Abstract
Obstructive sleep apnea (OSA) is a common disorder that describes recurrent collapse of the upper airway during sleep. Animal models have been pivotal to the understanding of OSA pathogenesis, consequences, and treatment. In this review, we highlight the history of OSA research in animals and include the discovery of animals with spontaneous OSA, the induction of OSA in animals, and the emulation of OSA using exposures to intermittent hypoxia and sleep fragmentation.
Keywords: sleep apnea, animal, intermittent hypoxia, fragmentation
Obstructive sleep apnea (OSA) describes recurrent complete (apnea) or partial (hypopnea) collapse of the upper airway during sleep. The estimated prevalence of OSA in North America is 20–30% in males and 10–15% in females (1, 2), a figure rapidly on the rise with obesity. Additional risk factors for OSA include advanced age, male sex, and craniofacial or upper airway soft tissue abnormalities. During sleep, efforts of breathing against an occluded upper airway lead to impaired gas exchange, swings in intrathoracic pressure, and sleep fragmentation (SF). OSA severity is quantified by the apnea–hypopnea index (AHI), which measures the frequency of disordered events. Mild, moderate, and severe OSA are classified according to whether the AHI is 5–15/h, 15–30/h, or greater than 30/h, respectively (3). OSA is associated with significant cardiovascular, metabolic, and neurocognitive consequences. Continuous positive airway pressure (CPAP) therapy is first-line treatment for OSA. Other therapies for sleep apnea include dental appliances, weight loss, upper airway surgery, and upper airway electrical stimulation.
The core defect leading to OSA is susceptibility of the upper airway to collapse during sleep. This susceptibility is governed both by passive anatomical properties and by active control of airway dilator muscles. During sleep, the susceptibility of collapse is quantified by determining the luminal collapsing pressure, called the critical pressure (Pcrit) (4, 5). In normal subjects, substantial negative pressure may be necessary to induce airway collapse during sleep (e.g., Pcrit = −10 cm H20), whereas, in OSA, the Pcrit may be greater than 0, reflecting the need for CPAP to maintain airway patency. Pcrit is modified by both static anatomical factors (“passive” Pcrit) and dynamic activation of upper airway dilator muscles (“active” Pcrit). Muscle tone to airway structures is high during wakefulness, but may significantly decrease during sleep, leading to OSA in susceptible individuals. Besides Pcrit, OSA is influenced by several other factors. For example, the tendency to hyperventilate against changes in blood oxygenation (loop gain), or to awaken easily from sleep (arousal threshold) may aggravate OSA. An integrative scale using Pcrit, arousal threshold, loop gain, and muscle responsiveness proposed by Owens and colleagues provides a framework for dividing patients between those who may require CPAP and those who might be treated with non-CPAP therapies (6). Patients with OSA can have different clinical presentations, ranging from insomnia to excessive daytime sleepiness (7). For example, a recent cluster analysis identified patients with OSA with sleep-related complaints, daytime sleepiness, or asymptomatic status associated with discrete cardiovascular risk profiles (7).
Over the last few decades, the consequences of OSA, including neurologic, cardiovascular, and metabolic function, have been studied. However, OSA is often accompanied by comorbidities, such as obesity. Animal models of OSA have furthered our understanding of OSA pathogenesis and its consequences. The purpose of the current review is to highlight milestones in the use of animals for OSA research. The review first addresses spontaneous sleep apnea in animals and surgical techniques that induce OSA. Next, we focus on the animal models that deal with the principle components of OSA—intermittent hypoxia (IH) and SF.
Animals with Spontaneous Sleep Apnea
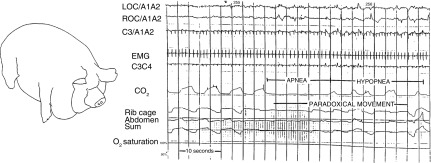
Sleep apnea was first described in humans in 1965 (8, 9), and it has since been discovered in some of our mammalian counterparts. In 1987, Hendricks and colleagues (10) described mild OSA in the English bulldog. With its large soft palate and narrow oropharynx, this breed is prone to snoring and hypopneas in contrast to other breeds studied. These animals have both central and obstructive events, with worsening during rapid eye movement (REM) sleep accompanied by daytime hypersomnolence (10). The AHI ranged from 0.5 to 21 during sleep, whereas the REM AHI ranged from 2 to 40. Interestingly, these bulldogs experienced OSA even though they were not obese. Other animals develop OSA with the development of obesity, in an analogous manner to humans. For example, Lonergan and colleagues (11) reported that female Yucatan miniature pigs developed OSA (Figure 1). Two obese pigs exhibited OSA with an overall AHI of approximately 9 and a REM AHI of approximately 27, whereas a third pig exhibited severe central sleep apnea. In 2006, using magnetic resonance imaging techniques, Brennick and colleagues noted that obese Zucker rats exhibited small airway cross-sectional areas during inspiration, together with increases in tongue fat infiltration. He found similar features in obese, leptin-resistant New Zealand obese mice, which accumulate fat in pharyngeal soft tissues (12).
Figure 1.
Sleep apnea occurs in obese Yucatan miniature pigs. Capnography tracing indicates periods of apnea and hypopnea with paradoxical movements of the rib cage and abdomen during sleep. Reprinted with permission from Lonergan and colleagues (11). EMG, electromyogram; LOC, left outer canthus; ROC, right outer canthus.
The actual demonstration of OSA in rodents was made possible by the development of a plethysmography system by Hernandez and colleagues (13) in 2012. Their system built upon existing technology to measure respiration in unrestrained mice, but with modifications, including continuous measurements of breathing, tidal volume, and respiratory effort. Using this system, it was revealed that New Zealand obese, leptin-deficient mice, or diet-induced obese mice exhibit flow-limited breathing. Furthermore, the chamber pressure could be modulated to determine rodent Pcrit (14).
Surgical/Mechanical Models
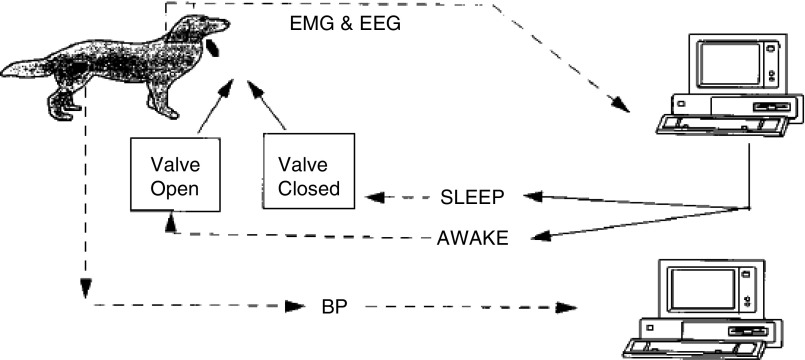
OSA has also been induced in experiments since the 1990s by mechanically occluding or altering the upper airway. Many experiments involve a tracheotomy with an intermittently occluded endotracheal tube. Thus far, these techniques have been used in dogs (15), lambs (16), baboons (17), piglets (18, 19), and rats (20). A dog model of OSA is depicted in Figure 2, in which a computer-controlled valve would close upon detection of a predetermined period of sleep. Farre and colleagues developed two versions of airway obstruction in the rat: one that involves tracheostomy (20), and another that involves occlusion of a valve external to the animal (21). In monkeys, intradermal liquid collagen injections in the uvula, tongue, and pharyngeal walls resulted in significant hypopneas (22). As an example of what these animal models have taught us, acute airway obstruction in dogs was shown to cause surges in blood pressure during sleep (23). Interestingly, the development of sustained hypertension was only observed with chronic sleep-induced upper airway obstruction, but not in animals subjected to recurrent arousals without airway obstruction. Thus, repetitive asphyxic stimulation is a stressor that can uniquely induce hypertension, as has been observed in OSA clinical studies (24, 25). Animals without intrinsic OSA have also been used to study the feasibility of various treatment modalities, including hypoglossal nerve stimulation in dogs (26), and radiofrequency thermal ablation to the base of the tongue in pigs (27). Overall, these techniques have been used to study effects of OSA on control of breathing and cardiovascular function. However, these models are labor intensive, with low throughput, making them challenging to use in studying OSA complications.
Figure 2.
Schematic of a dog model of obstructive sleep apnea (OSA). After a sleep was detected via computer, an occlusion valve in the tracheal tube was closed. The valve would subsequently open upon detection of awakening. Reprinted with permission from Brooks and colleagues (15). BP, blood pressure; EEG, electroencephalogram; EMG, electromyogram.
IH
IH is one of the hallmark features of OSA. In 1992, Fletcher and colleagues (28) developed a system to deliver hypoxic gases to rats in a cyclic pattern resembling the oxygen profile of severe OSA. Rats exposed to IH for 3 weeks during their sleep phase developed hypertension in a manner dependent upon sympathetic reflexes. In some cases, IH has been delivered only with the onset of electroencephalogram-monitored sleep (29). However, due to the labor-intensive nature of this approach, most experiments using IH forego sleep/awake detection systems in favor of high throughput. IH has since become the most widely used OSA model, as it is believed to be one of the more noxious stimuli of OSA (30), and because IH can be easily adjusted to induce OSA of varying severity. An example of one such IH system is shown in Figure 3. Over the last 25 years, numerous studies have examined the effects of IH in rodents (31). For example, chronic IH has been shown to cause or accelerate atherosclerosis (32, 33), oxidative stress (34, 35), neurocognitive dysfunction (36), hyperlipidemia (37, 38), and glucose intolerance (39). Furthermore, genetic interventions have been applied to rodents that can yield novel mechanistic insights. Experiments using knockout mice have demonstrated pathologic interactions between IH and nicotinamide adenine dinucleotide reduced oxidase (40), adipose angiopoietin-like 4 (41), and hypoxia-inducible factor-1α (42, 43) in mediating downstream consequences of IH.
Figure 3.
An intermittent hypoxia (IH) system used in the Johns Hopkins animal sleep laboratory. The gas controller unit (left) provides timed flows of air, nitrogen, and oxygen into mouse cages. Cages are kept at a constant ambient temperature (30°C) in a neonatal incubator to ensure maintenance of basal metabolic conditions. Ambient oxygen, CO2 level, and temperature are continuously monitored.
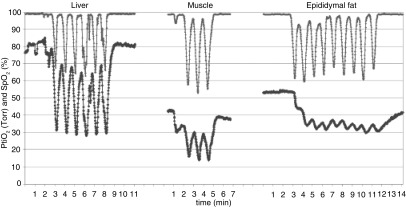
Most IH models have used severe IH, and protocols have been summarized in a previous review (44). A common technique lowers the ambient cage oxygen to 5%, 60 times/h, resulting in a mouse arterial oxygen saturation nadir ranging from less than 50% (45) to 67% (33). Lee and colleagues measured arterial blood gases during this type of IH at 10-second intervals and reported a milder nadir of 85%, perhaps related to averaging between sampling intervals (46). Using an electrode to probe tissue oxygen levels in anesthetized mice, Reinke and colleagues (45) noted that IH induced large amplitude fluctuations in liver oxygen, with attenuated or nearly absent fluctuations in muscle and adipose tissue, respectively (Figure 4). Almendros and colleagues measured tissue oxygen in rats during mechanical airway obstruction compared with IH, with each stimulus occurring for 15 s/min. Interestingly, obstructive apneas actually increased cerebral oxygen content, reflecting autoregulation (47). Hence, IH can have highly tissue-specific effects on oxygen delivery.
Figure 4.
IH in mice results in variable levels and fluctuations of tissue oxygen tension. Note that, in some tissues, such as epididymal fat, the amplitude of fluctuations is blunted and resembles sustained hypoxia. Reprinted with permission from Reinke and colleagues (46). PtiO2, tissue oxygen pressure; SpO2, oxygen saturation as measured by pulse oximetry.
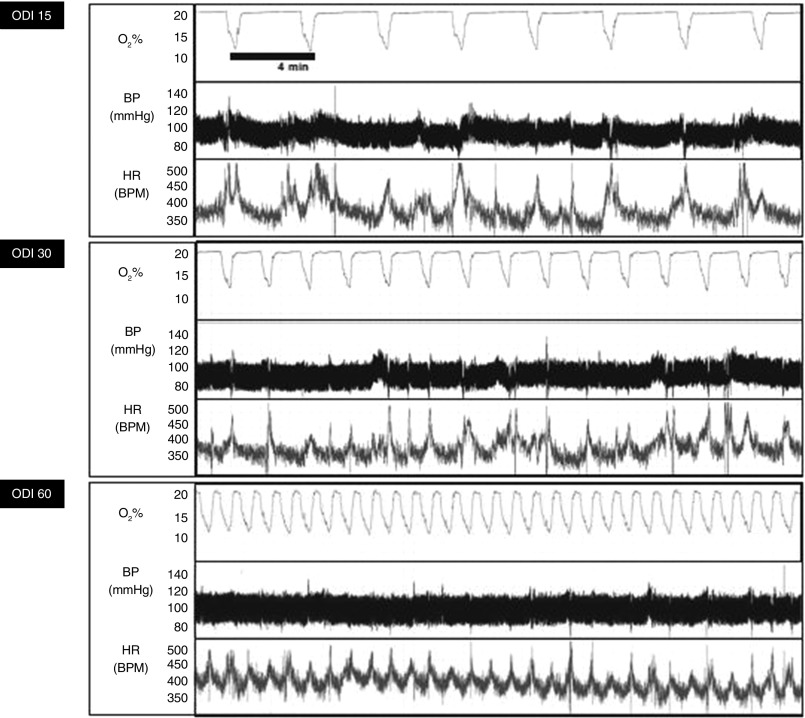
Most of the IH models discussed previously here induce severe hypoxia that may be significantly more severe than that typically experienced by patients with OSA. Several investigators have made refinements to IH to improve the clinical realism of experiments. Many of these changes were made possible by implementing technology to measure real-time pulse oximetry in mice (e.g., MouseOx; STARR Life Sciences, Oakmont, PA; MouseSTAT, Kent Scientific Corp., Torrington, CT) during IH exposure. Chodzyński and colleagues (48) developed an IH system allowing independent control of oxygen nadir and frequency in individual animals. Lim and colleagues observed that the sinusoidal IH pattern induced in rodents bears little resemblance to the IH of OSA, which usually involves a more rapid recovery of oxygen saturation as measured by pulse oximetry. Using a model of IH characterized by rapid oxygen restoration kinetics, they detected a greater increase in oxidative stress (49). Jun and colleagues developed an IH system capable of inducing 0, 15, 30, or 60 desaturations per hour with a fixed oxygen saturation, as measured by pulse oximetry, nadir of 80% (Figure 5). Using this model, it was shown that IH induces frequency-dependent glucose intolerance and hyperglycemia that could be abolished by sympatholytic interventions (34). These next-generation IH models may yield additional important insights into consequences of OSA in the near future.
Figure 5.
Mouse arterial oxygen saturation, BP, and heart rate (HR) during IH delivered at variable frequencies simulating OSA with an oxygen desaturation index (ODI) of 15, 30, or 60/h using the apparatus shown in Figure 3. The nadir fraction of inspired oxygen of 11% was empirically determined, targeting an arterial oxygen saturation nadir of 80%. Note the low basal HR due to thermoneutral conditions of the study and the acceleration of HR during each desaturation. Reprinted with permission from Jun and colleagues (34). BPM, beats per minute.
In OSA, IH follows from periodic asphyxia leading to either a stable or subtly increased arterial carbon dioxide pressure (PaCO2) level (50). By contrast, the delivery of hypoxic gases to animals results in compensatory hyperventilation with a mild depression of PaCO2. Based on physics of gas mixing and variable hypoxic ventilatory responses, it is difficult to control PaCO2 during IH. In a few experiments, mixtures of gas enriched with CO2 were delivered during IH. For example, McGuire and colleagues delivered flows of mixed gas targeting an ambient cage CO2 of 10–14% CO2 (ambient CO2 = 0.03%) and O2 of 6–8% for 15 seconds, twice per minute. This induced severe hypercapnia in rats with the end-tidal CO2 rising from approximately 36 to approximately 63 mm Hg as the arterial oxygen pressure decreased to 50–60 mm Hg during each IH cycle (51). This stimulus induced significant pulmonary hypertension, erythrocytosis, and right ventricular remodeling. More recently, Dergacheva and colleagues (52) exposed rats to IH/hypercapnia by delivering a mixture of 6% O2 and 5% CO2 10 times per hour for 8 hours using a commercially available chamber (BioSpherix, Parish, NY).
Although IH research has yielded valuable insights into OSA consequences, there are significant caveats. IH is but one of several potentially injurious aspects of OSA that also include upper airway obstruction with intrathoracic pressure swings, and SF (30). In addition, most of the currently employed models of IH resemble very severe OSA, and the findings should be applied to clinical OSA with caution. Another potential confounder of IH has been lack of attention to experimental temperature. Most IH experiments have been performed at unadjusted ambient temperatures around 22°C, well below the thermoneutral zone of mice (∼30°C). These cool temperatures significantly increase stress, thermogenesis, and metabolic rate in a hypoxia-suppressible manner (53). This phenomenon explains why hypoxia increases triglyceride levels in mice housed at 22°C, but not at 30°C (54). To date, only one IH study has been performed at thermoneutrality (34).
SF
In OSA, apneas or hypopneas can induce frequent, brief arousals from sleep, inducing what has been termed SF. Various apparatuses have been developed to prevent or disrupt animal sleep. For example, Rechtschaffen’s laboratory (55) published studies beginning in 1989 using a rotating disk over water to prevent sleep in rats. Other devices have since been developed such as rotating wheels or treadmills (56), whereas short-term sleep restriction can also be achieved by gentle handling (57). To selectively prevent REM sleep, an inverted “flower pot” technique was developed by Mendelson and colleagues (58) in 1974 in which animals were placed on a platform immersed in water. With the onset of REM muscle atonia, the animals would awaken after falling into water. In 2009, Arrigoni and colleagues (59) used lesions of the ventrolateral preoptic area to induce chronic partial sleep loss in rats. This novel approach does not induce forced animal locomotion or other environmental stress.
SF may occur in many sleep disorders, such as insomnia or restless leg syndrome. The arousals from sleep in OSA tend to be brief, triggered frequently by recurrent apneas and hypopneas. Some of the above methods have therefore been adapted to induce SF in a pattern similar to that of OSA. For example, a rotating disc, equipped with a center divider that must be avoided by the mouse within, can be triggered to turn at programmed intervals (60). The timing and direction of disc rotation can also be randomized to prevent behavioral adaptation (61) (Figure 6). A timed orbital shaker has also been used to induce SF. In one study exemplifying this technique, cages were shaken at 100 rpm for 30 seconds every 2 minutes (62). Commercial SF systems have also been developed using these principles (no. 8400-rotating platform [Pinnacle Technology, Lawrence, KS]; no. 80390—sweeper device [Lafeyette Instruments, Lafayette, IN]). Consequences of long-term SF have provided a convincing argument that this aspect of OSA is a potent detrimental stimulus. Outcomes after chronic SF have included weight gain and hyperphagia (63), insulin resistance (64), endothelial dysfunction (64), and accelerated tumor growth (65). A disadvantage of these models is that they introduce forced locomotion, which may have independent effects on the outcomes of interest.
Figure 6.
Sleep fragmentation device using a rotating disk. Reprinted with permission from Trammell and colleagues (60).
More sophisticated techniques of SF, not requiring forced animal locomotion, have been developed over the last few years. Optogenetics uses light to activate genetically targeted neurons expressing a light-sensitive receptor. This technique enables precise and timed activation of selective neuron populations without affecting surrounding tissues (66). Adamantidis and colleagues used a fiber optic cable to deliver light directly into the brain of mice, and found that stimulation of hypocretin neurons could induce awakenings in a hypocretin-dependent manner (67). Subsequently, they showed that optic stimulation (once every 60 s) caused frequent, brief arousals, without altering the total sleep time or architecture. After 4 hours of this stimulus, mice exhibited deficits in memory consolidation (68).
Future Directions
Animal models have been pivotal to the understanding of OSA pathogenesis, consequences, and treatment. Recent milestones in this field include the refinement of IH models to simulate OSA in a more clinically realistic manner, recognition that SF imparts severe systemic consequences, and the pursuit of SF models that minimize locomotor stimulation. Beyond OSA research, advances in understanding sleep–wake homeostasis are also becoming possible with tools such as optogenetics.
The rising prevalence of OSA and increasing evidence of its association with cardiometabolic diseases demands further research to address the true impact of this disorder. Going forward, we advocate for increasing attention to clinical realism in modeling OSA. First, IH laboratories should attempt to standardize protocols using clinically realistic conditions, and detailed parameters of oxyhemoglobin profile should be provided in all experiments. Second, unrecognized cold stress at usual laboratory temperatures affects all aspects of rodent research (69), and is especially problematic for sleep (70) and hypoxia (54) research. Thermoneutrality should be maintained in sleep experiments, unless cold stress is deliberately being examined as an additional variable. Third, there is growing recognition that OSA is a complex syndrome caused by multiple factors. New methods of monitoring sleeping mice should be leveraged to understand how the interplay of Pcrit, arousal threshold, loop gain, and muscle responsiveness lead to OSA. Rodents are uniquely suited to this type of work, because invasive or genetic interventions can be used to probe each of these variables. Finally, as novel approaches to inducing sleep arousal and SF become possible without forced locomotion, the complex interplay between metabolic health and sleep (71) may become clearer. The history of OSA research in animals has been fruitful, but the field is poised for even greater discoveries.
Footnotes
Author Contributions: S.C., V.Y.P., and J.C.J. contributed equally to drafting the manuscript for intellectual content.
Originally Published in Press as DOI: 10.1165/rcmb.2015-0218TR on October 8, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Young T, Palta M, Dempsey J, Peppard PE, Nieto FJ, Hla KM. Burden of sleep apnea: rationale, design, and major findings of the Wisconsin Sleep Cohort study. WMJ. 2009;108:246–249. [PMC free article] [PubMed] [Google Scholar]
- 2.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Report of an American Academy of Sleep Medicine Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 4.Smith PL, Wise RA, Gold AR, Schwartz AR, Permutt S. Upper airway pressure–flow relationships in obstructive sleep apnea. J Appl Physiol (1985) 1988;64:789–795. doi: 10.1152/jappl.1988.64.2.789. [DOI] [PubMed] [Google Scholar]
- 5.Boudewyns A, Schwartz AR, Van de Heyning PH. Upper airway collapsibility: measurement techniques and therapeutic implications. Acta Otorhinolaryngol Belg. 2002;56:121–125. [PubMed] [Google Scholar]
- 6.Owens RL, Edwards BA, Eckert DJ, Jordan AS, Sands SA, Malhotra A, White DP, Loring SH, Butler JP, Wellman A. An integrative model of physiological traits can be used to predict obstructive sleep apnea and response to non positive airway pressure therapy. Sleep. 2015;38:961–970. doi: 10.5665/sleep.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye L, Pien GW, Ratcliffe SJ, Björnsdottir E, Arnardottir ES, Pack AI, Benediktsdottir B, Gislason T. The different clinical faces of obstructive sleep apnoea: a cluster analysis. Eur Respir J. 2014;44:1600–1607. doi: 10.1183/09031936.00032314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gastaut H, Tassinari CA, Duron B. Polygraphic study of diurnal and nocturnal (hypnic and respiratory) episodal manifestations of Pickwick syndrome [Article in French] Rev Neurol (Paris) 1965;112:568–579. [PubMed] [Google Scholar]
- 9.Jung R, Kuhlo W. Neurophysiological studies of abnormal night sleep and the Pickwickian syndrome. Prog Brain Res. 1965;18:140–159. doi: 10.1016/s0079-6123(08)63590-6. [DOI] [PubMed] [Google Scholar]
- 10.Hendricks JC, Kline LR, Kovalski RJ, O’Brien JA, Morrison AR, Pack AI. The English bulldog: a natural model of sleep-disordered breathing. J Appl Physiol (1985) 1987;63:1344–1350. doi: 10.1152/jappl.1987.63.4.1344. [DOI] [PubMed] [Google Scholar]
- 11.Lonergan RP, III, Ware JC, Atkinson RL, Winter WC, Suratt PM. Sleep apnea in obese miniature pigs. J Appl Physiol (1985) 1998;84:531–536. doi: 10.1152/jappl.1998.84.2.531. [DOI] [PubMed] [Google Scholar]
- 12.Brennick MJ, Pickup S, Cater JR, Kuna ST. Phasic respiratory pharyngeal mechanics by magnetic resonance imaging in lean and obese zucker rats. Am J Respir Crit Care Med. 2006;173:1031–1037. doi: 10.1164/rccm.200505-705OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hernandez AB, Kirkness JP, Smith PL, Shneider H, Polotsky M, Richardson RA, Hernandez WC, Schwartz AR. Novel whole body plethysmography system for the continuous characterization of sleep and breathing in a mouse. J Appl Physiol. 2012;112:671–680. doi: 10.1152/japplphysiol.00818.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Polotsky M, Elsayed-Ahmed AS, Pichard L, Harris CC, Smith PL, Schneider H, Kirkness JP, Polotsky V, Schwartz AR. Effects of leptin and obesity on the upper airway function. J Appl Physiol. 2012;112:1637–1643. doi: 10.1152/japplphysiol.01222.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks D, Horner RL, Kozar LF, Render-Teixeira CL, Phillipson EA. Obstructive sleep apnea as a cause of systemic hypertension. Evidence from a canine model. J Clin Invest. 1997;99:106–109. doi: 10.1172/JCI119120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fewell JE, Williams BJ, Szabo JS, Taylor BJ. Influence of repeated upper airway obstruction on the arousal and cardiopulmonary response to upper airway obstruction in lambs. Pediatr Res. 1988;23:191–195. doi: 10.1203/00006450-198802000-00013. [DOI] [PubMed] [Google Scholar]
- 17.White SG, Fletcher EC, Miller CC., 3rd Acute systemic blood pressure elevation in obstructive and nonobstructive breath hold in primates. J Applied Physiol. 1995;79:324–330. doi: 10.1152/jappl.1995.79.1.324. [DOI] [PubMed] [Google Scholar]
- 18.Launois SH, Averill N, Abraham JH, Kirby DA, Weiss JW. Cardiovascular responses to nonrespiratory and respiratory arousals in a porcine model. J Appl Physiol (1985) 2001;90:114–120. doi: 10.1152/jappl.2001.90.1.114. [DOI] [PubMed] [Google Scholar]
- 19.Pinto JM, Garpestad E, Weiss JW, Bergau DM, Kirby DA. Hemodynamic changes associated with obstructive sleep apnea followed by arousal in a porcine model. J Appl Physiol (1985) 1993;75:1439–1443. doi: 10.1152/jappl.1993.75.4.1439. [DOI] [PubMed] [Google Scholar]
- 20.Nácher M, Serrano-Mollar A, Farré R, Panés J, Seguí J, Montserrat JM. Recurrent obstructive apneas trigger early systemic inflammation in a rat model of sleep apnea. Respir Physiol Neurobiol. 2007;155:93–96. doi: 10.1016/j.resp.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Farre R, Rotger M, Montserrat JM, Calero G, Navajas D. Collapsible upper airway segment to study the obstructive sleep apnea/hypopnea syndrome in rats. Resp Physiol Neurobi. 2003;136:199–209. doi: 10.1016/s1569-9048(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 22.Philip P, Gross CE, Taillard J, Bioulac B, Guilleminault C. An animal model of a spontaneously reversible obstructive sleep apnea syndrome in the monkey. Neurobiol Dis. 2005;20:428–431. doi: 10.1016/j.nbd.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 23.O’Donnell CP, Ayuse T, King ED, Schwartz AR, Smith PL, Robotham JL. Airway obstruction during sleep increases blood pressure without arousal. J Appl Physiol (1985) 1996;80:773–781. doi: 10.1152/jappl.1996.80.3.773. [DOI] [PubMed] [Google Scholar]
- 24.Fava C, Dorigoni S, Dalle Vedove F, Danese E, Montagnana M, Guidi GC, Narkiewicz K, Minuz P. Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta-analysis. Chest. 2014;145:762–771. doi: 10.1378/chest.13-1115. [DOI] [PubMed] [Google Scholar]
- 25.Schein AS, Kerkhoff AC, Coronel CC, Plentz RD, Sbruzzi G. Continuous positive airway pressure reduces blood pressure in patients with obstructive sleep apnea; a systematic review and meta-analysis with 1000 patients. J Hypertens. 2014;32:1762–1773. doi: 10.1097/HJH.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 26.Sahin M, Durand DM, Haxhiu MA. Closed-loop stimulation of hypoglossal nerve in a dog model of upper airway obstruction. IEEE Trans Biomed Eng. 2000;47:919–925. doi: 10.1109/10.846686. [DOI] [PubMed] [Google Scholar]
- 27.Nour SG, Lewin JS, Gutman M, Hillenbrand C, Wacker FK, Wong JW, Mitchell IC, Armstrong CB, Hashim MM, Duerk JL, et al. Percutaneous MR imaging–guided radiofrequency interstitial thermal ablation of tongue base in porcine models: implications for obstructive sleep apnea syndrome. Radiology. 2004;230:359–368. doi: 10.1148/radiol.2302021056. [DOI] [PubMed] [Google Scholar]
- 28.Fletcher EC, Lesske J, Qian W, Miller CC, III, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992;19:555–561. doi: 10.1161/01.hyp.19.6.555. [DOI] [PubMed] [Google Scholar]
- 29.Hamrahi H, Stephenson R, Mahamed S, Liao KS, Horner RL. Selected Contribution: Regulation of sleep-wake states in response to intermittent hypoxic stimuli applied only in sleep. J Appl Physiol (1985) 2001;90:2490–2501. doi: 10.1152/jappl.2001.90.6.2490. [DOI] [PubMed] [Google Scholar]
- 30.Mesarwi OA, Sharma EV, Jun JC, Polotsky VY. Metabolic dysfunction in obstructive sleep apnea: A critical examination of underlying mechanisms. Sleep Biol Rhythms. 2014;13:2–17. doi: 10.1111/sbr.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prabhakar NR, Peng YJ, Kumar GK, Nanduri J, Di Giulio C, Lahiri S. Long-term regulation of carotid body function: acclimatization and adaptation–invited article. Adv Exp Med Biol. 2009;648:307–317. doi: 10.1007/978-90-481-2259-2_35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Savransky V, Nanayakkara A, Li J, Bevans S, Smith PL, Rodriguez A, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis. Am J Respir Crit Care Med. 2007;175:1290–1297. doi: 10.1164/rccm.200612-1771OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jun J, Reinke C, Bedja D, Berkowitz D, Bevans-Fonti S, Li J, Barouch LA, Gabrielson K, Polotsky VY. Effect of intermittent hypoxia on atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis. 2010;209:381–386. doi: 10.1016/j.atherosclerosis.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jun JC, Shin MK, Devera R, Yao Q, Mesarwi O, Bevans-Fonti S, Polotsky VY. Intermittent hypoxia-induced glucose intolerance is abolished by α-adrenergic blockade or adrenal medullectomy. Am J Physiol Endocrinol Metab. 2014;307:E1073–E1083. doi: 10.1152/ajpendo.00373.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dumitrascu R, Heitmann J, Seeger W, Weissmann N, Schulz R. Obstructive sleep apnea, oxidative stress and cardiovascular disease: lessons from animal studies. Oxid Med Cell Longev. 2013;2013:234631. doi: 10.1155/2013/234631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang SX, Wang Y, Gozal D. Pathological consequences of intermittent hypoxia in the central nervous system. Compr Physiol. 2012;2:1767–1777. doi: 10.1002/cphy.c100060. [DOI] [PubMed] [Google Scholar]
- 37.Yao Q, Shin MK, Jun JC, Hernandez KL, Aggarwal NR, Mock JR, Gay J, Drager LF, Polotsky VY. Effect of chronic intermittent hypoxia on triglyceride uptake in different tissues. J Lipid Res. 2013;54:1058–1065. doi: 10.1194/jlr.M034272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drager LF, Li J, Shin MK, Reinke C, Aggarwal NR, Jun JC, Bevans-Fonti S, Sztalryd C, O’Byrne SM, Kroupa O, et al. Intermittent hypoxia inhibits clearance of triglyceride-rich lipoproteins and inactivates adipose lipoprotein lipase in a mouse model of sleep apnoea. Eur Heart J. 2012;33:783–790. doi: 10.1093/eurheartj/ehr097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drager LF, Li J, Reinke C, Bevans-Fonti S, Jun JC, Polotsky VY. Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obesity. 2011;19:2167–2174. doi: 10.1038/oby.2011.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jun J, Savransky V, Nanayakkara A, Bevans S, Li J, Smith PL, Polotsky VY. Intermittent hypoxia has organ-specific effects on oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1274–R1281. doi: 10.1152/ajpregu.90346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Drager LF, Yao Q, Hernandez KL, Shin MK, Bevans-Fonti S, Gay J, Sussan TE, Jun JC, Myers AC, Olivecrona G, et al. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin-like 4. Am J Respir Crit Care Med. 2013;188:240–248. doi: 10.1164/rccm.201209-1688OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1α deficiency impairs carotid body–mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Semenza GL, Prabhakar NR. HIF-1–dependent respiratory, cardiovascular, and redox responses to chronic intermittent hypoxia. Antioxid Redox Signal. 2007;9:1391–1396. doi: 10.1089/ars.2007.1691. [DOI] [PubMed] [Google Scholar]
- 44.Foster GE, Poulin MJ, Hanly PJ. Intermittent hypoxia and vascular function: implications for obstructive sleep apnoea. Exp Physiol. 2007;92:51–65. doi: 10.1113/expphysiol.2006.035204. [DOI] [PubMed] [Google Scholar]
- 45.Reinke C, Bevans-Fonti S, Drager LF, Shin MK, Polotsky VY. Effects of different acute hypoxic regimens on tissue oxygen profiles and metabolic outcomes. J Appl Physiol (1985) 2011;111:881–890. doi: 10.1152/japplphysiol.00492.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee EJ, Woodske ME, Zou B, O'Donnell CP. Dynamic arterial blood gas analysis in conscious, unrestrained C57BL/6J mice during exposure to intermittent hypoxia. J Appli Physiol. 2009;107:290–294. doi: 10.1152/japplphysiol.91255.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Almendros I, Farre R, Planas AM, Torres M, Bonsignore MR, Navajas D, Montserrat JM. Tissue oxygenation in brain, muscle, and fat in a rat model of sleep apnea: differential effect of obstructive apneas and intermittent hypoxia. Sleep. 2011;34:1127–1133. doi: 10.5665/SLEEP.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chodzyński KJ, Conotte S, Vanhamme L, Van Antwerpen P, Kerkhofs M, Legros JL, Vanhaeverbeek M, Van Meerhaeghe A, Coussement G, Boudjeltia KZ, et al. A new device to mimic intermittent hypoxia in mice. PLoS One. 2013;8:e59973. doi: 10.1371/journal.pone.0059973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lim DC, Brady DC, Po P, Chuang LP, Marcondes L, Kim EY, Keenan BT, Guo X, Maislin G, Galante RJ, et al. Simulating obstructive sleep apnea patients' oxygenation characteristics into a mouse model of cyclical intermittent hypoxia. J Appl Physiol. 2015;118:544–557. doi: 10.1152/japplphysiol.00629.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ayappa I, Berger KI, Norman RG, Oppenheimer BW, Rapoport DM, Goldring RM. Hypercapnia and ventilatory periodicity in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2002;166:1112–1115. doi: 10.1164/rccm.200203-212OC. [DOI] [PubMed] [Google Scholar]
- 51.McGuire M, Bradford A. Chronic intermittent hypercapnic hypoxia increases pulmonary arterial pressure and haematocrit in rats. Eur Respir J. 2001;18:279–285. doi: 10.1183/09031936.01.00078801. [DOI] [PubMed] [Google Scholar]
- 52.Dergacheva O, Dyavanapalli J, Piñol RA, Mendelowitz D. Chronic intermittent hypoxia and hypercapnia inhibit the hypothalamic paraventricular nucleus neurotransmission to parasympathetic cardiac neurons in the brain stem. Hypertension. 2014;64:597–603. doi: 10.1161/HYPERTENSIONAHA.114.03603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hill JR. The oxygen consumption of new-born and adult mammals. Its dependence on the oxygen tension in the inspired air and on the environmental temperature. J Physiol. 1959;149:346–373. doi: 10.1113/jphysiol.1959.sp006344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jun JC, Shin MK, Yao Q, Devera R, Fonti-Bevans S, Polotsky VY. Thermoneutrality modifies the impact of hypoxia on lipid metabolism. Am J Physiol Endocrinol Metab. 2013;304:E424–E435. doi: 10.1152/ajpendo.00515.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bergmann BM, Kushida CA, Everson CA, Gilliland MA, Obermeyer W, Rechtschaffen A. Sleep deprivation in the rat: II. Methodology. Sleep. 1989;12:5–12. doi: 10.1093/sleep/12.1.5. [DOI] [PubMed] [Google Scholar]
- 56.McCoy JG, Tartar JL, Bebis AC, Ward CP, McKenna JT, Baxter MG, McGaughy J, McCarley RW, Strecker RE. Experimental sleep fragmentation impairs attentional set-shifting in rats. Sleep. 2007;30:52–60. doi: 10.1093/sleep/30.1.52. [DOI] [PubMed] [Google Scholar]
- 57.Franken P, Dijk DJ, Tobler I, Borbély AA. Sleep deprivation in rats: effects on EEG power spectra, vigilance states, and cortical temperature. Am J Physiol. 1991;261:R198–R208. doi: 10.1152/ajpregu.1991.261.1.R198. [DOI] [PubMed] [Google Scholar]
- 58.Mendelson WB, Guthrie RD, Frederick G, Wyatt RJ. The flower pot technique of rapid eye movement (REM) sleep deprivation. Pharmacol Biochem Behav. 1974;2:553–556. doi: 10.1016/0091-3057(74)90018-5. [DOI] [PubMed] [Google Scholar]
- 59.Arrigoni E, Lu J, Vetrivelan R, Saper CB. Long-term synaptic plasticity is impaired in rats with lesions of the ventrolateral preoptic nucleus. Eur J Neurosci. 2009;30:2112–2120. doi: 10.1111/j.1460-9568.2009.07001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trammell RA, Verhulst S, Toth LA. Effects of sleep fragmentation on sleep and markers of inflammation in mice. Comp Med. 2014;64:13–24. [PMC free article] [PubMed] [Google Scholar]
- 61.Ringgold KM, Barf RP, George A, Sutton BC, Opp MR. Prolonged sleep fragmentation of mice exacerbates febrile responses to lipopolysaccharide. J Neurosci Methods. 2013;219:104–112. doi: 10.1016/j.jneumeth.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sinton CM, Kovakkattu D, Friese RS. Validation of a novel method to interrupt sleep in the mouse. J Neurosci Methods. 2009;184:71–78. doi: 10.1016/j.jneumeth.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Carreras A, Lee S, Hakim F, Zhang SX, Nair D, Ye H, Gozal D. Chronic sleep fragmentation promotes obesity in young adult mice. Obesity. 2014;22:758–762. doi: 10.1002/oby.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang SX, Khalyfa A, Wang Y, Carreras A, Hakim F, Neel BA, Brady MJ, Qiao Z, Hirotsu C, Gozal D. Sleep fragmentation promotes NADPH oxidase 2–mediated adipose tissue inflammation leading to insulin resistance in mice. Int J Obes. 2014;38:619–624. doi: 10.1038/ijo.2013.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hakim F, Wang Y, Zhang SX, Zheng J, Yolcu ES, Carreras A, Khalyfa A, Shirwan H, Almendros I, Gozal D. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and TLR4 signaling. Cancer Res. 2014;74:1329–1337. doi: 10.1158/0008-5472.CAN-13-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Lecea L. Optogenetic control of hypocretin (orexin) neurons and arousal circuits. Curr Top Behav Neurosci. 2015;25:367–378. doi: 10.1007/7854_2014_364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rolls A, Colas D, Adamantidis A, Carter M, Lanre-Amos T, Heller HC, de Lecea L. Optogenetic disruption of sleep continuity impairs memory consolidation. Proc Natl Acad Sci USA. 2011;108:13305–13310. doi: 10.1073/pnas.1015633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maloney SK, Fuller A, Mitchell D, Gordon C, Overton JM. Translating animal model research: does it matter that our rodents are cold? Physiology (Bethesda) 2014;29:413–420. doi: 10.1152/physiol.00029.2014. [DOI] [PubMed] [Google Scholar]
- 70.Lo Martire V, Silvani A, Bastianini S, Berteotti C, Zoccoli G. Effects of ambient temperature on sleep and cardiovascular regulation in mice: the role of hypocretin/orexin neurons. PLoS One. 2012;7:e47032. doi: 10.1371/journal.pone.0047032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jun JC, Polotsky VY. Sleep and sleep loss: an energy paradox? Sleep. 2012;35:1447–1448. doi: 10.5665/sleep.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]