Abstract
Exposure to urban particulate matter (UPM) exacerbates asthmatic lung inflammation. Lung dendritic cells (DCs) are critical for stimulating T cell immunity and in maintaining airway tolerance, but they also react to airway UPM. The adjuvant role of UPM in enhancing primary immune responses by naive cells to allergen has been reported, but the direct effects of UPM-activated DCs on the functionality of human memory CD4 T cells (Tms), which constitute the majority of T cells in the lung, has not been investigated. Blood CD1c+ DCs were purified and activated with UPM in the presence or absence of house dust mite or tetanus toxoid control antigen. 5-(and -6)-Carboxyfluorescein diacetate succinimidyl ester–labeled blood Tms were cocultured with autologous DCs, T cell proliferation and effector function were assessed using flow cytometry, and secreted cytokines were measured by combined bead array. UPM-DCs elicited IFN-γ and IL-13 secretion and induced proliferation in Tms isolated from both allergic patients with asthma and healthy control subjects, whereas only IL-13 was produced by Tms from patients with atopic asthma stimulated by house dust mite–loaded DCs. UPM-DCs drove the expansion and differentiation of a mixed population of Th1, Th2, and Th17 cell effectors through a mechanism that was dependent on major histocompatibility class II but not on cytokine-driven expansion. The data suggest that UPM not only has adjuvant properties but is also a source of antigen that stimulates the generation of Th2, Th1, and Th17 effector phenotypes, which have been implicated in both exacerbations of asthma and chronic inflammatory diseases.
Keywords: dendritic cells, memory CD4 T cells, air pollution, cytokines, urban particulate matter
Clinical Relevance
Exposure to particulate air pollution is thought to exacerbate allergic asthma through direct and indirect activation of airway dendritic cells (DCs). This study shows that, instead of enhancing T cell responses to allergen, loading of human DCs with urban particulate matter alone induces resting autologous memory CD4 T cells to expand into Th1, Th2, and Th17 effectors through an antigen-dependent mechanism. Such responses might contribute to the development and exacerbation of asthma.
Asthma is a chronic inflammatory lung disease, which now affects 1 in 12 of the UK population (1). Accumulating epidemiological evidence suggests that environmental factors, particularly the particulate matter (PM) fraction of air pollution, and especially that derived from diesel exhaust particles (DEPs), have a substantial role in contributing to increased asthma rates and in triggering asthma exacerbation (2–4). Recent research has found associations between exposure to PM and severe asthma (5); such patients suffer considerable morbidity, and there is an unmet need for new therapies. Furthermore, exposure to PM is linked with increased risk of chronic obstructive pulmonary disease hospitalization and mortality (6) and suppressed immunity to mycobacterial infections (7).
The typical exhaust particulate, which makes up a large part of urban air pollution, consists of a carbonaceous core coated in metals, sulfates, and polyaromatic hydrocarbons (8) In addition, such particulates have the potential to bind allergens and adsorb LPS (9). In vivo studies have shown that inhaled PM stimulates innate immune responses by a protective layer of specialized airway epithelial cells (AECs). Located between and below this barrier are subsets of dendritic cells (DCs), which select and sequester particulates and pathogens, and are the most potent antigen presenting cells (APCs) in the immune system, being the only APCs capable of eliciting a primary response from naive T cells to neoantigen (10). These are recruited to the lung from the peripheral blood in response to stimuli, including allergen and PM (11, 12).
In vivo, exposure to pure DEPs, which constitute a large proportion of the organic mass of urban PM (UPM), induces maturation of mouse myeloid lung DCs, which express costimulatory molecules, migrate to mediastinal lymph nodes, and stimulate antigen-specific CD4 T cells to secrete proinflammatory cytokines (12). DEPs adsorb LPS and, in a mouse model of asthma, intranasal administration of allergen and low-dose LPS led to predominantly neutrophilic IL-17–mediated lung inflammation (13). Inhalation of DEPs by rats sensitized to allergen induces a mixed lung infiltrate dominated by neutrophils (14). Importantly, allergen-sensitized mice exposed to ambient PM instead of pure DEPs have exacerbated allergic airway inflammation characterized by both IL-17 and type 2 cytokines and a mixed eosinophilic and neutrophilic lung inflammation (15). However, PM alone in the absence of allergen sensitization can induce Th2 and Th17 effectors, which are essential for inducing airway hyperresponsiveness (16). Recently, a subset of Th17-secreting Th2 cells has been reported, with elevated frequency in patients with moderate/severe asthma (17), and has been implicated in more severe forms of neutrophilic asthma (18).
In vitro, DEP-treated human DCs have been reported to induce a Th1 cytokine profile by alloantigen-responder CD4 T cells, which become more Th0/2 when DCs are cocultured with DEP-treated bronchial epithelial cells through a mechanism involving thymic stromal lymphopoietin (19, 20). These DEP-induced Th0/2 effectors were proposed as the mechanism through which DEPs could promote asthma with the primary effect of DEPs being indirect through activation by DEP-induced epithelial-derived cytokines acting on stimulator DCs. However, histological examination of mediastinal lymph node tissue from animals that had undergone intratracheal instillation of DEPs clearly showed DEP-loaded DCs (12). These observations suggest that DEPs may also have a direct effect on lung DCs and on subsequent T cell priming, but no in vitro model with human cells has managed to recapitulate the Th2 and Th17 effector expansion seen in animal models of PM-induced asthma or in patients with severe asthma.
The studies reported here investigate how human DCs, and subsequent T cell priming, are affected by exposure to UPM using National Institute of Standards and Technology SRM 1648a, a standard reference material composed of real-world particulate air pollutants. We previously established that UPM directly matures human CD1c+ myeloid DCs, which are the precursors of DCs that populate the lung mucosa and submucosa, and enhance the maturation induced by granulocyte/macrophage colony–stimulating factor (GM-CSF), a product of UPM-stimulated AECs, that is essential for lung myeloid DC survival and differentiation (21, 22). We found that UPM-activated DCs are capable of priming alloantigen-specific human naive CD4 T cell proliferation, but suppress the capacity of newly primed cells to produce Th1 and Th2 cytokines (23).
We reasoned that, if UPM-activated DCs could affect naive CD4 T cell priming in the lymph node, then they might also have access to antigen-specific central memory T cells (Tcms), which have similar patterns of cell trafficking. Furthermore, UPM-activated DCs might affect the activation of effector memory CD4 T cells (Tems) in lung tissue (24). We hypothesized that the maturation of DCs by UPM would enhance the recall response of Tms to allergen with higher levels of cytokine production and T cell proliferation than by allergen-loaded DCs alone. To address this, we have used CD1c+ DCs from the blood, because these are the precursors of the DCs that migrate to and populate the lung and, once stimulated, migrate on to the lymph nodes. Similarly, we used the total CD4 Tms from the blood, as these contain antigen-specific central and effector memory. Our aims were to determine: (1) whether UPM-loaded DCs enhance the production of proinflammatory cytokines by Tms from patients with atopic asthma in response to allergen; (2) whether UPM-loaded DCs influence the generation of Tm effectors; and (3) the mechanisms through which UPM might exert its adjuvant effect.
Materials and Methods
Donor Cohorts
Patients with mild–moderate asthma with allergy to house dust mite (HDM), as defined by positive skin-prick tests (nine male and seven female; age range, 21–62 years; defined by Global Initiative for Asthma [GINA] guidelines) and healthy control subjects without asthma or atopy (five male and three female; age range, 27–56 years) were recruited after informed consent. The study was approved by the Research Ethics Committee at Guy’s Hospital (London, UK).
Cells and Culture
CD1c+ myeloid DCs (mDCs) were purified using the BDCA-1 DC isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) from Ficoll Hypaque (Nycomed, Oslo, Norway)–separated peripheral blood mononuclear cells (PBMC). DC (purity >99% CD11c+) were cultured overnight at a density of 1.0 × 104/ml in 96-well u-bottomed plates in complete medium (RPMI 1640; Invitrogen, Paisley, UK) supplemented with 10% human AB serum, 2 mM L-glutamine, and 250 μg/ml gentamicin. Myeloid DCs were activated for 18 hours with 5 μg/ml of UPM (23) from a stock of 500 μg/ml of Standard Reference Material 1648a (National Institute of Standards and Technology, Gaithersburg, MD), resuspended in 5% methanol/ultrapure water with or without 50 ng/ml GM-CSF (R&D Systems Ltd, Oxford, UK) in the absence or presence of antigens; HDM (Alk-Abello, Horsholm, Denmark) or Tetanus Toxoid (Calbiochem, Leicester, UK). To neutralize LPS, aliquots of UPM or tetanus toxoid (TT) were each incubated with polymyxin B (Sigma-Aldrich, Dorset, UK) for 60 minutes before adding to DCs (final polymyxin B concentration of 5 μg/ml). In some experiments, DC production of IL-6 was neutralized with anti–human IL-6 (1 μg/ml: R&D Systems) and T-cell receptor (TCR)–major histocompatibility class (MHC) II interactions between DCs and memory T cells were blocked with anti–MHC II DP, DQ, DR (BD Biosciences, Oxford, UK)
Memory CD4 T Cell Purification
CD4+CD45R0+ memory T cells were isolated by negative selection (Miltenyi Biotec) from cryopreserved aliquots of CD1c+- and CD19+-depleted PBMCs. T cell viability was 90% and CD4+CD45R0+ purity was 97–99%.
Flow Cytometric Analysis
DC maturation was assessed by flow cytometry. Myeloid DCs were removed using 2 mM EDTA/1% FCS in Dulbecco’s PBS and stained with FITC-labeled anti-CD80, APC-labeled anti-human leukocyte antigen (HLA)-DR and CD83-phycoerythrin (PE), or respective isotype controls (BD Biosciences) on ice. Flow cytometry was performed with a FACScalibur cytometer (Becton Dickinson, Mountain View, CA) using standard CellQuestPro acquisition software (Mountain View, CA). Cells were gated on forward and 90° side scatter to exclude cell debris.
CFSE Staining
Labeling of T cells with 5-(and -6)-carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen, Paisley, Scotland, UK) was performed as described previously (25).
Autologous Lymphocyte:DC Reaction
Aliquots of 1 × 105 CFSE-labeled memory CD4 T cells were cocultured with 1 × 104 autologous resting or activated DCs in complete media for 5 days before measurement of cell proliferation by flow cytometry and cytokine production in supernatants.
Intracellular Cytokine Staining
The 5-day T cell:DC cocultures were further expanded for 2 days in fresh media and IL-2 (10 μ/ml; MRC NIBSC, Potters Bar, UK) before the effector T cell cytokine profile was assessed after stimulation for 5 hours with 50 ng/ml phorbol-12-myristate-13-acetate (PMA) and 500 ng/ml ionomycin (Sigma-Aldrich). Cytokine secretion was blocked with 5 μg/ml Brefeldin-A (Sigma-Aldrich) during the last 4 hours of stimulation. Cells were surface stained with anti–CD3-PerCP (BD Biosciences) before being fixed and permeabilized using BD PermFix (BD Biosciences) and stained with PE- or APC-conjugated anti–IFN-γ (BD Biosciences) or PE- or APC-conjugated anti–IL-13 (Biolegend, San Diego, CA) antibodies or APC-conjugated anti–IL-17 (e-Bioscience, San Diego, CA).
Cytokine Analysis
Cell supernatants were analyzed for cytokines using Combined Bead Array (CBA) multiplex assays (BD Biosciences).
Statistical Analysis
Statistical analysis was performed with GraphPad Prism 5.0 (La Jolla, CA). Normality was tested by the D’Agostino and Pearson omnibus normality test. Paired and unpaired data were analyzed by the two-tailed paired or unpaired t test, respectively. Groups were analyzed by one-way ANOVA with repeated measures and Bonferroni’s multiple comparison test. Significance of interactions between UPM and HDM was tested by two-way ANOVA with repeated measures and Bonferroni’s multiple comparison test.
Results
UPM-Activated DCs Activate Autologous Memory CD4 T Cells
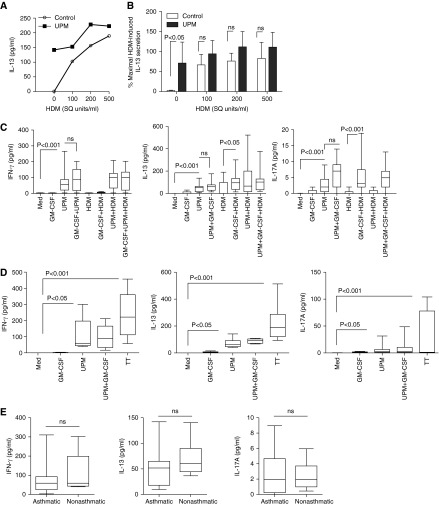
Myeloid DCs line the mucosa of the airway, and previous studies have shown that nasal challenge with DEPs enhances recall allergen-induced type 2 IL-4, -5, -6, and -13 cytokine production by individuals with allergy (26). To test the hypothesis that UPM stimulation of DCs (UPM-DCs) enhances T cell recall responses to allergen, Tms from patients with asthma with allergy to HDM were cocultured with autologous DCs pretreated overnight in the presence or absence of a fixed dose of UPM (previously optimized at 5 μg/ml [23]) with or without HDM (dose range 0–500 standardized-quality [SQ] units/ml). Cells were cultured for 5 days and IL-13 secretion in supernatants was measured by CBA. Figure 1A shows that HDM-loaded DCs induced dose-dependent IL-13 production by autologous Tms. Unexpectedly, DCs loaded with UPM alone also induced Tm IL-13 secretion in quantities equivalent to those induced by allergen (P < 0.05 compared with autologous DCs alone), but stimulation of HDM-DCs with UPM had no significant additive or synergistic effect (Figure 1B).
Figure 1.
Memory CD4 T cells produce proinflammatory cytokines in response to urban particulate matter (UPM)–loaded dendritic cells (DCs) in the presence and absence of antigen. (A) Memory CD4 T cells from donors with atopic asthma were cocultured in complete medium (Med) with autologous CD1c+ DCs loaded with or without house dust mite (HDM) at a T cell:DC ratio of 10:1 in the presence or absence of a fixed dose of UPM (5 μg/ml) for 5 days before supernatants were harvested for IL-13 analysis by Combined Bead Array (CBA). Data are representative of five experiments. (B) Summarized data showing effect of HDM and UPM loading of DCs on memory CD4 T cell (Tms) IL-13 secretion. Data are normalized to percentages of maximal IL-13 secretion induced by HDM alone and are presented as means ± SD (n = 5). Statistical analysis was by two-way ANOVA with repeated measures, followed by Bonferroni’s multiple comparison test (ns, nonsignificant). (C and D) Effect of coactivation with granulocyte/macrophage colony–stimulating factor (GM-CSF) on 5-day IFN-γ, IL-13, and IL-17A cytokine production by Tms from (C) a donor with atopic asthma (n = 10) and (D) a donor without atopy or asthma (n = 6) induced by UPM and HDM or tetanus toxoid (TT)–loaded DCs, respectively. Data are presented as the median, interquartile range (IQR), and 5th and 95th percentiles. Statistical analysis was by one-way ANOVA with repeated measures, followed by Bonferroni’s multiple comparison test. (E) Comparison of IFN-γ, IL-13, and IL-17A cytokine production by Tms from a donor with atopic asthma (n = 10) and (D) a donor without atopy or asthma (n = 6) induced by UPM-DCs. Statistical comparison was by two-tailed unpaired t test. SQ, standardized quality.
We next investigated whether UPM-DC induced Tm production of cytokines other than type 2 cytokines by analyzing production of IFN-γ and IL-17A and whether GM-CSF, a cytokine secreted by AECs that enhances UPM-induced DC maturation (23), enhances Tm responses to UPM-loaded DCs. Data summarized in Figure 1C show that UPM-DCs elicited a mixed inflammatory Tm cytokine profile characterized by high levels of IFN-γ and IL-13 (P < 0.001 compared with medium control), and low levels of IL-17A. Although coactivation of UPM-DCs with GM-CSF enhances DC maturation and subsequent capacity to stimulate allogeneic naive CD4 T cells (23), this did not significantly enhance Tm cytokine production. In accordance with the atopic status of the donors, HDM-DC–induced Tm responses were highly polarized toward Th2, as indicated by IL-13, but not IFN-γ or IL-17A secretion. Activation of HDM-DCs with GM-CSF significantly increased secretion of IL-13 (P < 0.05) compared with HDM-DCs alone and elicited the secretion of IL-17A (P < 0.01), although the latter remained comparatively low. Tms stimulated by HDM-DCs loaded with UPM secreted a mixture of all three cytokines, but there was no significant enhancement of any one cytokine compared with UPM-DCs or HDM-DCs alone.
PBMC from individuals with asthma produce higher levels of inflammatory cytokines in response to DEP stimulation than healthy patients without asthma (27). To investigate whether the stimulatory effect of UPM-DCs on Tm functionality was unique to subjects with asthma or a more generalized phenomenon, we conducted the same experiments on Tms from healthy individuals without atopy or asthma using the recall antigen, TT (1 μg/ml), instead of HDM as a positive control for antigen-specific responses.
Figure 1D shows that UPM-loaded DCs from patients without asthma, like those from subjects with asthma, also induced the secretion of IFN-γ and IL-13 and low quantities of IL-17A. TT antigen–specific Tms had a mixed cytokine profile, with relatively high production of all three cytokines compared with that induced by UPM-DCs, although more variable between individuals. Direct comparison of the amounts of IFN-γ, IL-13, and IL-17A secreted by UPM-DC–activated Tms from patients with asthma and healthy controls without asthma showed no significant difference (Figure 1E).
UPM-Activated DCs Activate Autologous Memory CD4 T Cell Proliferation from Donors with and Those without Asthma
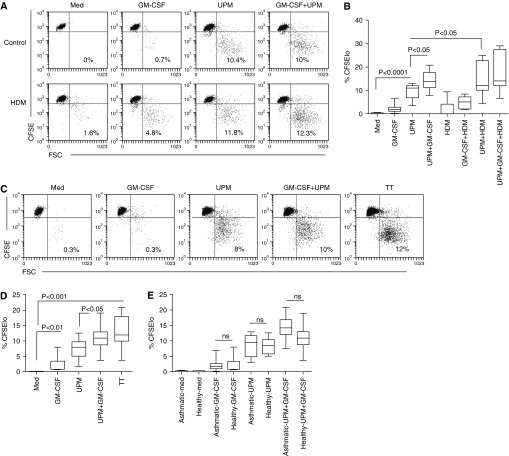
Mature DCs powerfully stimulate resting Tms to undergo antigen-specific proliferation. To determine the effect of UPM stimulation on the capacity of DCs to induce allergen-induced Tm proliferation, CFSE-labeled Tms were cocultured with HDM-loaded DCs (200 SQ units/ml) for 5 days and proliferation was assessed by CFSE dilution. Figure 2A and data summarized in Figure 2B show that UPM-loaded DCs from patients with asthma consistently induced autologous Tm proliferation (P < 0.0001 compared with medium alone), which was 5- to 10-fold higher than that induced by HDM-loaded DCs, and was significantly enhanced by costimulation with GM-CSF (P < 0.05). Under the same conditions, DCs loaded with both UPM and HDM generated significantly greater Tm proliferation than UPM-DCs alone (P < 0.05).
Figure 2.
Memory CD4 T cell proliferation induced by UPM-activated CD1c+ DCs in the presence or absence of GM-CSF or antigen. (A) 5-(and -6)-Carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled CD4+ T cells from donors with atopic asthma were stimulated with UPM-loaded DCs in the presence or absence of GM-CSF or HDM for 5 days at a T cell:DC ratio of 10:1. Values in the plots show the proportion of proliferating cells. Data are representative of 10 experiments. (B) Summarized data in terms of percentages of proliferating (CFSElo) cells are presented as the median, IQR, and 5th and 95th percentiles (n = 10). (C) Proliferative responses of CFSE-labeled CD4+ T cells from donors without asthma or atopy to UPM-DCs in the presence or absence of GM-CSF or TT. Data are representative of six experiments. (D) CFSElo cells are presented as the median, IQR, and 5th and 95th percentiles (n = 6). Statistical analysis was by one-way ANOVA with repeated measures, followed by Bonferroni’s multiple comparison test. (E) Comparison of Tm proliferation by donors with atopic asthma (n = 10) and donors without asthma or atopy (n = 6) induced by UPM-DCs. Statistical comparison was by two-tailed unpaired t test.
In individuals without asthma, Figure 2C and data summarized in Figure 2D show that UPM-DC stimulation alone induced Tm proliferation and that the coactivation of UPM-DCs with GM-CSF induced Tm proliferation almost as strongly under the same conditions as TT-loaded DCs. Comparison of the functional Tm responses from patients with asthma and healthy control subjects without asthma showed similar levels of UPM-DC–induced Tm proliferation (Figure 2E).
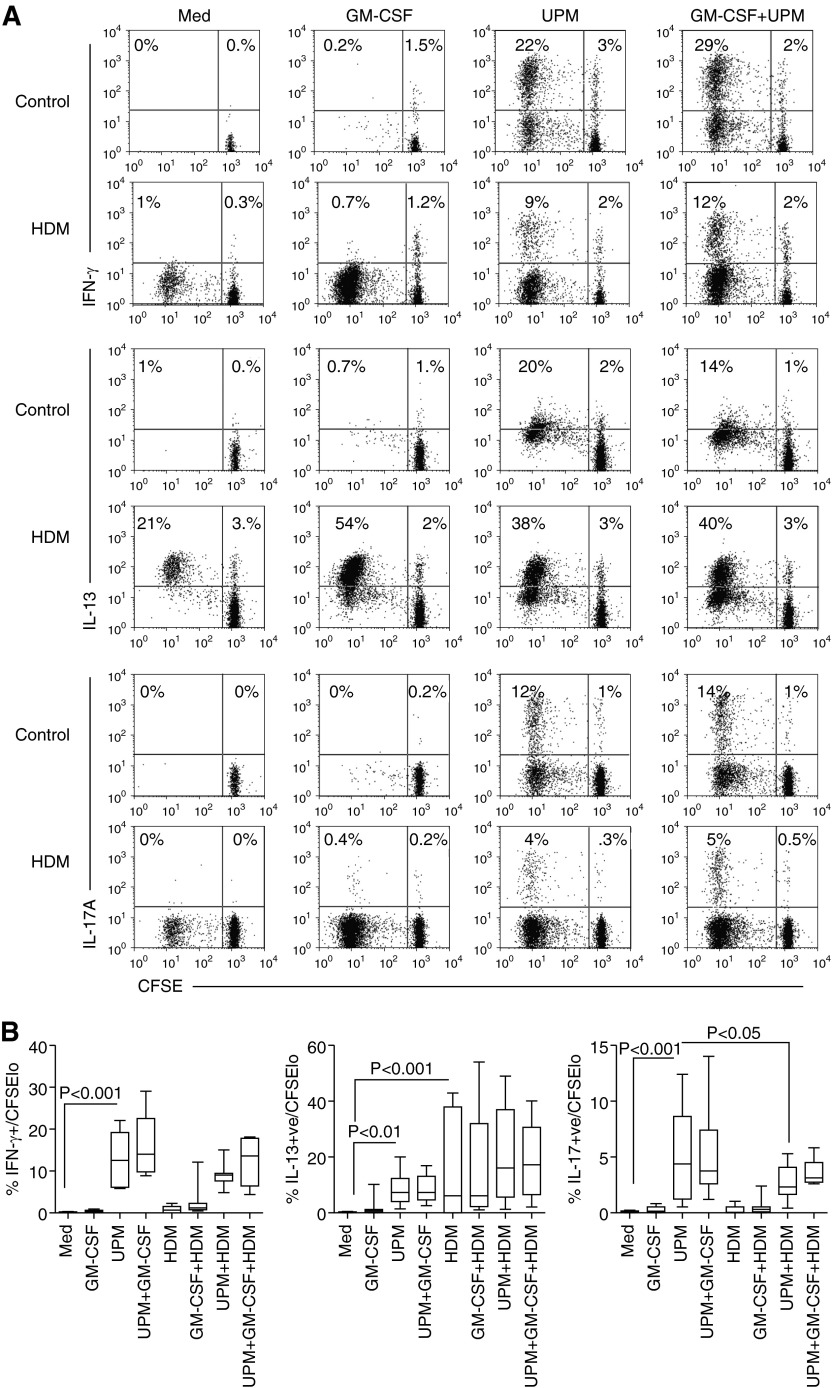
UPM-Activated DCs Induce the Differentiation of Potent Inflammatory Mixed IFN-γ–, IL-13–, and IL-17A–Producing Effectors
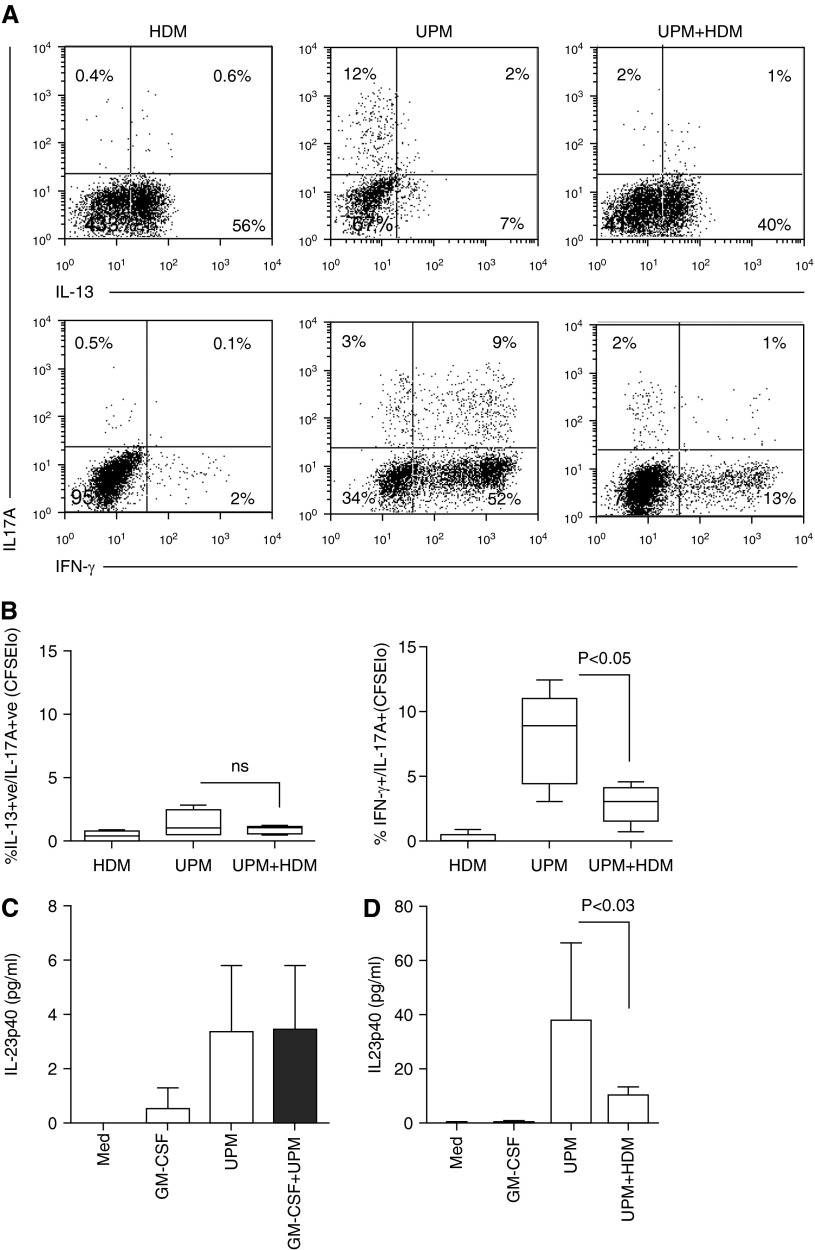
Tms are composed of effector and central memory cells, which possess great plasticity by differentiating in response to inflammatory or tolerizing signals in the microenvironment received either directly by the T cell or by direction from tissue DCs. To determine the proportions of Th1, Th2, and Th17 effector cells generated through stimulation by UPM-DCs, Day-5 cultures were expanded with IL-2 (10 μ/ml) for 2 days and effector phenotype was analyzed by intracellular cytokine staining. The data in Figure 3A and summarized in Figure 3B show that, upon re-stimulation by PMA and ionomycin, a substantial proportion of UPM-DC–stimulated proliferating (CFSElo) Tms from donors with asthma and atopy produced IFN-γ (P < 0.001 compared with control DC stimulation), whereas a lower proportion produced IL-13 (P < 0.01). Strikingly, a fraction of Tms had differentiated to produce high levels of IL-17A (P < 0.001, compared with control DCs alone). In contrast, although highly variable between individuals, the effector profile of HDM-specific Tms was dominated by IL-13–producing cells, with few IFN-γ and IL-17A producers. The effector profile of Tms generated by activation of DCs loaded with a combination of UPM and HDM was a mixture of IFN-γ–, IL-13–, and IL-17A–producing cells, with a significantly reduced frequency of the latter compared with that induced by UPM-DCs alone (P < 0.05).
Figure 3.
Cytokine-producing capacity and frequency of CD4 T cell effectors activated by UPM-DCs from donors with atopic asthma in the presence or absence of GM-CSF or HDM. (A) CFSE-labeled Tms were stimulated with DCs for 5 days before a 2-day expansion with IL-2. Intracellular production of IFN-γ, IL-13, and IL-17A was assessed 5 hours after restimulation with phorbol-12-myristate acetate (PMA) and ionomycin. Values in the upper left quadrant denote the frequency of divided cells that are cytokine +ve (positive). Values in the upper right quadrant denote the frequency of nondividing cells that are cytokine +ve. Data are representative of 10 independent experiments. (B) Summarized data in terms of percentages of total CFSElo-dividing cells producing IFN-γ, IL-13, and IL-17A. Data are presented as the median, IQR, and 5th and 95th percentiles (n = 10). Statistical analysis was by one-way ANOVA with repeated measures, followed by Bonferroni’s multiple comparison test.
Parallel analysis of the capacity of UPM-induced effector Tms to secrete cytokines upon short-term stimulation by PMA and ionomycin (24 hours) was quantified by CBA. Data summarized in Figure E1 in the online supplement suggest that, after further expansion in the presence of IL-2, Tms induced by UPM-DCs with or without HDM had differentiated into potent effectors capable of secreting high concentrations of IFN-γ, IL-13, and, most strikingly, IL-17A, which had been detected at only picogram concentrations after the initial 5 days of UPM-DC stimulation in coculture (Figure 1D).
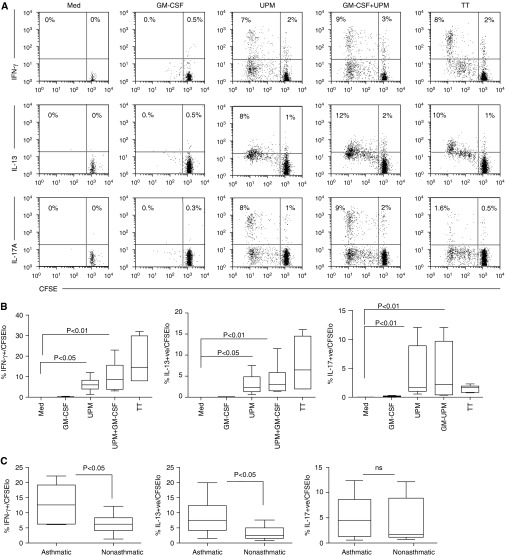
We then investigated whether or not UPM-DC–stimulated Tms from donors without asthma also differentiated into similar effector Tms. Figure 4A and data summarized in Figure 4B show that UPM-DCs from donors without asthma induced significant differentiation of Tm effectors capable of producing IFN-γ (P < 0.05), IL-13 (P < 0.05), and IL-17A (P < 0.01). However, comparison of Tm effector frequencies generated by stimulation with UPM-DCs showed that donors with atopic asthma generated significantly higher frequencies of IFN-γ– (P < 0.05) and IL-13 (P < 0.05) –secreting Tm effectors than patients without asthma, but similar frequencies of IL-17–secreting effectors (Figure 4C).
Figure 4.
Cytokine-producing capacity and frequency of CD4 T cell effectors activated by UPM-DCs from donors without asthma or atopy in the presence or absence of GM-CSF or TT. (A) CFSE-labeled Tms were stimulated with DCs for 5 days before a 2-day expansion with IL-2. Intracellular production of IFN-γ, IL-13, and IL-17A was assessed 5 hours after restimulation with PMA and ionomycin. Values in the upper left quadrant denote the frequencies of divided cells that are cytokine +ve. Values in the upper right quadrant denote the frequency of nondividing cells that are cytokine +ve. Data are representative of six independent experiments. (B) Summarized data in terms of percentages of total CFSElo-dividing cells producing IFN-γ, IL-13, and IL-17A. Data are presented as the median, IQR, and 5th and 95th percentiles (n = 6). Statistical analysis was by one-way ANOVA with repeated measures, followed by Bonferroni’s multiple comparison test. (C) Comparison between donors with atopic asthma (n = 10) and donors without asthma or atopy (n = 6) in frequencies of IFN-γ–, IL-13–, and IL-17A–secreting effector Tms induced by UPM-DCs. Statistical comparison was by two-tailed unpaired t test.
UPM-Activated DCs Induce the Differentiation of IFN-γ+IL-17A+ Coexpressing Effectors
Previous studies have reported that intratracheal DEP instillation, in combination with HDM sensitization, can induce Th17/Th2 coexpressing cells (5, 17). We therefore investigated whether UPM-DCs induce the differentiation of these effectors. Figure 5A and data summarized in Figure 5B show that, in the gated proliferating CFSElo fraction, the UPM-induced effectors were mainly IL-17+ and IL-13+ single secreting cells with few IL-13+/IL-17+ coexpressing cells, whereas HDM-DC–induced Tms were predominantly IL-13+ single secreting cells. In contrast, UPM-DCs induced the differentiation of IFN-γ+/IL-17+ coexpressing cells as well as IL-17 and IFN-γ single secreting cells. The combination of UPM and HDM decreased the proportion of IFN-γ+/IL-17+ coexpressing cells and IFN-γ single secreting cells. IFN-γ+/IL-17+ coexpression is a characteristic of the Th17.1 subset, which is expanded from precursors by TCR stimulation in the presence of IL-23 (28). Figure 5C shows that 20 hours of UPM stimulation of DCs induced very low IL-23 secretion; 10–20 times greater quantities were, however, measured in Day-5 Tm:UPM-DC coculture supernatants (Figure 5D), suggesting that most IL-23 production was elicited through subsequent activated Tm–DC interactions. In contrast, the cross-talk between Tms activated by UPM+ HDM-DCs elicited substantially less IL-23 secretion.
Figure 5.
UPM-DCs prime the generation of IFN-γ+/IL-17A+ Th17.1 effectors. (A) Cytokine-producing capacity and frequency of CD4 T cell effectors gated within the CFSElo fraction. CFSE-labeled Tms were stimulated with UPM-DCs from donors with atopic asthma in the presence or absence of HDM for 5 days before a 2-day expansion with IL-2. Intracellular production of IFN-γ, IL-13, and IL-17A was assessed 5 hours after restimulation with PMA and ionomycin. Values in the upper right quadrants denote the frequencies of cells that coproduce IL-17A and IL-13 (upper panel) or IL-17A and IFN-γ (lower panel), respectively. Values in the upper left and lower right quadrants denote the frequencies of cells that produce single cytokines. Data are representative of five independent experiments. (B) Summarized data in terms of percent of CFSElo-dividing cells coproducing IL-13 and IL-17A or IFN-γ and IL-17A. Data are presented as mean ± SD (n = 5). Statistical analysis was by paired t test. (C) UPM-DCs produce IL-23. Supernatants from DCs stimulated for 20 hours with UPM in the presence or absence of GM-CSF were analyzed by CBA. Data are presented as means ± SD (n = 5). (D) Enhancement of IL-23 production in 5-day Tm:UPM-DC cocultures. Data are presented as means ± SD (n = 5). Statistical analysis was by two-tailed paired t test.
UPM-DC–Induced Functional Tm Responses Are Mediated through IL-6–Independent MHC II–TCR Interactions
Stimulation of mDCs with UPM induces the production of a mixture of innate cytokines, such as IL-6, IL-10, and TNF-α (23). In combination with γ-chain cytokines, such as IL-2 and IL-15, these cytokines can induce the expansion and differentiation of naive CD4 T cells and Tcm into mixed Th1/Th2 Tems in an antigen-independent manner (29). Furthermore, IL-6 is an important cytokine for human Th17 differentiation (30, 31). We therefore investigated whether the expansion and differentiation of UPM-DC–induced Tms is IL-6 dependent.
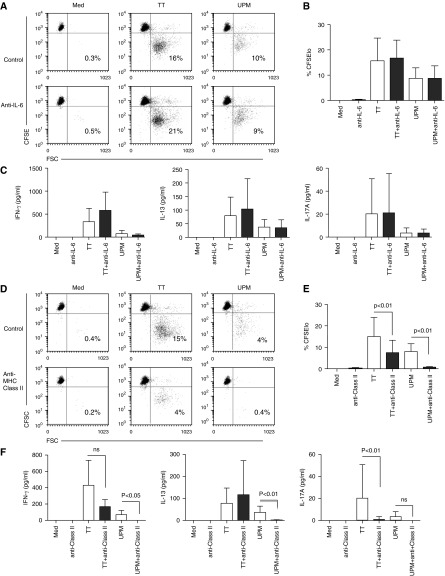
Data in Figure E3A show that, although IL-6 in Day-5 DC-Tm cocultures was efficiently neutralized by treatment of DCs with anti–IL-6 at Day 0, this had no inhibitory effect on UPM or TT-DC–induced Tm proliferation (Figure 6A and summarized in Figure 6B). Neither did IL-6 neutralization inhibit the secretion of IFN-γ, IL-13, or IL-17A by TM effectors induced by UPM-DCs or TT-DCs (Figure 6C).
Figure 6.
UPM-DC–induced CD4 memory T cell activation of cytokine secretion and proliferation is antigen dependent instead of through homeostatic expansion mediated by IL-6. (A) Proliferative responses of CFSE-labeled CD4+ T cells from donors without asthma or atopy after 5-day coculture with UPM-DCs or TT-DCs in the presence or absence of anti–IL-6. Data are representative of six experiments. (B) Summarized data in terms of percentages of proliferating CFSElo cells. Data are presented as means ± SD (n = 6). (C) Effect of anti–IL-6 on UPM-DC–induced IFN-γ, IL-13, and IL-17A secretion by Tm after 5 days of coculture. Data are presented as means ± SD. Statistical analysis was by two-tailed paired t test, followed by Bonferroni’s correction. (D) Proliferative responses of CFSE-labeled CD4+ memory T cells from donors without asthma or atopy after 5 days of coculture with UPM-DCs or TT-DCs in the presence or absence of anti–major histocompatibility class (MHC) II DP, DQ, DR. Data are representative of five experiments (E) Summarized data in terms of percentages of proliferating CFSElo cells. Data are presented as means ± SD (n = 5). (F) Effect of anti–MHC II DP, DQ, DR on UPM-DC–induced IFN-γ, IL-13, and IL-17A secretion by Tms after 5 days of coculture. Data are presented as means ± SD. Statistical analysis was by two-tailed paired t test, followed by Bonferroni’s correction.
We have previously found that, although UPM contains low levels of LPS, UPM-induced DC maturation is LPS independent (23). Because LPS-induced cytokine production might potentially influence Tm differentiation, residual LPS in UPM was blocked by preincubation with polymyxin B (PxB)—an antibiotic that strongly binds and neutralizes LPS—before DC loading. Figure E3 shows that IFN-γ secretion and Tm proliferation induced by UPM-DCs persisted after PxB pretreatment, whereas the same responses induced by TT-DCs were significantly inhibited (P < 0.05 and P < 0.01, respectively).
To test the alternative possibility, that UPM-DC–induced Tm expansion in these experiments was antigen dependent, DCs were treated overnight with anti–MHC II DP, DQ, DR (anti–class II) to block the recognition of MHC II–cognate peptide complexes by TCR-bearing Tms. Figure 6D and data summarized in Figure 6E show that UPM-DC induced Tm proliferation was abolished by anti–class II, although this was insufficient to fully block TT-DC–induced Tm responses, which were reduced by approximately 60% (P < 0.01). Equally, blockade of MHC II–TCR interactions efficiently prevented UPM-DC–induced inflammatory Tm cytokine secretion (P < 0.01), but was insufficient to completely inhibit TT-DC–induced Tm cytokine responses. These data suggest that the activation of Tms by UPM was by antigen rather than through inflammatory cytokines.
Discussion
Mounting epidemiological evidence suggests that exposure to the particulate component of traffic-related urban air pollution is linked with exacerbation of pre-existing asthma and suppression of lung immunity, and may contribute to the development of new-onset asthma (32), but the mechanisms remain unclear. Whereas a number of studies have investigated how pollution particulates might skew the priming of naive CD4 T cells to Th2, to the best of our knowledge, this is the first study to report that the loading of human myeloid DCs with UPM alone stimulates autologous Tms to secrete cytokines and expand and differentiate into a mixed population of potentially highly inflammatory Th1, Th2, and Th17 effector memory cells. The ability of UPM-DC to activate autologous Tms in the absence of overt exogenous antigen was nevertheless critically dependent on MHC II availability, suggesting an antigen-dependent mechanism. Furthermore, UPM-activated DCs induced this response equally in cells from both patients with asthma and healthy control patients without asthma, suggesting a common mechanism of Tm effector generation independent of asthma status.
The blood CD1c+ DCs used in these studies are the precursors of the CD11b− lung DCs lining the airway and the CD11b+ DCs in the lung parenchyma (33). Previous studies have revealed that instillation of DEPs causes increased numbers of lung CD11b− DCs to mature and migrate to the lung lymph nodes. These lymph node DCs were loaded with DEPs, demonstrating that there are direct interactions between lung DCs and particulates. DEP-loaded DCs were found to augment primary antigen-specific T cell responses from naive CD4 T cell precursors (12), but we reasoned that these DCs would also have the potential to stimulate antigen-experienced Tcms, which also traffic through the lymph nodes, as well as Tems either in or entering the lung mucosa from the periphery. Furthermore, the lung parenchyma is populated with Tems, and these could access resident UPM-activated CD11b+ DCs. Understanding which DC subset is involved in the generation of autologous UPM-DC–induced Tm effector cell subsets needs further investigation.
High levels of DEP exposure in children with severe atopic asthma has been linked with sixfold elevated levels of serum concentrations of IL-17A and significantly more asthma exacerbations (5). In vivo models show that, although intratracheal instillation of diesel particles alone is sufficient to induce the generation of Th17 cells, in the absence of allergen sensitization this is insufficient to cause asthma symptoms (5). Furthermore, inhalation of UPM during secondary challenge with allergen induces more Th2 and Th17 effectors and more severe symptoms of asthma exacerbation than allergen alone (15), whereas chronic inhalation of real-world air pollution particulates promotes the generation of Th1/Th17 cells (34). The induction of Th17 cells may be through DEP-induced production of Th17-polarizing cytokines, such as IL-1β (35), but Th17 cell differentiation is also promoted by activation of the arylhydrocarbon receptor by polyaromatic hydrocarbons (36), which are found in the organic coating of DEPs (37). Similarly to the effects of DEPs in vivo, UPM-DC–induced generation of Th17 effectors in vitro in the present study occurred in the absence of overt antigen (37), but, unlike DEPs, was accompanied by the generation of potential Th1 and Th2 effectors, which, in turn, was abolished by anti–MHC II blockade.
Our most striking observation is that UPM-DCs consistently induced functional Tm responses in the absence of overt exogenous antigen many fold higher than those induced by GM-CSF–treated DCs, although the latter had higher viability and expressed higher levels of maturation markers than UPM-DCs (data not shown, and Ref. 23). However, DCs stimulated by GM-CSF (which would be produced by UPM-stimulated AECs in vivo [38]) and loaded with UPM induced greater levels of T cell proliferation and subsequently greater numbers of T cell effectors.
The observation that anti–class II, but not anti–IL-6 or polymyxin-B, completely abolished Tm responses to autologous UPM-DCs suggests a TCR/antigen-dependent rather than a cytokine-driven mode of activation. Indeed, that Tm responses to TT-DCs were not completely abolished by anti–class II treatment, but were partially inhibited by PxB (Figure E3) suggests that some of the response to TT used as a positive control in these experiments was LPS-induced, cytokine-driven expansion.
Our original hypothesis had been that UPM-mediated maturation of DCs would enhance allergen-specific memory T cell cytokine responses. Instead, the lack of additive effect in eliciting IL-13 secretion from Tms stimulated by DCs loaded with both UPM and HDM, and the reduced frequencies of IL-17A+–secreting effectors, compared with those of Tms stimulated by UPM-DCs alone, suggests that the Tm responses to UPM and HDM are in competition with each other. Which Tm response dominates will probably differ from individual to individual according to T cell precursor frequency, TCR affinity for antigen, and MHC background. Yet it is the sum of the individual responses to UPM-associated antigens and HDM that generates the observed mixture of Th1,Th2, and Th17 effectors.
We speculate that, if UPM has the same effect on Tms in the lungs of patients with asthma allergic to HDM, as in the in vitro experiments described here, the overall result would be HDM-induced, Th2-mediated eosinophilia combined with UPM-induced, Th1- and Th17-mediated neutrophilia, and airway smooth muscle activation (39), manifest clinically as more severe asthma. Importantly, the majority of the UPM-DC–induced Th17 cells coexpressed IFN-γ rather than IL-13, suggesting that UPM-DCs drive the generation of the recently described inflammatory Th17.1 cell subset associated with autoimmune disease rather than asthma-promoting IL-17–secreting Th2 cells (17). This pathogenic Th17 subset expands from precursor memory cells through TCR stimulation coupled with IL-23 (28). Th17.1 cells are glucocorticoid resistant, and Th17 cells have been implicated in glucocorticoid-resistant asthma in humans (40). Furthermore, in addition to IL-17A, Tms from subjects with severe, glucocorticoid-resistant asthma also secrete high levels of IFN-γ (41), which has been implicated in the pathogenesis of nonatopic asthma (42), and is the primary mediator of airway hyperreactivity in models of chronic asthma (43). Strikingly, we detected IL-23 in our 5-day UPM-DC Tm coculture supernatants, and generated Tm effectors similarly capable of producing high levels of IFN-γ and IL-17A. The requirement for TCR stimulation for expansion of Th17.1 cells further suggests that antigen is being presented by UPM-DCs.
That UPM-DCs activated strong responses from Tm from healthy individuals without asthma as well as subjects with asthma suggests that the triggering antigen is ubiquitous, and it is tempting to speculate what its nature might be. Possible candidates include autoantigens (44), or self-antigens modified, for example, by reactive oxygen species generated by UPM (45), or by the structural distortion of the surface of self-peptide MHC complexes, via internal interaction with metallic cations, as has been reported for the HLA-DP2–restricted mechanism of chronic beryllium disease (46). This is of particular interest, because UPM contains a multitude of transition and alkali metals.
Another possibility is the “reprogramming” of self-antigen–specific FoxP3+ regulatory T cells (T-regs) into effectors, which has been described in mice and requires presentation of antigen by activated CD11b+ DCs and IL-6 (47). Although UPM-activated DCs secreted IL-6, complete neutralization of IL-6 had no effect on the generation of UPM-DC–induced IL-17–secreting Tems. Indeed, depletion of 90% of T-regs resulted in an enhancement of UPM-DC–induced IFN-γ and IL-13 effector generation, and did not prevent the generation of UPM-DC–induced Th17 cells (Figure E4), suggesting that “plastic” T-regs are not the source of UPM-DC–induced Tems.
An alternative explanation is that UPM contains exogenous antigen, for example, from aerial microbes, such as bacteria, fungal spores, pollen, and viruses. It may then be envisaged that the frequencies of Tms specific for such antigens may be increased in subjects with asthma because of impaired airway barrier function (48). This might also explain why our observed frequencies of Tms secreting IFN-γ and IL-13 from patients with asthma were significantly higher than those from healthy control subjects. Although we have no direct evidence that Tms are responding to environmental microbial antigens in UPM, it is noteworthy that a similarly collected UPM source was reported to contain gram-negative and gram-positive bacteria and fungal spores (49). Furthermore, typical UPM of PM2.5–PM10 size tend to deposit in bronchioles, which contribute significantly to airways obstruction in asthma and are also typical sites where bacterial infections are initiated (50). Whether the UPM (SRM 1648a) that we have used contains similar environmental microbes, and if so, whether Tm responses to UPM are antigen specific or cross-reactive, needs further investigation, as does the question of whether other components of UPM, such as PAHs or metal salts, act as adjuvants increasing the immunogenicity of microbes taken up by DCs. Together, these observations could explain why subjects without atopic asthma have Tm responses to UPM and why the cytokine profile is similar to that induced by TT. Furthermore, they may provide a mechanism for how exposure to UPM might contribute to systemic inflammation–induced exacerbation of chronic diseases and the development and exacerbation of asthma in both individuals with and those without atopy.
Acknowledgments
Acknowledgments
The authors thank David Richards for technical advice, the departmental nurses, and the donors for providing blood samples.
Footnotes
This work was supported by funding from the Him Lee Fund, the Lee Iu Cheung Fund, the Santander Foundation (T.H.L.) and the Wellcome Trust (P.E.P.). The authors acknowledge support from the Department of Health via the National Institute for Health Research Comprehensive Biomedical Research Centre award to Guy’s and St. Thomas’ National Health Service (NHS) Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
Disclaimer: The views expressed are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research, or the Department of Health.
Author Contributions: Concept and design—N.C.M., F.J.K., C.M.H., and T.H.L.; analysis and interpretation—N.C.M., P.E.P., E.H.M., C.J.C., and C.M.H.; drafting the manuscript for important intellectual content—N.C.M., P.E.P., E.H.M., C.J.C., C.M.H., and T.H.L.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0084OC on July 21, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Simpson CR, Sheikh A. Trends in the epidemiology of asthma in England: a national study of 333,294 patients. J R Soc Med. 2010;103:98–106. doi: 10.1258/jrsm.2009.090348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwartz J. Air pollution and daily mortality: a review and meta analysis. Environ Res. 1994;64:36–52. doi: 10.1006/enrs.1994.1005. [DOI] [PubMed] [Google Scholar]
- 3.Ilabaca M, Olaeta I, Campos E, Villaire J, Tellez-Rojo MM, Romieu I. Association between levels of fine particulate and emergency visits for pneumonia and other respiratory illnesses among children in Santiago, Chile. J Air Waste Manag Assoc. 1999;49:154–163. doi: 10.1080/10473289.1999.10463879. [DOI] [PubMed] [Google Scholar]
- 4.Norris G, YoungPong SN, Koenig JQ, Larson TV, Sheppard L, Stout JW. An association between fine particles and asthma emergency department visits for children in Seattle. Environ Health Perspect. 1999;107:489–493. doi: 10.1289/ehp.99107489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brandt EB, Kovacic MB, Lee GB, Gibson AM, Acciani TH, Le Cras TD, Ryan PH, Budelsky AL, Khurana Hershey GK. Diesel exhaust particle induction of IL-17A contributes to severe asthma. J Allergy Clin Immunol. 2013;132:1194–1204. doi: 10.1016/j.jaci.2013.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gan WQ, FitzGerald JM, Carlsten C, Sadatsafavi M, Brauer M. Associations of ambient air pollution with chronic obstructive pulmonary disease hospitalization and mortality. Am J Respir Crit Care Med. 2013;187:721–727. doi: 10.1164/rccm.201211-2004OC. [DOI] [PubMed] [Google Scholar]
- 7.Sarkar S, Song Y, Sarkar S, Kipen HM, Laumbach RJ, Zhang J, Strickland PA, Gardner CR, Schwander S. Suppression of the NF-κB pathway by diesel exhaust particles impairs human antimycobacterial immunity. J Immunol. 2012;188:2778–2793. doi: 10.4049/jimmunol.1101380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008;26:339–362. doi: 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- 9.Hopke PK, Rossner A. Exposure to airborne particulate matter in the ambient, indoor, and occupational environments. Clin Occup Environ Med. 2006;5:747–771. doi: 10.1016/j.coem.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony–stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarthy NE, Jones HA, Marks NA, Shiner RJ, Ind PW, Al-Hassi HO, English NR, Murray CM, Lambert JR, Knight SC, et al. Inhaled allergen-driven CD1c up-regulation and enhanced antigen uptake by activated human respiratory-tract dendritic cells in atopic asthma. Clin Exp Allergy. 2007;37:72–82. doi: 10.1111/j.1365-2222.2006.02631.x. [DOI] [PubMed] [Google Scholar]
- 12.Provoost S, Maes T, Willart MA, Joos GF, Lambrecht BN, Tournoy KG. Diesel exhaust particles stimulate adaptive immunity by acting on pulmonary dendritic cells. J Immunol. 2010;184:426–432. doi: 10.4049/jimmunol.0902564. [DOI] [PubMed] [Google Scholar]
- 13.Wilson RH, Whitehead GS, Nakano H, Free ME, Kolls JK, Cook DN. Allergic sensitization through the airway primes Th17-dependent neutrophilia and airway hyperresponsiveness. Am J Respir Crit Care Med. 2009;180:720–730. doi: 10.1164/rccm.200904-0573OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li JZ, Yuan XZ, Chi YX, Jin ZY. Effects of diesel exhaust particles inhalation on immediate reaction in asthma rats [in Chinese] Zhonghua Er Ke Za Zhi. 2009;47:91–95. [PubMed] [Google Scholar]
- 15.Li N, Harkema JR, Lewandowski RP, Wang M, Bramble LA, Gookin GR, Ning Z, Kleinman MT, Sioutas C, Nel AE. Ambient ultrafine particles provide a strong adjuvant effect in the secondary immune response: implication for traffic-related asthma flares. Am J Physiol Lung Cell Mol Physiol. 2010;299:L374–L383. doi: 10.1152/ajplung.00115.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saunders V, Breysse P, Clark J, Sproles A, Davila M, Wills-Karp M. Particulate matter–induced airway hyperresponsiveness is lymphocyte dependent. Environ Health Perspect. 2010;118:640–646. doi: 10.1289/ehp.0901461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YH, Voo KS, Liu B, Chen CY, Uygungil B, Spoede W, Bernstein JA, Huston DP, Liu YJ. A novel subset of CD4(+) T(H)2 memory/effector cells that produce inflammatory IL-17 cytokine and promote the exacerbation of chronic allergic asthma. J Exp Med. 2010;207:2479–2491. doi: 10.1084/jem.20101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poon AH, Eidelman DH, Martin JG, Laprise C, Hamid Q. Pathogenesis of severe asthma. Clin Exp Allergy. 2012;42:625–637. doi: 10.1111/j.1365-2222.2012.03983.x. [DOI] [PubMed] [Google Scholar]
- 19.Bleck B, Tse DB, Curotto de Lafaille MA, Zhang F, Reibman J. Diesel exhaust particle–exposed human bronchial epithelial cells induce dendritic cell maturation and polarization via thymic stromal lymphopoietin. J Clin Immunol. 2008;28:147–156. doi: 10.1007/s10875-007-9149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bleck B, Tse DB, Gordon T, Ahsan MR, Reibman J. Diesel exhaust particle–treated human bronchial epithelial cells upregulate Jagged-1 and OX40 ligand in myeloid dendritic cells via thymic stromal lymphopoietin. J Immunol. 2010;185:6636–6645. doi: 10.4049/jimmunol.1000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bleck B, Tse DB, Jaspers I, Curotto de Lafaille MA, Reibman J. Diesel exhaust particle–exposed human bronchial epithelial cells induce dendritic cell maturation. J Immunol. 2006;176:7431–7437. doi: 10.4049/jimmunol.176.12.7431. [DOI] [PubMed] [Google Scholar]
- 22.Greter M, Helft J, Chow A, Hashimoto D, Mortha A, Agudo-Cantero J, Bogunovic M, Gautier EL, Miller J, Leboeuf M, et al. GM-CSF controls nonlymphoid tissue dendritic cell homeostasis but is dispensable for the differentiation of inflammatory dendritic cells. Immunity. 2012;36:1031–1046. doi: 10.1016/j.immuni.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matthews NC, Faith A, Pfeffer P, Lu H, Kelly FJ, Hawrylowicz CM, Lee TH. Urban particulate matter suppresses priming of T helper type 1 cells by granulocyte/macrophage colony–stimulating factor–activated human dendritic cells. Am J Respir Cell Mol Biol. 2014;50:281–291. doi: 10.1165/rcmb.2012-0465OC. [DOI] [PubMed] [Google Scholar]
- 24.Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS One. 2011;6:e16245. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyons AB, Parish CR. Determination of lymphocyte division by flow cytometry. J Immunol Methods. 1994;171:131–137. doi: 10.1016/0022-1759(94)90236-4. [DOI] [PubMed] [Google Scholar]
- 26.Fujieda S, Diaz-Sanchez D, Saxon A. Combined nasal challenge with diesel exhaust particles and allergen induces in vivo IgE isotype switching. Am J Respir Cell Mol Biol. 1998;19:507–512. doi: 10.1165/ajrcmb.19.3.3143. [DOI] [PubMed] [Google Scholar]
- 27.Mamessier E, Nieves A, Vervloet D, Magnan A. Diesel exhaust particles enhance T-cell activation in severe asthmatics. Allergy. 2006;61:581–588. doi: 10.1111/j.1398-9995.2006.01056.x. [DOI] [PubMed] [Google Scholar]
- 28.Ramesh R, Kozhaya L, McKevitt K, Djuretic IM, Carlson TJ, Quintero MA, McCauley JL, Abreu MT, Unutmaz D, Sundrud MS. Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med. 2014;211:89–104. doi: 10.1084/jem.20130301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benwell RK, Lee DR. Essential and synergistic roles of IL1 and IL6 in human Th17 differentiation directed by TLR ligand–activated dendritic cells. Clin Immunol. 2010;134:178–187. doi: 10.1016/j.clim.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Zielinski CE, Mele F, Aschenbrenner D, Jarrossay D, Ronchi F, Gattorno M, Monticelli S, Lanzavecchia A, Sallusto F. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 32.Guarnieri M, Balmes JR. Outdoor air pollution and asthma. Lancet. 2014;383:1581–1592. doi: 10.1016/S0140-6736(14)60617-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Plantinga M, Hammad H, Lambrecht BN. Origin and functional specializations of DC subsets in the lung. Eur J Immunol. 2010;40:2112–2118. doi: 10.1002/eji.201040562. [DOI] [PubMed] [Google Scholar]
- 34.Deiuliis JA, Kampfrath T, Zhong J, Oghumu S, Maiseyeu A, Chen LC, Sun Q, Satoskar AR, Rajagopalan S. Pulmonary T cell activation in response to chronic particulate air pollution. Am J Physiol Lung Cell Mol Physiol. 2012;302:L399–L409. doi: 10.1152/ajplung.00261.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Provoost S, Maes T, Pauwels NS, Vanden Berghe T, Vandenabeele P, Lambrecht BN, Joos GF, Tournoy KG. NLRP3/caspase-1–independent IL-1beta production mediates diesel exhaust particle–induced pulmonary inflammation. J Immunol. 2011;187:3331–3337. doi: 10.4049/jimmunol.1004062. [DOI] [PubMed] [Google Scholar]
- 36.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links Th17-cell–mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 37.van Voorhis M, Knopp S, Julliard W, Fechner JH, Zhang X, Schauer JJ, Mezrich JD. Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PLoS One. 2013;8:e82545. doi: 10.1371/journal.pone.0082545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takano H, Ichinose T, Miyabara Y, Shibuya T, Lim HB, Yoshikawa T, Sagai M. Inhalation of diesel exhaust enhances allergen-related eosinophil recruitment and airway hyperresponsiveness in mice. Toxicol Appl Pharmacol. 1998;150:328–337. doi: 10.1006/taap.1998.8437. [DOI] [PubMed] [Google Scholar]
- 39.Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT, Atabai K, et al. IL-17A produced by αβ T cells drives airway hyper-responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med. 2012;18:547–554. doi: 10.1038/nm.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nanzer AM, Chambers ES, Ryanna K, Richards DF, Black C, Timms PM, Martineau AR, Griffiths CJ, Corrigan CJ, Hawrylowicz CM. Enhanced production of IL-17A in patients with severe asthma is inhibited by 1alpha,25-dihydroxyvitamin D3 in a glucocorticoid-independent fashion. J Allergy Clin Immunol. 2013;132:297–304. doi: 10.1016/j.jaci.2013.03.037. [DOI] [PubMed] [Google Scholar]
- 41.Chambers ES, Nanzer AN, Pfeffer PE, Richards DF, Timms PM, Martineau AR, Griffiths CJ, Corrigan CJ, Hawrylowicz CM.Distinct endotypes of steroid-resistant asthma characterised by IL-17Ahigh and IFN-γ high immunophenotypes: potential benefits of calcitriol J Allergy Clin Immunol 2015136628–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zoratti E, Havstad S, Wegienka G, Nicholas C, Bobbitt KR, Woodcroft KJ, Ownby DR, Johnson CC. Differentiating asthma phenotypes in young adults through polyclonal cytokine profiles. Ann Allergy Asthma Immunol. 2014;113:25–30. doi: 10.1016/j.anai.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar RK, Webb DC, Herbert C, Foster PS. Interferon-gamma as a possible target in chronic asthma. Inflamm Allergy Drug Targets. 2006;5:253–256. doi: 10.2174/187152806779010909. [DOI] [PubMed] [Google Scholar]
- 44.Lohse AW, Dinkelmann M, Kimmig M, Herkel J, Meyer zum Büschenfelde KH. Estimation of the frequency of self-reactive T cells in health and inflammatory diseases by limiting dilution analysis and single cell cloning. J Autoimmun. 1996;9:667–675. doi: 10.1006/jaut.1996.0087. [DOI] [PubMed] [Google Scholar]
- 45.Ciencewicki J, Trivedi S, Kleeberger SR. Oxidants and the pathogenesis of lung diseases. J Allergy Clin Immunol. 2008;122:456–468. doi: 10.1016/j.jaci.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clayton GM, Wang Y, Crawford F, Novikov A, Wimberly BT, Kieft JS, Falta MT, Bowerman NA, Marrack P, Fontenot AP, et al. Structural basis of chronic beryllium disease: linking allergic hypersensitivity and autoimmunity. Cell. 2014;158:132–142. doi: 10.1016/j.cell.2014.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma MD, Huang L, Choi JH, Lee EJ, Wilson JM, Lemos H, Pan F, Blazar BR, Pardoll DM, Mellor AL, et al. An inherently bifunctional subset of Foxp3+ T helper cells is controlled by the transcription factor eos. Immunity. 2013;38:998–1012. doi: 10.1016/j.immuni.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Davies DE. Epithelial barrier function and immunity in asthma. Ann Am Thorac Soc. 2014;11:S244–S251. doi: 10.1513/AnnalsATS.201407-304AW. [DOI] [PubMed] [Google Scholar]
- 49.Alexis NE, Lay JC, Zeman K, Bennett WE, Peden DB, Soukup JM, Devlin RB, Becker S. Biological material on inhaled coarse fraction particulate matter activates airway phagocytes in vivo in healthy volunteers. J Allergy Clin Immunol. 2006;117:1396–1403. doi: 10.1016/j.jaci.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 50.Kim CS, Hu SC, DeWitt P, Gerrity TR. Assessment of regional deposition of inhaled particles in human lungs by serial bolus delivery method. J Appl Physiol (1985) 1996;81:2203–2213. doi: 10.1152/jappl.1996.81.5.2203. [DOI] [PubMed] [Google Scholar]