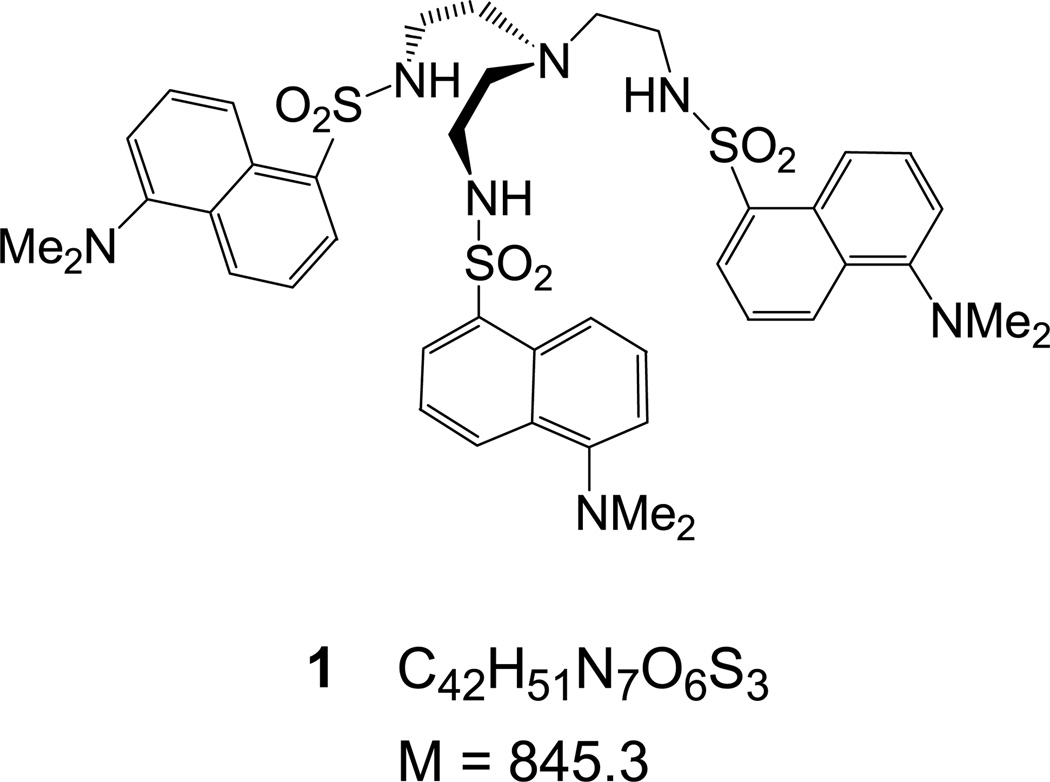
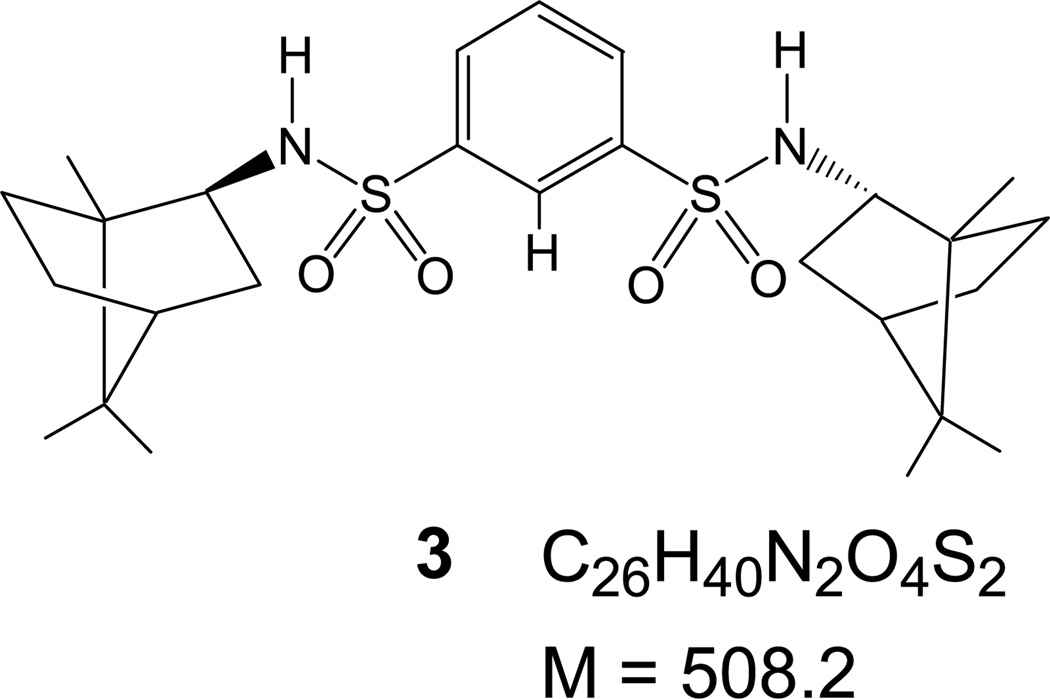
Abstract
As part of a mass spectrometric investigation of the binding properties of sulfonamide anion receptors, an atmospheric pressure chemical ionization mass spectrometric (APCI-MS) method involving direct infusion followed by thermal desorption was employed for identification of anionic supramolecular complexes in dichloromethane (CH2Cl2). Specifically, the dansylamide derivative of tris(2-aminoethyl)amine (tren) (1), the chiral 1,3-benzenesulfonamide derivatives of (1R,2S)-(+)-cis-1-amino-2-indanol (2), and (R)-(+)-bornylamine, (3), were shown to bind halide and nitrate ions in the presence of (n − Bu)4N+X− (X− = Cl−, , Br−, I−). Solutions of receptors and anions in CH2Cl2 were combined to form the anionic supramolecular complexes, which were subsequently introduced into the mass spectrometer via direct infusion followed by thermal desorption. The anionic supramolecular complexes [M + X]−, (M = 1–3, X− = Cl−, , Br−, I−) were observed in negative mode APCI-MS along with the deprotonated receptors [M − H]−. Full ionization energy of the APCI corona pin (4.5 kV) was necessary for obtaining mass spectra with the best signal-to-noise ratios.
Mass spectrometric methods [1–3] have been extensively used as analytical tools because they are sensitive, selective, and reliable techniques for the analysis of organic compounds, including explosives [4], biomolecules [5], and pollutants [6]. Inorganic ions with small mass-to-charge ratios are typically analyzed by other techniques, such as ion chromatography and capillary electrophoresis, because under normal MS ionization conditions many compounds can produce small ions that can interfere with their detection. There are, however, some notable examples [7, 8] that demonstrate the potential of soft ionization methods [9], such as atmospheric pressure chemical ionization mass spectrometry (APCI-MS) and electrospray ionization mass spectrometry (ESI-MS) [10] for analysis of inorganic ions, and ion pairs, such as ammonium nitrate [11].
Increased sensitivity for MS analysis of inorganic ions has been achieved by a relatively new and promising strategy, which bridges the fields of mass spectrometry and supramolecular chemistry [12–14]. This approach is based on selective host molecules for specific cationic or anionic guests, which can form ionic adducts with higher molecular mass. Most of the supramolecular complexes investigated by MS involve macrocycles, such as crown ethers and cryptands, which form cationic metal-receptor complexes detectable in positive ion mode [15–19]. Anion supramolecular chemistry [20] is relatively less well developed and oftentimes anion receptors are not as strong as cation receptors in terms of thermodynamic binding constants. Moreover, negative ionization is more susceptible to corona discharge [21, 22]. Therefore, negative-ion mode MS detection of noncovalent anion-receptor complexes is less common than positive-ion mode, with the examples reported [23–28] utilizing almost exclusively ESI-MS. Yinon and coworkers, [29] and very recently Mathis and McCord, [30] have reported anionic adduct formation by ESI-MS [29, 30] and APCI-MS [29] for the detection of organic explosives. APCI presents some advantages compared with ESI in quantitative analysis such as higher dynamic range, and is considered rugged, easy to operate, and more tolerant of higher buffer concentrations (fewer matrix effects) [31]. Multiply charged species are typically not observed in APCI-MS spectra [1]. Even though APCI is considered a soft ionization method, it has not found the same application in anion supramolecular chemistry as ESI because the larger supramolecular ions are generally not observed. Therefore, successful identification of noncovalent complexes by APCI-MS could lead to selective detection of only the relatively more stable supramolecular complexes, thus offering a potentially powerful screening tool for evaluation of anion receptor libraries, in a similar fashion as it has been reported for mixtures of cation receptors by ESI-MS [32, 33].
In both APCI-MS and ESI-MS, polar solvents with high dielectric constants such as water, acetonitrile, and methanol are typically used. In anion supramolecular chemistry there is an interest in applying negative-ion mode MS in relatively less polar solvents such as CH2Cl2, in which the anion-receptor complexes (formed via hydrogen bonding) exhibit higher formation constants and are involved in separation applications based on liquid-liquid extraction. There are only a few ESI-MS examples of such studies in these solvents reported by other investigators [34, 35] as well as ourselves [36–38]. Herein, we wish to communicate a unique application of APCI-MS in CH2Cl2 for detection of simple halide anions, and , a common component of inorganic explosives, via complexation with receptors Schemes 1–3. The noncovalent anionic complexes were detected in the negative ion mode along with the [M − H]− anions corresponding to the deprotonated receptors.
Scheme 1.
Scheme 3.
Experimental Section
Materials and Methods
All chemicals were purchased from Aldrich Chemical Co. (Milwaukee, WI) or ACROS Organics (Morris Plains, NJ), and used without further purification unless otherwise noted. Spectroscopic grade CH2Cl2 was used for all MS experiments. Receptor 1 [38, 39] and chiral receptors 2 and 3 [40] were synthesized by small modifications of procedures reported elsewhere and characterized spectroscopically [41]. All compounds were dried under vacuum, and 1 was kept in the dark. APCI mass spectra were obtained on a Finnigan ThermoQuest Navigator (San Jose, CA) aQa single-quadrupole LC-MS instrument.
APCI-MS Experiments
The mass spectrometer was tuned for m/z = 200–1000. CH2Cl2 was run through the instrument before each analysis. 1.0 × 10−3 M solutions of 1–3 and 1.0 × 10−3 M (for 1) or 2.0 × 10−3 M (for 2, 3) solutions of (n − Bu)4N+X− (, Cl−, Br−, I−) were prepared in CH2Cl2. Spectra were obtained in APCI negative ion detection mode after mixing the solutions at 1:1 volume ratios. The resulting solutions were introduced directly into the mass spectrometer by a thermal desorption technique (ramping the probe temperature). Typical experiment settings were: sample infusion flow rate 100 µL/min; cone voltage −11 V; corona pin voltage 4.5 kV; thermal desorption by probe temperature ramping from 100 to 350 °C at 18 °C/s. After spectral collection, it was assured that the sample was fully desorbed before the next analysis was attempted, and CH2Cl2 was used to rinse the system between sample analyses. No carryover effects were observed. For the corona pin variation experiments for the [1 + NO3]− complex, the cone voltage of the instrument was set at −5 V, and the probe temperature was ramped from 25 to 400 °C. The experiments were repeated for corona pin voltage set at 0 kV, 2.25 kV, and 4.5 kV.
Results and Discussion
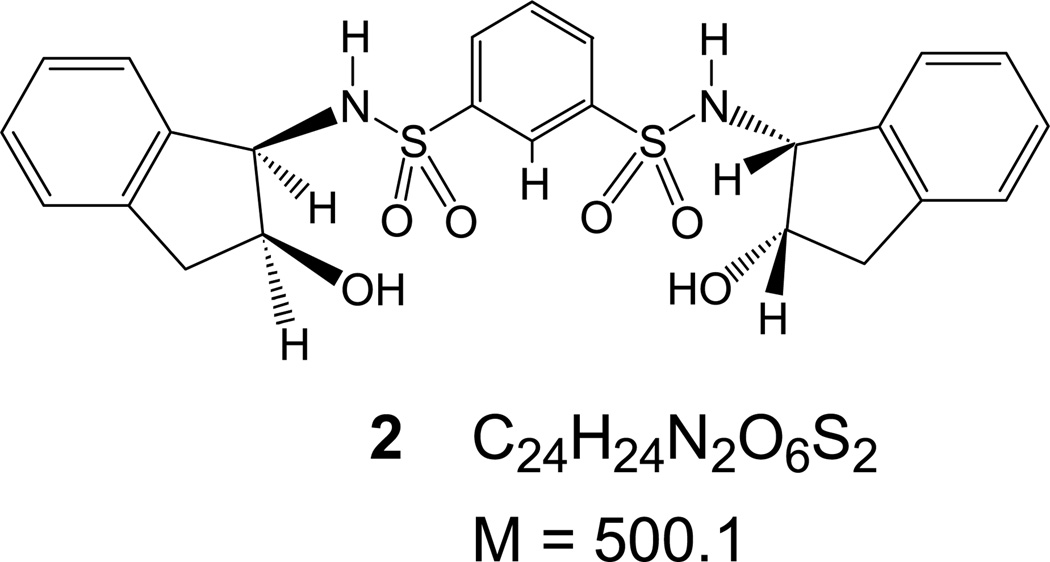
Sulfonamides, such as the dansylamide derivative of tris(2-aminoethyl)amine (tren) 1 and the chiral derivatives 2 and 3 have been known to bind anions via hydrogen bonding forming 1:1 anion/receptor complexes [38, 42, 43]. Association constants from 1H-NMR titrations indicate the general stability trend [M + Cl]− > [M + Br]− ≈ [M + NO3]− > [M + I]− for 1 [38], 2, and 3 [41–43], and other similar sulfonamides. When comparing the different receptors to each other, the stability of receptor-anion complexes from 1H-NMR titrations shows the trend: [2 + X]− > [3 + X]− ≈ [1 + X]− [38, 41].
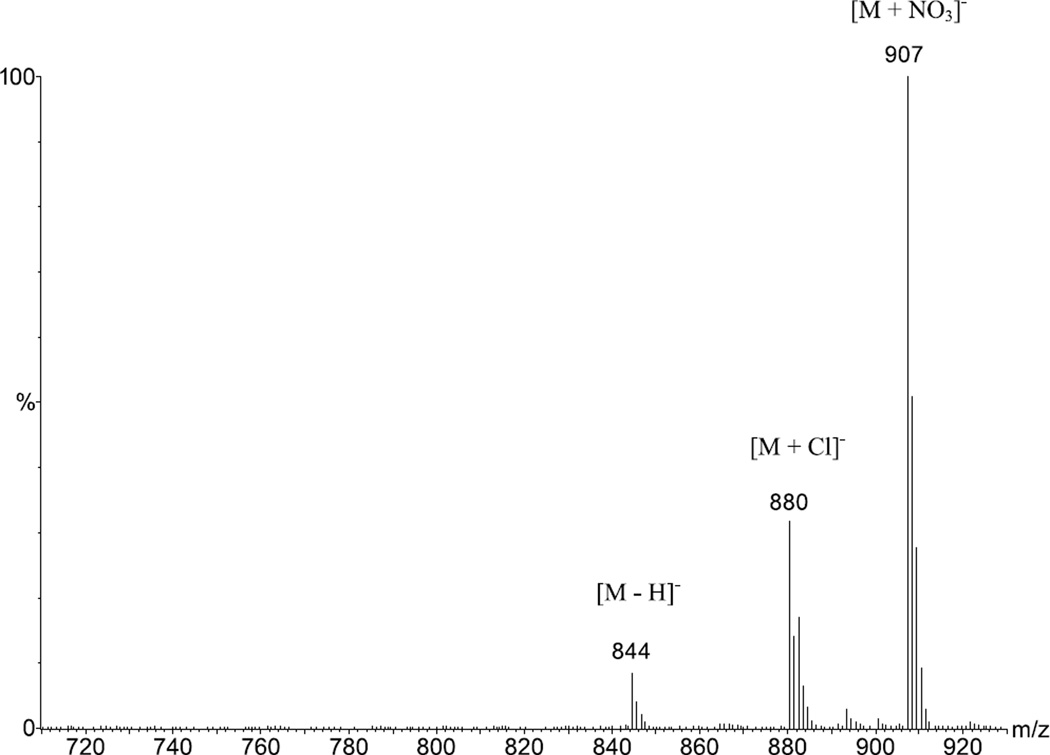
Anion complexation of 1 with Cl− and was observed by APCI-MS in anion detection mode (Figure 1) after the essential thermal desorption. Both the [1 + Cl]− and [1 + NO3]− complexes at m/z = 880 and m/z = 907, respectively, were identified, along with the deprotonated receptor [1 − H]− at m/z = 844, as expected for a weak acid [34, 44]. The [1 + Cl]− signal at m/z = 880 was always prominent even when (n − Bu)4NCl had not been added to the solution; formation of [M + Cl]− anionic adducts in chlorinated solvents has been extensively discussed for ESI-MS by Cole and Zhu [34] and for APCI-MS by Zhao and Yinon [45]. Since the [M + Cl]− adduct signal in nonchloride-containing samples is attributed to the solvent and shows consistent intensity for samples of similar composition, it is used in the following discussions for comparing the signal intensities of the various formed anionic adducts. Complexes of 1 with Br− and I− were also detected after addition of the corresponding tetrabutylammonium salts, but with significantly reduced intensities compared with [1 + Cl]− and [1 + NO3]−.
Figure 1.
APCI-MS spectrum of 1 and (n − Bu)4NNO3 (1:1) in CH2Cl2 showing signals for [1 + Cl]− at m/z = 880, [1 + NO3]− at m/z = 907, and the deprotonated receptor [1 − H] − at m/z = 844.
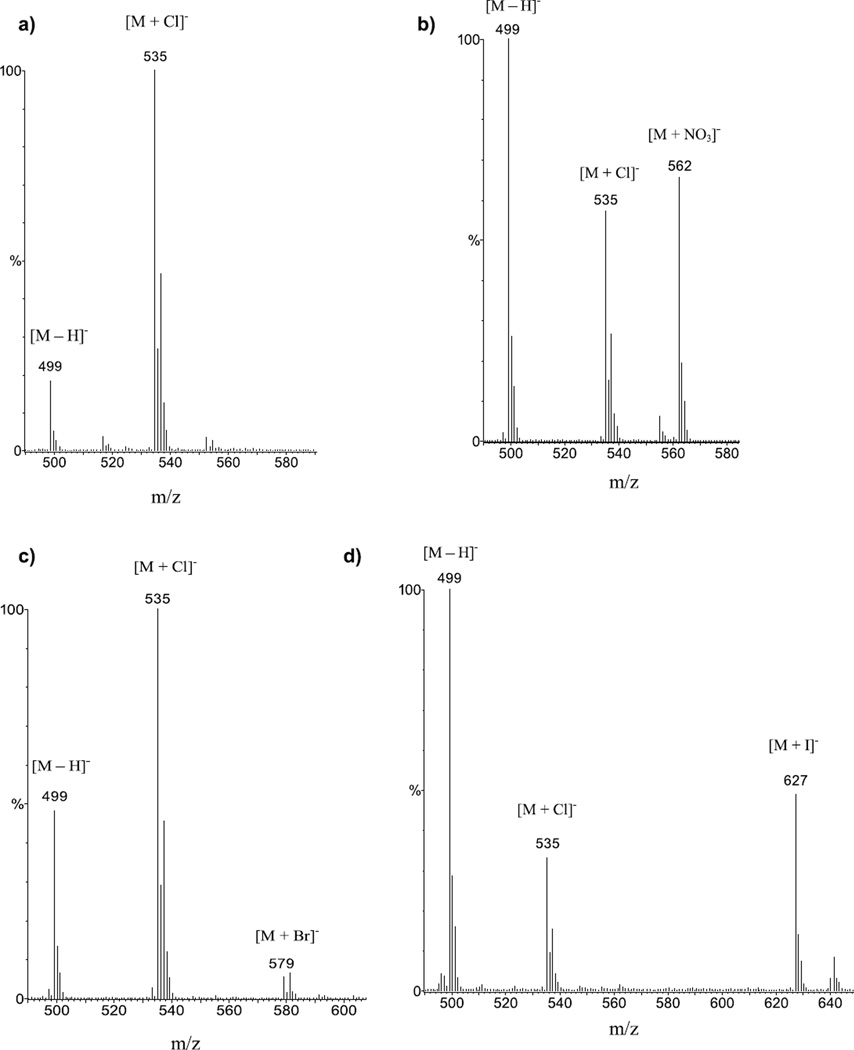
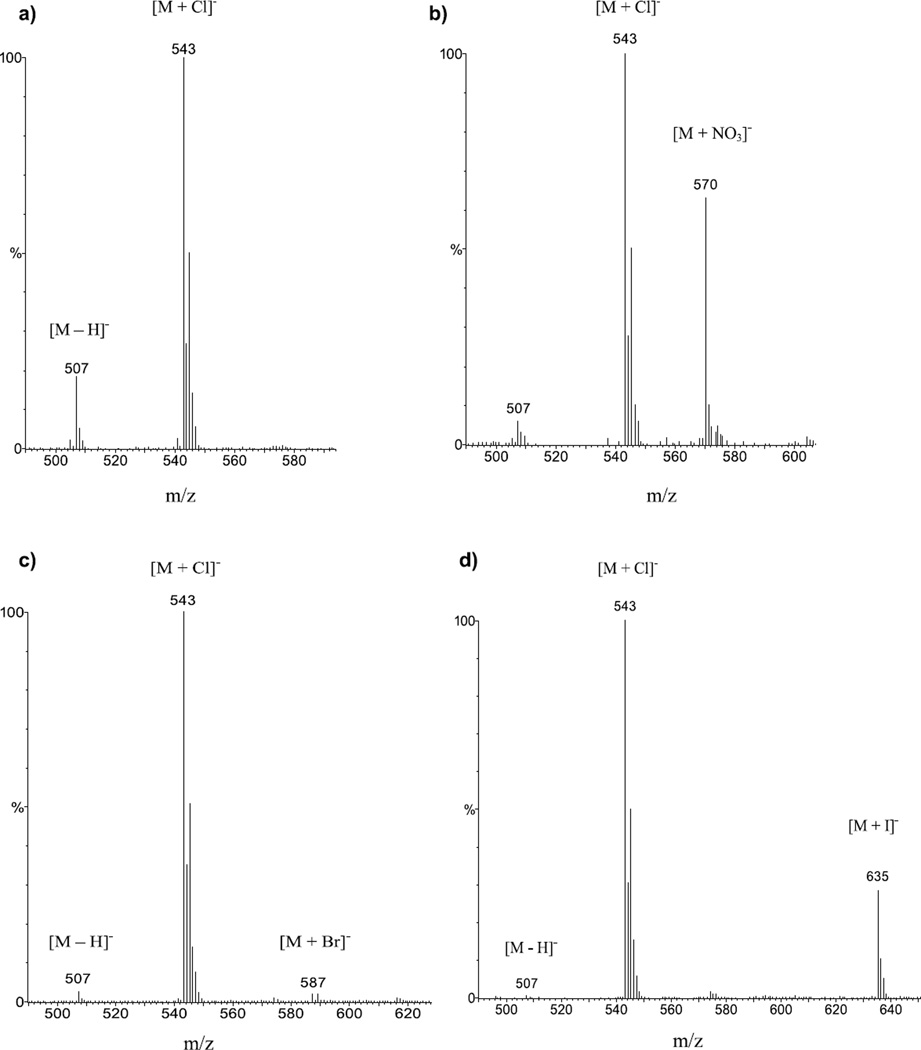
For the APCI-MS detection of anionic complexes of 2 and 3, the experiments were carried out in a similar fashion: anionic complexes of 2 with Cl−, , Br−, and I− at m/z of 535, 562, 579, and 627, respectively, were detected with strong to medium intensities (Figure 2). The anionic complexes of 3 with Cl−, , Br−, and I− were detected at m/z of 543, 570, 587, and 635, respectively (Figure 3). As with the case of 1, the [2 + Cl]− and [3 + Cl]− species consistently gave high-intensity signals even without the presence of (n − Bu)4NCl indicating formation of anionic adducts due to CH2Cl2 [34, 45]. Comparing the signal intensities in reference to [M + Cl]− shows the trend [M + Cl]− > [M + NO3]− ≈ [M + I]− > [M + Br]− for 2 and 3. For 2, a prominent signal was observed at all times (Figure 2) for m/z = 499 corresponding to the deprotonated receptor [2 − H]−. For 3 the [3 − H]− at m/z = 507 was generally observed at much reduced intensities (Figure 3), indicating possibly lower acidity. Even though a systematic quantitative study was not attempted, the approximate adduct intensities for the anion series of both 2 and 3 are generally consistent with the general anion-binding trend observed for such sulfonamides, indicating stronger affinities for the more hydrophilic anions, as expected for hydrogen bonding receptors [43]. There is, however, an obvious “inversion” for the [M + I]− complexes, which show stronger signal intensities than it would be expected based on their thermodynamic stability. Conversely, the [M + Br]− complexes appear consistently with weaker intensities than someone would expect.
Figure 2.
APCI-MS spectra in CH2Cl2 of 2 and (a) (n − Bu)4NCl (1:2) showing signals for [2 + Cl] − at m/z = 535, and for the deprotonated receptor [2 − H] − at m/z = 499. (b) (n − Bu)4NNO3 (1:2) showing signals for [2 + Cl]− at m/z = 535, [2 + NO3]− at m/z = 562, and for the deprotonated receptor [2 − H]− at m/z = 499. (c) (n − Bu)4NBr (1:2) showing prominent signals for [2 + Cl]− at m/z = 535 and for the deprotonated receptor [2 − H]− at m/z = 499, and a weaker signal for [2 + Br]− at m/z = 579. (d) (n − Bu)4NI (1:2) showing signals for [2 + Cl]− at m/z = 535, [2 + I]− at m/z = 627, and for the deprotonated receptor [2 − H]− at m/z = 499.
Figure 3.
APCI-MS spectra in CH2Cl2 of 3 and (a) (n − Bu)4NCl (1:2) showing signals for [3 + Cl]− at m/z = 543, and for the deprotonated receptor [3 − H]− at m/z = 507. (b) (n − Bu)4NNO3 (1:2) showing prominent signals for [3 + Cl]− at m/z = 543, [3 + NO3]− at m/z = 570, and a weaker signal for the deprotonated receptor [3 − H]− at m/z = 507. (c) (n − Bu)4NBr (1:2) showing a prominent signal for [3 + Cl]− at m/z = 543 and weaker signals for [3 + Br]− at m/z = 587, and for the deprotonated receptor [3 − H]− at m/z = 507. (d) (n − Bu)4NI showing prominent signals for [3 + Cl]− at m/z = 543, [3 + I]− at m/z = 635, and a weaker signal for the deprotonated receptor [3 − H]− at m/z = 507.
In an attempt to elucidate the mechanism of ionization, a 1.0 × 10−3 M solution of (n − Bu)4NNO3 and 1 was analyzed using three different instrument settings to test whether or not the anionic complexes can be introduced into the mass spectrometer using only simple thermal desorption from the needle with reduced or no APCI corona pin voltage. For this set of experiments, the cone voltage of the instrument was set at −5 V and the probe temperature was ramped from 25 to 400 °C. With a corona pin ionization energy of 0 kV, the signal for the anion/receptor complexes was indistinguishable from the background noise of the spectrum. With an ionization energy of 2.25 kV, all signals showed low abundance, with only the [1 + Cl]− (m/z 880) signal being clearly distinguishable from background noise (S/N ratio = 9). When full ionization energy of 4.5 kV was used, all adducts were detected in good abundance, with a prominent [1 + Cl]− signal (S/N ratio = 34). The [1 + NO3]− signal (m/z 907) showed an abundance of 16% relative to [1 + Cl]−. Anionic complexes of 1 are already present in solution before being introduced into the mass spectrometer. Thus, theoretically, it could be possible to introduce the adducts directly by simple thermal desorption without any ionization. Since the anionic adduct detection was dependent on the corona pin voltage, it is possible that ion pairing [38, 46] between the anionic complex and the tetrabutylammonium cation is rather strong in CH2Cl2. APCI-driven ionization disrupts this ion pairing and facilitates the detection of the anion-receptor complexes, possibly via a solvent-mediated mechanism, as it would be indicated by the increase in the [1 + Cl]− signal intensity [34]. ESI-MS and APCI-MS analysis of supramolecular complexes is traditionally carried out in polar solvents with high dielectric constants. The application of negative ion mode APCI-MS for detection of supramolecular complexes in less polar solvents promoting ion pairing, such as CH2Cl2, is unique and could be potentially used in combination with liquid-liquid extraction as a novel evaluation tool for anion binding and for extraction-based sensor discovery [47, 48].
Conclusions
A unique application of a simple thermal desorption APCI-MS method for the identification of anionic supramolecular adducts was demonstrated. Future quantification and optimization of this technique could offer a potentially powerful tool for anion detection and for evaluation of receptors and receptor libraries in water immiscible solvents, such as CH2Cl2. This advance may lead to future wider application of APCI-MS in anion host–guest chemistry, discovery of efficient and selective anion sensors, and characterization of supramolecular phenomena in complex systems involving solvent extraction by synthetic hosts.
Scheme 2.
Acknowledgments
The authors thank Myron Georgiadis for help with the upkeep of the Advanced Mass Spectrometry Facility (AMSF) at FIU, and Drs. Piero R. Gardinali and J. Martin E. Quirke for their useful comments and suggestions. This project was funded in part by NIH (NIEHS/ARCH Pilot Project S11-ES11181), Florida International University, and the FIU Foundation. JMR was supported by a scholarship from the National Institutes of Health (MBRS-RISE program R25-GM061347).
References
- 1.De Hoffmann E, Stroobant V. Mass Spectrometry. 2nd. Chichester: Wiley; 2002. [Google Scholar]
- 2.Niessen WMA. LC-MS in quantitative analysis. Rev. Anal. Chem. 2000;19:289–301. [Google Scholar]
- 3.Chapman JR. Practical Organic Mass Spectrometry, A Guide for Chemical and Biochemical Analysis. 2nd. New York: Wiley; 1995. [Google Scholar]
- 4.Yinon J, editor. Advances in Forensic Applications of Mass Spectrometry. Boca Raton, FL: GRC Press; 2004. [Google Scholar]
- 5.Van Berkel GJ. An Overview of Some Recent Developments in Ionization Methods for Mass Spectrometry. Eur. J. Mass Spectrom. 2003;9:539–562. doi: 10.1255/ejms.586. [DOI] [PubMed] [Google Scholar]
- 6.Richardson SD. Environmental Mass Spectrometry: Emerging Contaminants and Current Issues. Anal. Chem. 2004;76:3337–3364. doi: 10.1021/ac040060d. [DOI] [PubMed] [Google Scholar]
- 7.Fuerstenau SD, Fenn JB. 5523566. US Patent. 1996 Jun 4;
- 8.Ahrer W, Buchberger W. Analysis of Low-Molecular-Mass Inorganic and Organic Anions by Ion Chromatography-Atmospheric Pressure Ionization Mass Spectrometry. J. Chromatogr. A. 1999;854:275–287. doi: 10.1016/s0021-9673(99)00396-9. [DOI] [PubMed] [Google Scholar]
- 9.Rosenberg E. The Potential of Organic (Electrospray- and Atmospheric Pressure Chemical Ionization) Mass Spectrometric Techniques Coupled to Liquid-Phase Separation for Speciation Analysis. J. Chromatogr. A. 2003;1000:841–889. doi: 10.1016/s0021-9673(03)00603-4. and references therein. [DOI] [PubMed] [Google Scholar]
- 10.Cole RB. Electrospray Ionization Mass Spectrometry: Fundamentals, Instrumentation, and Applications. New York: Wiley; 1997. [Google Scholar]
- 11.Zhao X, Yinon J. Characterization of Ammonium Nitrate by Electrospray Ionization Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2001;15:1514–1519. doi: 10.1002/rcm.309. [DOI] [PubMed] [Google Scholar]
- 12.Brodbelt JS, Dearden DV. Mass Spectrometry. In: Davies JED, Ripmeester JA, editors. Comprehensive Supramolecular Chemistry. VIII. Oxford: Pergamon, Elsevier; 1996. pp. 567–591. [Google Scholar]
- 13.Brodbelt JS. Probing Molecular Recognition by Mass Spectrometry. Int. J. Mass Spectrom. 2000;200:57–69. [Google Scholar]
- 14.Schalley CA. Molecular Recognition and Supramolecular Chemistry in the Gas Phase. Mass Spectrom. Rev. 2002;20:253–309. doi: 10.1002/mas.10009. [DOI] [PubMed] [Google Scholar]
- 15.Blair SM, Kempen CE, Brodbelt JS. Determination of Binding Selectivities in Host–Guest Complexation by Electrospray/Quadrupole Ion Trap Mass Spectrometry. J. Am. Soc. Mass Spectrom. 1998;9:1049–1059. [Google Scholar]
- 16.Kempen EC, Brodbelt JS, Bartsch RA, Jang Y, Kim JS. Investigation of Alkali Metal Cation Selectivities of Lariat Ethers by Electrospray Ionization Mass Spectrometry. Anal. Chem. 1999;71:5493–5500. [Google Scholar]
- 17.Bartoszek M, Graubaum H, Wendland D, Dambowski R. Investigation of Metal-Ion Complexes of Triazino Crown Ethers with Podand Arms by Electrospray Ionization. Eur. Mass Spectrom. 1999;5:81–88. [Google Scholar]
- 18.Ralph SF, Sheil MM, Hick LA, Geue RJ, Sargeson AM. An Electrospray Mass Spectrometry Study of Some Metal-Ion Cage Complexes. J. Chem. Soc. Dalton Trans. 1996:4417–4424. [Google Scholar]
- 19.Milman BL. Cluster Ions of Diquat and Paraquat in Electrospray Ionization Mass Spectra and Their Collision-Induced Dissociation Spectra. Rapid Commun. Mass Spectrom. 2003;17:1344–1349. doi: 10.1002/rcm.1056. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi A, Bowman-James K, Garcia-Espana E, editors. The Supramolecular Chemistry of Anions. New York: Wiley-VCH; 1997. and references therein. [Google Scholar]
- 21.Cole RB, Harrata AK. Solvent Effect on Analyte Charge State, Signal Intensity, and Stability in Negative Ion Electrospray Mass Spectrometry; Implications for the Mechanism of Negative Ion Formation. J. Am. Soc. Mass Spectrom. 1993;4:546–556. doi: 10.1016/1044-0305(93)85016-Q. [DOI] [PubMed] [Google Scholar]
- 22.Wampler FM, Blades AT, Kebarle P. Negative Ion Electrospray Mass Spectrometry of Nucleotides: Ionization from Water Solution with Sulfur Hexafluoride Discharge Suppression. J. Am. Soc. Mass Spectrom. 1993;4:289–295. doi: 10.1016/1044-0305(93)85050-8. [DOI] [PubMed] [Google Scholar]
- 23.Cai Y, Cole RB. Stabilization of Anionic Adducts in Negative Ion Electrospray Mass Spectrometry. Anal. Chem. 2002;74:985. doi: 10.1021/ac0108818. [DOI] [PubMed] [Google Scholar]
- 24.Bieske EJ. Spectroscopic Studies of Anion Complexes and Clusters: A Microscopic Approach to Understanding Anion Solvation. Chem. Soc. Rev. 2003;32:231–237. doi: 10.1039/b106752b. [DOI] [PubMed] [Google Scholar]
- 25.Bossee A, Fournier F, Tasseau O, Bellier B, Tabet J-C. Electrospray Mass Spectrometric Study of Anion Complexes of Enkephalins with Cu(II): Regioselective Distinction of Leu/Ile at the C-Terminus Induced by Metal Reduction. Rapid Commun. Mass Spectrom. 2003;17:1229–1239. doi: 10.1002/rcm.1041. [DOI] [PubMed] [Google Scholar]
- 26.Mollah S, Pris AD, Johnson SK, Gwizdala AB, III, Houk RS. Identification of Metal Cations, Metal Complexes, and Anions by Electrospray Mass Spectrometry in the Negative Ion Mode. Anal. Chem. 2000;72:985–991. doi: 10.1021/ac9908647. [DOI] [PubMed] [Google Scholar]
- 27.Deery MJ, Fernandez T, Howarth OW, Jennings KR. Aqueous Coordination Chemistry Using Electrospray Mass Spectrometry of Anions: Metal(VI)-Monosaccharide Complexes (M = W or Mo) J. Chem. Soc. Dalton Trans. 1998:2177–2183. [Google Scholar]
- 28.Urbansky ET, Magnuson ML, Freeman D, Jelks C. Quantitation of Perchorate Ion by Electrospray Ionization Mass Spectrometry (ESI-MS) Using Stable Association Complexes with Organic Cations and Bases to Enhance Selectivity. J. Anal. At. Spectrom. 1999;14:1861–1866. [Google Scholar]
- 29.Gapeev A, Sigman M, Yinon J. Liquid Chromatography/Mass Spectrometric Analysis of Explosives: RDX Adduct Ions. Rapid Commun. Mass Spectrom. 2003;17:943–948. doi: 10.1002/rcm.1006. [DOI] [PubMed] [Google Scholar]
- 30.Mathis JA, McCord BR. The Analysis of High Explosives by Liquid Chromatography/Electrospray Ionization Mass Spectrometry: Multiplexed Detection of Negative Ion Adducts. Rapid Commun. Mass Spectrom. 2005;19:99–104. doi: 10.1002/rcm.1752. [DOI] [PubMed] [Google Scholar]
- 31.Bakhtiar R, Ramos L, Tse FLS. Use of Atmospheric Pressure Ionization Mass Spectrometry in Enantioselective Liquid Chromatography. Chirality. 2001;13:63–74. doi: 10.1002/1520-636X(2001)13:2<63::AID-CHIR1000>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 32.Kempen EC, Brodbelt JS, Bartsch RA, Blanda MT, Farmer DB. Screening Metal Binding Selectivities of Macrocycle Mixtures by HPLC-ESI-MS and Postcolumn Reactions. Anal. Chem. 2001;73:384–390. doi: 10.1021/ac0010476. [DOI] [PubMed] [Google Scholar]
- 33.Zadmard R, Kraft A, Schrader T, Linne U. Relative Binding Affinities of Molecular Capsules Investigated by ESI-Mass Spectrometry. Chem. Eur. J. 2004;10:4233–4239. doi: 10.1002/chem.200400034. [DOI] [PubMed] [Google Scholar]
- 34.Cole RB, Zhu J. Chloride Anion Attachment in Negative Ion Electrospray Ionization Mass Spectrometry. Rapid Commun. Mass Spectrom. 1999;13:607. [Google Scholar]
- 35.Cheng X, Gao Q, Smith RD, Simanek EE, Mammen M, Whitesides GM. Detection of Hydrogen-Bonded Supramolecular Complexes Using Electrospray Ionization from Chloroform. Rapid Commun. Mass Spectrom. 1995;9:312–316. [Google Scholar]
- 36.Kavallieratos K, Sachleben RA, Van Berkel GJ, Moyer BA. Novel Dual-Host Approach in Ion Pair Extraction: A simple Tripodal Nitrate Host Facilitates CsNO3 Transfer to 1,2-Dichloroethane by a Large Crown Ether. Chem. Commun. 2000:187–188. [Google Scholar]
- 37.Kavallieratos K, Danby A, Van Berkel GJ, Kelly MA, Sachleben RA, Moyer BA, Bowman-James K. Enhancement of CsNO3 Extraction in 1,2-Dichloroethane by Tris(2-Aminoethyl)Amine Triamide Derivatives via a Dual-Host Strategy. Anal. Chem. 2000;72:5258–5264. doi: 10.1021/ac0005971. [DOI] [PubMed] [Google Scholar]
- 38.Kavallieratos K, Bryan JC, Sachleben RA, Van Berkel GJ, Espetia OE, Kelly MA, Danby A, Bowman-James K, Moyer BA. Dual-Host Combinations: Using Tripodal Amides to Enhance Cesium Nitrate Extraction by Crown Ethers. In: Moyer BA, Singh R, editors. Fundamentals and Applications of Anion Separations. New York: Kluwer; 2004. [Google Scholar]
- 39.Prodi L, Bolletta F, Montalti M, Zaccheroni N. Searching for New Luminescent Sensors. Synthesis and Photophysical Properties of a Tripodal Ligand Incorporating the Dansyl Chromophore and of its Metal Complexes. Eur. J. Inorg. Chem. 1999;3:455–460. [Google Scholar]
- 40.Ghosh AK, Chen Y. Highly Stereoselective Reduction of α-Keto Esters: Utility of cis-1-Arylsulfonamido-2-Indanols as Chiral Auxiliaries. Tetrahedron Lett. 1995;36:6811–6814. doi: 10.1016/0040-4039(95)01385-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rodriguez JM, Galarza PJ, Sabucedo AJ, Kavallieratos K. Chiral Recognition: Anion Receptors Derived from Isophthaloyl Dichloride, 2,6-Pyridinedicarbonyl Chloride and 1,3-Benzenedisulfonyl Chloride, and Readily Available Chiral Amines; Proceedings of the 225th ACS National Meeting; March, 2003; New Orleans, LA. [Google Scholar]
- 42.Kavallieratos K, Bertao CM, Crabtree RH. Hydrogen Bonding in Anion Recognition: A Family of Versatile, Nonpreorganized Neutral and Acyclic Receptors. J. Org. Chem. 1999;64:1675–1683. doi: 10.1021/jo982382l. [DOI] [PubMed] [Google Scholar]
- 43.Kavallieratos K, Moyer BA. Attenuation of Hofmeister Bias in Ion-Pair Extraction by a Disulfonamide Anion Host Used in Strikingly Effective Synergistic Combination with a Calix-Crown Cs+ Host. Chem. Commun. 2001:1620–1621. doi: 10.1039/b102152b. [DOI] [PubMed] [Google Scholar]
- 44.Dauphin G, Kergomard A. The Acid Dissociation of Some Sulfonamides. Bull. Soc. Chim. Fr. 1961;3:486–492. [Google Scholar]
- 45.Zhao X, Yinon J. Identification of Nitrate Ester Explosives by Liquid Chromatography-Electrospray Ionization and Atmospheric Pressure Chemical Ionization Mass Spectrometry. J. Chromatogr. A. 2002;977:59–68. doi: 10.1016/s0021-9673(02)01349-3. [DOI] [PubMed] [Google Scholar]
- 46.Marcus Y. Ion Properties. New York: Marcel Dekker; 1997. [Google Scholar]
- 47.Kavallieratos K, Rosenberg JM, Bryan JC. Pb(II) Coordination and Synergistic Ion-Exchange Extraction by Combinations of Sulfonamide Chelates and 2,2′-Bipyridine. Inorg. Chem. 2005;44:2753–2575. doi: 10.1021/ic050047r. [DOI] [PubMed] [Google Scholar]
- 48.Kavallieratos K, Rosenberg JM, Chen W-Z, Ren T. Fluorescent Sensing and Selective Pb(II) Extraction by a Dansylamide Ion-Exchanger. J. Am. Chem. Soc. 2005;127:6514–6515. doi: 10.1021/ja050296e. [DOI] [PMC free article] [PubMed] [Google Scholar]