Abstract
The lung is enveloped by a layer of specialized epithelium, the pulmonary mesothelium. In other organs, mesothelial cells undergo epithelial–mesenchymal transition and contribute to organ stromal cells. The contribution of pulmonary mesothelial cells (PMCs) to the developing lung has been evaluated with differing conclusions. PMCs have also been indirectly implicated in lung fibrosis in the progressive, fatal lung disease idiopathic pulmonary fibrosis. We used fetal or postnatal genetic pulse labeling of PMCs to assess their fate in murine development, normal lung homeostasis, and models of pulmonary fibrosis. We found that most fetal PMC-derived mesenchymal cells (PMCDCs) expressed markers of pericytes and fibroblasts, only a small minority expressed smooth muscle markers, and none expressed endothelial cell markers. Postnatal PMCs did not contribute to lung mesenchyme during normal lung homeostasis or in models of lung fibrosis. However, fetal PMCDCs were abundant and actively proliferating within fibrotic regions in lung fibrosis models, suggesting that they actively participate in the fibrotic process. These data clarify the role of fetal and postnatal PMCDCs in lung development and disease.
Keywords: idiopathic pulmonary fibrosis, pulmonary mesothelium, epithelial–mesenchymal transition
Clinical Relevance
This article shows that the fetal pulmonary mesothelium contributes to mesenchymal cells of the adult lung. These mesenchymal cells form fibroblasts and myofibroblasts that activate and expand during lung fibrosis.
Fibrosis is a common component of disease processes of many organs. Fibroblast expansion and activation, to form myofibroblasts, leads to increased deposition of extracellular matrix, which distorts normal organ architecture and function. In the lung, obliterative fibrosis is the hallmark of interstitial lung diseases, the most common of which is idiopathic pulmonary fibrosis (IPF), a progressive, fatal lung disease that affects approximately 0.04% of the population (1). The origin of fibroblasts and myofibroblasts that secrete extracellular matrix proteins in interstitial lung diseases, such as IPF, is incompletely defined. Because the origin of these lineages cannot be definitively evaluated in human IPF, investigators have turned to animal models, which mimic some, but not all, features of the human disease (2).
Among the proposed sources has been the mesothelial lining of the lung (3). The mesothelium is a polarized epithelial sheet that covers the surface of most visceral organs and is required for their normal development. During organ development, the mesothelium undergoes epithelial–mesenchymal transition (EMT) to form mesenchymal cells that contribute to organogenesis (4, 5). Some of these mesenchymal cells retain their plasticity and become resident mesenchymal stem cells (6), whereas others differentiate into stromal cell types, such as fibroblasts, smooth muscle cells (SMCs), and endothelial cells (ECs) (4, 5, 7). This EMT process becomes quiescent at the completion of organogenesis, but can be reactivated by disease processes. For example, myocardial infarction partially reactivates EMT and causes thickening of the cardiac mesothelium (known as the epicardium), resulting in formation of fibroblasts, myofibroblasts, and SMCs that remain within the thickened epicardium and contribute to the myocardial injury response (8).
The developing lung is also enveloped within a mesothelial lining, the visceral pleura, composed of pulmonary mesothelial cells (PMCs). These cells, like other mesothelial cells, express the transcription factor, Wilms’ tumor 1 (Wt1), and Wt1-driven expression of constitutive Cre or inducible CreERT2 alleles has been used to trace the fate of PMCs during lung development, postnatal lung homeostasis, and lung disease, with conflicting results (3, 9–11). During lung development, one study using a constitutive Wt1-Cre transgene reported that PMCs contribute to vascular SMCs (11). A second study using a different constitutive Wt1-Cre transgene showed that PMCs contribute to a significant fraction of pulmonary endothelial cells, CD34+ fibroblasts, bronchial SMCs, and tracheal and bronchial cartilage, as well as vascular SMCs (9). A third study used pulse mesothelial labeling of the fetal lung mesothelium to show that its differentiation is restricted to subpopulations of bronchial and vascular SMCs and desmin+ fibroblasts, whereas differentiation into ECs was not reported (10). Thus, although several studies concur that PMCs form mesenchymal cells during lung development via EMT, the fate of these mesenchymal cells is uncertain.
PMCs have also been proposed to contribute to a subset of myofibroblasts in IPF (3). In the early stages of IPF, fibrosis is localized to distal subpleural regions and progresses proximally. Cultured PMCs exposed to transforming growth factor (TGF) β1 (12), a growth factor highly up-regulated in IPF, undergo phenotypic changes consistent with EMT (13). PMCs labeled in adulthood with Wt1CreERT2 appeared to migrate into the submesothelial region in the 4 hours immediately after TGF-β1 stimulation (3). However, these studies did not determine the extent to which PMC-derived fibroblasts or myofibroblasts contribute to lung fibrosis. In this study we assessed the fate of PMC-derived cells during lung development, homeostasis, and lung fibrosis in murine lung fibrosis models.
Materials and Methods
Please see the Materials and Methods in the online supplement for additional details.
Mice
Studies were performed under protocols approved by the Boston Children’s Hospital Institutional Animal Care and Use Committee. Wt1CreERT2, Wt1GFPCre, Rosa26mTmG, and Rosa26fsTdTomato mouse lines (Jackson Labs, Bar Harbor, MA) were described previously (5, 14, 15). Tamoxifen was administered by gavage at 0.12 mg/g body weight (fetal labeling at Embryonic Day [E] 10.5) or 0.1 mg/g body weight (postnatal labeling, on Postnatal Days 4, 5, and 6).
Models of Lung Fibrosis
We induced pulmonary fibrosis by chronic intranasal bleomycin (modified from Ref. 16) or intratracheally administered activated TGF-β1 adenovirus (12). We obtained adenovirus expressing activated TGF-β1 from J. Gauldie (17).
Histology
Lungs were instilled with 4% paraformaldehyde (PFA), fixed overnight, and embedded in optimal cutting temperature medium (OCT). Cryosections were stained with picrosirius-red and fast green. Immunostaining was performed with antibodies listed in the Materials and Methods in the online supplement and imaged using a confocal microscope. At least three mice were observed per group, and, for each mouse, we analyzed at least 10 sections from each of two macroscopically affected lobes.
Results
Fate of PMC-Derived Cells in the Developing Lung
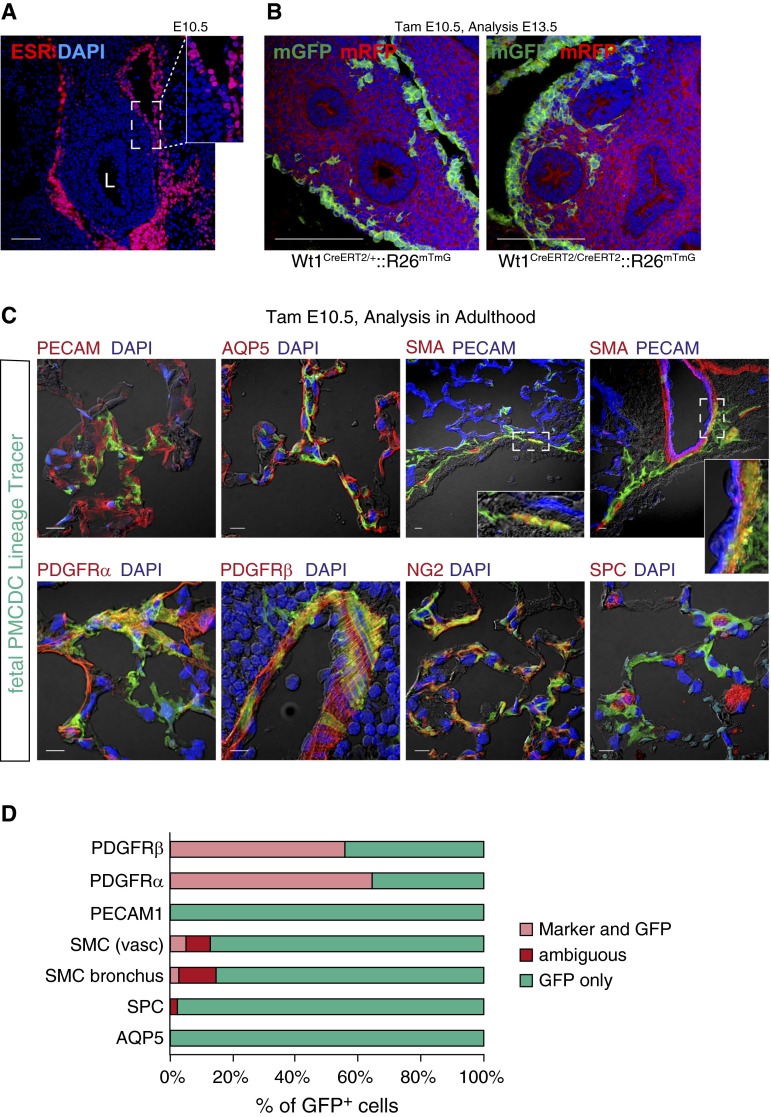
We used Cre-loxP lineage tracing technology to study the fate of PMC-derived cells in the developing lung (see Figure E1A in the online supplement). Consistent with prior reports (3, 9–11), lung WT1 and Wt1-driven Cre expression was confined to the mesothelium (Figure 1A and Figure E1B). Pulse activation of Wt1CreERT2/+ by administration of tamoxifen at E10.5 selectively and indelibly recombined Cre-activated reporter alleles, labeling lung mesothelium and its derivatives (Figures 1B and E1C). We used the highest tamoxifen dose that was compatible with survival of embryos to term, and labeled the large majority (81 ± 1.7%) of mesothelial cells. PMC-derived mesenchymal cells (PMCDCs) were found within the lung mesenchyme (Figure 1B), consistent with having undergone an EMT, as reported previously (9–11). The entry of fetal PMCs into lung mesenchyme was confirmed using cultured fetal lung explants, where mesothelium was selectively labeled using the cell-tracking dye, 5-chloromethylfluorescein diacetate (CMFDA) (Figure E2). In the heart, mesothelial EMT required Wt1 (18). Interestingly, PMCDCs in the lung formed in the absence of Wt1 (Figure 1B, right panel), suggesting that regulation of this process differs between heart and lung.
Figure 1.
Fetal pulmonary mesothelial cells (PMCs) in the developing lung. (A) WT1CreERT2 was selectively expressed in PMCs at Embryonic Day (E) 10.5. ESR is the estrogen receptor, which is fused to Cre in CreERT2. L, lung. (B) Genetic lineage tracing of PMCs and PMC-derived mesenchymal cells (PMCDCs), green fluorescent protein (GFP)-labeled by Wt1CreERT2 activated by tamoxifen at E10.5. PMCDCs were observed within lung mesenchyme at E13.5. Wt1CreERT2/CreERT2 PMCDCs, which lack functional WT1, continued to enter lung mesenchyme. (C) Fate of fetal PMCDCs in the adult lung. PMCDCs were labeled by tamoxifen administration at E10.5. Mice were raised to adulthood, and lungs were examined by immunostaining with markers characteristic of major lung lineages. (D) Quantitation of the fraction of fetally labeled PMCDCs that coexpressed the indicated lineage markers in the adult lung. Scale bar = 100 μm (A and B), 10 μm (C). AQP5, acquaporin 5; DAPI, 4′,6-diamidino-2-phenylindole; ESR, estrogen receptor; mGFP, membrane-localized green fluorescent protein; mRFP, membrane-localized red fluorescent protein; NG2, neuron-glial antigen 2; PDGFR, plateled-derived growth factor receptor; PECAM, platelet endothelial cell adhesion molecule; PMCDC, pulmonary mesothelial cell-derived cells; RFP, red fluorescent protein; SMA, α-smooth muscle actin; SMC, smooth muscle cells; SPC, surfactant protein C; WT1, Wilm's tumor gene 1.
Several studies have examined the fates of PMCDCs, with divergent conclusions (3, 9–11). To re-evaluate this question, we pulse labeled pulmonary mesothelium by administering tamoxifen at E10.5 to Wt1CreERT2/+::R26mTmG embryos. We raised embryos to adulthood and analyzed lungs using confocal microscopy (Figures 1C and E3). Labeled PMCDCs were distributed throughout the mesenchyme of the adult lung, but not in the lung epithelia. PMCDCs, marked by the membrane-localized green fluorescent protein (mGFP) lineage tracer, were quantitatively analyzed for coexpression of differentiation markers of ECs platelet endothelial cell adhesion molecule (PECAM1), SMCs (smooth muscle myosin heavy chain [SM-MHC], alpha smooth muscle actin [SMA], calponin), and lung epithelium (surfactant protein C [SPC], aquaporin 5 [AQP5]; Figures 1C and 1D, Figure E4A). A minority of cells were classified as “ambiguous” because we could not resolve if the PMCDC was expressing the marker or adjacent to the expressing cell (Figure E4B). Lung epithelium did not express the mGFP lineage tracer, although a small fraction of PMCDCs (2.3%) were ambiguous for expression of surfactant protein C (SPC). Although a small fraction of ECs in heart and gut derive from mesothelium, we did not observe this in the lung. A small fraction (∼15%) of mGFP+ cells were intimately related to bronchial or vascular SMCs. In some cases the PMCDCs clearly expressed the SMC lineage marker, but a greater fraction were ambiguous, as we could not resolve coexpression versus adjacency (Figures E4B and 1D). Thus, whereas prior studies reported that pulmonary mesothelium differentiates into subsets of lung endothelial and SMCs, our study shows that the large majority of PMCDCs do not acquire these fates during normal lung development and homeostasis.
We investigated other potential PMC fates using this lineage tracing system. Most mGFP+ cells coexpressed plateled-derived growth factor receptor β (PDGFRβ) or plateled-derived growth factor receptor α (PDGFRα) (Figures 1C and 1D). PDGFRα is expressed by fibroblasts, and, in the heart, the fetal mesothelium (epicardium) is a major source of fibroblasts (7). PDGFRβ is a marker of pericytes, and many mGFP+ cells also coexpressed the pericyte marker, neuron-glial antigen 2 (NG2). PMCDC differentiation into NG2-expressing cells was confirmed with an alternate Cre reporter line, R26fsTdTomato, which expresses red fluorescent protein (RFP) after Cre recombination. Consistent with our observations using R26mTmG reporter, most PMCDCs, marked by RFP, coexpressed pericyte marker NG2, whereas few coexpressed SMA, and none coexpressed platelet endothelial cell adhesion molecule (PECAM) (Figure E5).
Together, our data show that the majority of fetal PMCDCs differentiate into mesenchymal cells that express PDGFRα or PDGFRβ/NG2 in the adult lung, suggestive of their differentiation into fibroblasts and pericytes, respectively.
PMCDCs Do Not Contribute to Lung Parenchyma in the Normal Postnatal Lung
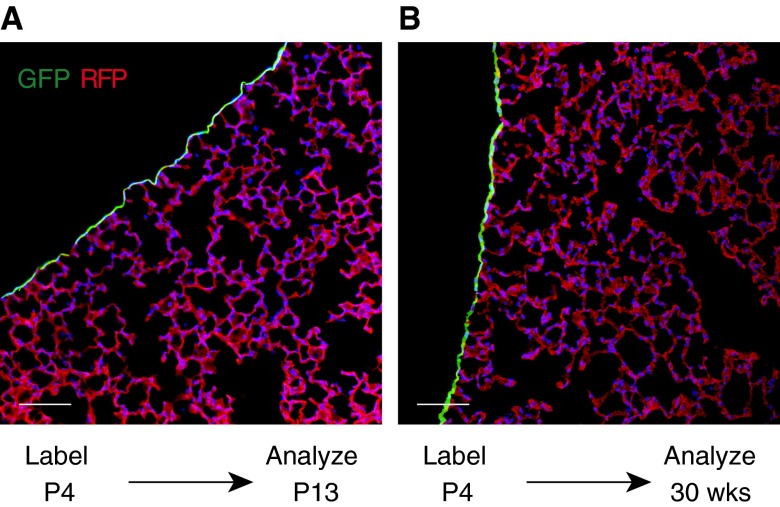
Next, we asked if postnatal PMCs undergo EMT and make cellular contributions to the homeostasis of the postnatal lung. To address this question, we labeled lung mesothelium in newborn Wt1CreERT2/+::Rosa26mTmG mice by administering tamoxifen intragastrically at P4–P6. This selectively and efficiently labeled PMCs (Figure E1C; 88.6 ± 6% of mesothelial cells labeled). We then analyzed the normal mouse lungs at 8–20 weeks. Descendants of neonatal PMCs, genetically labeled by GFP expression, remained confined to the mesothelium (Figure 2). GFP+ cells were not observed within the lung parenchyma, indicating that EMT of PMCs ceases during postnatal life, and is not involved in normal lung homeostasis. However, the mesothelium may still be an important signaling center that regulates postnatal lung maturation and function through paracrine factor secretion, by analogy epicardium in heart development (8, 18).
Figure 2.
PMCs in postnatal lung homeostasis. (A) PMCs were labeled by tamoxifen administration at Postnatal Day (P) 4. Lungs, analyzed at P13, showed selective labeling of PMCs. (B) P4-labeled lungs were analyzed at 30 weeks of life. The PMC label was retained in the mesothelium. Labeled cells were not found within the lung mesenchyme. Scale bar = 100 μm.
Postnatal PMCDCs Do Not Contribute to Lung Parenchyma in Lung Fibrosis Models
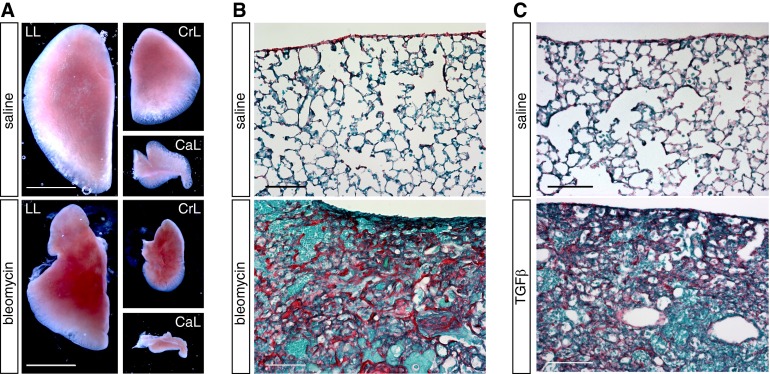
To examine the contribution of adult PMCDCs to pathological lung fibrosis, we used two independent models of lung injury, which recapitulate some features of IPF. First, we used chronic instillation of bleomycin (16). After bleomycin treatment, we observed gross distortion of lung morphology from atelectasis and scarring (Figure 3A), consistent with substantial lung injury. Histological sections revealed “honeycombing” characteristic of pulmonary fibrosis, along with cellular infiltration and collagen deposition (Figure 3B).
Figure 3.
Models of interstitial lung fibrosis. Chronic bleomycin or transforming growth factor (TGF) β1 adenovirus efficiently induced pulmonary fibrosis. (A) Whole-mount images showing atelectasis and scarring in bleomycin-treated lungs compared with saline-treated controls. CaL, caudal lobe; CrL, cranial lobe; LL, left lobe. (B and C) Picrosirius-red staining revealed collagen deposition, cell infiltration, and honeycombing after bleomycin (B) or transforming growth factor-β1 (TGF-β1) (C) treatment. Scale bar = 1 mm (A), 100 μm (B and C).
Second, we used intratracheal activated TGF-β1 adenovirus administration (12, 17). TGF-β1–induced fibrosis may be important for IPF pathogenesis (19). Moreover, TGF-β1 induced EMT of PMCs in culture (13), and stimulated PMCDCs to migrate into the lung in vivo (3). Intratracheal TGF-β1 adenovirus administration robustly stimulated pulmonary fibrosis, scarring, and collagen deposition in TGF-β1 adenovirus–treated lungs (Figure 3).
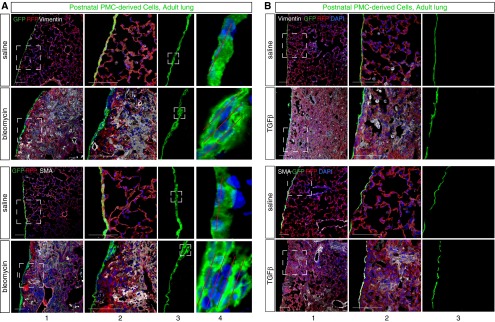
To evaluate the cellular contribution of postnatal PMCs to lung fibrosis in these models, we labeled PMCs before induction of lung injury by treating P4 Wt1CreERT2/+::Rosa26mTmG mice with tamoxifen. When the mice reached adulthood, we established pulmonary fibrosis by bleomycin treatment, and examined the contribution of PMCDCs to the cellular infiltrate found within the parenchyma of the fibrotic lung. Bleomycin-treated lung mesothelium had thickened foci that were multiple cell layers thick (Figure 4A, columns 3–4), reminiscent of, but less pronounced than, epicardial thickening observed after myocardial infarction (8). PMCDCs, marked by GFP expression, were not found within the lung parenchyma, even in areas with extensive infiltration by vimentin+ or SMA+ fibroblasts/myofibroblasts (Figure 4A). In 18 sections examined from lungs of 3 mice, GFP+ cells had detached from the lung mesothelium and migrated into the parenchyma. Thus, in this fibrotic injury model, cells from the postnatal pulmonary mesothelium do not make a significant contribution to the fibroblast/myofibroblast lineage.
Figure 4.
Bleomycin induced infiltration with myofibroblasts but did not stimulate formation of mesenchymal cells from postnatal PMCs. Neonatal administration of tamoxifen labeled postnatal PMCs with GFP. Pulmonary fibrosis was then induced with bleomycin (A) or TGF-β1 (B). Infiltrating myofibroblasts strongly expressing vimentin or SMA did not coexpress GFP. Boxed areas are magnified in adjacent columns. In bleomycin treatment, the mesothelium became focally thickened (boxed areas, [A] column 3). Red lines ([A] column 4) indicate multiple layers of mesothelial cells. Focal mesothelial thickening was not observed with TGF-β1 treatment ([B] column 3). Scale bar = 100 μm.
We evaluated the cellular contribution of postnatal PMCs to lung fibrosis in the independent TGF-β1–induced fibrosis model. As in the bleomycin injury model, postnatal PMCs did not make a cellular contribution to lung fibrosis induced by TGF-β1, including regions with dense infiltration by vimentin+ or SMA+ fibroblasts/myofibroblasts (Figure 4B). Interestingly, TGF-β1 did not induce mesothelial thickening that was observed in the bleomycin injury model (Figure 4B, column 3).
Together, our data indicate that postnatal PMCDCs do not contribute to the cells engaged in pulmonary fibrosis in adult lung injury by bleomycin or TGF-β1.
Fetal PMCDCs Participate in Adult Lung Fibrosis
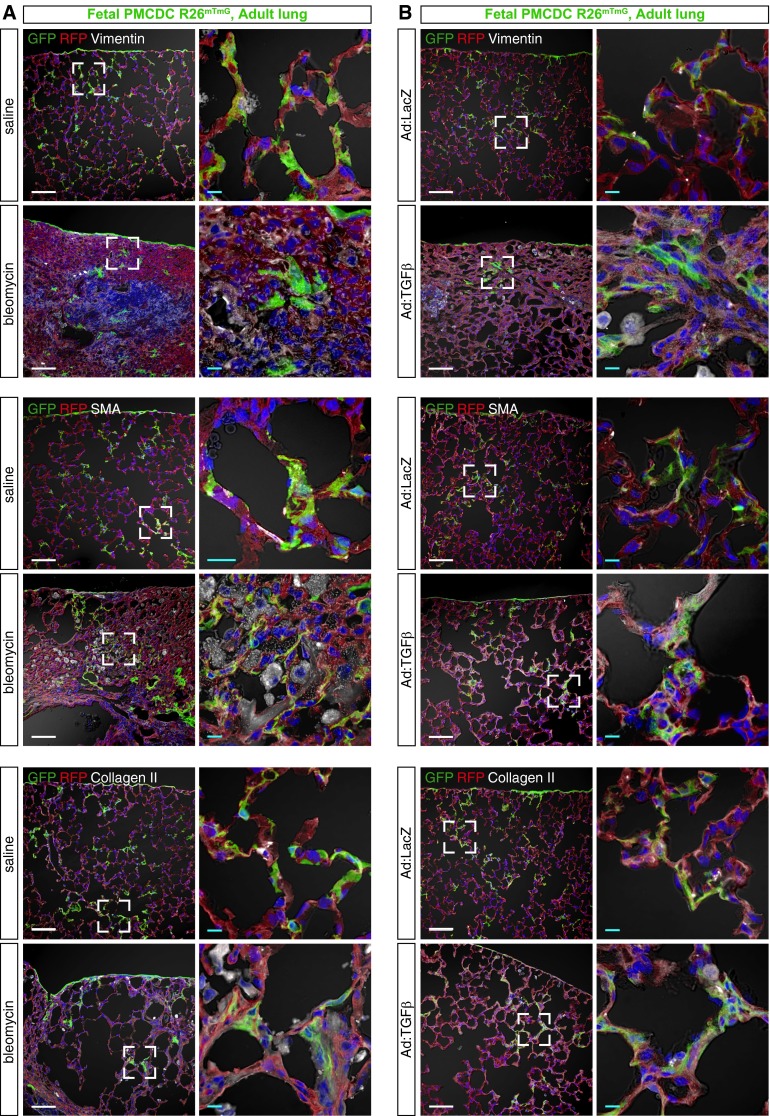
Our data suggested that fetal PMCs differentiate into adult lung mesenchymal cells that express PDGFRα or PDGFRβ. Therefore, we hypothesized that fetal PMCDCs contribute to adult lung fibrosis. We tested this hypothesis by generating adult mice in which fetal PMCDCs were genetically labeled and then subjecting them to bleomycin- or TGF-β1–induced lung injury. We found fetal PMCDCs within areas of lung fibrosis in both injury models, and these PMCDCs expressed vimentin, SMA, and collagen, markers of fibroblasts and myofibroblasts (Figures 5A and 5B and Figure E6). This result was independent of the Cre-activated lineage-tracing allele used, as we observed it using both the Rosa26mTmG (Figures 5 and E6) and Rosa26fsTdTomato reporters (Figure E7). In control experiments, we established that cells are not labeled during normal lung development or after lung injury in the absence of tamoxifen treatment (Figure E8), excluding the possibility that lineage-traced cells arise from “leakiness” of the genetic lineage–tracing system.
Figure 5.
Fetal PMCDCs participate in adult lung fibrosis. Fetal PMCDCs were labeled by treatment of Wt1CreERT2/+::Rosa26mTmG with tamoxifen at E10.5. Adult mice were then treated with bleomycin (A) or TGF-β1 (B) to induce lung fibrosis. Lung cryosections were imaged to identify GFP-labeled PMCDCs that coexpressed markers of fibroblasts and myofibrobasts (SMA, collagen II, and vimentin). Fibrotic regions contained PMCDCs that coexpressed these fibrosis markers. Boxed areas are magnified in adjacent columns. Scale bar = 100 μm (white), 10 μm (teal).
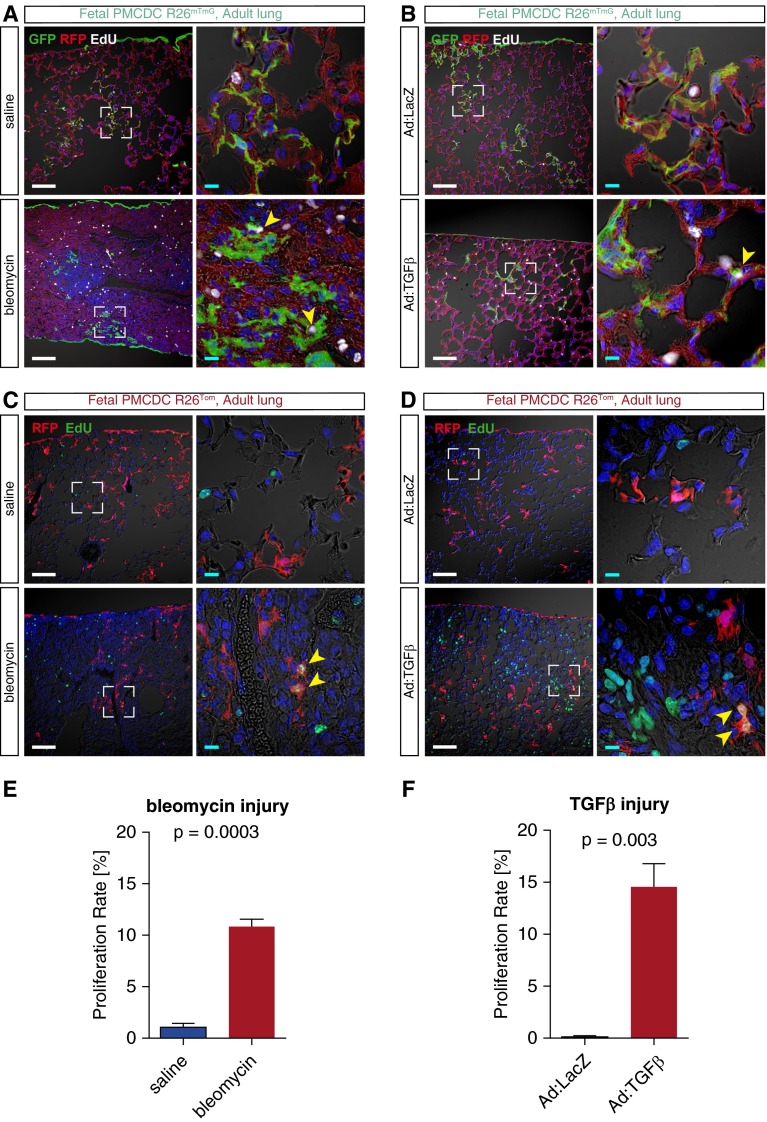
We asked if lung injury stimulates expansion of PMCDCs. After fetal pulse labeling of PMCs and adult lung injury by either bleomycin or TGF-β1, we marked proliferating cells by weekly injection of the S-phase marker, 5-ethynyl-2′-deoxyuridine (EdU). We then measured PMCDC proliferation as the fraction of these cells that incorporated EdU into their DNA. Both bleomycin- and TGF-β1–induced lung fibrosis increased the cell cycle activity of PMCDCs (Figure 6).
Figure 6.
Fibrotic injury stimulated fetal PMCDC proliferation in the adult lung. Wt1CreERT2/+::Rosa26mTmG (A and B) or Wt1CreERT2/+::Rosa26fsTdTomato mice (C and D) were treated with tamoxifen at E10.5. After reaching adulthood, the mice were treated with bleomycin (A and C) or TGF-β1 (B and D) to induce lung fibrosis. Proliferating cells were labeled with 5-ethynyl-2′-deoxyuridine (EdU) and injected intraperitoneally weekly for 8 weeks (bleomycin; A and C) or 4 weeks (TGF-β1; B and D), respectively. Boxed areas are magnified in adjacent columns. Yellow arrowheads highlight colocalization of EdU in genetically labeled PMCDCs (GFP+ in A and B; RFP+ in C and D). Scale bar = 100 μm (white), 10 μm (teal). Quantitation of proliferating PMCDC, data represent means ± SEM (E and F).
Together, these observations show activation and expansion of fetal PMCDCs in pulmonary fibrosis in these models.
Discussion
This study defined the contribution of PMCs and their derivatives to lung mesenchymal cells in development, homeostasis, and fibrotic disease. During lung development, PMCs actively formed mesenchymal cells, consistent with prior studies. However, our lineage-tracing data indicate that the predominant fate of PMCDCs differs from that reported previously (9–11). We show that this process becomes quiescent postnatally, and is not reactivated in two different lung injury and fibrosis models. Our observations exclude a substantial role for PMCs in the formation of lung fibroblasts or myofibroblasts in these disease models, unlike recent reports that reached the contrary conclusion using less definitive lineage-tracing approaches (3, 13).
In the developing lung, PMCs form mesenchymal cells that migrate into the lung parenchyma. Wt1 was dispensable for formation and migration of PMCDCs within the lung, whereas this EMT process required Wt1 in the heart. Sonic hedgehog signaling was previously shown to be required for EMT of PMCs, but not epicardium (10). Together, these studies indicate that mesothelial EMT is regulated through organ-specific mechanisms.
Several studies have come to different conclusions regarding the fate of PMCDCs in the lung (9–11). We evaluated this question using an inducible lineage-tracing system similar to the one used by Dixit and colleagues (10). A common finding of our study and the prior reports is that a minor subset of pulmonary vascular SMCs were labeled by Wt1-driven Cre, indicating that they arise from PMCs. However, PMCs form only a small fraction of pulmonary vascular SMCs, as most of these cells were not labeled by Wt1CreERT2. This situation is unlike the heart, where most vascular SMCs originate from the epicardium (4). Although Que and colleagues (11) did not observe Wt1-driven Cre labeling of bronchial SMCs, we observed, as did Cano and colleagues (9) and Dixit and colleagues (10), that a small subset of bronchial SMCs descended from PMCs. Whether these PMC-derived SMCs have distinct properties is an interesting area for future study. In addition to SMCs, PMCs also contributed to 5–15% of ECs in the heart and gut (4). Cano and colleagues (9) found that a constitutive Wt1-Cre transgene labeled 15–25% of ECs in the lung. However, using the inducible Wt1CreERT2 labeling system, we did not observe significant contribution of fetal PMCDCs to the endothelial lineage. This difference might be due to incomplete labeling of a subset of PMCs that contributes to the endothelial lineage, or it might reflect capture of nonmesothelial (and even endothelial) populations by the constitutive Cre, as discussed previously (8).
In our study, most fetal PMCDCs did not express SMC or EC markers, even when we chased these cells into the adult lung. Rather, a majority of PMCDCs expressed PDGFRβ/NG2 or PDGFRα. PDGFRβ and NG2 are both expressed by pericytes, suggesting that a subset of PMCDCs differentiate into pericytes. However, neither PDGFRβ nor NG2 are specific pericyte markers, and additional experiments will be needed to further define the characteristics of this fetal PMCDC population. PDGFRα is expressed by fibroblasts, and recent studies show that fetal epicardial cells become PDGFRα+ fibroblasts in the adult heart (7). Indeed, we observed that fetal PMCDCs contributed to fibrotic regions during adult pulmonary fibrosis, consistent with their contribution to the resident pulmonary fibroblast lineage. Additional PMCDCs may represent a pulmonary progenitor lineage, akin to mesothelium-derived mesenchymal stem cells in the heart (6).
In the postnatal lung, we did not detect significant ongoing contribution of PMCs to the lung parenchyma. Similarly, epicardium does not significantly form derivatives that migrate within the postnatal myocardium, in either heart homeostasis or injury (7, 8). However, in the heart, manipulation of paracrine signaling pathways promotes amplification, mobilization, and guided differentiation of epicardial progenitors so that they more robustly participate in cardiac repair and regeneration (20). In the future, it will be interesting to determine whether similar interventions could be used to drive PMCs to therapeutically participate in lung injury responses.
It has been proposed that postnatal PMCs contribute to the pathogenesis of pulmonary fibrosis by undergoing EMT to form fibroblasts or myofibroblasts (21). Evidence to support this hypothesis has come largely from analysis of cultured PMCs (13, 22) and from analyses of marker gene expression in histological sections of human IPF samples and murine models (22). More recently, studies pointed to migration of postnatal PMC-derived cells into the lung parenchyma in vivo during a 4 hour observation period after TGF-β1 stimulation (3). However, the extent or long-term contribution of postnatal PMCDCs to the fibroblast/myofibroblast lineages was not evaluated. Here, we show that postnatal PMCDCs make little to no long-term contribution to these lineages in two independent murine lung fibrosis models. This result does not exclude a role of PMCs to participate in the lung injury response through paracrine signaling, as we observed for the epicardium in cardiac injury models (8).
To the extent that the mouse model recapitulates some features of human IPF, our data suggest that EMT of postnatal PMCs is unlikely to significantly contribute to IPF pathogenesis. However, a limitation of our study is that the mouse models of IPF are imperfect, and results cannot be extrapolated with certainty to the human disease.
Footnotes
This work was supported by National Institutes of Health grants R01HL094683 and U01HL100401 (W.T.P.).
Author Contributions: Conception and design—A.v.G., B.Z., and W.T.P.; data collection—A.v.G., S.M.S., L.B.H., J.H.O., C.G., and B.Z.; analysis and interpretation—A.v.G., S.M.S., B.Z., and W.T.P.; drafting the manuscript for important intellectual content—A.v.G. and W.T.P.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0461OC on June 29, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 2.Degryse AL, Lawson WE. Progress toward improving animal models for idiopathic pulmonary fibrosis. Am J Med Sci. 2011;341:444–449. doi: 10.1097/MAJ.0b013e31821aa000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karki S, Surolia R, Hock TD, Guroji P, Zolak JS, Duggal R, Ye T, Thannickal VJ, Antony VB. Wilms’ tumor 1 (Wt1) regulates pleural mesothelial cell plasticity and transition into myofibroblasts in idiopathic pulmonary fibrosis. FASEB J. 2014;28:1122–1131. doi: 10.1096/fj.13-236828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilm B, Ipenberg A, Hastie ND, Burch JB, Bader DM. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development. 2005;132:5317–5328. doi: 10.1242/dev.02141. [DOI] [PubMed] [Google Scholar]
- 5.Zhou B, Ma Q, Rajagopal S, Wu SM, Domian I, Rivera-Feliciano J, Jiang D, von Gise A, Ikeda S, Chien KR, et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature. 2008;454:109–113. doi: 10.1038/nature07060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong JJ, Chandrakanthan V, Xaymardan M, Asli NS, Li J, Ahmed I, Heffernan C, Menon MK, Scarlett CJ, Rashidianfar A, et al. Adult cardiac-resident MSC-like stem cells with a proepicardial origin. Cell Stem Cell. 2011;9:527–540. doi: 10.1016/j.stem.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moore-Morris T, Guimarães-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, Stallcup WB, Gu Y, Dalton ND, Cedenilla M, et al. Resident fibroblast lineages mediate pressure overload–induced cardiac fibrosis. J Clin Invest. 2014;124:2921–2934. doi: 10.1172/JCI74783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou B, Honor LB, He H, Ma Q, Oh JH, Butterfield C, Lin RZ, Melero-Martin JM, Dolmatova E, Duffy HS, et al. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J Clin Invest. 2011;121:1894–1904. doi: 10.1172/JCI45529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cano E, Carmona R, Muñoz-Chápuli R. Wt1-expressing progenitors contribute to multiple tissues in the developing lung. Am J Physiol Lung Cell Mol Physiol. 2013;305:L322–L332. doi: 10.1152/ajplung.00424.2012. [DOI] [PubMed] [Google Scholar]
- 10.Dixit R, Ai X, Fine A. Derivation of lung mesenchymal lineages from the fetal mesothelium requires hedgehog signaling for mesothelial cell entry. Development. 2013;140:4398–4406. doi: 10.1242/dev.098079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Que J, Wilm B, Hasegawa H, Wang F, Bader D, Hogan BL. Mesothelium contributes to vascular smooth muscle and mesenchyme during lung development. Proc Natl Acad Sci USA. 2008;105:16626–16630. doi: 10.1073/pnas.0808649105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonniaud P, Margetts PJ, Kolb M, Schroeder JA, Kapoun AM, Damm D, Murphy A, Chakravarty S, Dugar S, Higgins L, et al. Progressive transforming growth factor beta1-induced lung fibrosis is blocked by an orally active ALK5 kinase inhibitor. Am J Respir Crit Care Med. 2005;171:889–898. doi: 10.1164/rccm.200405-612OC. [DOI] [PubMed] [Google Scholar]
- 13.Nasreen N, Mohammed KA, Mubarak KK, Baz MA, Akindipe OA, Fernandez-Bussy S, Antony VB. Pleural mesothelial cell transformation into myofibroblasts and haptotactic migration in response to TGF-beta1 in vitro. Am J Physiol Lung Cell Mol Physiol. 2009;297:L115–L124. doi: 10.1152/ajplung.90587.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- 15.Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Degryse AL, Tanjore H, Xu XC, Polosukhin VV, Jones BR, McMahon FB, Gleaves LA, Blackwell TS, Lawson WE. Repetitive intratracheal bleomycin models several features of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2010;299:L442–L452. doi: 10.1152/ajplung.00026.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Adenovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Gise A, Zhou B, Honor LB, Ma Q, Petryk A, Pu WT. WT1 regulates epicardial epithelial to mesenchymal transition through β-catenin and retinoic acid signaling pathways. Dev Biol. 2011;356:421–431. doi: 10.1016/j.ydbio.2011.05.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol. 2014;9:157–179. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zangi L, Lui KO, von Gise A, Ma Q, Ebina W, Ptaszek LM, Später D, Xu H, Tabebordbar M, Gorbatov R, et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat Biotechnol. 2013;31:898–907. doi: 10.1038/nbt.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Batra H, Antony VB. The pleural mesothelium in development and disease. Front Physiol. 2014;5:284. doi: 10.3389/fphys.2014.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mubarak KK, Montes-Worboys A, Regev D, Nasreen N, Mohammed KA, Faruqi I, Hensel E, Baz MA, Akindipe OA, Fernandez-Bussy S, et al. Parenchymal trafficking of pleural mesothelial cells in idiopathic pulmonary fibrosis. Eur Respir J. 2012;39:133–140. doi: 10.1183/09031936.00141010. [DOI] [PubMed] [Google Scholar]