Abstract
Lysophosphatidic acid (LPA) is a pleiotropic lipid signaling molecule associated with asthma pathobiology. LPA elicits its effects by binding to at least six known cell surface G protein–coupled receptors (LPA1–6) that are expressed in the lung in a cell type–specific manner. LPA2 in particular has emerged as an attractive therapeutic target in asthma because it appears to transduce inhibitory or cell-protective signals. We studied a novel and specific small molecule LPA2 agonist (2-[4-(1,3-dioxo-1H,3H-benzoisoquinolin-2-yl)butylsulfamoyl] benzoic acid [DBIBB]) in a mouse model of house dust mite–induced allergic airway inflammation. Mice injected with DBIBB developed significantly less airway and lung inflammation compared with vehicle-treated controls. Levels of lung Th2 cytokines were also significantly attenuated by DBIBB. We conclude that pharmacologic activation of LPA2 attenuates Th2-driven allergic airway inflammation in a mouse model of asthma. Targeting LPA receptor signaling holds therapeutic promise in allergic asthma.
Keywords: asthma, house dust mite, periostin, lysophosphatidic acid
Clinical Relevance
Lysophosphatidic acid (LPA) is associated with asthma pathobiology. A novel LPA2 agonist attenuates allergic inflammation, highlighting that targeting LPA receptor signaling holds therapeutic promise in allergic asthma.
Allergic asthma is a complex and heterogeneous disease caused by aberrant immune responses to allergens and other inhaled irritants. Although available therapies can effectively control asthma symptoms in many patients, there is an unmet need for new treatments that target the underlying disease. Biologic compounds targeting specific molecular endotypes are under active development (1), but approaches that inhibit new effector pathways in asthma would be an important advance.
Lysophosphatidic acid (LPA) is a pleiotropic lipid signaling molecule associated with asthma pathobiology (2, 3). LPA is a normal component of epithelial lining fluids, and lung LPA concentrations increase significantly after segmental allergen challenge (especially polyunsaturated species) (4, 5). The majority of extracellular LPA is generated by hydrolysis of the choline moiety from lysophosphatidylcholine by the enzyme autotaxin (ATX), which was recently shown to play an important role in a mouse model of allergic airway inflammation (5). Relevant to the pathophysiology of asthma, LPA can enhance smooth muscle growth and contractility (6–9), induce mast cell activation (10–13), induce chemotaxis and migration of inflammatory cells (14–17), and elicit chemokine and cytokine release from bronchial epithelial cells (18–22). Because LPA can also exert antiinflammatory or homeostatic effects in other contexts (2, 23, 24), the net effect of this molecule on airway inflammation and hyperreactivity will depend on where and when it is produced in the airway, and on the target cell types and receptors engaged.
LPA elicits its effects by binding to at least six known cell surface G protein–coupled receptors (LPA1–6) that are expressed in the lung in a cell type–specific manner (reviewed in Ref. 25). LPA receptor signaling regulates diverse cellular processes, such as activation, proliferation, survival, and migration. LPA2, in particular, has emerged as an attractive therapeutic target in asthma for several reasons. First, the intracellular C-terminal domain of LPA2 couples with unique downstream modules distinct from other LPA receptors that appear to transduce cell-protective signals (26–29). Second, LPA2 deficiency in mice attenuates allergen-driven airway inflammation and hyperreactivity, at least in part by inhibiting dendritic cell activation (30). Third, novel small-molecule LPA2 agonists were recently synthesized that demonstrate robust receptor selectivity without inhibiting ATX activity (a weakness of earlier-generation compounds [31]). These LPA2 agonists demonstrated remarkable efficacy in protecting mice from acute radiation syndromes, indicating that they have favorable bioactivity in the setting of tissue inflammation and damage (32). Here, we report that 2-[4-(1,3-dioxo-1H,3H-benzoisoquinolin-2-yl)butylsulfamoyl] benzoic acid [DBIBB]), a novel and specific LPA2-specific sulfamoyl benzoic acid agonist, strongly inhibits house dust mite (HDM)–driven allergic inflammation in mice.
Materials and Methods
Mice
Wild-type BALB/c mice were purchased from Charles River Laboratories (Wilmington, MA). All mice were maintained at the University of Rochester and age- and sex-matched littermate controls were used in all experiments. The studies were performed in strict accordance with guidelines from the National Institutes of Health. The protocol was reviewed and approved by the University of Rochester Committee of Animal Resources, and studies were conducted in accordance with institutional guidelines.
HDM Asthma Protocol and Sample Collection
Female BALB/c mice were used at 8–10 weeks of age. HDM (Dermatophagoides pteronyssinus) extract (endotoxin 156.2 EU/ml; Greer Laboratories, Lenoir, NC) was resuspended in sterile PBS and 30 μg/30 μl was administered to isoflurane-anesthetized mice intranasally for 10 consecutive days. In addition, mice were administered either vehicle (PBS, 1% ethanol, 2% propanediol) or 30 μg of an LPA2 agonist (DBIBB) intraperitoneally each day (Figure 1). At 48 hours after the last challenge, mice were killed. Bronchoalveolar lavage (BAL) was performed by exposing the trachea and gently instilling 0.75 ml of PBS into the lungs twice using a Teflon cannula. Serum was collected after centrifugation of whole blood. The mice were perfused with 5 ml of PBS and then the left lobe was tied off and cut out for lung digests. The remaining lung was inflated with 10% formalin for histological analyses. Total cell counts from the BAL fluid were determined using a hemocytometer and cytospins were prepared by cytocentrifugation and staining with Protocol Hema 3 Stain Set (Fisher Scientific, Waltham, MA) to count differentials.
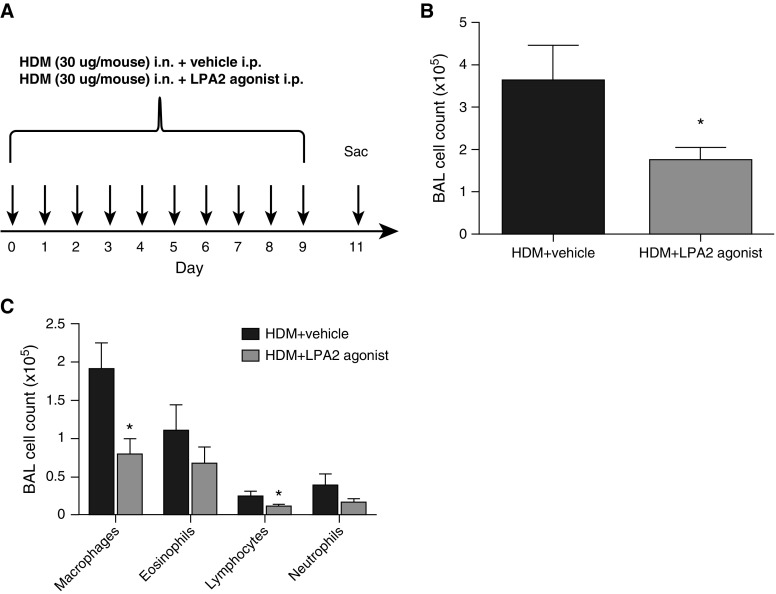
Figure 1.
Less airway inflammation in lysophosphatidic acid (LPA) 2 agonist–treated mice. (A) Diagram of the house dust mite (HDM) allergen dosing regimen. Mice were administered HDM extract (30 μg/30 μl) intranasally (i.n.) for 10 consecutive days. Mice were also administered either vehicle (PBS, 1% ethanol, 2% propanediol) or 30 μg of an LPA2 agonist (2-[4-(1,3-dioxo-1H,3H-benzoisoquinolin-2-yl)butylsulfamoyl] benzoic acid [DBIBB]) intraperitoneally (i.p.) each day. At 48 hours after the last challenge, mice were killed. (B) Total bronchoalveolar lavage (BAL) counts were determined by hemocytometry. (C) Absolute number of BAL macrophages, eosinophils, lymphocytes, and neutrophils. Data are mean ± SEM, n = 8 mice/group. *P < 0.05.
Histological Analysis
Lung tissue was embedded in paraffin and 5-μm sections were mounted on slides and stained with hematoxylin and eosin. Lung sections were scored by blinded observers using a semiquantitative scoring system, on a 0–4 scale, which takes into account the extent and severity of inflammation, as described in the online supplement.
Lung Digest Preparation
The excised left lobe was finely chopped and incubated in media containing collagenase type 2 (1 mg/ml; Worthington Biochemical, Lakewood, NJ) and DNase (30 μg/ml) for 30 minutes. The pieces were then gently mashed through a 100-μm strainer, washed, red blood cells lysed, and cells plated in a 24-well plate with media. The cells were incubated at 37°C and the supernatants were collected 24 hours later.
ATX Activity Assay
Lysophospholipase D activity of ATX was measured using an enzyme-coupled assay, as described in the online supplement.
Results
To test the LPA2 agonist (DBIBB) in a physiologically relevant model of allergic inflammation, wild-type BALB/c mice (8- to 10-week-old females) were administered intranasal HDM extract (30 μg in 30 μl normal saline; D. pteronyssinus, endotoxin 156.2 EU/ml) for 10 consecutive days (Figure 1A). This protocol reproduces many features of allergic asthma, including eosinophilic airway inflammation and Th2 cytokine production (33). Control mice received vehicle (PBS, 1% ethanol, 2% propanediol) by intraperitoneal injection daily, whereas test mice received 30 μg of DBIBB resuspended in vehicle in parallel (Figure 1A). At 48 hours after the last challenge, mice were killed. To ensure that the vehicle itself was not having deleterious effects, HDM-treated mice were compared with HDM plus vehicle-treated mice, and no differences were observed (see Figure E1 in the online supplement).
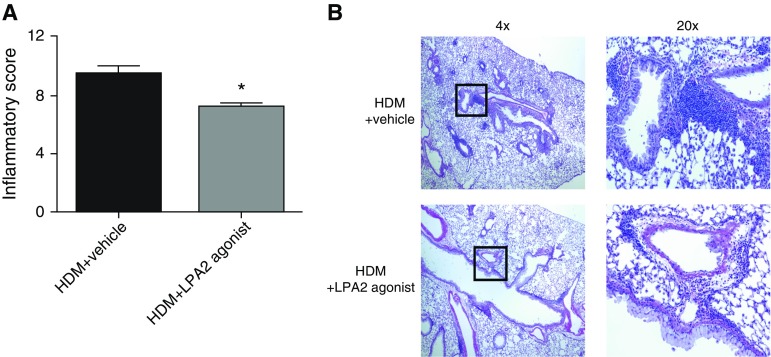
After HDM exposure, vehicle-treated mice developed substantial airway inflammation, as demonstrated by increased BAL cell counts, which was significantly attenuated in mice administered the LPA2 agonist (3.61 ± 0.86 versus 1.74 ± 0.30 × 105 total cells [mean ± SEM], n = 8, P = 0.0353; Figure 1B). BAL fluid differentials demonstrated a trend toward lower numbers of eosinophils and neutrophils, and a statistically significant reduction in macrophages and lymphocytes after LPA2 activation (Figure 1C; macrophages: 1.9 ± 0.35 and 0.79 ± 0.21 × 105 [P = 0.01], eosinophils: 1.1 ± 0.34 and 0.67 ± 0.22 × 105 [P = 0.16], lymphocytes: 0.24 ± 0.07 and 0.10 ± 0.03 × 105 [P = 0.05], neutrophils: 0.38 ± 0.15 and 0.15 ± 0.05 × 105 [P = 0.10], and in vehicle-treated and LPA2 agonist-treated mice, respectively). We also observed decreased tissue inflammation in response to LPA2 activation, as determined by semiquantitative scoring of hematoxylin and eosin–stained lung sections in a blinded manner (9.5 ± 0.53 versus 7.25 ± 0.25 inflammatory score [mean±SEM]; n = 4–8; P = 0.0039; Figure 2A). Vehicle-treated mice displayed robust pulmonary inflammation with perivascular and peribronchial cellular infiltration. The extent and severity of these inflammatory markers were significantly attenuated in the LPA2 agonist treated mice (Figure 2B).
Figure 2.
Less tissue inflammation in LPA2 agonist–treated mice. (A) Scoring of inflammation severity from hematoxylin and eosin–stained lung sections (scoring system described in the online supplement) and (B) representative images (4× and 20× [zoomed-in view of boxes in the 4× images]). Data are mean ± SEM, n = 4–8 mice/group. *P < 0.05.
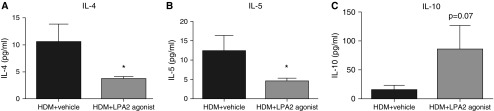
Analysis of BAL supernatants revealed marked attenuation of proinflammatory cytokines in LPA2 agonist–treated mice after HDM exposure. The levels of Th2 cytokines IL-4 and IL-5 were decreased in response to LPA2 activation, whereas the levels of IL-10 tended to increase compared with vehicle-treated mice (Figures 3A–3C). These data suggest that the LPA2 agonist changes the Th2 cytokine milieu in the airways of the treated mice, leading to a more antiinflammatory environment. However when we scored PAS+-stained lung sections, we did not observe any differences in mucus production in the airways between the two groups (Figure E2).
Figure 3.
Fewer Th2 cytokines in the BAL fluid of LPA2 agonist–treated mice. BAL supernatants from HDM + vehicle and HDM + LPA2 agonist–treated mice were assayed by cytokine multiplex for (A) IL-4, (B) IL-5, and (C) IL-10. Data are mean ± SEM, n = 8 mice/group; *P < 0.05.
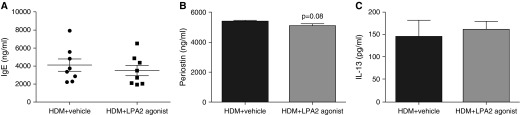
The titer of total serum IgE was not significantly different between the two groups (Figure 4A; 4.1 ± 0.69 and 3.5 ± 0.58 × 103 ng/ml; P = 0.26). These data suggest that pharmacologic activation of LPA2 during HDM allergen sensitization and challenge reduced lung and airway inflammation, but did not affect the class switching of Igs synthesized by B cells. In addition, we measured serum levels of periostin, which is secreted by airway epithelial cells in response to stimulation by IL-4 and IL-13, and has recently been identified as a surrogate biomarker of Th2-driven asthma. Periostin levels in mice treated with the LPA2 agonists were slightly, but not significantly, reduced compared with vehicle-treated controls (P = 0.08; Figure 4B). There was also no difference in serum IL-13 concentrations (Figure 4C).
Figure 4.
No difference in total serum IgE, periostin, or IL-13 after LPA2 agonist administration. Total serum IgE (A), periostin (B), and IL-13 (C) concentrations from HDM + vehicle and HDM + LPA2 agonist–treated mice were measured by ELISA. Data are mean ± SEM, n = 8 mice/group.
To determine if tonic engagement of LPA2 by DBIBB leads to changes in LPA2 expression in the lung epithelial cells, we treated human bronchial epithelial (16HBE) cells with DBIBB (10 μM) or vehicle (1:500 DMSO) for 0, 2, 6, and 24 hours and measured LPA2 expression by Western blot. LPA2 expression was not different between groups (Figure E3).
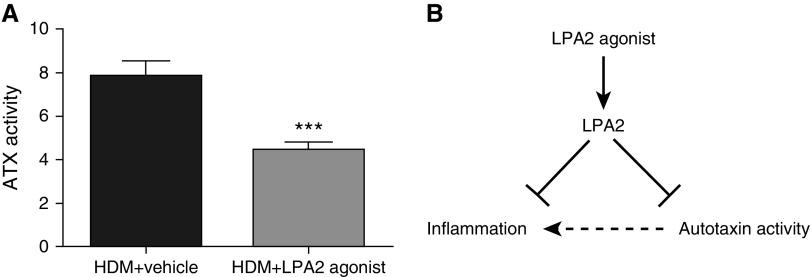
Finally, we also noted that DBIBB decreased ATX activity in lung digests, suggesting a previously unsuspected negative-feedback loop between LPA2 and ATX (Figure 5). However, ATX protein concentrations in lung digest supernatants were below the limit of detection, and ATX protein and activity were unchanged in the serum of vehicle-treated and LPA2 agonist–treated mice (Figure E4). Therefore, the mechanisms linking LPA2 activation to reduction of ATX activity will require further study.
Figure 5.
LPA2 agonist decreases autotaxin (ATX) activity in the lung. (A) The lysophospholipase D activity of ATX was indirectly measured in lung digest supernatants using an enzyme-coupled assay using N-ethyl-N-(2-hydroxyl-3-sulfoproryl)-3-methylaniline (described in the online supplement). Data are mean ± SEM, n = 8 mice /group. ***P < 0.001. (B) Proposed model: the LPA2 agonist (DBIBB) activates the LPA2 receptor, which leads to a dampening of allergen-driven inflammation.
Discussion
In this article, we show, for the first time, that activating LPA2 has therapeutic potential in a murine allergic airway inflammation model. Mice that were administered the novel LPA2 agonist, DBIBB, developed significantly less lung inflammation after 10 days of HDM challenges compared with vehicle-treated mice. The antiinflammatory effect of DBIBB was evident through total numbers of cells recovered from the BAL fluid, analysis of lung histology, and measurement of Th2 cytokine production. Collectively, these data support a model in which altering the ATX/LPA axis by activating LPA2 leads to a dampened immune response upon allergen challenge.
Several lines of evidence point to a role for the ATX/LPA axis in the pathogenesis of asthma (2–5). First, subjects with asthma have an increase in the LPA species 20:4 and 22:5 in their BAL fluid after segmental allergen challenge (4). Park and colleagues (5) corroborated these findings and showed an increase in LPA 22:5 and 22:6 in allergen-challenged subjects with asthma and an increase in LPA 22:5 in the BAL fluid of mice in a triple-allergen dust mite, ragweed, Aspergillus mouse model of allergic asthma. Second, in preclinical models, LPA has been implicated in many processes associated with asthma pathophysiology, including leukocyte recruitment and activation, as well as bronchial hyperreactivity (reviewed in Ref. 2). Because LPA can exert both proinflammatory and antiinflammatory effects in the lung (reviewed in Ref. 25), its precise role in asthma will be dependent on timing and location of LPA generation, as well as LPA receptors engaged.
Our results build on preclinical data that showed that LPA2 signaling is antiinflammatory and a negative regulator of dendritic cell activation (30). We have shown previously that dendritic cells from mice that are deficient in lpa2 were hyperactive compared with their wild-type counterparts in vitro, as seen by increased ability to stimulate allogeneic CD4+ T cells to proliferate and produce IL-13. When lpa2-deficient dendritic cells were allergen pulsed and intratracheally administered to wild-type mice, followed by aerosol allergen challenge, the recipient mice succumbed to greater lung eosinophilia. In addition, lpa2-deficient mice developed more airway inflammation and hyperreactivity than wild-type mice in mouse models of asthma involving both systemic and mucosal sensitization (with Ova as model allergen) (30). In contrast to these findings, Zhao and colleagues (34) have previously reported that mice heterozygous for lpa2 (lpa2+/−) were partially protected from lung inflammation after Schistosoma egg antigen challenge. Likewise, in the dust mite, ragweed, Aspergillus model of allergic lung inflammation, lpa2−/− mice were more resistant to lung inflammation than wild-type mice (5). Reasons for these discrepancies are unknown, but may be due to differences in the antigens used or genetic backgrounds of the mice.
In addition to dendritic cells, LPA2 is expressed on mouse CD4+ (35, 36) and CD8+ (36) T cells, neutrophils (36), lung endothelial cells (36), fibroblasts (36), and tracheal epithelial cells (34). Due to a lack of an antibody that detects extracellular surface LPA2, LPA receptor expression has been analyzed at the mRNA and protein level by quantitative PCR or Western blot analysis, respectively. LPA2 is unique from the other LPA receptors in its intracellular C terminus, which contains distinct protein–protein interaction domains. The last four amino acids interact with proteins such as the Na+/H+ exchange regulatory factor 2 (NHERF-2) and membrane-associated guanylate kinase with inverted orientation-3 (MAGI-3) (37, 38), which can activate extracellular signal-regulated kinases and Ras homolog gene family member A (RhoA) to promote cell migration (39). In the proximal region, LPA2 has a dileucine motif and several putative palmitoylated cysteine residues that associate with the LIM domains of thyroid receptor interacting protein 6 (TRIP6) (40) and Siva-1 protein (26, 27). Activating LPA2 leads to the recruitment of TRIP6, a focal adhesion molecule, to the C terminus of LPA2 at the plasma membrane. This association leads to its targeting to focal adhesions and colocalization with actin, and is involved with regulating LPA-induced cell adhesion and migration (27, 40–42). LPA2 also binds the Siva-1 protein, which is typically thought of as proapoptotic, because it activates caspases (43) and is up-regulated during the DNA damage response (44–46). However, upon it’s binding to activated LPA2, Siva-1 is polyubiquitinated and degraded in the proteasome, thereby down-regulating its proapoptotic activity (26, 27). Likewise, studies have shown that LPA2 is necessary and sufficient to protect cells from radiation-induced cell death and gut injury (28, 29). To this end, recent research has aimed to identify small-molecule drug candidates that target LPA2 to protect against radiation-induced apoptosis. The DBIBB compound was recently reported to reduce mortality of high-dose (15.69 Gy) γ-irradiated mice and mitigated gastrointestinal acute radiation syndrome, highlighted by increased crypt survival, enterocyte proliferation, and reduced apoptosis (32). DBIBB represents the first radiomitigator small-molecule compound to effectively treat tissue damage after radiation, and future studies investigating the mechanisms of this protective effect will be beneficial.
In our model, we administered DBIBB at the same time as HDM allergen. Whether or not DBIBB has mitigative effects after allergen-induced inflammation is established is unknown, and warrants future studies. Our data show that, when the LPA2 agonist is administered to mice during HDM challenges, significantly less allergen-induced inflammation developed compared with vehicle-treated mice. The striking efficacy of DBIBB in dampening tissue inflammation and Th2 cytokine production could be due to attenuation of cell recruitment to the lung, either indirectly by inhibiting chemokine production, or directly by affecting cell migration. One possibility is that the LPA2 agonist acts on bronchial epithelial cells and changes their cytokine and/or chemokine expression, thus inhibiting the recruitment or activation of immune cells to the airways. LPA has been shown to stimulate the expression of thymic stromal lymphopoietin (TSLP), involved in dendritic cell maturation, and CCL20, a dendritic cell and T cell chemoattractant, from bronchial epithelial cells (22). Whether this results from LPA2 signaling is not known. Another possibility is that epithelial chemokine production is dependent on LPA2, and, if tonic engagement of LPA2 by DBIBB leads to receptor desensitization and/or internalization, then DBIBB-exposed epithelial cells might be less responsive to endogenously produced LPA. Little is known about receptor recycling or trafficking of LPA receptors, except that there are different mechanisms that regulate LPA-dependent and PMA-dependent internalization of LPA1 (47). In addition, Lee and colleagues (39) have shown that LPA does not induce LPA2 internalization, although rapid internalization or recycling might have escaped detection. We also did not see any difference in LPA2 expression via Western blot after DBIBB treatment (Figure E3). Another explanation is that DBIBB inhibits DC activity via LPA2, which would support our previous observation that LPA2-deficient DCs have a hyperactive phenotype (30). However, because serum IgE was not significantly inhibited in DBIBB-treated mice, we concluded that early sensitization steps of the HDM-dependent immune response were intact in our model. Future studies investigating the effects of DBIBB on maturation and activation markers on lung DC subsets after HDM challenge will be needed to address this issue.
LPA also increases soluble ST2 (sST2) (24) and IL-13Rα2 (23) expression from mouse and human bronchial epithelial cells. These are both decoy receptors that bind to IL-33 and IL-13, respectively, and are considered antiinflammatory mediators. Modulating the levels of available IL-33 could have an impact on innate immune cell activation, such as IL-33–responsive type 2 innate lymphoid cells (ILC2s) (48). In the lung, ILC2s make IL-5 and IL-13 (49). In Figure 3, we note a significant decrease in IL-5, whereas IL-13 was immeasurable in both groups. However, when we measured sST2 levels in the serum of both vehicle-treated and DBIBB-treated mice, we did not detect any differences (Figure E5). In addition, IL-13Rα2 expression in BAL fluid was not significantly different between vehicle-treated and DBIBB-treated mice (Figure E6). Consequently, we concluded that the antiinflammatory effects of DBIBB are likely independent of ST2 and IL-13Rα2 in our experiments. Consequently, we concluded that the antiinflammatory effects of DBIBB are likely independent of ST2 and IL-13Rα2 in our experiments.
The LPA2 agonist could also be binding directly to the immune cells and inhibiting their migration to the lung in response to HDM. LPA2 signals through Gα12/13, which activates Rho kinases and leads to cytoskeletal remodeling, thus regulating cell migration (50). Human and mouse immune cells, such as eosinophils, neutrophils, macrophages, dendritic cells, CD4+ and CD8+ T cells, and mast cells, have been shown to express LPA2, albeit at differing levels (reviewed in Ref. 25). We only saw a trend toward fewer eosinophils and neutrophils in the BAL fluid after LPA2 agonist administration (Figure 1), but a significant decrease in IL-5 levels (Figure 3). A reason for this disconnect could be timing, and future experiments that look earlier and later after the last HDM challenge would be interesting. Furthermore, DBIBB could be affecting the recruitment or expansion of ILC2s, which are another producer of IL-5 in the lung (49, 51). In Figure 3, we show that DBIBB administration leads to an increase in IL-10 in the BAL fluid. We have not dissected the source of this antiinflammatory cytokine, but we can speculate that it is being produced from regulatory T cells or apoptotic macrophages in response to DBIBB (52, 53). We can measure other antiinflammatory cytokines, such as prostaglandin E2 or transforming growth factor-β1 to investigate whether DBIBB is enhancing resolution from HDM-induced lung inflammation (54). Future studies using mice deficient in lpa2 and adoptive transfer experiments will be needed to define specific effects of DBIBB on the recruitment of different cell types in vivo.
Periostin is an evolving biomarker of Th2 inflammation that is secreted by airway epithelial cells and lung fibroblasts in response to IL-4 and IL-13 (55–59). Gene expression profiling of humans with asthma have shown a greater than fourfold increase in periostin gene expression compared with healthy control subjects (60). However, data in periostin-deficient mice have not been as well defined (61–63). Blanchard and colleagues (61) noted decreased eosinophilic inflammation in the mouse lung after Aspergillus fumigatus challenge in periostin-deficient mice, whereas Gordon and colleagues (62) noted increased airway hyperresponsiveness and serum IgE after allergen challenge. In another study, Sehra and colleagues (63) saw no difference in eosinophils or Th2 cytokines between wild-type and periostin-deficient mice, but did report enhanced mucus production by goblet cells in periostin-deficient mice in an ovalbumin sensitization model. In our study, we noted a decrease in serum periostin after DBIBB treatment, but the difference was not statistically significant. Whether LPA signaling plays a direct role in periostin expression or if less periostin was induced due to the decreased levels of IL-4 remains to be determined.
Taken together, our data identify LPA2 as a novel therapeutic target in asthma. It will be interesting in future studies to determine how DBIBB attenuates tissue inflammation and Th2 cytokine production in the lung, and if these correlate with physiological outcomes, such as airway hyperresponsiveness. It will also be exciting to decipher the mechanisms and consequences of LPA2-dependent inhibition of ATX activity (Figure 5). Our results should add to the growing enthusiasm for new therapies that target the ATX/LPA axis in inflammatory diseases in general and allergic asthma in particular.
Footnotes
This work was supported by National Institutes of Health grants R01 HL071933 (S.N.G.), and T32 AI007285 and T32 HL66988A-14 (S.A.K.).
Author Contributions: Conception and design—S.A.K., T.J.C., and S.N.G.; performed experiments—S.A.K. and S.E.H.; analysis and interpretation—S.A.K., T.J.C., G.T., and S.N.G.; synthesis of 2-[4-(1,3-dioxo-1H,3H-benzoisoquinolin-2-yl)butylsulfamoyl] benzoic acid compound—R.P. and D.D.M.; drafting the manuscript—S.A.K., G.T., and S.N.G.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0124OC on August 6, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Fajt ML, Wenzel SE. Asthma phenotypes and the use of biologic medications in asthma and allergic disease: the next steps toward personalized care. J Allergy Clin Immunol. 2015;135:299–310, quiz 311. doi: 10.1016/j.jaci.2014.12.1871. [DOI] [PubMed] [Google Scholar]
- 2.Knowlden S, Georas SN. The autotaxin–LPA axis emerges as a novel regulator of lymphocyte homing and inflammation. J Immunol. 2014;192:851–857. doi: 10.4049/jimmunol.1302831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Georas SN. Allergic to autotaxin: a new role for lysophospholipase D and lysophosphatidic acid in asthma? Am J Respir Crit Care Med. 2013;188:889–891. doi: 10.1164/rccm.201309-1597ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Georas SN, Berdyshev E, Hubbard W, Gorshkova IA, Usatyuk PV, Saatian B, Myers AC, Williams MA, Xiao HQ, Liu M, et al. Lysophosphatidic acid is detectable in human bronchoalveolar lavage fluids at baseline and increased after segmental allergen challenge. Clin Exp Allergy. 2007;37:311–322. doi: 10.1111/j.1365-2222.2006.02626.x. [DOI] [PubMed] [Google Scholar]
- 5.Park GY, Lee YG, Berdyshev E, Nyenhuis S, Du J, Fu P, Gorshkova IA, Li Y, Chung S, Karpurapu M, et al. Autotaxin production of lysophosphatidic acid mediates allergic asthmatic inflammation. Am J Respir Crit Care Med. 2013;188:928–940. doi: 10.1164/rccm.201306-1014OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toews ML, Ustinova EE, Schultz HD. Lysophosphatidic acid enhances contractility of isolated airway smooth muscle. J Appl Physiol (1985) 1997;83:1216–1222. doi: 10.1152/jappl.1997.83.4.1216. [DOI] [PubMed] [Google Scholar]
- 7.Cerutis DR, Nogami M, Anderson JL, Churchill JD, Romberger DJ, Rennard SI, Toews ML. Lysophosphatidic acid and EGF stimulate mitogenesis in human airway smooth muscle cells. Am J Physiol. 1997;273:L10–L15. doi: 10.1152/ajplung.1997.273.1.L10. [DOI] [PubMed] [Google Scholar]
- 8.Ediger TL, Toews ML. Synergistic stimulation of airway smooth muscle cell mitogenesis. J Pharmacol Exp Ther. 2000;294:1076–1082. [PubMed] [Google Scholar]
- 9.Ediger TL, Toews ML. Dual effects of lysophosphatidic acid on human airway smooth muscle cell proliferation and survival. Biochim Biophys Acta. 2001;1531:59–67. doi: 10.1016/s1388-1981(01)00084-1. [DOI] [PubMed] [Google Scholar]
- 10.Bagga S, Price KS, Lin DA, Friend DS, Austen KF, Boyce JA. Lysophosphatidic acid accelerates the development of human mast cells. Blood. 2004;104:4080–4087. doi: 10.1182/blood-2004-03-1166. [DOI] [PubMed] [Google Scholar]
- 11.Lin DA, Boyce JA. IL-4 regulates MEK expression required for lysophosphatidic acid–mediated chemokine generation by human mast cells. J Immunol. 2005;175:5430–5438. doi: 10.4049/jimmunol.175.8.5430. [DOI] [PubMed] [Google Scholar]
- 12.Lundequist A, Boyce JA. LPA5 is abundantly expressed by human mast cells and important for lysophosphatidic acid induced MIP-1β release. PLoS One. 2011;6:e18192. doi: 10.1371/journal.pone.0018192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hashimoto T, Ohata H, Momose K, Honda K. Lysophosphatidic acid induces histamine release from mast cells and skin fragments. Pharmacology. 2005;75:13–20. doi: 10.1159/000085784. [DOI] [PubMed] [Google Scholar]
- 14.Idzko M, Laut M, Panther E, Sorichter S, Dürk T, Fluhr JW, Herouy Y, Mockenhaupt M, Myrtek D, Elsner P, et al. Lysophosphatidic acid induces chemotaxis, oxygen radical production, CD11b up-regulation, Ca2+ mobilization, and actin reorganization in human eosinophils via pertussis toxin–sensitive G proteins. J Immunol. 2004;172:4480–4485. doi: 10.4049/jimmunol.172.7.4480. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto T, Yamashita M, Ohata H, Momose K. Lysophosphatidic acid enhances in vivo infiltration and activation of guinea pig eosinophils and neutrophils via a Rho/Rho–associated protein kinase–mediated pathway. J Pharmacol Sci. 2003;91:8–14. doi: 10.1254/jphs.91.8. [DOI] [PubMed] [Google Scholar]
- 16.Panther E, Idzko M, Corinti S, Ferrari D, Herouy Y, Mockenhaupt M, Dichmann S, Gebicke-Haerter P, Di Virgilio F, Girolomoni G, et al. The influence of lysophosphatidic acid on the functions of human dendritic cells. J Immunol. 2002;169:4129–4135. doi: 10.4049/jimmunol.169.8.4129. [DOI] [PubMed] [Google Scholar]
- 17.Chan LC, Peters W, Xu Y, Chun J, Farese RV, Jr, Cases S. LPA3 receptor mediates chemotaxis of immature murine dendritic cells to unsaturated lysophosphatidic acid (LPA) J Leukoc Biol. 2007;82:1193–1200. doi: 10.1189/jlb.0407221. [DOI] [PubMed] [Google Scholar]
- 18.Cummings R, Zhao Y, Jacoby D, Spannhake EW, Ohba M, Garcia JG, Watkins T, He D, Saatian B, Natarajan V. Protein kinase Cδ mediates lysophosphatidic acid–induced NF-κB activation and interleukin-8 secretion in human bronchial epithelial cells. J Biol Chem. 2004;279:41085–41094. doi: 10.1074/jbc.M404045200. [DOI] [PubMed] [Google Scholar]
- 19.Zhao Y, Usatyuk PV, Cummings R, Saatian B, He D, Watkins T, Morris A, Spannhake EW, Brindley DN, Natarajan V. Lipid phosphate phosphatase-1 regulates lysophosphatidic acid–induced calcium release, NF-κB activation and interleukin-8 secretion in human bronchial epithelial cells. Biochem J. 2005;385:493–502. doi: 10.1042/BJ20041160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saatian B, Zhao Y, He D, Georas SN, Watkins T, Spannhake EW, Natarajan V. Transcriptional regulation of lysophosphatidic acid–induced interleukin-8 expression and secretion by p38 MAPK and JNK in human bronchial epithelial cells. Biochem J. 2006;393:657–668. doi: 10.1042/BJ20050791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalari S, Zhao Y, Spannhake EW, Berdyshev EV, Natarajan V. Role of acylglycerol kinase in LPA-induced IL-8 secretion and transactivation of epidermal growth factor-receptor in human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2009;296:L328–L336. doi: 10.1152/ajplung.90431.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Medoff BD, Landry AL, Wittbold KA, Sandall BP, Derby MC, Cao Z, Adams JC, Xavier RJ. CARMA3 mediates lysophosphatidic acid-stimulated cytokine secretion by bronchial epithelial cells. Am J Respir Cell Mol Biol. 2009;40:286–294. doi: 10.1165/rcmb.2008-0129OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao Y, He D, Zhao J, Wang L, Leff AR, Spannhake EW, Georas S, Natarajan V. Lysophosphatidic acid induces interleukin-13 (IL-13) receptor alpha2 expression and inhibits IL-13 signaling in primary human bronchial epithelial cells. J Biol Chem. 2007;282:10172–10179. doi: 10.1074/jbc.M611210200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao J, Chen Q, Li H, Myerburg M, Spannhake EW, Natarajan V, Zhao Y. Lysophosphatidic acid increases soluble ST2 expression in mouse lung and human bronchial epithelial cells. Cell Signal. 2012;24:77–85. doi: 10.1016/j.cellsig.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magkrioti C, Aidinis V. Autotaxin and lysophosphatidic acid signaling in lung pathophysiology. World Journal of Respirology. 2013;3:77–103. [Google Scholar]
- 26.Lin FT, Lai YJ, Makarova N, Tigyi G, Lin WC. The lysophosphatidic acid 2 receptor mediates down-regulation of Siva-1 to promote cell survival. J Biol Chem. 2007;282:37759–37769. doi: 10.1074/jbc.M705025200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.E S, Lai YJ, Tsukahara R, Chen CS, Fujiwara Y, Yue J, Yu JH, Guo H, Kihara A, Tigyi G, et al. Lysophosphatidic acid 2 receptor-mediated supramolecular complex formation regulates its antiapoptotic effect. J Biol Chem. 2009;284:14558–14571. doi: 10.1074/jbc.M900185200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deng W, Shuyu E, Tsukahara R, Valentine WJ, Durgam G, Gududuru V, Balazs L, Manickam V, Arsura M, VanMiddlesworth L, et al. The lysophosphatidic acid type 2 receptor is required for protection against radiation-induced intestinal injury. Gastroenterology. 2007;132:1834–1851. doi: 10.1053/j.gastro.2007.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng W, Balazs L, Wang DA, Van Middlesworth L, Tigyi G, Johnson LR. Lysophosphatidic acid protects and rescues intestinal epithelial cells from radiation- and chemotherapy-induced apoptosis. Gastroenterology. 2002;123:206–216. doi: 10.1053/gast.2002.34209. [DOI] [PubMed] [Google Scholar]
- 30.Emo J, Meednu N, Chapman TJ, Rezaee F, Balys M, Randall T, Rangasamy T, Georas SN. Lpa2 is a negative regulator of both dendritic cell activation and murine models of allergic lung inflammation. J Immunol. 2012;188:3784–3790. doi: 10.4049/jimmunol.1102956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patil R, Fells JI, Szabó E, Lim KG, Norman DD, Balogh A, Patil S, Strobos J, Miller DD, Tigyi GJ. Design and synthesis of sulfamoyl benzoic acid analogues with subnanomolar agonist activity specific to the LPA2 receptor. J Med Chem. 2014;57:7136–7140. doi: 10.1021/jm5007116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patil R, Szabó E, Fells JI, Balogh A, Lim KG, Fujiwara Y, Norman DD, Lee SC, Balazs L, Thomas F, et al. Combined mitigation of the gastrointestinal and hematopoietic acute radiation syndromes by an LPA2 receptor–specific nonlipid agonist. Chem Biol. 2015;22:206–216. doi: 10.1016/j.chembiol.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Llop-Guevara A, Colangelo M, Chu DK, Moore CL, Stieber NA, Walker TD, Goncharova S, Coyle AJ, Lundblad LK, O’Byrne PM, et al. In vivo-to-in silico iterations to investigate aeroallergen–host interactions. PLoS One. 2008;3:e2426. doi: 10.1371/journal.pone.0002426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y, Tong J, He D, Pendyala S, Evgeny B, Chun J, Sperling AI, Natarajan V. Role of lysophosphatidic acid receptor LPA2 in the development of allergic airway inflammation in a murine model of asthma. Respir Res. 2009;10:114. doi: 10.1186/1465-9921-10-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knowlden SA, Capece T, Popovic M, Chapman TJ, Rezaee F, Kim M, Georas SN. Regulation of T cell motility in vitro and in vivo by LPA and LPA2. PLoS One. 2014;9:e101655. doi: 10.1371/journal.pone.0101655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, et al. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med. 2008;14:45–54. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 37.Oh YS, Jo NW, Choi JW, Kim HS, Seo SW, Kang KO, Hwang JI, Heo K, Kim SH, Kim YH, et al. NHERF2 specifically interacts with LPA2 receptor and defines the specificity and efficiency of receptor-mediated phospholipase C-β3 activation. Mol Cell Biol. 2004;24:5069–5079. doi: 10.1128/MCB.24.11.5069-5079.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yun CC, Sun H, Wang D, Rusovici R, Castleberry A, Hall RA, Shim H. LPA2 receptor mediates mitogenic signals in human colon cancer cells. Am J Physiol Cell Physiol. 2005;289:C2–C11. doi: 10.1152/ajpcell.00610.2004. [DOI] [PubMed] [Google Scholar]
- 39.Lee SJ, Ritter SL, Zhang H, Shim H, Hall RA, Yun CC. MAGI-3 competes with NHERF-2 to negatively regulate LPA2 receptor signaling in colon cancer cells. Gastroenterology. 2011;140:924–934. doi: 10.1053/j.gastro.2010.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu J, Lai YJ, Lin WC, Lin FT. TRIP6 enhances lysophosphatidic acid-induced cell migration by interacting with the lysophosphatidic acid 2 receptor. J Biol Chem. 2004;279:10459–10468. doi: 10.1074/jbc.M311891200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lai YJ, Chen CS, Lin WC, Lin FT. c-Src–mediated phosphorylation of TRIP6 regulates its function in lysophosphatidic acid–induced cell migration. Mol Cell Biol. 2005;25:5859–5868. doi: 10.1128/MCB.25.14.5859-5868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lai YJ, Lin WC, Lin FT. PTPL1/FAP-1 negatively regulates TRIP6 function in lysophosphatidic acid-induced cell migration. J Biol Chem. 2007;282:24381–24387. doi: 10.1074/jbc.M701499200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Py B, Slomianny C, Auberger P, Petit PX, Benichou S. Siva-1 and an alternative splice form lacking the death domain, Siva-2, similarly induce apoptosis in T lymphocytes via a caspase-dependent mitochondrial pathway. J Immunol. 2004;172:4008–4017. doi: 10.4049/jimmunol.172.7.4008. [DOI] [PubMed] [Google Scholar]
- 44.Prasad KV, Ao Z, Yoon Y, Wu MX, Rizk M, Jacquot S, Schlossman SF. CD27, a member of the tumor necrosis factor receptor family, induces apoptosis and binds to Siva, a proapoptotic protein. Proc Natl Acad Sci USA. 1997;94:6346–6351. doi: 10.1073/pnas.94.12.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Daoud SS, Munson PJ, Reinhold W, Young L, Prabhu VV, Yu Q, LaRose J, Kohn KW, Weinstein JN, Pommier Y. Impact of p53 knockout and topotecan treatment on gene expression profiles in human colon carcinoma cells: a pharmacogenomic study. Cancer Res. 2003;63:2782–2793. [PubMed] [Google Scholar]
- 46.Fortin A, MacLaurin JG, Arbour N, Cregan SP, Kushwaha N, Callaghan SM, Park DS, Albert PR, Slack RS. The proapoptotic gene SIVA is a direct transcriptional target for the tumor suppressors p53 and E2F1. J Biol Chem. 2004;279:28706–28714. doi: 10.1074/jbc.M400376200. [DOI] [PubMed] [Google Scholar]
- 47.Urs NM, Kowalczyk AP, Radhakrishna H. Different mechanisms regulate lysophosphatidic acid (LPA)–dependent versus phorbol ester–dependent internalization of the LPA1 receptor. J Biol Chem. 2008;283:5249–5257. doi: 10.1074/jbc.M710003200. [DOI] [PubMed] [Google Scholar]
- 48.Barlow JL, Peel S, Fox J, Panova V, Hardman CS, Camelo A, Bucks C, Wu X, Kane CM, Neill DR, et al. IL-33 is more potent than IL-25 in provoking IL-13–producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013;132:933–941. doi: 10.1016/j.jaci.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 49.Klein Wolterink RG, Kleinjan A, van Nimwegen M, Bergen I, de Bruijn M, Levani Y, Hendriks RW. Pulmonary innate lymphoid cells are major producers of IL-5 and IL-13 in murine models of allergic asthma. Eur J Immunol. 2012;42:1106–1116. doi: 10.1002/eji.201142018. [DOI] [PubMed] [Google Scholar]
- 50.Yamada T, Ohoka Y, Kogo M, Inagaki S. Physical and functional interactions of the lysophosphatidic acid receptors with PDZ domain–containing Rho guanine nucleotide exchange factors (RhoGEFs) J Biol Chem. 2005;280:19358–19363. doi: 10.1074/jbc.M414561200. [DOI] [PubMed] [Google Scholar]
- 51.Nussbaum JC, Van Dyken SJ, von Moltke J, Cheng LE, Mohapatra A, Molofsky AB, Thornton EE, Krummel MF, Chawla A, Liang HE, et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature. 2013;502:245–248. doi: 10.1038/nature12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr, et al. Regulatory T cell–derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 53.Zhao Y, Xiong Z, Lechner EJ, Klenotic PA, Hamburg BJ, Hulver M, Khare A, Oriss T, Mangalmurti N, Chan Y, et al. Thrombospondin-1 triggers macrophage IL-10 production and promotes resolution of experimental lung injury. Mucosal Immunol. 2014;7:440–448. doi: 10.1038/mi.2013.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fadok VA, Bratton DL, Konowal A, Freed PW, Westcott JY, Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lopez-Guisa JM, Powers C, File D, Cochrane E, Jimenez N, Debley JS. Airway epithelial cells from asthmatic children differentially express proremodeling factors. J Allergy Clin Immunol. 2012;129:990–997. doi: 10.1016/j.jaci.2011.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118:98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 57.Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, Toda S, Inagaki N, Kurihara Y, Hayashida S, et al. Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest. 2012;122:2590–2600. doi: 10.1172/JCI58978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, Shikotra A, Carter R, Audusseau S, Hamid Q, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol. 2012;130:647–654. doi: 10.1016/j.jaci.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kanemitsu Y, Matsumoto H, Izuhara K, Tohda Y, Kita H, Horiguchi T, Kuwabara K, Tomii K, Otsuka K, Fujimura M, et al. Increased periostin associates with greater airflow limitation in patients receiving inhaled corticosteroids. J Allergy Clin Immunol. 2013;132:305–312. doi: 10.1016/j.jaci.2013.04.050. [DOI] [PubMed] [Google Scholar]
- 60.Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, Ellwanger A, Sidhu SS, Dao-Pick TP, Pantoja C, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA. 2007;104:15858–15863. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blanchard C, Mingler MK, McBride M, Putnam PE, Collins MH, Chang G, Stringer K, Abonia JP, Molkentin JD, Rothenberg ME. Periostin facilitates eosinophil tissue infiltration in allergic lung and esophageal responses. Mucosal Immunol. 2008;1:289–296. doi: 10.1038/mi.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gordon ED, Sidhu SS, Wang ZE, Woodruff PG, Yuan S, Solon MC, Conway SJ, Huang X, Locksley RM, Fahy JV. A protective role for periostin and TGF-β in IgE-mediated allergy and airway hyperresponsiveness. Clin Exp Allergy. 2012;42:144–155. doi: 10.1111/j.1365-2222.2011.03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sehra S, Yao W, Nguyen ET, Ahyi AN, Tuana FM, Ahlfeld SK, Snider P, Tepper RS, Petrache I, Conway SJ, et al. Periostin regulates goblet cell metaplasia in a model of allergic airway inflammation. J Immunol. 2011;186:4959–4966. doi: 10.4049/jimmunol.1002359. [DOI] [PMC free article] [PubMed] [Google Scholar]