in typical distribution (aortic/common iliac); or features in the primary tumor implying very high risk for lymph node dissemination (“suspected”), including at least 1 of the following: (1) Gleason score ≥8 plus PSA ≥25 ng/mL, (2) clinical (c)T3 disease together with Gleason score ≥7, or (3) cT4 disease. Patients with small-cell/sarcomatoid variants, clinical/radiological evidence of bone or visceral metastatic disease (M1b or M1c) or with prior chemotherapy or >6 months of ADT were excluded. All patients signed an institutional review board-approved written informed consent form before treatment.
ADT was combined with docetaxel and followed by surgical consolidation in eligible patients. Luteinizing hormone–releasing hormone agonist therapy was given for approximately 1 year until the actual surgical date. Oral bicalutamide (50 mg daily) was permitted. Docetaxel (35 mg/m2) was administered intravenously on days 1, 8, 15, and 22 every 6 weeks for a total of three cycles in the first 6 months of the preoperative period.
Patients with PSA <1 ng/mL at the end of the systemic treatment period and no clinical progression were offered open or robot-assisted laparoscopic radical prostatectomy (per surgeon’s preference) and extended bilateral pelvic LN dissection, scheduled within 6 weeks of completion of 1 year of ADT (from study enrollment). Surgery was not attempted in patients with grossly unresectable–appearing disease.
Disease evaluation and monitoring
PSA concentrations were assessed before initiation of each chemotherapy cycle, every 3 months, and within 6 weeks of surgery. Tumor imaging (bone scan, chest x-ray, computed tomography or magnetic resonance imaging of abdomen-pelvis) was performed before treatment and surgery and whenever felt indicated. After surgery, PSA and testosterone measurements were obtained every 3 months (first 12 months) and every 6 months thereafter until progression (in the case of testosterone, until recovery or return to baseline levels).
Toxicity
Toxicity was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events v3.0. Two docetaxel dose reductions (30 mg/m2 and 25) were allowed. Patients needing more than two reductions or delays beyond 2 weeks owing to toxicity were withdrawn.
Surgical complications were assessed during and within 90 days of the procedure by using Clavien grading.20 Urinary continence was evaluated by patient-reported daily pad usage 6 months postoperatively, and potency at 12 months.
End points and definition of treatment failure
The primary end point of the study was to define the rate of event-free survival (ie, freedom from treatment failure) 1 year postoperatively. Secondary end points included pathological complete response (pCR) rate, perioperative/postoperative morbidity, and tissue biomarker analysis.
Treatment failure was defined as objective tumor progression during therapy or in the year after surgery, confirmed postoperative PSA ≥1 ng/mL, or any postoperative radiation, hormonal, or other systemic therapy. The PSA ≥1 ng/mL level to determine failure was chosen because of (1) the very low probability of lower concentrations to relate to clinically visible metastases, and (2) its use in clinical practice as threshold to initiate ADT after recurrence following surgery. Patients who did not undergo surgery within 8 weeks of completing 1 year of therapy on protocol (for any reason, including patient refusal) were counted as treatment failure, as were patients whose surgery was begun and aborted.
Statistical analyses
The study was designed to enroll a maximum of 40 patients and to be stopped early if the data suggested that p (θ>0.50 | data) <5% (θ being the proportion of patients PSA-progression-free at 1 year postoperatively), assuming prior beta distribution with mean 0.5 and variance 0.083. Time to treatment failure (TTF) was estimated using the Kaplan-Meier method and calculated from the date of study enrollment to the date of progression or off-treatment date (or last follow-up, if missing), whichever first. All statistical analyses were performed with SAS 9.3 (SAS Institute Inc., Cary, NC) and S-Plus 8.2 (TIBCO Software Inc., Palo Alto, CA).
Results
Patients’ characteristics
Forty patients were enrolled. Table 1 details demographics and baseline tumor features. Twenty-one patients (52.5%) had cN1 disease, 12 (30%) suspected LN involvement, and 7 (17.5%) clinical disease extension to nonregional LNs (M1a). Pathologic LN involvement at presentation was demonstrated on biopsy in 18/28 patients (64%) with N1/M1a disease. One patient (2.5%) declined presurgical therapy after enrollment and was excluded from subsequent analyses.
Table 1.
Patients’ characteristics
| Characteristic | Patients (n=40) |
|---|---|
| Median age at enrollment, years (range) | 61.5 (38–75) |
| Race/ethnicity, no. (%) | |
| Caucasian | 36 (90) |
| African American | 3 (7.5) |
| Hispanic | 1 (2.5) |
| Clinical T stage, no. (%)* | |
| T2b | 5 (12.5) |
| T2c | 5 (12.5) |
| T3a | 9 (22.5) |
| T3b | 14 (35) |
| T4 | 7 (17.5) |
| Lymph nodes status, no. (%)* | |
| Suspected** | 12 (30) |
| N1 | 21 (52.5) |
| M1a | 7 (17.5) |
| Biopsy Gleason score, no. (%) | |
| 7 | 3 (7.5) |
| 8 | 7 (17.5) |
| 9 | 27 (67.5) |
| 10 | 3 (7.5) |
| PSA before presurgical therapy, ng/mL (range) | 26.7 (1.4–479.5) |
| >25 | 15 (37) |
| ≤25 | 26 (63) |
2002 American Joint Committee on Cancer (AJCC) staging system.
Features in the primary tumor implying very high risk for lymph node dissemination (see Materials and Methods, Patients and Treatment section for description.
Presurgical therapy
Systemic treatment took place as planned in 39 patients. Of these, 37 (95%) completed the intended 12 weekly doses of docetaxel; the other 2 patients missed a single dose. Two patients (5%) required docetaxel dose reduction to 30 mg/m2 during cycle 1. No dose reductions occurred during cycles 2–3. The most common treatment-related events were anemia (15 patients, all grade 1), fatigue (11), hyperglycemia (7), aspartate and/or alanine aminotransferase elevation (5), and limb edema (5). Table 2 shows grade >2 chemotherapy–related side effects. No patient withdrew for toxicity. Fourteen of the 39 patients (36%) received bicalutamide.
Table 2.
Chemotherapy-related grade >2 preoperative medical toxicity (n=39)
| Toxicity | Grade 3 | Grade 4 | Total |
|---|---|---|---|
| Hyponatremia | 0 | 1 | 1 |
| Atrial fibrillation | 1 | 0 | 1 |
| Pericardial effusion | 1 | 0 | 1 |
| ALT/AST elevation | 1 | 0 | 1 |
Common Terminology Criteria for Adverse Events v3.0.
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Surgery
Three patients (7.7%) voluntarily declined surgery following completion of the systemic therapy portion of the study. After approximately 1 year of presurgical therapy, 28 of the remaining 36 patients (78%) demonstrated no clinical progression and had PSA <1 ng/mL, moving to prostatectomy and pelvic LN dissection. However, surgery could not be completed in 2 of those patients (dense pelvic adhesions and intraoperative hypoxemia, one patient each). Twenty of the 26 patients (77%) underwent open procedures; the remaining 6 (23%) had robot-assisted surgery. The median hospitalization length was 2 days (range, 1–16).
Four patients (11%) of the 39 had no clinical progression, but their PSA was ≥1 ng/mL (mean, 2.9 μg/L; range, 1.6–4.4). All 4 elected to have surgery off study. Another 4 patients (11%) experienced clinical progression preoperatively and discontinued treatment; of these, 2 eventually underwent cystoprostatectomy for symptom control. Figure 1 shows the study profile.
Figure 1. CONSORT diagram showing study’s profile.
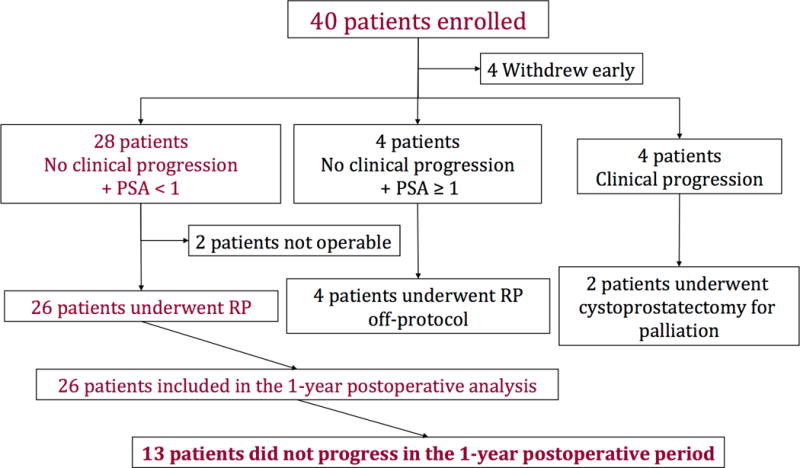
RP, radical prostatectomy with bilateral pelvic lymph node dissection.
Perioperative complications
The median intraoperative blood loss was 0.7 L (range, 0.1–2.9 L). Four of the 26 patients who underwent surgery on study (11%) required intraoperative blood transfusion (one unit, n=1; two, n=2; six, n=1). One patient experienced an intraoperative rectal injury that was successfully repaired. No perioperative deaths occurred. Clavien-graded complications and functional recovery data are respectively shown in Supplementary Table and Supplementary Results.
Pathology
Table 3 summarizes the surgical specimens’ pathological characteristics. The median number of lymph nodes excised was 17 (range 4–49). Although most (20/26; 77%) demonstrated extraprostatic extension, the negative surgical margins rate was high (24/26; 92%). Two patients (8%) experienced pCRs. We reported the biomarker analysis in a separate publication.19
Table 3.
Surgical pathology results (n=26)
| Parameter | No. (%) |
|---|---|
| Pathological T stage | |
| ypT0* | 2 (8) |
| ypT2 | 4 (15) |
| ypT3a | 2 (8) |
| ypT3b | 18 (69) |
| ypT4 | 0 (0) |
| Surgical margins | |
| Involved | 2 (8) |
| Free | 24 (92) |
| Pelvic lymph node involvement | |
| Present | 12 (46) |
| Absent | 14 (54) |
Includes one patient with a cluster of residual tumor cells within a vascular space.
Postsurgical outcomes
Among the 26 patients who completed chemohormonal therapy and underwent surgery per protocol, 13 (50% [33% of the initial 39]) experienced treatment success, ie, PSA <1 ng/mL for at least 1 year postoperatively without clinical progression/additional treatment. PSA remained <0.1 ng/mL at 1 year in 8 of these patients (31% [21% of the initial 39]). The disease of 6 (23% [15% of the initial 39]) has not yet recurred (median follow-up 52.9 months); 4 continue to have undetectable PSA. These 6 patients include 5 with locally advanced adenocarcinoma at diagnosis, 2 of them with PSA >120 ng/mL and a separate 1 with high-volume Gleason score 10 (5+5) in the diagnostic biopsies; and 1 patient with biopsy-confirmed lymphadenopathy up to retrocrural space who achieved a pCR.
Total testosterone measurements for the first 4 months postoperatively were available for 22/26 patients who completed treatment. In 11/22 (50%), testosterone had recovered to >150 ng/dL. Twelve of the 13 patients who experienced treatment success had determinations available 1 year postoperatively: testosterone was >150 ng/dL in 11 (92%).
Of the 20 patients whose disease recurred after completion of treatment (Table 4), 4 (15% of the 26) experienced local recurrence; only 2 (8%) were symptomatic (including ureteral obstruction). The surgical margins were negative in all 4 patients.
Table 4.
Patterns of disease recurrence among patients completing treatment (n=20)
| Pattern | No. (%) |
|---|---|
| Biochemical (PSA only) | 10 (50) |
| Local* | 4 (20) |
| Asymptomatic | 2 (10) |
| Symptomatic | 2 (10) |
| Systemic* | 7 (35) |
| Lymph nodes | 4 (20) |
| Lymph nodes + bone | 1 (5) |
| Liver + lymph nodes + peritoneum | 1 (5) |
| Brain† | 1 (5) |
| Unknown | 1 (5) |
Two patients experienced synchronous local and systemic disease recurrence.
Prostatic adenocarcinoma in resected cerebellar mass
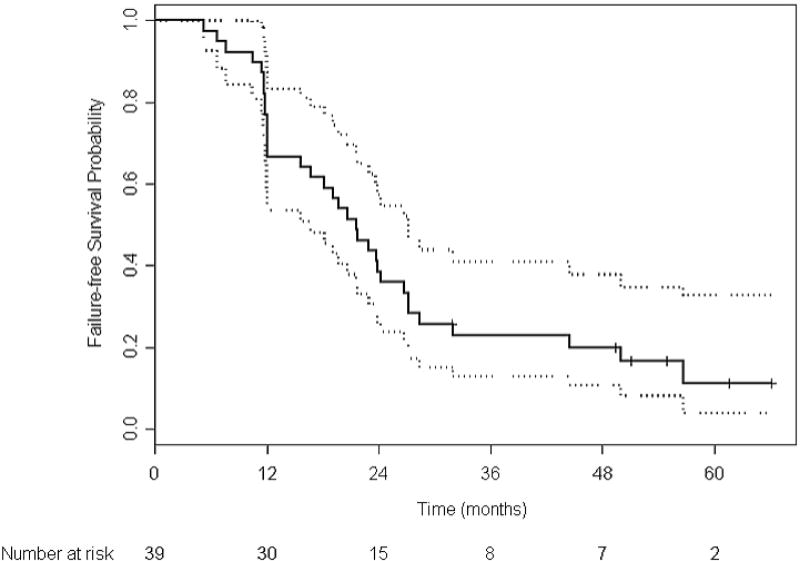
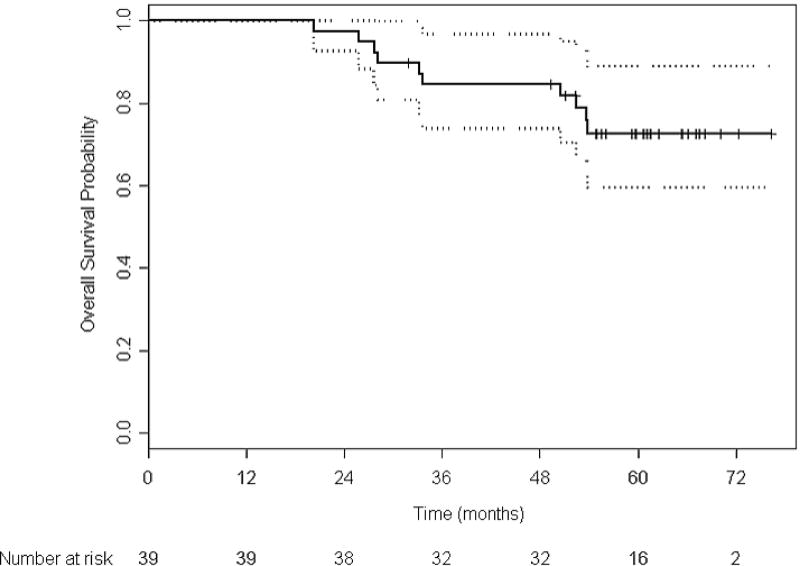
With a median follow-up of 61 months, the overall median TTF in the 39 patients receiving presurgical therapy was 21.6 months (95% CI 12.1–26.7) (Figure 2a). The median TTF in the 26 patients who underwent surgery per protocol was 27.0 months (95% CI 21.7–44.4). With 10 deaths (9 prostate cancer-related, 1 due to an accident), the median overall survival has not been reached (Figure 2b). Four of the deaths occurred in patients who had completed protocol treatment.
Figure 2. Kaplan-Meier estimates of event-free survival (A) and overall survival (B) in the 39 patients receiving presurgical therapy.
The median time to treatment failure was 21.6 months (95% CI 12.1–26.7 months [dashed lines]). Among the six patients who were failure free at their last assessment, the median follow-up time was 52.9 months.
Discussion
Participants in this clinical trial presenting with clinically evident nodal disease or advanced primaries not meeting criteria for curative surgery upfront (70% and 30% of the patients, respectively), received systemic treatment for 1 year combining ADT and chemotherapy. We reasoned that this strategy would help identify those most likely to benefit from consolidative surgery. Analysis of the residual tumors also allowed characterization of molecular changes associated with the development of the lethal castrate-resistant phenotype.19
We envisioned a twofold benefit from this approach: to allow for a meaningful temporal break in androgen ablation (favored by setting the PSA threshold to pronounce failure at 1 ng/mL) and, more importantly, to prevent or delay prostate cancer progression. Withholding hormonal therapy for at least a year probably resulted in decreased morbidity and improved quality of life for the individuals in whom it was withheld, although this was not formally tested in the study. Regarding disease progression, our results show a respectable TTF >2 years for the patients completing surgery, a significant number of whom (50% here) may remain with PSA <1 ng/mL and clinically disease-free for a minimum of 1 year while at very low risk for recurrence at the local site. Our 15% total and 8% symptomatic local relapse rates are better than those reported in retrospective series of prostatectomy alone8,14 and comparable to prostatectomy and adjuvant ADT and/or radiotherapy.8,21 Moreover, that 15% of patients remained free from recurrence with almost four-and-a-half years median follow-up is worth noting for this prostate cancer set. Our limited sample size, follow-up time and lack of comparator nonsurgical arm, though, warrant caution in making comparisons.
Tolerance to the combination of weekly docetaxel and ADT was good and predictable. This study was designed at a time when docetaxel was the only systemic therapy having shown a survival advantage for castrate-resistant prostate cancer and its preoperative use still considered promising. Unfortunately, the results of studies of taxanes as single agents or in combination with ADT in previously untreated high-risk patients have suggested no enhanced benefit over the use of ADT alone concerning pathologic as well as long-term outcomes, even when the docetaxel was administered every 3 weeks and at doses higher than that in our trial.22–26 Consistently, >75% and approximately 50% of our patients had evidence of respectively residual locally advanced and pelvic LN–metastatic disease after completing treatment, and 9 have died of progressive prostate cancer. Very recent data from a randomized phase III clinical trial (CHAARTED) suggests that, among patients newly diagnosed with metastatic hormone sensitive prostate cancer, those presenting with visceral disease and/or ≥4 bone lesions are the ones that benefit the most from the early use of docetaxel.27
In current clinical practice, the decision to offer surgery and/or radiation to patients presenting with LN metastasis after a period of ADT is not well established. It depends on careful assessment of the quality of the achieved response and the patient’s context, including general health, comorbidities, and treatment preferences. We chose 1 year of presurgical therapy to allow for slow responders to be considered for prostatectomy but, in retrospect, 6- 9 months would have probably been enough since chemotherapy was completed in the first half and few additional symptomatic/PSA responses occurred in the second (not shown). Six months is also a useful time frame for identifying prostate cancers with an “anaplastic” phenotype, which demonstrate decreased dependence on androgen-regulated pathways by their behavior.28 In considering consolidative treatment, it is important to notice that the PSA response alone is not enough and can be misleading, as demonstrated by our observed 77% patients with PSA <1 and high residual (extraprostatic) volumes of cancer.
To the best of our knowledge, this is the first prospective study of presurgical therapy and consolidative surgery primarily directed to patients newly diagnosed with lymph node-metastatic prostate cancer. Although significantly limited by a relatively small patient set and lack of a comparator ADT-only arm, precluding any practical conclusions, our results suggest that such integrative strategy is feasible, and at a minimum likely to enhance disease control in carefully selected prostate cancer patients presenting with LN metastasis. The use of weekly docetaxel did not result in better pathologic or survival outcomes than might be seen with ADT alone, but the presurgical therapy still helped select patients for surgery. Removal of residual treatment-resistant foci in the primary site and lymph nodes may retard metastatic progression in some patients, thus allowing for the opportunity to delay hormonal therapy after surgery, or cure others. However, the effectiveness of presurgical therapies must be improved before this approach can be considered for phase III testing, since the greatest survival benefit is to likely be obtained in those achieving major responses.
Supplementary Material
Acknowledgments
Funding: This work was supported in part by the National Cancer Institute at the National Institutes of Health through MD Anderson’s Support Grant P30-CA016672.
Footnotes
Financial disclosure: None.
Supplementary information is available on the Prostate Cancer and Prostatic Diseases website
References
- 1.Swanson GP, Thompson IM, Basler J. Current status of lymph node-positive prostate cancer: Incidence and predictors of outcome. Cancer. 2006;107(3):439–450. doi: 10.1002/cncr.22034. [DOI] [PubMed] [Google Scholar]
- 2.Cheng L, Zincke H, Blute ML, Bergstralh EJ, Scherer B, Bostwick DG. Risk of prostate carcinoma death in patients with lymph node metastasis. Cancer. 2001;91(1):66–73. doi: 10.1002/1097-0142(20010101)91:1<66::aid-cncr9>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 3.von Bodman C, Godoy G, Chade DC, Cronin A, Tafe LJ, Fine SW, et al. Predicting biochemical recurrence-free survival for patients with positive pelvic lymph nodes at radical prostatectomy. J Urol. 2010;184(1):143–148. doi: 10.1016/j.juro.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofer MD, Kuefer R, Huang W, Li H, Bismar TA, Perner S, et al. Prognostic factors in lymph node-positive prostate cancer. Urology. 2006;67(5):1016–1021. doi: 10.1016/j.urology.2005.10.055. [DOI] [PubMed] [Google Scholar]
- 5.Cheng L, Bergstralh EJ, Cheville JC, Slezak J, Corica FA, Zincke H, et al. Cancer volume of lymph node metastasis predicts progression in prostate cancer. Am J Surg Pathol. 1998;22(12):1491–1500. doi: 10.1097/00000478-199812000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Daneshmand S, Quek ML, Stein JP, Lieskovsky G, Cai J, Pinski J, et al. Prognosis of patients with lymph node positive prostate cancer following radical prostatectomy: long-term results. J Urol. 2004;172(6 Pt 1):2252–2255. doi: 10.1097/01.ju.0000143448.04161.cc. [DOI] [PubMed] [Google Scholar]
- 7.Briganti A, Karnes JR, Da Pozzo LF, Cozzarini C, Gallina A, Suardi N, et al. Two positive nodes represent a significant cut-off value for cancer specific survival in patients with node positive prostate cancer. A new proposal based on a two-institution experience on 703 consecutive N+ patients treated with radical prostatectomy, extended pelvic lymph node dissection and adjuvant therapy. Eur Urol. 2009;55(2):261–270. doi: 10.1016/j.eururo.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 8.Wiegand LR, Hernandez M, Pisters LL, Spiess PE. Surgical management of lymph-node-positive prostate cancer: improves symptomatic control. BJU Int. 2011;107(8):1238–1242. doi: 10.1111/j.1464-410X.2010.09657.x. [DOI] [PubMed] [Google Scholar]
- 9.Verhagen PC, Schroder FH, Collette L, Bangma CH. Does local treatment of the prostate in advanced and/or lymph node metastatic disease improve efficacy of androgen-deprivation therapy? A systematic review. Eur Urol. 2010;58(2):261–269. doi: 10.1016/j.eururo.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Grimm MO, Kamphausen S, Hugenschmidt H, Stephan-Odenthal M, Ackermann R, Vogeli TA. Clinical outcome of patients with lymph node positive prostate cancer after radical prostatectomy versus androgen deprivation. Eur Urol. 2002;41(6):628–634. doi: 10.1016/s0302-2838(02)00134-3. [DOI] [PubMed] [Google Scholar]
- 11.Boorjian SA, Thompson RH, Siddiqui S, Bagniewski S, Bergstralh EJ, Karnes RJ, et al. Long-term outcome after radical prostatectomy for patients with lymph node positive prostate cancer in the prostate specific antigen era. J Urol. 2007;178(3 Pt 1):864–870. doi: 10.1016/j.juro.2007.05.048. [DOI] [PubMed] [Google Scholar]
- 12.Engel J, Bastian PJ, Baur H, Beer V, Chaussy C, Gschwend JE, et al. Survival benefit of radical prostatectomy in lymph node-positive patients with prostate cancer. Eur Urol. 2010;57(5):754–761. doi: 10.1016/j.eururo.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 13.Steuber T, Budaus L, Walz J, Zorn KC, Schlomm T, Chun F, et al. Radical prostatectomy improves progression-free and cancer-specific survival in men with lymph node positive prostate cancer in the prostate-specific antigen era: a confirmatory study. BJU Int. 2011;107(11):1755–1761. doi: 10.1111/j.1464-410X.2010.09730.x. [DOI] [PubMed] [Google Scholar]
- 14.Dorin RP, Lieskovsky G, Fairey AS, Cai J, Daneshmand S. Outcomes after radical prostatectomy for patients with clinical stages T1-T2 prostate cancer with pathologically positive lymph nodes in the prostate-specific antigen era. Urol Oncol. 2012 doi: 10.1016/j.urolonc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Schroder FH, Kurth KH, Fossa SD, Hoekstra W, Karthaus PP, De Prijck L, et al. Early versus delayed endocrine treatment of T2-T3 pN1-3 M0 prostate cancer without local treatment of the primary tumour: final results of European Organisation for the Research and Treatment of Cancer protocol 30846 after 13 years of follow-up (a randomised controlled trial) Eur Urol. 2009;55(1):14–22. doi: 10.1016/j.eururo.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Messing EM, Manola J, Yao J, Kiernan M, Crawford D, Wilding G, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7(6):472–479. doi: 10.1016/S1470-2045(06)70700-8. [DOI] [PubMed] [Google Scholar]
- 17.Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373(9660):301–308. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- 18.Mottet N, Peneau M, Mazeron JJ, Molinie V, Richaud P. Addition of radiotherapy to long-term androgen deprivation in locally advanced prostate cancer: an open randomised phase 3 trial. Eur Urol. 2012;62(2):213–219. doi: 10.1016/j.eururo.2012.03.053. [DOI] [PubMed] [Google Scholar]
- 19.Tzelepi V, Efstathiou E, Wen S, Troncoso P, Karlou M, Pettaway CA, et al. Persistent, biologically meaningful prostate cancer after 1 year of androgen ablation and docetaxel treatment. J Clin Oncol. 2011;29(18):2574–2581. doi: 10.1200/JCO.2010.33.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 21.Bolla M, van Poppel H, Collette L, van Cangh P, Vekemans K, Da Pozzo L, et al. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366(9485):572–578. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 22.Konety BR, Eastham JA, Reuter VE, Scardino PT, Donat SM, Dalbagni G, et al. Feasibility of radical prostatectomy after neoadjuvant chemohormonal therapy for patients with high risk or locally advanced prostate cancer: results of a phase I/II study. J Urol. 2004;171(2 Pt 1):709–713. doi: 10.1097/01.ju.0000108122.36893.5a. [DOI] [PubMed] [Google Scholar]
- 23.Febbo PG, Richie JP, George DJ, Loda M, Manola J, Shankar S, et al. Neoadjuvant docetaxel before radical prostatectomy in patients with high-risk localized prostate cancer. Clin Cancer Res. 2005;11(14):5233–5240. doi: 10.1158/1078-0432.CCR-05-0299. [DOI] [PubMed] [Google Scholar]
- 24.Dreicer R, Magi-Galluzzi C, Zhou M, Rothaermel J, Reuther A, Ulchaker J, et al. Phase II trial of neoadjuvant docetaxel before radical prostatectomy for locally advanced prostate cancer. Urology. 2004;63(6):1138–1142. doi: 10.1016/j.urology.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 25.Chi KN, Chin JL, Winquist E, Klotz L, Saad F, Gleave ME. Multicenter phase II study of combined neoadjuvant docetaxel and hormone therapy before radical prostatectomy for patients with high risk localized prostate cancer. J Urol. 2008;180(2):565–570. doi: 10.1016/j.juro.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Mellado B, Font A, Alcaraz A, Aparicio LA, Veiga FJ, Areal J, et al. Phase II trial of short-term neoadjuvant docetaxel and complete androgen blockade in high-risk prostate cancer. Br J Cancer. 2009;101(8):1248–1252. doi: 10.1038/sj.bjc.6605320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sweeney C, Chen Y-H, Carducci MA, Liu G, Jarrard DF, Eisenberger MA. Impact on overall survival (OS) with chemohormonal therapy versus hormonal therapy for hormone-sensitive newly metastatic prostate cancer (mPrCa): An ECOG-led phase III randomized trial. ASCO Annual Meeting. 2014:LBA2. [Google Scholar]
- 28.Aparicio AM, Harzstark AL, Corn PG, Wen S, Araujo JC, Tu SM, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res. 2013;19(13):3621–3630. doi: 10.1158/1078-0432.CCR-12-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.