Abstract
While clinical trials have now solidified the role of thrombectomy in emergent large vessel occlusive stroke, additional therapies are needed to optimize patient outcome. Using our previously described experimental ischemic stroke model for evaluating adjunctive intra-arterial drug therapy after vessel recanalization, we studied the potential neuroprotective effects of verapamil. A calcium channel blocker, verapamil is often infused intra-arterially by neurointerventionalists to treat cerebral vasospasm. Such a direct route of administration allows for both focused targeting of stroke-impacted brain tissue and minimizes potential systemic side effects. Intra-arterial administration of verapamil at a flow rate of 2.5 µl/min and injection volume of 10 µl immediately after middle cerebral artery recanalization in C57/Bl6 mice was shown to be profoundly neuroprotective as compared to intra-arterial vehicle-treated stroke controls. Specifically, we noted a significant (P ≤ 0.05) decrease in infarct volume, astrogliosis, and cellular apoptosis as well as a significant increase in neuronal survival and functional outcome over seven days. Furthermore, intra-arterial administration of verapamil was well tolerated with no hemorrhage, systemic side effects, or increased mortality. Thus, verapamil administered intra-arterially immediately following recanalization in experimental ischemic stroke is both safe and neuroprotective and merits further study as a potential therapeutic adjunct to thrombectomy.
Keywords: Ischemic stroke, cerebral ischemia, recanalization, intra-arterial, verapamil, neuroprotection
Introduction
Ischemic stroke is the fifth leading cause of death with 137,000 deaths annually in the U.S., but the leading cause of morbidity with 800,000 affected each year; with such a high incidence of morbidity and mortality, new therapies are needed.1–4 The only FDA-approved pharmacotherapy for acute ischemic stroke is tissue plasminogen activator (t-PA), but with a therapeutic window of 4.5 h and further exclusion criteria, few patients receive treatment.5,6 The most disabling and life-threatening ischemic stroke is emergent large vessel occlusion (ELVO). For this condition, endovascular thrombectomy (mechanical removal of a clot by threading a catheter through the patient’s vasculature to the site of occlusion and removing the clot) in addition to intravenous (IV) t-PA is now the standard of care.1,6,7 While both treatments have improved patient survival and rate of good clinical outcomes, the magnitude of outcome advancement has not mirrored the increasing improvements in the ability to reopen occluded vessels.7–9 This disconnect highlights an urgent need for adjunctive therapies to further improve outcomes after effective thrombolysis with t-PA and thrombectomy.7,10,11
Using the tandem transient middle cerebral/common carotid artery occlusion (MCAo) technique to model ischemic stroke in mice, we have developed an intra-arterial (IA) method of pharmacotherapy administration that mimics the human clinical stroke condition to better test potential neuroprotective compounds.12–14 This method mirrors the clinical scenario of a recanalized artery immediately following thrombectomy; it allows us to test the feasibility, safety, and efficacy of IA pharmacotherapy immediately following thrombectomy and provides an animal model to reflect the present clinical opportunity to infuse agents intra-arterially immediately following successful thrombectomy with the patient still on the angiography procedure table. Verapamil, a calcium channel blocker, is FDA approved for angina and arrhythmia.15 Previous verapamil stroke studies had mixed results; while some studies support its neuroprotective potential, other experiments failed to show a benefit.16–19 However, in experiments that failed to show benefit, animal models were induced with permanent vessel occlusion and/or given the drug in a time interval far beyond its likely therapeutic potential.16,17 Clinically, we believe treatment for ELVO strokes should consist of rapid recanalization of the occluded vessel combined with selective pharmacotherapy, i.e. IV or IA t-PA administration directly to the site of ischemia.20,21 We hypothesize that such a stroke-targeted approach could greatly optimize the therapeutic potential of neuroprotective agents. Therefore, in this study we investigated the therapeutic potential of IA verapamil in our experimental ischemic stroke.
Material and methods
All experiments conformed to protocols on file with the University of Kentucky Division of Laboratory Animal Research, Institutional Animal Care and Use Committee and ARRIVE Guidelines.22 Briefly, 16-week-old male C57/Bl6 mice (25–30 g) from Jackson Laboratories were separated into three groups (10 per group for control and treated, five for naïve) in a blinded fashion using randomized selection. Groups were divided into naïve (age-matched litter mate controls excluded from anesthesia and surgery), control (MCAo surgery with 10 µl saline injection at 2.5 µl/minute IA through internal carotid artery (ICA)), and treated (MCAo surgery with 10 µl saline + verapamil injection IA through ICA). Verapamil was administered at a dose of 0.15 mg/kg.23,24 This dose was calculated to be similar to 10 mg in a 70 kg person, which is the most clinically relevant current IA dose. Prior studies determined the optimal flow rate of 2.5 µl/min with an injection volume of 10 µl per animal.14 Physiological measurements, blood flow measurements, behavioral, infarct volume, and immunohistochemistry analyses were conducted in a blinded fashion with experimental subjects numerically labeled. Animals were excluded from the study if the middle cerebral or common carotid artery (CCA) was punctured during wire and clamp insertion or removal, died following surgery in recovery, or were euthanized before the end of the study due to poor health. Overall, there is <5% death rate for our stroke model following both surgery and during behavior.
Animal numbers
We conducted the experiment using an N of 65 animals; an N of 45 were used for behavior, perfusion, and infarct volume with N of 25 for treated and N of 20 for control, the second N of 20 was used for immunohistochemistry with an N of 10 for treated and N of 10 for control. Additionally, an N of 5 naïve age-matched controls was used for behavioral testing.
Vital signs
Vital signs were monitored using the MouseOx Plus (Starr Life Science). A thigh sensor, placed on the shaved left thigh, was used to measure heart rate and pulse distention (blood pressure) with measurements taken from 0 to 5 min for baseline measurements, at 65 min (drug administration) after un-occlusion/reperfusion following 60 MCAo surgery, at 70 and 75 min reperfusion.
Perfusion
Blood flow through the MCA was measured pre- and post-occlusion and after reperfusion using the Laser Doppler Periflux System 5000 with a 2 mm tip and Laser Speckle PeriCam PSI System—Blood Perfusion Imager High Resolution (Perimed). To measure occlusion and reperfusion of the MCA, measurements were taken before drilling the burr hole (0 min), after metal wire insertion under MCA (5 min), and following 60 min occlusion with un-occlusion/reperfusion measurements at 70 (drug administration), 75, and 80 min. Care was taken to ensure the probe was placed in the same location for each measurement. To measure hemispheric blood flow, the Laser Speckle was positioned 8 cm above the exposed pronated mouse skull and set to measure at regions on interest and time points of interest. The laser guidance system positioned along the sagittal suture acted as a landmark to ensure consistent measurements. Measurements were taken before occlusion at 0 min, at 5 min after occlusion, and after reperfusion at 80 min which included drug administration and 10 min of reperfusion.
Middle cerebral artery occlusion and IA injection
Animals were anesthetized using a ketamine and xylazine mixture in a weight-based ratio. To induce focal cerebral ischemia, we used the previously described MCAo stroke model.12,13 The occlusion was for a 1 h interval, and recanalization was confirmed via Laser Doppler and Speckle. Animals were warmed on a heating pad throughout surgery and core temperature measurements (rectal thermometer) were monitored using the MouseOx Plus to ensure body temperature did not drop below recommended levels. Post-surgery, animals were warmed on a heating pad with overhead light and checked every 5 min to ensure hyper/hypothermia did not occur.
To infuse IA verapamil, we followed our previously published protocol.14 Briefly, we exposed the CCA bifurcation and its branches the internal and external carotid arteries (ICA/ECA). The distal ECA was permanently occluded, the ICA was temporarily occluded, and a nick was made with micro scissors in the ECA to allow for micro-angio tube insertion and secured with suture. Following the 1 h occlusion, the CCA and ICA clamps and metal filament under the MCA were removed restoring blood flow. After 5 min of reperfusion, the study agent (saline or verapamil) was infused intra-arterially at a rate of 2.5 µl/min at a volume of 10 µl.14 After the injection the proximal ECA was permanently occluded and the incisions in the thoracic and temporal region were sutured.
Behavioral testing
Two behavior tests were performed to assess functional outcome after surgery and pharmacotherapy administration: the rotor rod (used to measure forced movement) and open field (used to measure free roam). For the rotor rod, animals, naïve (age-matched controls, no anesthesia or surgery), control (MCAo surgery with IA injection of saline), and treated (MCAo surgery with IA injection of verapamil) were tested for three days prior to stroke surgery and tested for four days following stroke surgery: the mice were placed on the rotor rod for 5 min with an increasing acceleration from 0 to 40 r/min for three trials. Distance covered by each animal was measured in centimeters. The open field consists of a squared box measuring 50 cm by 50 cm by 30 cm with an infrared camera positioned above the box, which tracks the movement of the animals. Our parameters were set to measure distance covered in centimeters. Behavioral testing occurred on the rotor rod for three days prior to stroke (-3, -2, and -1) and on post-stroke day (PSD) 1, 3, 5, and 7, while the open field testing occurred one day prior to stroke (-1) and on PSD 1 and 7.
Infarct volume analysis
The mice were euthanized via cervical dislocation on PSD 7, and the whole brain was removed, sectioned using a brain mold into 2 mm sections and stained using triphenyl tetrazolium chloride (TTC) for 10 min. Infarct volume was analyzed from scanned TTC stained brain sections with Image J software (NIH). Whole brains were flash frozen, sectioned on the cryostat, and stained with Cresyl Violet for additional infarct volume analysis as described below under immunohistochemistry.
Immunohistochemistry
Flash frozen whole brains were sectioned on a Leica CM 1950 cryostat at 20 µm and mounted on slides. Infarct volume and overall neuronal health were assessed by TUNEL stain (Millipore). Astrogliosis was assessed with glial fibrillary activating protein (GFAP 1:1000 antibody dilution, Sigma) immunohistochemistry. Mature neuron survival within the stroke affected area was assessed via NeuN (1:1000 antibody dilution, Abcam) immunohistochemistry. Area of infarct analysis corresponds to cortical region that was the epicenter of the stroke morphologically identified based on cryostat sectioning to include greatest affected area. Stains were visualized with a Nikon Ecliplse Ti microscope at 20X magnification and images collected via CCD camera and attached to computer qualified with Adobe Photoshop. Nikon NIS Element BR Analysis imaging software was used to analyze brain sections from control and treated groups, sections were plated 4 to a slide with 10 slides per animal for a total of 40 sections.
Statistical analysis
All measured variables are presented as mean ± SEM. We selected rotor rod as the primary endpoint for power calculations because it had the smallest anticipated effect size. Based on an anticipated effect size of 1.0 (Cohen’s d) and significance level of 0.05, we expected to have 81% power with 17 animals per group to detect improvement from day 1 to day 7 post-surgery. We increased the sample sizes in each group to bolster power and account for potential losses. Based on preliminary data, we expected close to 100% power to detect differences in infarct volume (anticipated Cohen’s d = 3.4) using these same groups. Analysis of results for comparison between treatment groups (infarct volume, immunohistochemistry) was performed using a Student’s t-test. For time course comparisons (behavior, vital signs, and perfusion), a two-way repeated measures ANOVA was used. Significance is defined as a *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001.
Compliance with STAIR criteria
To maximize the applicability of our results, we designed the study with reference to the STAIR recommendations for preclinical neuroprotection research.25 With regard to dose of drug, we selected a weight-based dose equivalent to 10 mg in a 70 kg human. This is the dose used currently by endovascular neurointerventionalists to treat patients in the angiography suite. We have not yet conducted preclinical dose–response studies. In terms of window of opportunity, we administered the drug in immediate sequence after vessel perfusion in order to simulate the clinical scenario of IA administration immediately following thrombectomy. We conducted the experiments in a blinded fashion, with randomization of assignment to treatment groups. We conducted a power analysis to ensure adequate subject numbers as detailed. We performed physiologic monitoring as detailed and achieved Laser Doppler flow reduction ≥60% as detailed in results. Regarding outcome measures, we evaluated functional outcome and infarct volume, as well as immunohistochemistry analysis of infarct. We did not analyze gender or age differences in this study, but such analysis is planned for future study.
Results
Vital signs
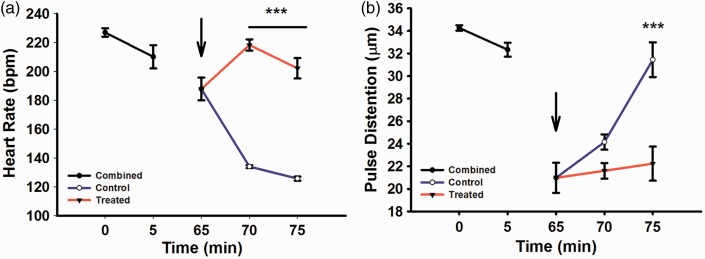
Measurements of baseline heart rate (beats per min, bpm) for control and treated groups (Figure 1(a)), combined prior to drug injection at 0 min (227 ± 2.92) and 5 min (210 ± 8.08), demonstrated a non-significant decrease of 67 bpm over this time period. Five minutes after recanalization and initiation of IA drug administration (i.e. at 70 min), the verapamil-treated group had a significantly (P < 0.001) higher heart rate (218.37 ± 3.83 versus 134.00 ± 1.02 in controls), a difference that largely persisted at the time (75 min) of final measurements (202.20 ± 7.04 versus 125.70 ± 1.36).
Figure 1.
MouseOx plus physiological measurements for combined (black), treated (red) and control (blue) groups. Arrow indicates drug administration after reperfusion/un-occlusion for 5 min. (a) Heart rate in beats per minute (bpm), 0–5 min (baseline measurements), 65 min (reperfusion/un-occlusion and drug administration), 70 and 75 min (reperfusion). (b) Pulse distention measuring vessel diameter (µm), 0–5 min (baseline), 65 min (reperfusion/un-occlusion and drug administration), 70 and 75 min (reperfusion). Combined (N = 30), control (N = 15), treated (N = 15).
Similarly, combined baseline pulse distention measurements (µm) at 0 min (34 ± 0.23) and 5 min (32 ± 0.61) decreased slightly by 2 µm over this time period. At 70 min the control group had a non-significant increase in pulse distention (24.15 ± 0.68 versus 21.60 ± 0.69), a difference that became significant (P < 0.001) at 75 min (31.45 ± 1.54 versus 22.23 ± 1.51).
Cerebral blood flow
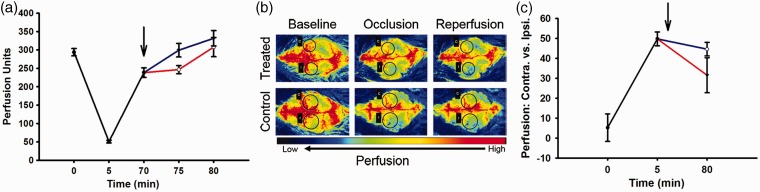
Five minutes after vessel occlusion, Laser Doppler (Figure 2(a)) blood flow measurements (Perfusion Units) through the MCA were reduced to approximately 17% of the pre-occlusion value. Reperfusion at 65 min demonstrated a 78.71% restoration of flow with a gradual and similar (i.e. not significantly different) return to pre-occlusion levels by 75 min after occlusion regardless of control or verapamil treatment.
Figure 2.
Perfusion measurements for combined (black), treated (red), and control (blue) groups. Arrow indicates drug administration after reperfusion/un-occlusion for 5 min. (a) Laser Doppler blood perfusion through middle cerebral artery at time points 0 min (baseline measurements), 5 min (occlusion), 70 min (reperfusion/un-occlusion), and 75 and 80 min (reperfusion). Treated (N = 9), control (N = 9). (b) Laser Speckle whole brain perfusion images through contralateral and ipsilateral cortex at time points 0 min (baseline), 5 min (MCA occlusion) and 80 min (reperfusion) for treated (N of 3) and control (N of 3). Black circles indicate regions of interest, ipsilateral (I) and corresponding contralateral (C) for MCA occlusion at aforementioned time points. (c) Laser Speckle graph for whole brain perfusion through contralateral and ipsilateral cortex at time points 0 minutes (baseline measurements), 5 min (MCA occlusion), and 80 (15 min reperfusion/un-occlusion) treated (N = 3), control (N = 3).
Similarly, Laser Speckle images (Figure 2(b)) and corresponding graph (Figure 2(c)) for equilateral cerebral blood flow measurements (perfusion units) demonstrated an expected reduction of the ipsilateral cerebral hemisphere 5 min after occlusion with non-significantly different restoration in blood flow measurements in the two treatment groups at 80 min post-occlusion.
Behavioral testing
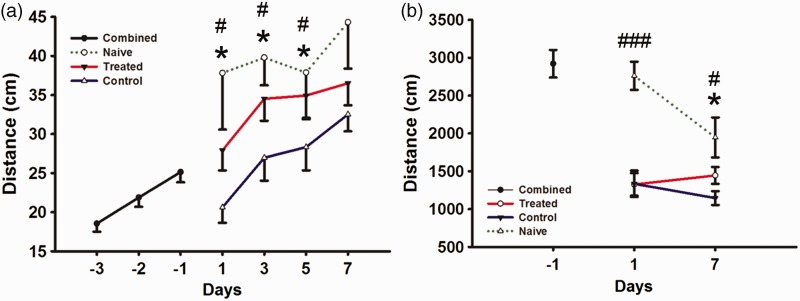
Behavioral testing for forced motor (rotor rod) movement (Figure 3(a)) measured in distance covered (cm) on training day -3, -2, and -1 demonstrated gradual improvement in this task as expected (18.55 ± 1.03, 21.87 ± 1.19, and 25.13 ± 1.29). On the day following surgery and drug administration, the two treatment groups showed a significant (P < 0.05) difference in performance (control 20.62 ± 1.97 versus treated 27.92 ± 2.56), a difference that continued on PSD 3 (control 26.97 ± 2.94 versus treated 34.52 ± 2.81) and 5 (control 28.34 ± 2.98 versus treated 34.93 ± 3.02). However, by PSD 7 the lower performance in the control group was no longer significantly different than the treated group (control 32.52 ± 2.17 versus treated 36.51 ± 2.82).
Figure 3.
Behavioral measurements for rotor rod and open field for combined (black), naïve (green dashed), treated (red), and control (blue) groups. (a) Rotor rod forced motor movement test for combined groups to learn task on days (-3, -2, and -1), groups separated following stroke surgery into control, treated, and naïve groups and were tested on days (1, 3, 5, and 7). (b) Open field free roam movement for combined groups on day (-1) and separated into control, treated, and naïve groups (1 and 7). Combined (N = 49), treated (N = 25), control (N = 20), naïve (N = 5). * indicates P ≤ 0.05 for treated versus control. # indicates P ≤ 0.05 for naïve versus control and ### indicates P ≤ 0.001 for naïve versus control and treated.
However, while both groups continued to improve after stroke, the verapamil-treated group exhibited greater improvement (11% versus -18% for controls) on PSD 1, (37% versus 7% for controls) on PSD 3, (39% versus 13% for controls) on PSD 5, and (45% versus 29% for controls) on PSD 7 when compared to baseline measurements (-1). Finally, while control-stroked mice rotor rod performance was significantly (P < 0.05) worse than naïve, aged-matched control mice on PSD 1 (37.81 ± 7.23), 3 (39.79 ± 3.55), 5 (37.87 ± 5.80), and 7 (44.32 ± 5.94), IA verapamil-treated group performance did not significantly differ from naïve group on these days.
In the free roam motor (open field) movement (Figure 3(b)), with analysis measured in distance (cm) covered over 5 min, both groups had a similarly significant (P < 0.001) decrease in free roam movement on PSD 1 (control 1334.09 ± 173.93 versus treated 1325.94 ± 149.00) when compared to baseline (-1) (2921.08 ± 182.74), but by PSD 7 the treated group moved significantly (P < 0.001) more (control 1146.28 ± 91.38 versus treated 1445.40 ± 111.76).
Both groups significantly (P < 0.001) decreased in overall distance covered on PSD 1 when compared to baseline (-1) and naïve, there was no significant difference between baseline and naïve (-6%) from baseline to PSD 1. Finally, while control stroked mice open field performance was significantly (P < 0.002) worse than naïve, aged-matched control mice (data not shown) on PSD 7, IA verapamil-treated group performance did not significantly differ from naïve group.
Infarct volume
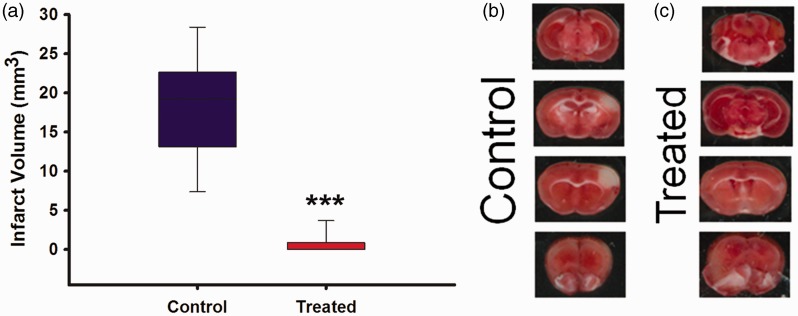
Infarct volume was analyzed using two forms of measurements: TTC and Cresyl Violet infarcts were combined and analyzed on PSD 7 (Figure 4). Mean infarct volumes (mm3) were significantly different (P < 0.001) between the control group (17.46 ± 2.66) and treated (0.64 ± 0.35).
Figure 4.
(a) Infarct volume analysis for control versus treated using TTC and cresyl violet stained stroked tissue. Treated (N = 18), control (N = 15); (b) TTC image for control group; (c) TTC image for treated. *** indicates P ≤ 0.001.
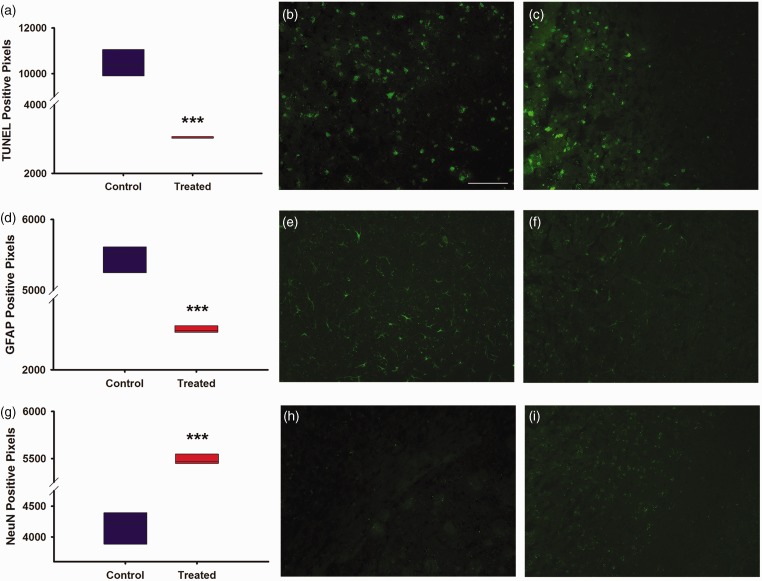
Immunohistochemistry
Specific antibodies were used for proteins related to overall cellular health (TUNEL stain), astrocytic inflammation/infiltration (GFAP), and neuron survival (NeuN, Figure 5). Compared to treated animals, there was a significantly greater increase in TUNEL positive pixel density in control groups (P < 0.001). Likewise, GFAP immunohistochemistry demonstrated significantly more GFAP immunoreactivity in the infarcted brain of control animals (P < 0.001). Lastly, NeuN immunohistochemistry demonstrated significantly decreased NeuN immunoreactivity in the infarcted brains of control animals (P < 0.001). Importantly, as mentioned in “Material and methods” section, the area of brain analyzed in these studies corresponds to the cortical region that was the epicenter of the stroke morphologically identified based on cryostat sectioning to include the greatest affected area.
Figure 5.
Graphs and images for immunohistochemistry, magnification at 20X with quantification of positive pixel density. (a) Graph depicting positive pixel for TUNEL stain of control versus treated in infarcted region. (b) Image of control TUNEL stain from infarcted region at magnification 20X. (c) Image of treated TUNEL stain from infarcted region at magnification 20X. (d) Graph depicting positive pixels for GFAP stain of control versus treated in infarcted region. (e) Image of control GFAP stain from infarcted region at magnification 20X. (f) Image of treated GFAP stain from infarcted region at magnification 20X. (g) Graph depicting positive pixels for NeuN stain of control versus treated in infarcted region. (h) Image of control NeuN stain from infarcted region at magnification 20X. (i) Image of control and treated NeuN stain from infarcted region at magnification 20X. Treated (N = 10), control (N = 10). White scale bar, 100 µm. *** indicates P ≤ 0.001.
Discussion
Stroke remains a leading cause of morbidity and mortality in the United States. However, with advances in vessel recanalization with t-PA and endovascular thrombectomy there is a greater chance of stroke survival and opportunity to decrease long-term disability. We believe that targeted IA delivery of a potential neuroprotective compound into the just recanalized large cerebral blood vessel is the next logical step in clinically acute ischemic stroke care. Our lab uses a clinically relevant mouse model of stroke to induce a focused, reliable, and reproducible cortical infarct that similarly affects motor function as seen clinically. Following vessel recanalization, we IA administered the potentially neuroprotective compound verapamil to mimic IA administration as a potential clinical therapeutic approach following endovascular thrombectomy. This was done at a specific flow rate (2.5 µl/min) and injection volume (10 µl) to maximize ipsilateral hemispheric targeting of the agent of interest while minimizing systemic exposure (no significant effects were noted in heart rate and pulse distention between baseline measurements and the treated group post-drug injection). This is an important result inasmuch as IV, systemically administered verapamil, an anti-hypertensive medication, would be expected to lower heart rate and pulse distention which can potentially cause bradycardia and hypotension by way of its venous return to the heart and thereby further exacerbate stroke symptoms by decreasing post-recanalization cerebral blood flow and negating verapamil’s intended neurological effects.
Verapamil is routinely administered IA to the brain by neurointerventionalists to treat cerebral artery vasospasm after subarachnoid hemorrhage. Given its recognized safety profile in the clinical setting, along with speculation of its neuroprotective properties, we chose to study its potential as an adjunctive therapeutic in stroke models. While its proposed primary mechanism has been vasodilation of intracranial arteries, we propose that it may have direct neuroprotective effects through L-type calcium channels beyond the vascular bed. To address this question of mechanism, we examined perfusion using Laser Doppler (testing the focal MCA) and Laser Speckle (testing whole brain cortex perfusion). Following IA administration, the Laser Doppler showed transiently higher perfusion in the control group. However, overall, the administration of verapamil did not significantly affect perfusion whether focally in the MCA or globally for cortical perfusion in the short term. This contrasted with the control group after drug administration that showed a significant drop in heart rate but a significant increase in perfusion units (blood pressure). Thus, the Laser Doppler and perfusion units correlate in the control group, showing an elevation relative to the verapamil-treated group. Given the role in maintaining adequate cerebral perfusion in ischemic stroke, we would not expect an elevation in blood pressure after reperfusion to explain the highly significant difference between the control and treatment group. While reperfusion injury is a recognized issue after recanalization, the transient differences (relative tachycardia and lower pulse distention) seen in the treated group do not adequately explain the benefit of verapamil in neuroprotection. One limitation of this assessment was a lack of follow-up assessments in mice 12–24 h after the procedure. Performing such an assessment would be challenging but could provide additional data on the perfusion mechanisms for neuroprotection by verapamil in the setting of recanalized ischemic stroke.
Acute stroke therapies are aimed at preventing death and minimizing functional disability. In our experimental strokes, death rates were not significantly affected with treatment, but were universally low in all conditions (less than 5% in both groups), a particular advantage of this experimental stroke model over others. Functional outcome was measured using two behavioral tests, rotor rod for forced motor movement and open field for free roam to determine the effect of IA verapamil on post-stroke function. Similarly, in the open field testing, although both groups reduced their movement on PSD 1, the treated group improved by PSD 7, while the control group continued to decline. Collectively, these results suggest that acutely administered IA verapamil is very effective in preventing behavioral decline in experimental stroke. However, this experiment may be confounded on PSD 1 by the recent surgical procedure. Recovery can be seen when comparing naïve, treated, and control PSD 1 measurements to baseline measurements; we found variable rates of improvement from baseline for the naïve- and verapamil-treated groups (77 and 11%, respectively) while the control group decreased 18% from baseline. However, such an effect should be dissipated by PSD 7. Finally, this analysis is somewhat limited by a lack of more long-term ( >30 day) functional assessment which is planned for future study. Therefore, the possibility that spontaneous functional recovery in the control stroked mice might eventually reach or plateau at the level of the stroked verapamil-treated mice cannot be excluded. However, in the setting of our planned methods for gross and immunohistochemistry assessment, we feel that seven day post-stroke testing was reasonable for initial assessment of verapamil’s treatment effect.
Verapamil was further tested to determine its effect on brain tissue preservation. Brain TTC and immunohistochemistry showed a large reduction in infarct, with reduction in cellular apoptosis and preservation of mature neurons. We chose these experiments as markers of step-wise processing in ischemic stroke–ischemic volume, inflammatory cell activation and infiltration, cell death and apoptosis, and neuronal survival. In each case, our data support the hypothesis that verapamil is neuroprotective on brain tissue when administered early and focally after reperfusion. Verapamil’s neuroprotective mechanism(s) of action remain to be elucidated. Indeed, as it is clear from these experiments that verapamil has significant potential as a therapeutic adjunct to thrombectomy in ischemic stroke, further study is needed to focus on the mechanism by which verapamil acts in this setting as well as its efficacy in different clinical models of ischemia such as the embolic model.
Indeed, while there are a number of animal models to mimic stroke with their differing advantages and disadvantages, our model mimics distal M1/M2 middle cerebral artery vessel occlusion and produces the hallmark motor and physiological deficits one would expect to see in a patient presenting with an occlusion in the M1/M2 middle cerebral artery. By comparison, injected thrombus models with t-PA have variable rates of recanalization, and technology does not exist to perform mechanical intracranial thrombectomy in a rodent model. While we do not inject a clot or administer t-PA in our ischemic stroke model, we are able to significantly decrease the flow of blood through the MCA as is seen clinically and un-occlude guaranteeing recanalization. For these reasons, we conclude that our experimental approach reasonably mimics selective IA neuroprotective administration after clinical ELVO thrombectomy.
Recent clinical trials such as MR CLEAN, ESCAPE, and EXTEND-IA have shown that endovascular thrombectomy is the new standard of care for ELVO stroke to ensure recanalization of the vessel and reperfusion of the ischemic area.6,7,9,10 Incorporating IA administration of a potential neuroprotective agent following recanalization and reperfusion would be a simple adjunctive process, as the thrombectomy procedure inherently provides timely and selective arterial access to the cerebrovascular bed. While multiple neuroprotective agents have shown promise in animal studies, a lack of positive clinical data limited their adoption in patient care.26 These past studies did not have rapid access to selective arterial infusion after recanalization; our model provides this translational testing ground. Using our mouse model of acute ischemic stroke with IA administration we have shown verapamil is physiologically safe, improves functional outcome, decreases infarct volume, decreases astrogliosis activation, and increases mature neuron survival. The exact mechanism of these actions for verapamil is yet unknown and will be the focus of further study that further elucidate additional efficacy for this drug and for neuropharmacology in ischemic stroke. However, this study provides the necessary data to support clinical evaluation of this therapy.
Acknowledgements
The authors would like to thank Dr Erin Abner of Sanders Brown Center on Aging and College of Public Health for her assistance with statistical analysis.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: NIH UL1TR000117 to JFF and NIH 5T32 NS077889 to MM
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Study design was based on collaborative efforts of MEM, JMR, JFF, and GJB. MEM and JMR performed stroke surgeries and behavior. MEM, JMR, and IA performed infarct volume analysis, immunohistochemistry, and image quantification. MEM and IA compiled and analyzed behavioral and perfusion data. MEM, JMR, JFF, and GJB compiled manuscript, image, and figure preparation. JFF and GJB provided oversight for the project. A completed copy of the manuscript was provided to all authors for their input and approval.
References
- 1.Saver JL, Jahan R, Levy EI, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet 2012; 380: 1241–1249. [DOI] [PubMed] [Google Scholar]
- 2.Bretz MN, Graves A, West A, et al. Steps against recurrent stroke plus: patient transition program. J Neurosci Nurs 2014; 46: E3–13. quiz E1-2. [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation 2014; 129: e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thacker C. Stroke falls to No. 5 cause of death in U.S. American Heart Association Media Alert. 12/30/2014 ed2014.
- 5.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 2013; 368: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsons MW, Albers GW. MR RESCUE: is the glass half-full or half-empty? Stroke 2013; 44: 2055–2057. [DOI] [PubMed] [Google Scholar]
- 7.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 8.Fransen PS, Beumer D, Berkhemer OA, et al. MR CLEAN, a multicenter randomized clinical trial of endovascular treatment for acute ischemic stroke in the Netherlands: study protocol for a randomized controlled trial. Trials 2014; 15: 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 10.Fargen KM, Meyers PM, Khatri P, et al. Improvements in recanalization with modern stroke therapy: a review of prospective ischemic stroke trials during the last two decades. J Neurointerv Surg 2013; 5: 506–511. [DOI] [PubMed] [Google Scholar]
- 11.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 12.Aronowski J, Cho KH, Strong R, et al. Neurofilament proteolysis after focal ischemia; when do cells die after experimental stroke? J Cerebral Blood Flow Metab 1999; 19: 652–660. [DOI] [PubMed] [Google Scholar]
- 13.Lee B, Clarke D, Al Ahmad A, et al. Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J Clin Invest 2011; 121: 3005–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maniskas M, Bix G, Fraser J. Selective intra-arterial drug administration in a model of large vessel ischemia. J Neurosci Methods 2015; 240: 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodsky SJ, Cutler SS, Weiner DA, et al. Treatment of stable angina of effort with verapamil: a double-blind, placebo-controlled randomized crossover study. Circulation 1982; 66: 569–574. [DOI] [PubMed] [Google Scholar]
- 16.Roy MW, Dempsey RJ, Meyer KL, et al. Effects of verapamil and diltiazem on acute stroke in cats. J Neurosurg 1985; 63: 929–936. [DOI] [PubMed] [Google Scholar]
- 17.Berger JR, Busto R, Ginsberg MD. Verapamil – failure of metabolic amelioration following global forebrain ischemia in the rat. Stroke 1984; 15: 1029–1032. [DOI] [PubMed] [Google Scholar]
- 18.Ueda T, Yamamoto YL, Diksic M. Transvenous perfusion of the brain with verapamil during focal cerebral ischemia in rats. Stroke 1989; 20: 501–506. [DOI] [PubMed] [Google Scholar]
- 19.Wauquier A, Melis W, Janssen PA. Long-term neurological assessment of the post-resuscitative effects of flunarizine, verapamil and nimodipine in a new model of global complete ischaemia. Neuropharmacology 1989; 28: 837–846. [DOI] [PubMed] [Google Scholar]
- 20.Investigators IMSS. Combined intravenous and intra-arterial recanalization for acute ischemic stroke: the Interventional Management of Stroke Study. Stroke 2004; 35: 904–911. [DOI] [PubMed] [Google Scholar]
- 21.del Zoppo GJ, Higashida RT, Furlan AJ, et al. PROACT: a phase II randomized trial of recombinant pro-urokinase by direct arterial delivery in acute middle cerebral artery stroke. PROACT Investigators. Prolyse in Acute Cerebral Thromboembolism. Stroke 1998; 29: 4–11. [DOI] [PubMed] [Google Scholar]
- 22.Kilkenny C, Browne WJ, Cuthill IC, et al. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 2010; 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sung RJ, Elser B, McAllister RG., Jr Intravenous verapamil for termination of re-entrant supraventricular tachycardias: intracardiac studies correlated with plasma verapamil concentrations. Ann Intern Med 1980; 93: 682–689. [DOI] [PubMed] [Google Scholar]
- 24.Waxman HL, Myerburg RJ, Appel R, et al. Verapamil for control of ventricular rate in paroxysmal supraventricular tachycardia and atrial fibrillation or flutter: a double-blind randomized cross-over study. Ann Intern Med 1981; 94: 1–6. [DOI] [PubMed] [Google Scholar]
- 25.Stroke Therapy Academic Industry R. Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke 1999; 30: 2752–2758. [DOI] [PubMed] [Google Scholar]
- 26.Grupke S, Hall J, Dobbs M, et al. Understanding history, and not repeating it. Neuroprotection for acute ischemic stroke: from review to preview. Clin Neurol Neurosurg 2015; 129: 1–9. [DOI] [PubMed] [Google Scholar]