Abstract
Neurovascular coupling reflects the close temporal and regional linkage between neural activity and cerebral blood flow. Although providing mechanistic insight, our understanding of neurovascular coupling is largely limited to non-physiological ex vivo preparations and non-human models using sedatives/anesthetics with confounding cerebrovascular implications. Herein, with particular focus on humans, we review the present mechanistic understanding of neurovascular coupling and highlight current approaches to assess these responses and the application in health and disease. Moreover, we present new guidelines for standardizing the assessment of neurovascular coupling in humans. To improve the reliability of measurement and related interpretation, the utility of new automated software for neurovascular coupling is demonstrated, which provides the capacity for coalescing repetitive trials and time intervals into single contours and extracting numerous metrics (e.g., conductance and pulsatility, critical closing pressure, etc.) according to patterns of interest (e.g., peak/minimum response, time of response, etc.). This versatile software also permits the normalization of neurovascular coupling metrics to dynamic changes in arterial blood gases, potentially influencing the hyperemic response. It is hoped that these guidelines, combined with the newly developed and openly available software, will help to propel the understanding of neurovascular coupling in humans and also lead to improved clinical management of this critical physiological function.
Keywords: Automated software, cerebral blood flow, cerebrovascular, functional hyperemia, neuronal activation
Introduction
The human brain comprises only 2% of body weight, yet consumes more than 20% of oxygen and glucose at rest, with almost all adenosine triphosphate in the brain being produced by oxidative metabolism of glucose.1 In addition to a great need for substrate provision and by-product clearance, the metabolic circumstances in the brain are compromised by a limited intra-cellular capacity for energy storage. These two characteristics, combined with the paramount importance of brain function in comparison with other end-organs, create a situation where coupling of blood flow requires careful matching to changes in neuronal activation. The brain is well equipped to provide suitable blood flow for a given metabolic demand due to two components: extremely high vascularization, as well as redundant and sophisticated regulation of blood flow.2 One primary factor responsible for ensuring appropriate blood supply within the brain is neurovascular coupling, which describes a close temporal and regional linkage between neural activity and cerebral blood flow (CBF) responses. This manuscript will review the major issues related to neurovascular coupling including: the anatomic structure of cerebral vasculature, organ-level mechanisms regulating CBF; provide insight into the mechanisms underlying the neurovascular response; outline the various methods used to measure neurovascular coupling in humans; present new guidelines for standardizing the assessment of neurovascular coupling in humans; and highlight the related alterations in neurovascular coupling in clinical conditions. Furthermore, this manuscript will describe a new analytical process to enable reliable and consistent assessments of human neurovascular coupling and provide directions for future research into this exciting and fascinating line of inquiry.
Anatomic structure
The internal carotid and vertebral arteries (ICA, VA), which branch off of the subclavian arteries, are the two conduit vessels providing blood delivery to the brain. The internal carotid arteries transmit approximately 70% of total CBF, while the remainder is provided by the VAs. In healthy individuals under typical scenarios, the VAs primarily provide blood flow to the brainstem, cerebellum, and occipital cortex. The VAs converge to form the basilar artery which then serves as a tributary to the circle of Willis and emerge as the posterior cerebral arteries. The circle of Willis is a crucially redundant anastomotic safety net evolutionarily commissioned to maintain perfusion if blood flow if one or more extracranial cerebral arteries is disrupted. It also represents the terminus of the internal carotid, which then contributes to the middle cerebral artery (MCA). The anterior cerebral arteries branch off the anterior aspect of the circle of Willis. The anterior, middle, and posterior cerebral arteries branch extensively before wrapping the brain surface in an array of vascularized tissue within the pia mater. Pial arteries then penetrate into the cortex. Upon penetration, arterioles are located within the Virchow-Robin space (i.e., pia mater protrudes into the brain with the arteriole). Once the arteries completely penetrate the parenchyma, they are considered “parenchymal” and are completely enveloped by astrocytic end-feet.3
Extracranial arteries (e.g. internal and vertebral arteries), large arteries of the brain (e.g. MCA), as well as pial arteries on the brain surface, are richly innervated by “extrinsic” perivascular postganglionic neurons from both sympathetic and parasympathetic origins.4,5 On the other hand, parenchymal arterioles are regulated by “intrinsic” factors within the parenchyma, which are mediated by neuronal activation and astrocytic modulation.6 Parenchymal arteries have greater basal tone and do not respond effectively to neurotransmitters, including norepinephrine and serotonin, which do elicit changes in the tone of upstream pial arteries.6 All arteries in the brain contain a specialized endothelial layer, which functions as a barrier in an attempt to tightly regulate exchange between the blood supply and the brain (i.e., blood-brain barrier). In addition to this, tight junctions prevent paracellular passage and connect the innermost layer of epithelial cells.
Regulation of cerebral blood flow
Regulation of CBF is achieved through several factors including metabolic, myogenic, neurogenic, and systemic control.7 Specifically, the primary controllers of CBF are partial pressure of arterial blood gases (CO2 and O2), cerebral metabolism, and the autonomic nervous system.8,9 Under conditions of sufficient perfusion pressure, the remaining factors interact to regulate CBF by modulations in cerebrovascular resistance.10 Cerebral blood flow is particularly sensitive to partial pressure of arterial CO2, as reflected in a 1-mm Hg increase or decrease from eupnoeic CO2 levels leading to a 3−6% elevation or a 1−3% reduction in CBF, respectively.
The large conduit arteries of the neck provide a large degree of influence in the regulation of CBF. Early canine in situ work showed that the arteries of the neck (i.e., ICA and VA) may provide as much as 50% of cerebrovascular resistance at rest.11–13 These large arteries actively change diameters in response to various stimuli including alterations in arterial blood gas partial pressures,14 and orthostatic challenges.15 Both extracranial and pial arteries are innervated by sympathetic neurons originating from the superior cervical ganglion, which may serve to regulate CBF through the release of norepinephrine and neuropeptide Y.4,16–19 Parasympathetic neurons from cranial nerves of the otic and pterygopalatine ganglia also modulate cerebrovascular tone by releasing acetylcholine, vasoactive intestinal peptide (VIP), and nitric oxide (NO), while the trigeminal ganglion fibres release a variety of other vasorelaxant mediators.4,20 This is in contrast to the vast majority of systemic vasculature where the parasympathetic nervous system does not actively regulate tone. Despite extensive autonomic innervation in the brain, findings in humans support only a modest and somewhat frequency-dependent role of sympathetic21–23 and parasympathetic activity24 in influencing dynamic cerebral autoregulation. The details of the complex interactions involved in human CBF regulation have recently been reviewed.9 The focus of this article is on the relatively unexplored topic of neurovascular coupling in humans. Herein, particular focus will be made on the physiology and pathophysiology of neurovascular coupling, methodological limitations, and advances in this field.
Mechanisms of neurovascular coupling
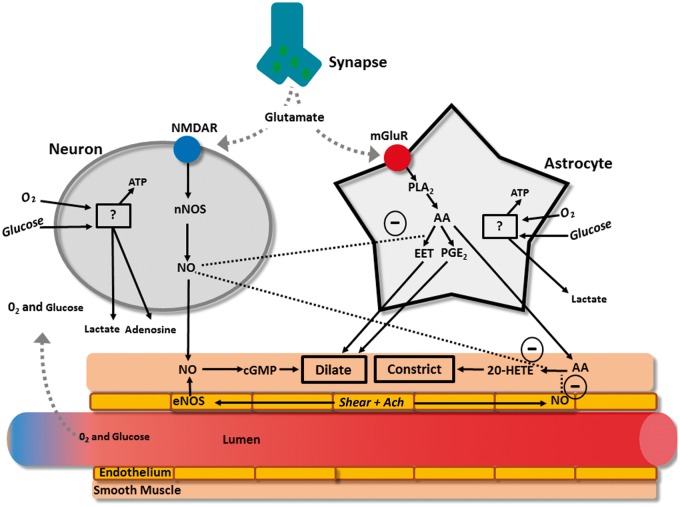
The neurovascular unit is made up of three major components: (1) the vascular smooth muscle, (2) the neuron, and (3) the astrocyte glial cell (Figure 1). At a rudimentary level of detail, modulations in neuronal activity cause changes in local blood flow mediated by transmission through the astrocyte. This interaction is termed “neurovascular coupling,” which describes coupling between neuronal activity and the vasculature. Tight temporal and amplitudinal linkage between neuronal activity and CBF delivery has been observed for >120 years.25–27 There is much nuance and redundancy in this simplistic explanation of the neurovascular unit’s interactions. For example, the neuron also alters vascular tone independently of astrocytic mediation, and these two cells play a synergistic or antagonistic role depending on the physiological environment. Readers are referred to recent reviews on this topic for further details.2,28,29
Figure 1.
Primary glutaminergic pathways involved in cerebral blood flow regulation. Glutamate release by the synapse activates N-methyl-D-aspartate receptors (NMDAR) on neurons as well as metabotropic glutamate receptors (mGluR) on astrocytes. Both of these receptors act by increasing cytosolic calcium concentration. In the neuron, Ca2+ activated neuronal nitric oxide synthase (nNOS) produces nitric oxide (NO). Increases in Ca2+ within the astrocytes activate phospholipase A2 (PLA2), which generates arachodonic acid (AA), and therefore both epoxyeicosatrienoic acid (EET) and prostaglandin E2 (PGE2), both of which dilate cerebral arteries.128–130 Nitric oxide exerts diverse effects including: 1) activating cyclic guanosine monophosphate (cGMP) in smooth muscle leading to dilation,131 2) inhibiting 20-hydroxyeicosatetraenoic acid (20-HETE) in smooth muscle to inhibit constriction,132,133 and 3) inhibiting production of EET in astrocytes which inhibits the vasodilatory action of EET on smooth muscle.129
Both neurons and astrocytes respond to increased extracellular glutamate to transmit direct and indirect vasoactive signals for the appropriate delivery and distribution of CBF.2 For example, glutamate released from presynaptic terminals activates N-methyl-D-aspartate receptors on neurons, which in the presence of sufficient intracellular oxygen and glucose, will stimulate the activation of neuronal NO synthase that provides NO to act directly on the parenchymal arterioles as a dilator.30 Adenosine will also be directly released to act as a vasodilator when neuronal adenosine triphosphate is low.31 Similarly to neurons, astrocytes respond to glutamate through metabotropic glutamate receptors, which again in the presence of sufficient intracellular oxygen and glucose, activate a cascading pathway involving the production of arachidonic acid which then produces epoxyeicosatrienoic acid and prostaglandins.32 Both prostaglandin and EET serve to dilate parenchymal arterioles, while arachidonic acid, after diffusing out of the astrocytes and into the smooth muscle, produces vasoconstriction.33 There also appears to be a complex relationship between astrocytes, incoming neuronal signals, and GABA interneurons that are thought to play a local integrating role precisely regulating local CBF delivery.29,34,35 These interneurons are ideally placed between glutaminergic pyramidal cells and project onto microvessels.35,36 Indeed, evoked firing of GABA interneurons resulted in increased concentration of a variety of vasoactive substances (e.g., NO, acetylcholine, neuropeptide-Y, VIP) that led to both constriction and dilation of cerebral microvasculature.34,35
Under normal conditions, the double stimuli (i.e., neuronal and astrocytic) leads to a 4-fold greater increase in CBF relative to the increase in ATP consumption.37 This overcompensation of functional hyperemia appears to support the feed-forward mechanism.37 It should be noted however that a feed-backward system capable of functional hyperemia has also been proposed as a compensatory mechanism in a model incorporating capillary transit time heterogeneity, maximum oxygen extraction fraction, metabolic rate of oxygen and CBF.38 Together, these factors demonstrated that more homogenous capillary flow and exaggerated CBF response compensated for reduced oxygen extraction during functional hyperemia.38 This overabundance of blood flow relative to metabolism is the theoretical foundation of blood-oxygen level dependent (BOLD) magnetic resonance imaging; which relies on the assumption that increases in deoxygenated blood are associated with increased neuronal activity.39
Although astrocytes are sufficient to evoke a neurovascular coupling response, it is unclear whether astrocytes are necessary for functional hyperemia to occur.40,41 Recently, it has been proffered that the role of astrocytes during neurovascular coupling is isolated to maintaining blood flow suitable for basal neuronal activity and to producing a “slow-onset” hyperemia (i.e., 3−4 s post neuronal activation) but not to be active in the immediate hyperemic effect that occurs in <1 s.41 Recent and sophisticated assessments of the neurovascular coupling response to visual stimulation showed that astrocytic endfeet/Ca2+ signalling was not responsible for the rapid functional hyperemia;40 readers are referred to more in-depth reviews on this topic.28,42 This is a somewhat emerging but compelling contention, such that it deserves mention in the context of the present review.
It is not just the arteriole smooth muscle that regulates neurovascular coupling, although this has been the primary understanding until very recently. Pericytes (i.e., small cells expressing contractile tissue located at 50 µm intervals along the length of capillaries)43 likely also play a large role in neurovascular regulation, as suggested by their anatomic arrangement.44 For example, neurons are more often closer to pericytes than arterioles, creating the plausible scenario whereby neuronal activation first alters resistance through modulations in pericyte tone on capillaries, and signals are then transmitted ‘up-stream’ to arterioles.45 Pericytes are theorized to be regulated in much the same way as arterioles.44 Brain slice preparations show that glutamate and norepinephrine lead to dilation and constriction of pericytes, respectively, and blocking glutaminergic receptors leads to vasoconstriction.46 The intervals between pericytes necessitate the transmission of vasoactive signals through gap junctions; however, whether these travel between pericytes/endothelial gaps remains to be elucidated.44,46 In any case, there is a huge capacity for the pericytes to influence neurovascular coupling. For example, it has been modelled that anywhere from 16−70% of resistance within the brain parenchyma is produced by capillaries.47,48 In reality this may be even larger, as capillaries are so narrow that white and red blood cells have to deform to pass through, creating even more resistance than Poiseuille's law predicts.49
The combination of incongruent findings,40,50 the elucidation of highly redundant mechanistic pathways,41 and limited translational capacity has led to the acknowledgment that the mechanisms of neurovascular coupling need to be better explored in vivo/human models rather than just in animals and isolated well-controlled preparations (e.g., two-photon imaging, brain slices, etc.).41,51 Establishing a standardized protocol for evaluating human neurovascular coupling in vivo would allow for insight into not only the mechanisms underlying this fundamental response but also how neurovascular coupling is altered in diverse populations (e.g., clinical populations, aging, etc.).
Human neurovascular coupling
Our present understanding of neurovascular coupling in humans is limited, and the available knowledge is generated from a small number of laboratories, using a variety of unstandardized assessments.52–63 Although there is mechanistic insight provided by the isolated preparations of neurovascular coupling,41 little is known about which mechanisms are sufficient versus necessary for a functional and effective neurovascular coupling response in humans. The available literature related to this topic is reviewed below.
Data indicate that the neurovascular coupling response is comprised of highly redundant mechanistic regulation and is well maintained under a variety of physiological and pharmacological stressors. Such mechanisms are largely unexplored in humans to date. Recently, we have attempted to specifically antagonize cerebrovascular L-type calcium channels using nimodipine, which theoretically blunts the capacity for the cerebrovasculature to respond to an array of vasoactive mediators thought to play a role in neurovascular coupling from isolated preparations (i.e., bradykinin, vasopressin, dopamine, epinephrine, norepinephrine, histamine, glutamate, acetylcholine, serotonin, and prostaglandin).64 Even with a clinically relevant dose of 60 mg, which is used to treat vasospasm, the neurovascular coupling response was normal as compared to a placebo control trial.64 Although impaired in various pathologies (as described in detail below), neurovascular coupling is also maintained during exercise when a number of physiological parameters a significantly altered.55 Together, these data indicate that human neurovascular coupling is remarkably robust and demonstrate the inability of isolated models to predict in vivo responses.
Outside of using physiological and pharmacological stressors, another approach to provide insight into the mechanisms underlying neurovascular coupling has been to use mathematical dynamic multivariate/autoregressive modelling.54 Through subcomponent modelling, alterations in critical closing pressure (i.e., the pressure inside a blood vessel below which the vessel will collapse) and resistance area product (i.e., resistance with acknowledgement of unknown vessel diameter; inverse slope of instantaneous beat-by-beat relationship between CBF velocity and blood pressure) during neurovascular coupling are thought to provide selective indices of metabolic and myogenic cerebrovascular regulation, respectively, while the influence of blood pressure and changes in end-tidal partial pressure CO2 (ETCO2) on neurovascular coupling are also modelled.65–67 Using this approach, a number of interesting studies have been completed with the following conclusions: (1) hypercapnia impairs the metabolic regulation of neurovascular coupling, (2) breath-by-breath changes in ETCO2 during cognition can impact the neurovascular coupling response, and (3) the blood pressure response to cognitive activation plays a large role in the neurovascular coupling response, particularly the initial spike.53,66,68 These interesting studies have yet to confirm a direct mechanistic link between the physiological regulatory pathways of interest (i.e., metabolic versus myogenic influences) and the subcomponent models/metrics being utilized (i.e. critical closing pressure versus resistance area product).
Some insight into neurovascular coupling in humans has been provided by unique models measuring concurrent cerebral tissue oxygenation and CBF. Although technically and environmentally challenging, these studies can provide detailed insight into flow-metabolism coupling. For example, using functional MRI, it is now known that a consistent BOLD signal over childhood development is underpinned by decreasing neuronal activation/metabolism counterbalanced by increasing neurovascular coupling.39 Also when using this approach in the healthy human brain, oxygenated venous blood rises (i.e., increased BOLD signal) to a greater extent than CBF during visual stimulation, indicating the effect of exaggerated hyperemia beyond cerebral metabolic demand.69 Furthermore, although reduced neural activity to a given stimulus has been reported after ischemic stroke,70 a recent study combining BOLD, fluid attenuation inversion recovery, and arterial spin labelling has shown a variety of neurovascular coupling phenotypes in this population.69 For example, individuals with ischemic stroke can exhibit a blunted, exaggerated or reduced functional MRI BOLD signal during visual stimulation, with exaggerated or blunted hyperemia.69 Although these data indicate a mismatch between CBF delivery and oxygenation extraction/metabolism,69 the mechanisms explaining the variability in the functional MRI BOLD signal responses during visual stimulation are unknown.
Clinical implications
Despite inconsistent methodologies, a number of studies have characterized neurovascular coupling in a variety of models of clinical conditions (and in some cases human populations), as summarized in Table 1. These conditions likely impact neurovascular coupling through a variety of diverse mechanistic pathways. For example, one fundamental factor underlying impairments of neurovascular coupling in clinical populations (i.e. high level spinal cord injury, autonomic dysfunction, and generalised hypotension) is the insufficient cerebral perfusion pressure, which likely renders any reductions in cerebrovascular tone secondary to neuronal activation futile for producing hyperemia.52,71–73 On the other hand, conditions such as stroke and traumatic brain injury are likely impaired due to alterations in hyperemic signalling from glutamate release secondary to neuronal death and astrocyte scarring.67,74–78 Furthermore, oxidative stress is suspected to play a particularly deleterious role in human neurovascular coupling and is implicated in the declines seen in hypertension (through angiotensin receptor type 1 agonism), Alzheimer’s (through amyloid β accretion), diabetes (through chronic hyperglycemia), and healthy aging (Table 1). It may be that reducing oxidative stress is a viable therapeutic target for improving neurovascular coupling in humans, although this possibility needs to be clearly examined.
Table 1.
Neurovascular coupling in various clinical conditions.
| Pathology | Impact on neurovascular coupling | Mechanism |
|---|---|---|
| Stroke | Impaired – particularly in hemisphere of insult | Brain edema, inflammation, impaired neurotransmission, and neuronal death impairs normal neuronal activation.a Also, activation of non-specific brain structures further complicates interpretation. 67,74,75 |
| Hypertension | Impaired | Elevated circulating angiotensin II leads to: 1) activation of AT1 receptors on cerebral blood vessels, 2) increased oxidative stress which inhibits neuronal and astrocytic vascular dilators.a,134,135 |
| Hypotension | Impaired | Inadequate perfusion pressure inhibits efficacious hyperemic response, although neuronal signalling may be normal.52 |
| Autonomic Dysfunction | Impaired | Impaired blood pressure responses, as well as altered neurogenic regulation of neurovascular coupling.b,52,73 |
| High level spinal cord injury | Impaired | Unknown.b Persistent dysfunction of neurovascular coupling after restoring normal blood pressure.52 |
| Traumatic Brain Injury | Impaired | Neuronal death and astrocytic scar formation precludes normal neurovascular response.a,76–78 |
| Alzheimer’s | Impaired neurovascular coupling and impaired resting cerebral perfusion. | Hypercontractility (phenylephrine) of smooth muscle, increased basal and enhanced occurrence of spontaneous Ca2+ waves.a Amyloid Beta also directly inhibits functional hyperemia by promoting oxidative stress which inhibits neuronal and astrocytic vascular dilators. 136–138 |
| Smoking | Impaired | Unclear – but does not appear to reverse after cessation: indicating long term alterations in structure/function of the neurovascular unit.b,82 |
| Diabetes | Impaired | Impaired astrocyte function due to high circulating glucose and increased oxidative stress.a Prolonged hyperglycemia interferes with astrocytic gap junctional communication.139,140 |
| Healthy Aging | Impaired | Reduced with healthy aging,59 however exercise may be viable intervention to slow the decline.b,141 Dynamic lateralization of cerebral blood flow may also be impaired with health aging.142 |
Mechanistic insight provided only by animal models/isolated preparations.
Mechanisms not clearly established.
An interesting relationship between cognitive activation and neurovascular coupling in humans has been demonstrated,79 which provides a mechanistic link in conditions where cognition is impaired (Table 1). For example, chronic hypotension depresses cognitive activity and resting CBF, which is directly mediated by neurovascular coupling.72,80 Conversely, cognitive activity can be improved with pharmacological treatment of hypotension, through improvements in neurovascular coupling;52,81 demonstrating that cognitive function is positively related to neuronal tissue oxygen delivery. This link between impaired neurovascular coupling and cognitive decline needs to be further established in other clinical conditions, but if confirmed, may be particularly alarming in the smoking population, where neurovascular coupling does not normalize after cessation.82 In order to increase the clinical utility of neurovascular coupling as an end-point treatment target, there is a need for an automated and standardized protocol for analysis, which produces interpretable metrics.
Finally, although not a feasible technique in most scenarios, treatment of traumatic brain injury (where the brain is exposed) can allow for direct measurement of CBF, local metabolism (via microdialysis) and/or oxygenation across brain tissue.83 Under these conditions, it has been demonstrated that during cortical spreading depolarization, the neurovascular coupling response often becomes inverse with neuronal activation, leading to a hypo-emic response.77 The mechanisms underlying this inversion are unknown; however, this response likely further compromises survival of cerebral tissue which is already ischemic. Clearly, a plethora of future studies are required to delineate the major factors and redundancies in the human in vivo neurovascular coupling response.
Assessing neurovascular coupling in humans
In the following sections, we will illustrate how transcranial Doppler (TCD) and/or near-infrared spectroscopy (NIRS) approaches are used for neurovascular coupling evaluation in humans and describe a standardized approach for eliciting the neurovascular coupling response. Furthermore, we will outline a suggested standardized protocol for neurovascular coupling evaluation, description and quantification in humans, which employs newly developed automated software.
Transcranial Doppler
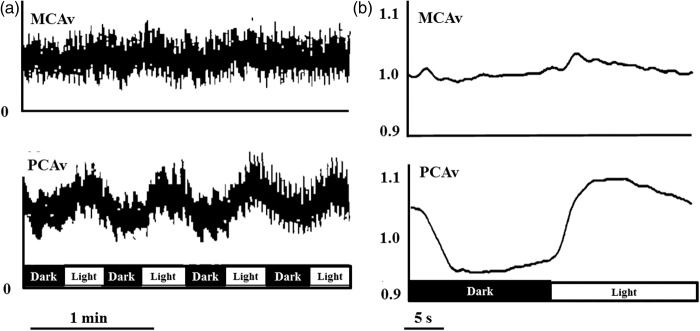
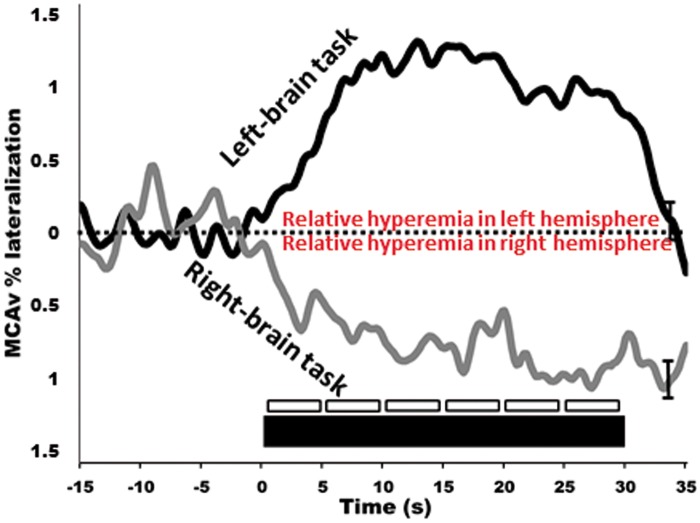
In humans, the most commonly used approach to study neurovascular coupling involves the use of transcranial Doppler (TCD). The high temporal resolution and non-invasive nature of TCD make it a useful tool in the assessment of integrative cerebrovascular function in terms of neurovascular coupling. New technologies further increase the utility of TCD. A TCD machine is relatively inexpensive ($25,000–$50,000 USD); moreover, TCD is easy to use and is safe in healthy and disease states alike. Therefore, it is practical to use TCD in the clinical setting to assess a variety of different cerebrovascular pathologies. Although the diameter of major arteries may change with fluctuations in arterial blood gases,14,84 the range of arterial CO2 at which TCD accurately estimates changes in CBF is not exceeded during standard neurovascular coupling protocols.52,67 The functional anatomy of the brain allows neurovascular coupling to be easily and reliably examined by measurement of the sensorimotor or cognitive stimulatory effects on cerebral blood velocity; a method termed functional TCD. This technique was first utilized by Aaslid in 1987, who showed that blood velocity in the posterior cerebral artery (PCA) changed with visual stimulation (Figure 2),85 but there are numerous studies in the neuro-cognitive literature that demonstrate consistent CBF changes in response to cognitive, verbal and motor tasks.85–88 In healthy individuals, this response will lead to an average of 10−20% increase in CBF in the PCA and a 5−8% increase in the MCA (Figures 2 and 3).59,86,89 Further, our group and others have explored hemispheric lateralization of CBF delivery using either right-handed/verbal tasks or left-handed motor paradigms to preferentially activate the left and right MCA.66,90 This latter technique has shown that indeed verbal-tasks combined with right-handed motor function lead to hyperemia preferentially in the left hemisphere, while spatial-tasks combined with left-handed motor function lead to hyperemia preferentially in the right hemisphere (Figure 4).66,90
Figure 2.
Traditionally reported hyperemia of the visual cortex to light stimulation in humans. (a) Middle cerebral artery blood velocity (MCAv), and posterior cerebral artery blood velocity (PCAv) during repetitive cycles of light exposure and darkness to the retina. Data is presented as relative changes. (b) Average responses to at least 16 repetitive dark and light sequences from each of 10 normal individuals for MCAv and PCAv. Figure adapted from Aaslid.85
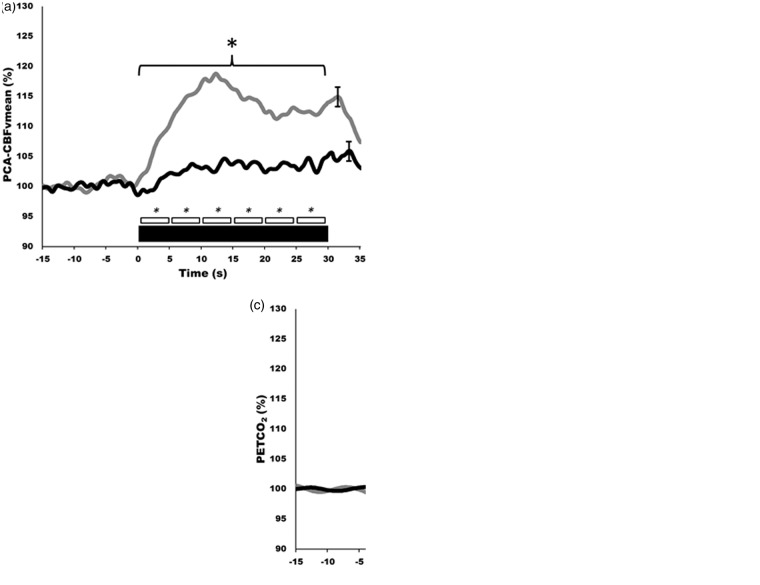
Figure 3.
Neurovascular coupling in humans generated from automated software using established standardized eyes-open/eyes-closed protocol. (a–c) Hyperemic responses, during visual stimulation, of the posterior cerebral artery (PCA; a), middle cerebral artery (MCA; b), and partial pressure end-tidal carbon dioxide (PETCO2; c). Note the selective activation of the PCA of around 20% vs. just 4−5% in MCA. Grey lines indicate healthy control group, black bars indicate high-level spinal cord injured group. Thick black bar indicates 30 s of eyes-open reading, being immediately preceded by eyes-closed. Smaller boxes represent 5 s bins which were averaged and compared quantitatively. These contours represent the response of 10 trials for each of 10 participants.
Figure 4.
Dynamic lateralization of hyperemic responses. In healthy individuals left-brain tasks lead to selective hyperemia of the left MCA, and the opposite occurs for right-brain tasks. The left-brain task involved using the right-hand to complete a verbal fluency test, whereas the right-brain task involved using the left-hand to build presented two-dimensional images as three-dimensional structures. Thick black bar indicates 30 s of left/right-brain activation preceded by eyes-closed. Smaller boxes represent 5 s bins which were averaged and compared quantitatively. These contours represent the response of 10 trials for each of 10 participants.
Detailed instructions on insonating the MCA and PCA have been provided elsewhere.91 Briefly, MCA blood velocity is generally viewed at 35−60 mm depth, after placing the Doppler probe on the middle transtemporal window oriented in line with the coronal axis. The PCA can then be viewed most commonly at 60−70 mm using the anterior transtemporal window by tilting the Doppler probe slightly posteriorly from the MCA. The P1 segment of the PCA will result in blood flow toward the Doppler probe, while the P2 segment curves away from the probe. Both the P1 and P2 segments have been utilized with similar results, however a consistent segment should be maintained throughout a given study.52,55,73,92,93 Lastly, often only the posterior window is suitable for insonating intracranial vessels in the elderly, due to skull thickening with advancing age.94 Some studies have reported that 10−15% of individuals will have inadequate windows for insonating intracranial cerebral vessels;95 as such, it may be advantageous to utilize other techniques such as NIRS (see next section) in addition to TCD for studies in the elderly or those with related cerebrovascular pathology.
It is now clear that the diameter of the MCA (and hence possibly the PCA) is influenced by marked changes in arterial PCO2 (>8 mmHg above or below resting) and arterial PO2 (<50 mmHg)84,96 (reviewed in literature9,97). It is less clear that changes in blood pressure will affect the diameter of these intra-cranial arteries. However, it would seem highly unlikely that hyperemic responses noted in the MCA/PCA are being affected by changes in arterial diameter over the neurovascular coupling response. The major reason is that changes in vasoactive influencers (i.e., arterial blood gases) do not occur and the NVC response is very rapid (∼1 s). For example, no discernable change in PETCO2 has been noted during 30−60 s of stimulation. 52,98 Furthermore, if the diameter of the MCA (or PCA) was dilating during the NVC response and being directly impacted by metabolic demand/hypoxia, this would be reflected in a blunted or absent hyperemic response (i.e., the dilation of the MCA/PCA would lead to a reduction in blood velocity). Thus, the major site driving the NVC response seems to be the neurovascular unit rather than the larger intra-cranial arteries.
Near infrared spectroscopy
In addition to TCD, NIRS can also be used for assessing neurovascular coupling in humans.99–102 Incorporating NIRS can indirectly quantify changes in oxygenated and deoxygenated hemoglobin, based on the relative transparency of tissue to near-infrared light, and oxygen-dependent light absorption caused by hemoglobin chromophores. As such, both quantitative and regional indexes of CBF can be provided utilizing the blood-oxygen level dependent signal to detect changes in oxygenated (O2Hb), deoxygenated, (HHb) and total hemoglobin (tHb) within for the most part, the venous vessels of the brain (i.e. <1 mm).103 Like the TCD, NIRS has very high temporal resolution and can most commonly provide analog outputs at >20 Hz. Moreover, NIRS is also similar to TCD in terms of cost and ease of use. As NIRS also provides some insight into changes in CBF, the underlying arterial CO2 must be considered, as alterations will affect CBF independently from changes in neuronal activity.104 Also similar to TCD, NIRS can evaluate CBF responses to neuronal activation using a variety of strategies of activation including various emotional, sensory, motor, and pure cognitive tasks.105,106 The history of the development of NIRS is well documented (reviewed in the literature103). The NIRS response during NVC is similar to that generated using TCD and typically results in increased O2Hb and tHb with a concurrent small decrease in HHb; these responses are similarly regionally specific (i.e., visual stimulation leads to posterior cortex hyperemia).99 During repeated squat-stand maneuvers where blood pressure rapidly increases and decreases, changes in NIRS and TCD were of similar amplitude however changes in NIRS signals trailed those from TCD by 1.8−2.6 s, which may be the result of arterial transit time and fundamental differences in anatomic location between TCD and NIRS.107 Further, when using other vasoactive stimuli, such as hypercapnia, the NIRS-derived percent hyperemic response is markedly less (1−15% increases vs. 20−100% increases) of that demonstrated by NIRS.108 This discrepancy, likely reflecting different physiological mechanisms, potentially impacts the sensitivity of NIRS to detect significant differences between clinical and control populations.109 Clearly, more studies are needed to address the congruency of TCD and NIRS signals during NVC. Care must be taken to ensure that the optodes are located in appropriate locations of the participant’s head to allow for the evaluation of hyperemic responses in relevant portions of the brain, and that the sources and detectors are appropriately spaced. These guidelines are somewhat less stringent as compared to TCD and involve placing the optodes over the general area of interest (i.e., posterior cortex, frontal, partietal lobe, etc.).99 Apart from the frontal cortex, however, hair will impede signal quality in other brain regions unless the hair is removed. Nevertheless, the simultaneous assessment of TCD (as an index of blood flow delivery) with NIRS (as an index of cerebral tissue oxygenation, the key end-point of interest for neuronal viability) during NVC will provide complimentary insight into integrated cerebrovascular physiology and will permit the inclusion of more subjects, especially when TCD is not possible.
Although TCD and NIRS are by far the most widely used tools for neurovascular coupling measurement in humans, there still exists significant variability in the software and strategies used to analyze these metrics. For example, many studies simply report the maximal change in CBF recorded during the “activation” period with55,92,110 or without111,112 addressing the temporal characteristics, while others attribute specific components and characteristics of the hyperemic contour to specific influences (i.e., perfusion pressure, neurogenic, autoregulation, blood gas concentration).54,74 Our approach is to incorporate both amplitudinal and temporal characteristics by plotting the entire hyperemic contour, while extracting data from temporal subdivisions (i.e., early versus late/5 s duration bins, etc.) for further analysis.52 The amount of data analysis required for the latter two approaches is significant, with a variety of processing steps required to coalesce contours into interpretable metrics.
Eliciting the neurovascular coupling response
A robust neurovascular coupling response can be assessed by simple visual stimulation (i.e., opening eyes/reading) during beat-by-beat measurement of blood flow/velocity in the PCA relative to the MCA, the prior of which is associated with perfusion of the visual cortex.52 The selective activation of the occipital lobe is achieved using repeatable and reliable stimuli involving an eyes-open task against a bright visual stimuli (reading, flashing screen, eyes tracking tester’s moving hand, etc.). The visual stimulation provides a strong mechanistic model of neurovascular coupling that is somewhat independent of variable neuronal activation schemes (i.e., a variety of different visual spatial tasks elicit similar PCA hyperemic responses);73 however, less is understood about the hyperemia response in MCA or anterior cerebral arteries to tasks of varying difficulties or under different states of motivation. Conventionally, after a 10−15 min period of quiet rest (to allow stabilization of blood volume and hemodynamic signals), 5−10 cycles are repeated where each cycle consists of a 20−30 s period of eyes-closed time followed by 30−40 s of eyes-open time.52,73 The greater the number of cycles, the higher the signal-to-noise ratio; however, this obviously must be balanced with the time constraints of the study and the impact on the participants whom may be from a clinical population. Patient positioning can vary depending on study design. Often for clinical relevance, the seated position is used; however, participants may be positioned supine if studying different loading conditions. Similarly, the left or right PCA can be chosen while assessing the contralateral MCA, although this must be consistent throughout the study as perfusion properties can vary between the left and right sides and often CBF is elevated to the left hemisphere.113
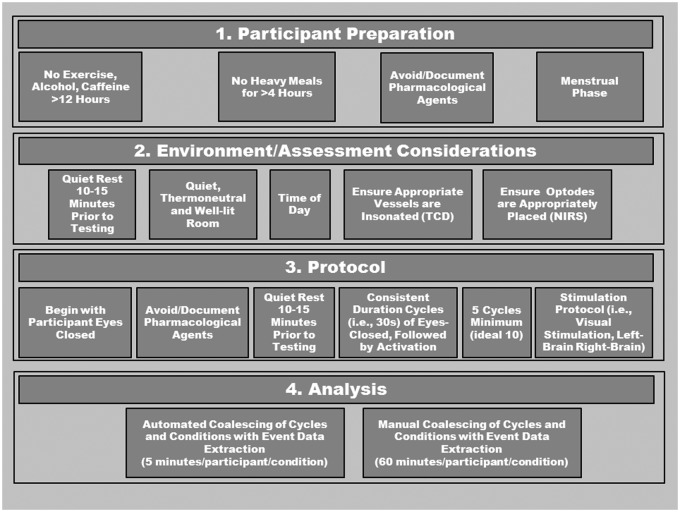
All testing should take place in a quiet, temperature-controlled and well-lit room. Depending on the study design, participants should avoid alcohol, caffeine, and strenuous exercise at least 12 h prior to testing, and be fasted for at least 4 h as these factors can influence vascular tone and function.114–125 Furthermore, assessments should take place at the same time of day, and in the same menstrual phase for premenopausal women.115 Additionally, medications should be avoided for at least four half-lives if possible, whereas non-steroidal anti-inflammatories should be discontinued for 2−3 days.126,127 This latter recommendation may be problematic in many clinical conditions; however, in any case, all medications should be carefully documented and considered during data interpretation. Due to the ease of administering the test, if artifact occurs within a cycle, another cycle should be added and used to replace the aberrant cycle. If a trial with an artifact needs to be included, the problematic data points should be linearly interpolated using one data point on either side of aberrant data. If more than two aberrant data points occur, this cycle should be removed. Please see Figure 5 for a detailed description of proposed standardization protocol.
Figure 5.
Recommendations for the assessment of neurovascular coupling in humans using transcranial Doppler (TCD) or near-infrared spectroscopy (NIRS). After ensuring appropriate participant preparation, testing should take place in a suitable environment with confidence that vessels/brain regions are accurately detected. Testing should also take place at consistent time of day between participants and conditions. A 10−15 min period of quiet rest in the testing position should precede the actual testing, allowing for stable hemodynamics. The protocol itself should begin with the participants’ eyes closed, and consist of 5−10 repeated cycles of 30 s eyes-closed, followed by eyes-open, followed by eyes-closed, with no time between. The timing of the cycles should be precise, and the assessor should ensure initiation of the “task” (i.e., eyes-open, closed, cognitive task) is immediate. Manual analysis can be extremely time-consuming; however, automated analysis is now available.
Analysis considerations
Hemodynamic data from each original hyperemic recording are extracted on a beat-by-beat basis using a variety of acquisition systems available (i.e., LabChart, LabView, etc.). The required outputs from the data acquisition programs include RR interval, systolic, diastolic, and mean arterial pressure as well as mean, peak and minimum velocity from the TCD/NIRS. After extraction, data from each subject and condition are placed in a separate spreadsheet that includes all of the 5−10 repeated neurovascular coupling cycles collected. After this, the software automatically coalescences all trials from that participant into one contour for each outcome metric of interest (see Table 2) over the “eyes-closed” and “eyes-open” period. From this average response within a participant, metrics over a fully adjustable range of “bin” durations (i.e., 1 s, 5 s, 10 s, etc.) can be extracted for statistical analysis (see Table 2). Finally, a single group-averaged (± standard error/deviation) contour at a 5 Hz sampling frequency is output for production in graphing software of the users' choice for any of the above metrics (Table 1, Figure 3). End-tidal gases are extracted on breath-by-breath basis and are also interpolated at 5 Hz and can be used to normalize CBF metrics for changes/differences in arterial blood gases. These data are then plotted identically.
Table 2.
List of metrics and binning options available with current software.
| Vessels | Measurement Techniques | Outcome metrics | Formula | Theoretic physiology associated with outcome metrics |
|---|---|---|---|---|
| ACA | TCD | vmax | ||
| MCA | Beat-by-beat blood pressurea | vmin | ||
| PCA | vmean | (2x(vmin) + (vmax))/3 | ||
| VA | CVC | vmean/MAP | Cerebral blood flow for given cerebral perfusion pressure indicates the ease of blood flow through the brain. With increasing vascular tone, conductance decreases. Because of the reciprocal relationship between conductance and resistance, it is recommended to use conductance in high flow situations, where resistance vessels are relatively relaxed, and modulations in tone lead to very little change in resistance but large changes in conductance. Due to this, if using CVR instead of CVC, the CVR response could be underestimating the decrease in tone during the increase in flow, as further increases in flow above the resting state may not result in a concomitant reduction in resistance although conductance is substantially increasing.143,144 | |
| ICA | CVR | MAP/vmean | Cerebral perfusion pressure for a given cerebral blood flow indicates the resistive forces acting on blood flow through the brain. With increasing vascular tone, resistance increases. 143,144 | |
| RAP | (SBP-DBP)/(vmean-vmin) | The instantaneous (i.e., over a single heart beat) theoretical relationship between blood pressure and flow. Indicates the change in perfusion pressure for a given change in cerebral blood flow. Describes how much perfusion pressure increases or decreases for a given change in cerebral blood flow. 145,146 | ||
| CCP | MAP – (vmean × RAP) | The instantaneous (i.e., over a single heart beat) theoretical perfusion pressure at which cerebral blood flow ceases (i.e., becomes zero). Generally calculated as the pressure corresponding to zero cerebral blood flow when calculating the instantaneous relationship between blood pressure and cerebral blood flow. This value is generally above zero due to significant collapsing forces exerted on the vasculature from intracranial pressure and tension within the vessel walls themselves. 145–147 | ||
| PI | (vmax-vmin)/vmean | Ratio of cerebral pulsatile blood flow to average cerebral blood flow. This is considered to be related to small cerebral vessel elasticity and tone. As small vessel tone increases, there is reduction in vmean resulting in increased PI; whereas as when vessels dilate, vmean increases resulting in a low PI. 148 | ||
| PR | ((vmax-vmin)/mean)/ ((SBP-DBP)/MAP) | Cerebral pulsatility index normalized for systemic pulsatility. It may not be appropriate to examine PI alone to represent cerebral small vessel tone, as a primary influence on PI is the systemic pulsatility coming into the cerebrovasculature. 149 | ||
| T-Max/Min | Time of peak/min values | Unknown. Time of peak is calculated for all of the above metrics. As such, it likely provides insight into ‘speed’ of the NVC response. | ||
| Lateralization | Leftvmean/ (Leftvmean + Rvmean) × 100 | Relative hyperemic responses between hemispheres. vmean is used here as an example;, however, the percent lateralization is calculated for any of the above metrics. |
Note: All variables can be calculated normalized to breath-by-breathe changes in PaCO2/ETCO2, and presented as absolute or percent changes. Furthermore, all values can be extracted from any time segment of interest (i.e., 5/15 s “bins”). ACA, anterior cerebral artery; MCA, middle cerebral artery; PCA, posterior cerebral artery; VA, vertebral artery; ICA, internal carotid artery; TCD, transcranial Doppler; NIRS, near-infrared spectroscopy; vmean, mean blood velocity from one cardiac cycle; vmax, maximal velocity/flow from one cardiac cycle; vmin, minimum velocity/flow from one cardiac cycle; CVC, cerebrovascular conductance; MAP, mean arterial pressure; CVR, cerebrovascular resistance; RAP, resistance area product; CCP, critical closing pressure; PI, pulsatility index; PR, pulsatility ratio.
Beat-by-beat blood pressure can be recorded from a variety of available devices including finometer, intra-arterial pressure, etc. Lateralization refers to the difference in hyperemic responses between the left and right hemispheres, which is calculated by relative percent change (left vs. right). Essentially, any permutation of the above variables can be calculated.
In order to normalize the time-scale to when heart/breathing rates are different between subjects/trials or within trials (which is critical when input values are sampled at different frequencies (i.e., heart rate and breathing frequency)), y-axis data are cubic spline interpolated and re-sampled in the x-axis at 5 Hz. As opposed to linear interpolation, which resamples data from a constructed straight line between data points, the cubic spline function has contributions from second derivative and degree-3 polynomial pieces that together allow for prediction of data points transitioning between extracted data points. Due to increased accuracy, a cubic spline interpolation likely provides more physiologically relevant transitions between data points.
Future directions
In an attempt to propagate our understanding of neurovascular coupling in humans, we have taken the step of developing standardized and automated software for neurovascular coupling analysis. Using the high temporal resolution of TCD or NIRS, this program automatically detects and coalesces multiple trials within participants and generates a hyperemia contour for each condition (see Figure 3). Furthermore, this software extracts a vast array of metrics for statistical analysis between groups and/or conditions (Table 2). Moreover, this software generates group mean hyperemia contours for presentation. The use of an approach such as this would serve to standardize the field interested in human neurovascular coupling, increase sensitivity of studies in laboratories currently limited to rudimentary peak responses, and provide a platform for between-laboratory comparisons. The software can be used with data collected on any charting software where data extraction can occur on a beat-by-beat basis and should ideally be gated by the QRS component of the ECG. Furthermore, this software merges with breath-by-breath PETCO2 values. Beat-by-beat, and breath-by-breath data is cubic spline interpolated at 5 Hz to generate a single contour for cycles of stimulus on/off tasks (i.e., eyes-open/closed). Once interpolated and re-sampled, this software then calculates a number of metrics such as conductance, normalized to changes in PETCO2 on a beat-by-beat basis. A list of the key metrics that can be obtained, and the various time-bins are outlined in Table 2. Furthermore, the mathematical formulas and theoretical physiological underpinnings are presented in Table 2.
Conclusions
Neurovascular coupling is a critical component of CBF regulation, having implications for cerebrovascular, autonomic, and cognitive dysfunction. Detailed knowledge has been accumulated related to neurovascular coupling in vitro, or in non-human models that unfortunately suffer from non-physiological approaches and/or unintended secondary consequences of vasoactive sedatives/anaesthetics. We are at an embryonic stage regarding neurovascular coupling function using an in vivo human model. One primary reason for this lack of development is inappropriate and inconsistent analysis strategies and stimulation paradigms.
A major concern related to neurovascular coupling is the lack of association with primary risk factors and relatively small sample sizes in clinical studies. This is due partially to the extremely time-consuming manual data analysis required for each individual in a study. In order to progress beyond this issue, a streamlined analysis using standardized software is needed to allow for large data set incorporation with associated risk-factor prediction for neurovascular coupling impairment within healthy and clinical populations, as well as across the life-span. Further, more studies will be able to develop an adjunct and clinically relevant cerebrovascular outcome measure when evaluating therapeutic interventions; with the capacity to impact management strategies for a number of risk factors and clinical conditions. It is our vision that a widespread implementation and utilization of standardized software would rapidly progress the field and benefit our understanding of neurovascular coupling.
Funding
A.A.P. is a Research Fellow and Postdoctoral Fellow funded by the Craig H. Neilsen Foundation, the Heart and Stroke Foundation of Canada (Focus on Stroke), and Michael Smith Foundation for Health Research. M.M.Z. is funded by Canadian Institute for Health Research (Scholarship). A.V.K. is funded by a Chair in Rehabilitation Medicine, the Canadian Institute for Health Research (Team Grant), Rick Hansen Foundation Clinical Outcome Measures and Optimizing Neurorecovery Program, the Craig Neilson Foundation, Christopher and Dana Reeves Foundation, and the Heart and Stroke Foundation of Canada. P.N.A. is supported by a Canada Research Chair in Cerebrovascular Physiology and Natural Sciences and Engineering Research Council (Canada) Discovery Grant.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Aaron A. Phillips – Conception and design, drafting and editing manuscript; Franco Chan − Conception and design, drafting and editing manuscript; Mei Mu Zi Zheng − Conception and design, drafting and editing manuscript; Andrei V. Krassioukov − Conception and design, editing manuscript; Philip N. Ainslie − Conception and design, drafting and editing manuscript. All authors provided final approval of the present version for publication. Our proposed software is openly accessible for assessments, testing and further development by contacting Dr. Aaron Phillips: aaphill@interchange.ubc.ca
References
- 1.Dienel GA, Hertz L. Glucose and lactate metabolism during brain activation. J Neurosci Res 2001; 66: 824–838. [DOI] [PubMed] [Google Scholar]
- 2.Attwell D, Buchan AM, Charpak S, et al. Glial and neuronal control of brain blood flow. Nature 2010; 468: 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 2004; 431: 195–199. [DOI] [PubMed] [Google Scholar]
- 4.Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol 2006; 100: 1059–1064. [DOI] [PubMed] [Google Scholar]
- 5.Lincoln J. Innervation of cerebral arteries by nerves containing 5-hydroxytryptamine and noradrenaline. Pharmacol Ther 1995; 68: 473–501. [DOI] [PubMed] [Google Scholar]
- 6.Cipolla MJ, Li R, Vitullo L. Perivascular innervation of penetrating brain parenchymal arterioles. J Cardiovasc Pharmacol 2004; 44: 1–8. [DOI] [PubMed] [Google Scholar]
- 7.Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev 1990; 2: 161. [PubMed] [Google Scholar]
- 8.Phillips AA, Ainslie PN, Krassioukov AV, et al. Regulation of cerebral blood flow after spinal cord injury. J Neurotrauma 2013; 30: 1551–1563. [DOI] [PubMed] [Google Scholar]
- 9.Willie CK, Tzeng Y-C, Fisher JA, et al. Integrative regulation of human brain blood flow. J Physiol 2014. doi:10.1113/jphysiol.2013.268953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aaslid R, Lindegaard K-FF, Sorteberg W, et al. Cerebral autoregulation dynamics in humans. Stroke 1989; 20: 45–52. [DOI] [PubMed] [Google Scholar]
- 11.Heistad DD, Marcus ML, Gross PM. Effects of sympathetic nerves on cerebral vessels in dog, cat, and monkey. Am J Physiol 1978; 235: H544–H552. [DOI] [PubMed] [Google Scholar]
- 12.Faraci FM, Heistad DD, Mayhan WG. Role of large arteries in regulation of blood flow to brain stem in cats. J Physiol 1987; 387: 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faraci FM, Mayhan WG, Heistad DD. Segmental vascular responses to acute hypertension in cerebrum and brain stem. Am J Physiol 1987; 252: H738–H742. [DOI] [PubMed] [Google Scholar]
- 14.Willie CK, Macleod DB, Shaw AD, et al. Regional brain blood flow in man during acute changes in arterial blood gases. J Physiol 2012; 590: 3261–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis NC, Smith KJ, Bain AR, et al. Impact of transient hypotension on regional cerebral blood flow in humans. Clin Sci (London) 2015. doi:10.1042/CS20140751. [DOI] [PubMed] [Google Scholar]
- 16.Edvinsson L. Characterization of the contractile effect of neuropeptide Y in feline cerebral arteries. Acta Physiol Scand 1985; 125: 33–41. [DOI] [PubMed] [Google Scholar]
- 17.Edvinsson L. Neurogenic mechanisms in the cerebrovascular bed. Autonomic nerves, amine receptors and their effects on cerebral blood flow. Acta Physiol Scand Suppl 1975; 427: 1. [PubMed] [Google Scholar]
- 18.Bleys RL, Cowen T, Groen GJ, et al. Perivascular nerves of the human basal cerebral arteries: I. Topographical distribution. J Cereb Blood Flow Metab 1996; 16: 1034–1047. [DOI] [PubMed] [Google Scholar]
- 19.Sercombe R, Aubineau P, Edvinsson L, et al. Neurogenic influence on local cerebral blood flow. effect of catecholamines or sympathetic stimulation as correlated with the sympathetic innervation. Neurology 1975; 25: 954–963. [DOI] [PubMed] [Google Scholar]
- 20.Cerebral blood flow and metabolism, Lippincott Williams & Wilkins, 2002. [Google Scholar]
- 21.Zhang R, Zuckerman JH, Iwasaki K, et al. Autonomic neural control of dynamic cerebral autoregulation in humans. Circulation 2002; 106: 1814–1820. [DOI] [PubMed] [Google Scholar]
- 22.Hamner JW, Tan CO, Lee K, et al. Sympathetic control of the cerebral vasculature in humans. Stroke 2010; 41: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogoh S, Brothers RM, Eubank WL, et al. Autonomic neural control of the cerebral vasculature: acute hypotension. Stroke 2008; 39: 1979–1987. [DOI] [PubMed] [Google Scholar]
- 24.Hamner JW, Tan CO, Tzeng Y-CC, et al. Cholinergic control of the cerebral vasculature in humans. J Physiol 2012; 590: 6343–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donders FC. Die Bewegungen des Gehirns und die Veränderungen der Gefässfüllung der Pia mater. Schmid’s Fahrbucher 1851; 69: 16–20. [Google Scholar]
- 26.Mosso A. Sulla circolazione del cervello dell’uomo. Atti R Accad Lincei 1880; 5: 237–358. [Google Scholar]
- 27.Roy CS, Sherrington CS. On the regulation of the blood-supply of the brain. J Physiol 1890; 11: 85–158.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci 2007; 10: 1369–1376. [DOI] [PubMed] [Google Scholar]
- 29.Lecrux C, Hamel E. The neurovascular unit in brain function and disease. Acta Physiol (Oxf) 2011; 203: 47–59. [DOI] [PubMed] [Google Scholar]
- 30.Busija DW, Bari F, Domoki F, et al. Mechanisms involved in the cerebrovascular dilator effects of N-methyl-d-aspartate in cerebral cortex. Brain Res Rev 2007; 56: 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ko KR, Ngai AC, Winn HR. Role of adenosine in regulation of regional cerebral blood flow in sensory cortex. Am J Physiol 1990; 259: H1703–H1708. [DOI] [PubMed] [Google Scholar]
- 32.Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. J Neurosci 1996; 16: 5073–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon GRJ, Choi HB, Rungta RL, et al. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 2008; 456: 745–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cauli B, Tong X-K, Rancillac A, et al. Cortical GABA interneurons in neurovascular coupling: relays for subcortical vasoactive pathways. J Neurosci 2004; 24: 8940–8949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaucher E, Tong XK, Cholet N, et al. GABA neurons provide a rich input to microvessels but not nitric oxide neurons in the rat cerebral cortex: a means for direct regulation of local cerebral blood flow. J Comp Neurol 2000; 421: 161–171. [PubMed] [Google Scholar]
- 36.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex 1997; 7: 476–486. [DOI] [PubMed] [Google Scholar]
- 37.Lin A-L, Fox PT, Hardies J, et al. Nonlinear coupling between cerebral blood flow, oxygen consumption, and ATP production in human visual cortex. Proc Natl Acad Sci USA 2010; 107: 8446–8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jespersen SN, Østergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab 2012; 32: 264–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmithorst VJ, Vannest J, Lee G, et al. Evidence that neurovascular coupling underlying the BOLD effect increases with age during childhood. Hum Brain Mapp 2015; 36: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonder DE, McCarthy KD. Astrocytic Gq-GPCR-linked IP3R-dependent Ca2+ signaling does not mediate neurovascular coupling in mouse visual cortex in vivo. J Neurosci 2014; 34: 13139–13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosenegger DG, Gordon GR. A Slow or Modulatory Role of Astrocytes in Neurovascular Coupling. Microcirculation 2015. doi:10.1111/micc.12184. [DOI] [PubMed] [Google Scholar]
- 42.Tayebati SK, Tomassoni D, Amenta F. Spontaneously hypertensive rat as a model of vascular brain disorder: microanatomy, neurochemistry and behavior. J Neurol Sci 2012; 322: 241–249. [DOI] [PubMed] [Google Scholar]
- 43.Shepro D, Morel NM. Pericyte physiology. FASEB J 1993; 7: 1031–8. [DOI] [PubMed] [Google Scholar]
- 44.Puro DG. Physiology and pathobiology of the pericyte-containing retinal microvasculature: new developments. Microcirculation 2007; 14: 1–10. [DOI] [PubMed] [Google Scholar]
- 45.Lovick TA, Brown LA, Key BJ. Neurovascular relationships in hippocampal slices: physiological and anatomical studies of mechanisms underlying flow-metabolism coupling in intraparenchymal microvessels. Neuroscience 1999; 92: 47–60. [DOI] [PubMed] [Google Scholar]
- 46.Peppiatt CM, Howarth C, Mobbs P, et al. Bidirectional control of CNS capillary diameter by pericytes. Nature 2006; 443: 700–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lu K, Clark JW, Ghorbel FH, et al. Cerebral autoregulation and gas exchange studied using a human cardiopulmonary model. Am J Physiol Heart Circ Physiol 2004; 286: H584–H601. [DOI] [PubMed] [Google Scholar]
- 48.Boas DA, Jones SR, Devor A, et al. A vascular anatomical network model of the spatio-temporal response to brain activation. Neuroimage 2008; 40: 1116–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pries AR, Secomb TW, Gaehtgens P. Biophysical aspects of blood flow in the microvasculature. Cardiovasc Res 1996; 32: 654–667. [PubMed] [Google Scholar]
- 50.Takano T, Tian G-F, Peng W, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci 2006; 9: 260–267. [DOI] [PubMed] [Google Scholar]
- 51.Tran CHT, Gordon GR. Acute two-photon imaging of the neurovascular unit in the cortex of active mice. Front Cell Neurosci 2015; 9: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Phillips AA, Warburton DE, Ainslie PN, et al. Regional neurovascular coupling and cognitive performance in those with low blood pressure secondary to high-level spinal cord injury: improved by alpha-1 agonist midodrine hydrochloride. J Cereb Blood Flow Metab 2014; 34: 794–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salinet ASM, Robinson TG, Panerai RB. Active, passive, and motor imagery paradigms: component analysis to assess neurovascular coupling. J Appl Physiol 2013; 114: 1406–1412. [DOI] [PubMed] [Google Scholar]
- 54.Maggio P, Salinet ASM, Robinson TG, et al. Influence of CO2 on neurovascular coupling: interaction with dynamic cerebral autoregulation and cerebrovascular reactivity. Physiol Rep 2014; 2: e00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willie CK, Cowan EC, Ainslie PN, et al. Neurovascular coupling and distribution of cerebral blood flow during exercise. J Neurosci Methods 2011; 198: 270–273. [DOI] [PubMed] [Google Scholar]
- 56.Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol 2006; 100: 328–335. [DOI] [PubMed] [Google Scholar]
- 57.Janzarik WG, Ehmann R, Ehlers E, et al. Neurovascular coupling in pregnancy and the risk of preeclampsia. Stroke 2014; 45: 2792–2794. [DOI] [PubMed] [Google Scholar]
- 58.Gommer ED, Bogaarts G, Martens EGHJ, et al. Visually evoked blood flow responses and interaction with dynamic cerebral autoregulation: correction for blood pressure variation. Med Eng Phys 2014; 36: 613–619. [DOI] [PubMed] [Google Scholar]
- 59.Flück D, Beaudin AE, Steinback CD, et al. Effects of aging on the association between cerebrovascular responses to visual stimulation, hypercapnia and arterial stiffness. Front Physiol 2014; 5: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Noonan JE, Dusting GJ, Nguyen TT, et al. Flicker light-induced retinal vasodilation is unaffected by inhibition of epoxyeicosatrienoic acids and prostaglandins in humans. Invest Ophthalmol Vis Sci 2014; 55: 7007–7013. [DOI] [PubMed] [Google Scholar]
- 61.Stewart JM, Del Pozzi AT, Pandey A, et al. Oscillatory cerebral blood flow is associated with impaired neurocognition and functional hyperemia in postural tachycardia syndrome during graded tilt. Hypertension 2015; 65: 636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang N, Liu Z, He B, et al. Noninvasive study of neurovascular coupling during graded neuronal suppression. J Cereb Blood Flow Metab 2008; 28: 280–290. [DOI] [PubMed] [Google Scholar]
- 63.Rosengarten B, Huwendiek O, Kaps M. Neurovascular coupling and cerebral autoregulation can be described in terms of a control system. Ultrasound Med Biol 2001; 27: 189–193. [DOI] [PubMed] [Google Scholar]
- 64.Phillips AA, Smith KJ, Zheng MM, et al. Volumetric neurovascular coupling in man: the effect of cerebrovascular L-type calcium channel blockade. J Appl Physiol. [Google Scholar]
- 65.Chacon M, Araya C, Panerai RB. Non-linear multivariate modeling of cerebral hemodynamics with autoregressive support vector machines. Med Eng Phys 2011; 33: 180–187. [DOI] [PubMed] [Google Scholar]
- 66.Moody M, Panerai RB, Eames PJ, et al. Cerebral and systemic hemodynamic changes during cognitive and motor activation paradigms. Am J Physiol Regul Integr Comp Physiol 2005; 288: R1581–R1588. [DOI] [PubMed] [Google Scholar]
- 67.Maggio P, Salinet ASM, Panerai RB, et al. Does hypercapnia-induced impairment of cerebral autoregulation affect neurovascular coupling? a functional TCD study. J Appl Physiol 2013; 115: 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ances BM, Greenberg JH, Detre JA. The effects of graded hypercapnia on the activation flow coupling response due to forepaw stimulation in alpha-chloralose anesthetized rats. Brain Res 2001; 911: 82–88. [DOI] [PubMed] [Google Scholar]
- 69.Blicher JU, Stagg CJ, O’Shea J, et al. Visualization of altered neurovascular coupling in chronic stroke patients using multimodal functional MRI. J Cereb Blood Flow Metab 2012; 32: 2044–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bundo M, Inao S, Nakamura A, et al. Changes of neural activity correlate with the severity of cortical ischemia in patients with unilateral major cerebral artery occlusion. Stroke 2002; 33: 61–66. [DOI] [PubMed] [Google Scholar]
- 71.Duschek S, Schandry R. Cognitive performance and cerebral blood flow in essential hypotension. Psychophysiology 2004; 41: 905–913. [DOI] [PubMed] [Google Scholar]
- 72.Duschek S, Schandry R. Reduced brain perfusion and cognitive performance due to constitutional hypotension. Clin Auton Res 2007; 17: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Azevedo E, Castro P, Santos R, et al. Autonomic dysfunction affects cerebral neurovascular coupling. Clin Auton Res 2011; 21: 395–403. [DOI] [PubMed] [Google Scholar]
- 74.Salinet ASM, Robinson TG, Panerai RB. Cerebral blood flow response to neural activation after acute ischemic stroke: a failure of myogenic regulation? J Neurol 2013; 260: 2588–2595. [DOI] [PubMed] [Google Scholar]
- 75.Brouns R, De Deyn PP. The complexity of neurobiological processes in acute ischemic stroke. Clin Neurol Neurosurg 2009; 111: 483–495. [DOI] [PubMed] [Google Scholar]
- 76.Østergaard L, Engedal TS, Aamand R, et al. Capillary transit time heterogeneity and flow-metabolism coupling after traumatic brain injury. J Cereb Blood Flow Metab 2014; 34: 1585–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hinzman JM, Andaluz N, Shutter LA, et al. Inverse neurovascular coupling to cortical spreading depolarizations in severe brain trauma. Brain 2014; 137: 2960–2972. [DOI] [PubMed] [Google Scholar]
- 78.Lok J, Wang X-S, Xing CH, et al. Targeting the neurovascular unit in brain trauma. CNS Neurosci Ther 2014. doi:10.1111/cns.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guiney H, Lucas SJ, Cotter JD, et al. Evidence cerebral blood-flow regulation mediates exercise-cognition links in healthy young adults. Neuropsychology 2015; 29: 1–9. [DOI] [PubMed] [Google Scholar]
- 80.Jegede AB, Rosado-Rivera D, Bauman WA, et al. Cognitive performance in hypotensive persons with spinal cord injury. Clin Auton Res 2010; 20: 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Duschek S, Hadjamu M, Schandry R. Enhancement of cerebral blood flow and cognitive performance following pharmacological blood pressure elevation in chronic hypotension. Psychophysiology 2007; 44: 145–53. [DOI] [PubMed] [Google Scholar]
- 82.Boms N, Yonai Y, Molnar S, et al. Effect of smoking cessation on visually evoked cerebral blood flow response in healthy volunteers. J Vasc Res 2010; 47: 214–220. [DOI] [PubMed] [Google Scholar]
- 83.Sekhon MS, Griesdale DE, Czosnyka M, et al. The effect of red blood cell transfusion on cerebral autoregulation in patients with severe traumatic brain injury. Neurocrit Care 2015; 23: 210–216. [DOI] [PubMed] [Google Scholar]
- 84.Coverdale NS, Gati JS, Opalevych O, et al. Cerebral blood flow velocity underestimates cerebral blood flow during modest hypercapnia and hypocapnia. J Appl Physiol 2014. jap.00285.2014. [DOI] [PubMed] [Google Scholar]
- 85.Aaslid R. Visually evoked dynamic blood flow response of the human cerebral circulation. Stroke 1987; 18: 771–775. [DOI] [PubMed] [Google Scholar]
- 86.Azevedo E, Santos R, Freitas J, et al. Deep brain stimulation does not change neurovascular coupling in non-motor visual cortex: an autonomic and visual evoked blood flow velocity response study. Parkinsonism Relat Disord 2010; 16: 600–603. [DOI] [PubMed] [Google Scholar]
- 87.Silvestrini M, Caltagirone C, Cupini LM, et al. Activation of healthy hemisphere in poststroke recovery. a transcranial Doppler study. Stroke 1993; 24: 1673–1677. [DOI] [PubMed] [Google Scholar]
- 88.Klingelhöfer J, Matzander G, Sander D, et al. Assessment of functional hemispheric asymmetry by bilateral simultaneous cerebral blood flow velocity monitoring. J Cereb Blood Flow Metab 1997; 17: 577–585. [DOI] [PubMed] [Google Scholar]
- 89.Phillips AA, Krassioukov AV, Ainslie PN, et al. Perturbed and spontaneous regional cerebral blood flow responses to changes in blood pressure after high level spinal cord injury: the effect of midodrine. J Appl Physiol 2014; 116: 645–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Phillips AA, Warburton DE, Ainslie PN, et al. Dynamic cerebral blood flow regulation in those with schizophrenia. Unpubl Find 2015. [Google Scholar]
- 91.Willie CK, Colino FL, Bailey DM, et al. Utility of transcranial Doppler ultrasound for the integrative assessment of cerebrovascular function. J Neurosci Methods 2011; 196: 221–237. [DOI] [PubMed] [Google Scholar]
- 92.Yonai Y, Boms N, Molnar S, et al. Acetazolamide-induced vasodilation does not inhibit the visually evoked flow response. J Cereb Blood Flow Metab 2010; 30: 516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rosengarten B, Auch D, Kaps M. Effects of initiation and acute withdrawal of statins on the neurovascular coupling mechanism in healthy, normocholesterolemic humans. Stroke 2007; 38: 3193–3197. [DOI] [PubMed] [Google Scholar]
- 94.Aaslid R. Transcranial Doppler sonography, Springer Verlag: New York, 1987. [Google Scholar]
- 95.Purkayastha S, Sorond F. Transcranial Doppler ultrasound: technique and application. Semin Neurol 2012; 32: 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Verbree J, Bronzwaer A-SGT, Ghariq E, et al. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physiol 2014; 117: 1084–1089. [DOI] [PubMed] [Google Scholar]
- 97.Ainslie PN, Hoiland RL. Transcranial Doppler ultrasound: valid, invalid, or both? J Appl Physiol 2014; 117: 1081–1083. [DOI] [PubMed] [Google Scholar]
- 98.Salinet ASM, Robinson TG, Panerai RB. Effects of cerebral ischemia on human neurovascular coupling, CO2 reactivity, and dynamic cerebral autoregulation. J Appl Physiol 2015; 118: 170–177. [DOI] [PubMed] [Google Scholar]
- 99.Jasdzewski G, Strangman G, Wagner J, et al. Differences in the hemodynamic response to event-related motor and visual paradigms as measured by near-infrared spectroscopy. Neuroimage 2003; 20: 479–488. [DOI] [PubMed] [Google Scholar]
- 100.Dutta A, Jacob A, Chowdhury SR, et al. EEG-NIRS based assessment of neurovascular coupling during anodal transcranial direct current stimulation − a stroke case series. J Med Syst 2015; 39: 205. [DOI] [PubMed] [Google Scholar]
- 101.Mackert B-M, Wübbeler G, Leistner S, et al. Neurovascular coupling analyzed non-invasively in the human brain. Neuroreport 2004; 15: 63–66. [DOI] [PubMed] [Google Scholar]
- 102.Mackert B-M, Leistner S, Sander T, et al. Dynamics of cortical neurovascular coupling analyzed by simultaneous DC-magnetoencephalography and time-resolved near-infrared spectroscopy. Neuroimage 2008; 39: 979–986. [DOI] [PubMed] [Google Scholar]
- 103.Ferrari M, Quaresima V. A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 2012; 63: 921–935. [DOI] [PubMed] [Google Scholar]
- 104.Kimoto H, Ohno T, Takashima S, et al. The effect of acetazolamide and carbon dioxide on cerebral hemodynamic changes on near-infrared spectroscopy in young rabbits. Brain Dev, 17: 261–263. [DOI] [PubMed] [Google Scholar]
- 105.Strait M, Scheutz M. What we can and cannot (yet) do with functional near infrared spectroscopy. Front Neurosci 2014; 8: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cui X, Bray S, Bryant DM, et al. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage 2011; 54: 2808–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Van Beek AHEA, Lagro J, Olde-Rikkert MGM, et al. Oscillations in cerebral blood flow and cortical oxygenation in Alzheimer’s disease. Neurobiol Aging 2012; 33 428.e21–31. [DOI] [PubMed] [Google Scholar]
- 108.Vernieri F, Tibuzzi F, Pasqualetti P, et al. Increased cerebral vasomotor reactivity in migraine with aura: an autoregulation disorder? A transcranial Doppler and near-infrared spectroscopy study. Cephalalgia 2008; 28: 689–695. [DOI] [PubMed] [Google Scholar]
- 109.Palazzo P, Tibuzzi F, Pasqualetti P, et al. Is there a role of near-infrared spectroscopy in predicting the outcome of patients with carotid artery occlusion? J Neurol Sci 2010; 292: 36–39. [DOI] [PubMed] [Google Scholar]
- 110.Szabo K, Lako E, Juhasz T, et al. Hypocapnia induced vasoconstriction significantly inhibits the neurovascular coupling in humans. J Neurol Sci 2011; 309: 58–62. [DOI] [PubMed] [Google Scholar]
- 111.Yamaguchi Y, Ikemura T, Kashima H, et al. Effects of vasodilatation and pressor response on neurovascular coupling during dynamic exercise. Eur J Appl Physiol 2015; 115: 619–625. [DOI] [PubMed] [Google Scholar]
- 112.Fabjan A, Bajrović FF, Musizza B, et al. Study of neurovascular coupling during cold pressor test in patients with migraine. Cephalalgia 2014. doi:10.1177/0333102414554661. [DOI] [PubMed] [Google Scholar]
- 113.Schöning M, Walter J, Scheel P. Estimation of cerebral blood flow through color duplex sonography of the carotid and vertebral arteries in healthy adults. Stroke 1994; 25: 17–22. [DOI] [PubMed] [Google Scholar]
- 114.Jones H, Lewis NCS, Thompson A, et al. Diurnal variation in vascular function: role of sleep. Chronobiol Int 2012; 29: 271–277. [DOI] [PubMed] [Google Scholar]
- 115.Adkisson EJ, Casey DP, Beck DT, et al. Central, peripheral and resistance arterial reactivity: fluctuates during the phases of the menstrual cycle. Exp Biol Med (Maywood) 2010; 235: 111–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tyldum GA, Schjerve IE, Tjønna AE, et al. Endothelial dysfunction induced by post-prandial lipemia: complete protection afforded by high-intensity aerobic interval exercise. J Am Coll Cardiol 2009; 53: 200–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lavi T, Karasik A, Koren-Morag N, et al. The acute effect of various glycemic index dietary carbohydrates on endothelial function in nondiabetic overweight and obese subjects. J Am Coll Cardiol 2009; 53: 2283–2287. [DOI] [PubMed] [Google Scholar]
- 118.Hijmering ML, de Lange DW, Lorsheyd A, et al. Binge drinking causes endothelial dysfunction, which is not prevented by wine polyphenols: a small trial in healthy volunteers. Neth J Med 2007; 65: 29–35. [PubMed] [Google Scholar]
- 119.Papamichael CM, Aznaouridis KA, Karatzis EN, et al. Effect of coffee on endothelial function in healthy subjects: the role of caffeine. Clin Sci (London) 2005; 109: 55–60. [DOI] [PubMed] [Google Scholar]
- 120.Dawson EA, Whyte GP, Black MA, et al. Changes in vascular and cardiac function after prolonged strenuous exercise in humans. J Appl Physiol 2008; 105: 1562–1568. [DOI] [PubMed] [Google Scholar]
- 121.Tinken TM, Thijssen DHJ, Black MA, et al. Time course of change in vasodilator function and capacity in response to exercise training in humans. J Physiol 2008; 586: 5003–5012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rognmo O, Bjørnstad TH, Kahrs C, et al. Endothelial function in highly endurance-trained men: effects of acute exercise. J Strength Cond Res 2008; 22: 535–542. [DOI] [PubMed] [Google Scholar]
- 123.Harris RA, Nishiyama SK, Wray DW, et al. The effect of oral antioxidants on brachial artery flow-mediated dilation following 5 and 10 min of ischemia. Eur J Appl Physiol 2009; 107: 445–453. [DOI] [PubMed] [Google Scholar]
- 124.Taneva E, Borucki K, Wiens L, et al. Early effects on endothelial function of atorvastatin 40 mg twice daily and its withdrawal. Am J Cardiol 2006; 97: 1002–1006. [DOI] [PubMed] [Google Scholar]
- 125.Otto ME, Svatikova A, Barretto RB de M, et al. Early morning attenuation of endothelial function in healthy humans. Circulation 2004; 109: 2507–2510. [DOI] [PubMed] [Google Scholar]
- 126.Fan J-L, Burgess KR, Thomas KN, et al. Influence of indomethacin on ventilatory and cerebrovascular responsiveness to CO2 and breathing stability: the influence of PCO2 gradients. Am J Physiol Regul Integr Comp Physiol 2010; 298: R1648–R1658. [DOI] [PubMed] [Google Scholar]
- 127.Markus HS, Vallance P, Brown MM. Differential effect of three cyclooxygenase inhibitors on human cerebral blood flow velocity and carbon dioxide reactivity. Stroke 1994; 25: 1760–1764. [DOI] [PubMed] [Google Scholar]
- 128.Davis RJ, Murdoch CE, Ali M, et al. EP4 prostanoid receptor-mediated vasodilatation of human middle cerebral arteries. Br J Pharmacol 2004; 141: 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci 2006; 26: 2862–2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zonta M, Angulo MC, Gobbo S, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci 2003; 6: 43–50. [DOI] [PubMed] [Google Scholar]
- 131.Armstead WM. Role of ATP-sensitive K+ channels in cGMP-mediated pial artery vasodilation. Am J Physiol 1996; 270: H423–H426. [DOI] [PubMed] [Google Scholar]
- 132.Harder DR, Lange AR, Gebremedhin D, et al. Cytochrome P450 metabolites of arachidonic acid as intracellular signaling molecules in vascular tissue. J Vasc Res, 34: 237–243. [DOI] [PubMed] [Google Scholar]
- 133.Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev 2002; 82: 131–185. [DOI] [PubMed] [Google Scholar]
- 134.De Silva TM, Faraci FM. Effects of angiotensin II on the cerebral circulation: role of oxidative stress. Front Physiol 2013; 3: 484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Takeda S, Sato N, Takeuchi D, et al. Angiotensin receptor blocker prevented beta-amyloid-induced cognitive impairment associated with recovery of neurovascular coupling. Hypertension 2009; 54: 1345–1352. [DOI] [PubMed] [Google Scholar]
- 136.Nicolakakis N, Hamel E. Neurovascular function in Alzheimer’s disease patients and experimental models. J Cereb Blood Flow Metab 2011; 31: 1354–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chow N, Bell RD, Deane R, et al. Serum response factor and myocardin mediate arterial hypercontractility and cerebral blood flow dysregulation in Alzheimer’s phenotype. Proc Natl Acad Sci USA 2007; 104: 823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci 2005; 28: 202–208. [DOI] [PubMed] [Google Scholar]
- 139.Vetri F, Xu H, Paisansathan C, et al. Impairment of neurovascular coupling in type 1 diabetes mellitus in rats is linked to PKC modulation of BK(Ca) and Kir channels. Am J Physiol Heart Circ Physiol 2012; 302: H1274–H1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gandhi GK, Ball KK, Cruz NF, et al. Hyperglycaemia and diabetes impair gap junctional communication among astrocytes. ASN Neuro 2010; 2: e00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Davenport MH, Hogan DB, Eskes GA, et al. Cerebrovascular reserve: the link between fitness and cognitive function? Exerc Sport Sci Rev 2012; 40: 153–158. [DOI] [PubMed] [Google Scholar]
- 142.Bracco L, Bessi V, Alari F, et al. Cerebral hemodynamic lateralization during memory tasks as assessed by functional transcranial Doppler (fTCD) sonography: effects of gender and healthy aging. Cortex 2011; 47: 750–758. [DOI] [PubMed] [Google Scholar]
- 143.O’Leary DS. Regional vascular resistance vs. conductance: which index for baroreflex responses? Am J Physiol 1991; 260: H632–H637. [DOI] [PubMed] [Google Scholar]
- 144.Claassen JAHR, Zhang R, Fu Q, et al. Transcranial Doppler estimation of cerebral blood flow and cerebrovascular conductance during modified rebreathing. J Appl Physiol 2007; 102: 870–877. [DOI] [PubMed] [Google Scholar]
- 145.Panerai RB, Salinet ASM, Brodie FG, et al. The influence of calculation method on estimates of cerebral critical closing pressure. Physiol Meas 2011; 32: 467–482. [DOI] [PubMed] [Google Scholar]
- 146.Robertson AD, Edgell H, Hughson RL. Assessing cerebrovascular autoregulation from critical closing pressure and resistance area product during upright posture in aging and hypertension. Am J Physiol Heart Circ Physiol 2014; 307: H124–H133. [DOI] [PubMed] [Google Scholar]
- 147.Panerai RB. The critical closing pressure of the cerebral circulation. Med Eng Phys 2003; 25: 621–632. [DOI] [PubMed] [Google Scholar]
- 148.Woodcock JP, Gosling RG, Fitzgerald DE. A new noninvasive technique for assessment of superficial femoral artery obstruction. Br J Surg 1972; 59: 226–231. [DOI] [PubMed] [Google Scholar]
- 149.Levine BD, Giller CA, Lane LD, et al. Cerebral versus systemic hemodynamics during graded orthostatic stress in humans. Circulation 1994; 90: 298–306. [DOI] [PubMed] [Google Scholar]