Abstract
Inflammasomes are specialized inflammatory signaling platforms that govern the maturation and secretion of proinflammatory cytokines, such as IL-1β and IL-18, through the regulation of caspase-1–dependent proteolytic processing. Several nucleotide binding domain leucine-rich repeat-containing receptor (NLR) family members (i.e., NLR family, pyrin domain containing [NLRP] 1, NLRP3, and NLR family, caspase recruitment domain containing-4 [NLRC4]) as well as the pyrin and hemopoietic expression, interferon-inducibility, nuclear localization domain–containing family member, absent in melanoma 2, can form inflammasome complexes in human cells. In particular, the NLRP3 inflammasome is activated in response to cellular stresses through a two-component pathway, involving Toll-like receptor 4–ligand interaction (priming) followed by a second signal, such as ATP-dependent P2X purinoreceptor 7 receptor activation. Emerging studies suggest that the NLRP3 inflammasome can exert pleiotropic effects in human diseases with potentially both pro- and antipathogenic sequelae. Whereas NLRP3 inflammasome activation can serve as a vital component of host defense against invading bacteria and pathogens, excessive activation of the inflammasome can lead to inflammation-associated tissue injury in the setting of chronic disease. In addition, pyroptosis, an inflammasome-associated mode of cell death, contributes to host defense. Recent research has described the regulation and function of the NLRP3 inflammasome in various pulmonary diseases, including acute lung injury and acute respiratory distress syndrome, sepsis, respiratory infections, chronic obstructive pulmonary disease, asthma, pulmonary hypertension, cystic fibrosis, and idiopathic pulmonary fibrosis. The NLRP3 and related inflammasomes, and their regulated cytokines or receptors, may represent novel diagnostic or therapeutic targets in pulmonary diseases and other diseases in which inflammation contributes to pathogenesis.
Keywords: inflammasomes, inflammation, pulmonary diseases
The lung is a dynamic organ consisting of multiple cell types, including epithelial, mesenchymal, secretory, and inflammatory cells. In serving its primary function in gas exchange, the uptake and transfer of oxygen essential for metabolism, the lung is highly susceptible to intake of foreign matter, including allergens, dust, particulates, and invading micro-organisms, which can result in infection, inflammation, or tissue injury (1). The innate immune system provides a primary defense against invading microbial pathogens and exogenous irritants. The homeostatic functions of the innate immune system initiate with the recognition of either microbial-derived constituents, called pathogen-associated molecular patterns (PAMPs), released during infection, or endogenous damage-associated molecular patterns (DAMPs), produced during cellular or tissue injury. Molecular sensing of PAMPs/DAMPs occurs through interactions with germline-encoded pattern recognition receptors (PRRs), represented by members of the Toll-like receptor (TLR), retinoic acid–inducible gene-I–like receptor, and nucleotide binding domain leucine-rich repeat-containing receptor (NLR) families. Upon pattern recognition, activated PRRs initiate signal transduction events that regulate inflammatory and innate immune responses (2–4).
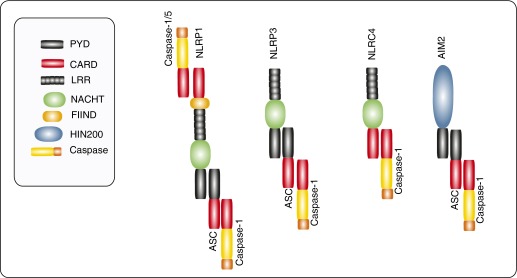
The cytosolic NLRs contain a three-domain homologous region that includes a central nucleotide-binding oligomerization domain and a C-terminal leucine-rich repeat domain (2–4). The variable N-terminal repeat domains of NLRs include a pyrin domain, as in NLR family, pyrin containing (NLRP) 1 or NLRP3, a caspase-recruitment domain, as in NLR family, caspase recruitment domain containing-4 (NLRC4), or a baculovirus inhibitor of apoptosis repeat (BIR) domain, as in NLR family, BIR domain containing-1 (NLRB1)/NLR family, apoptosis inhibitory protein (NAIP) (2, 4–5). Of the known NLR members, at least four NLRs (NLRP1, NLRP3, NLRC4, NLRB1) (2), as well as the pyrin and hemopoietic expression, interferon-inducibility, nuclear localization domain–containing family member, absent in melanoma 2 (6), can form multiprotein inflammasome complexes upon PRR stimulation (Figure 1).
Figure 1.
Structures of the major inflammasomes. The cytosolic nucleotide binding domain leucine-rich repeat-containing receptors (NLRs) typically contain a three-domain homologous region that includes a nucleotide-binding oligomerization domain (NACHT) and a leucine-rich repeat domain (LRR). NLR family, pyrin containing (NLRP) 1 and NLRP3 contain an N-terminal pyrin domain (PYD), whereas NLR family, caspase recruitment domain containing-4 (NLRC4) contains an N-terminal caspase-recruitment domain (CARD). In addition, NLRP1 contains a function to find (FIIND) domain and a C-terminal CARD. Apoptosis-associated speck-like protein containing caspase-recruitment domain (ASC) is an adaptor for caspase-1 binding that contains a PYD and CARD. The requirement for ASC in NLRC4 inflammasome assembly remains unclear. An additional inflammasome complex is represented by absent in melanoma (AIM) 2, which contains a CARD and HIN200 domain. The latter serves as a receptor for double-stranded DNA. Caspases-1/-5 interact with the inflammasomes through CARD–CARD interactions. HIN200, hemopoietic expression, interferon-inducibility, nuclear location, 200 amino-acid domain.
In addition to a core sensor protein (e.g., NLRP, such as NLRP3), inflammasomes contain the adaptor protein, apoptosis-associated speck-like protein containing caspase-recruitment domain (ASC), and pro–caspase-1. Inflammasome formation results in the autocatalysis and activation of the cysteine protease caspase-1 (7). Active caspase-1 subsequently catalyzes the proteolytic cleavage of proinflammatory cytokine precursors from their pro forms into their active forms (e.g., IL-1β and IL-18), which propagate inflammatory and innate immune responses (7).
Pyroptosis is a form of inflammation-associated programmed cell death that occurs in inflammatory cells, such as macrophages, and depends on the activation of caspase-1. This form of cell death occurs during host infection under conditions of inflammasome activation (8–10). During pyroptosis, cells are lysed to release their contents, which include high levels of proinflammatory cytokines that further propagate inflammation. During acute inflammation, pyroptosis acts as a host defense mechanism to curb infection through the elimination of infected macrophages (10). Excessive activation of pyroptosis can lead to tissue injury and pathogenic processes in the context of chronic inflammation (10).
Emerging studies suggest that inflammasomes exert important effector functions during the progression of human diseases, which can invoke adaptive or maladaptive outcomes. This review describes the regulation and function of the inflammasome, with an emphasis on the NLRP3 inflammasome, in human pulmonary diseases—in particular, those conditions that implicate inflammation as a key mechanism of pathogenesis.
Molecular and Metabolic Regulation of the NLRP3 Inflammasome
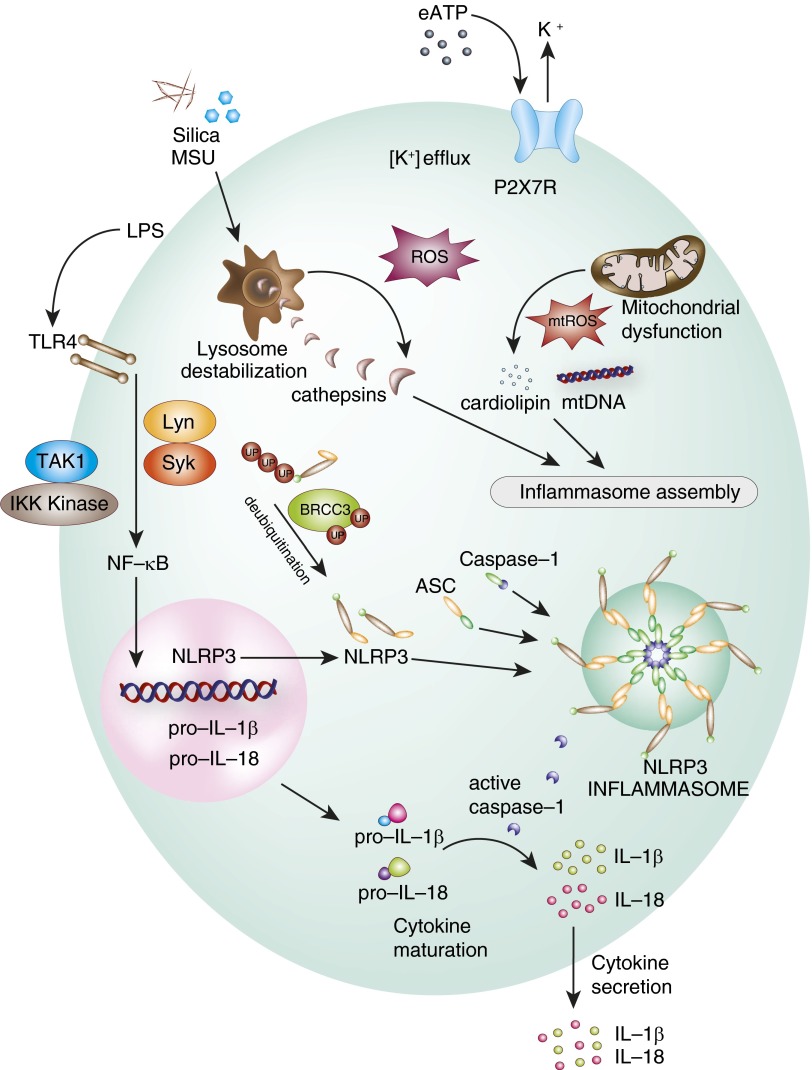
NLRP3 inflammasome formation and activation requires a canonical two-step mechanism involving the sequential ligand-dependent activation of TLRs (e.g., TLR4) or related receptors that, in turn, stimulates NF-κB–dependent transcription of NLRP3 (priming), leading to NLRP3 protein synthesis. This is followed by a second activation signal generated by ion flux, phagosomal destabilization, mitochondrial reactive oxygen species (mtROS) or mitochondrial DAMPs, that results in NLRP3–inflammasome assembly (multiprotein complex formation between NLRP3 protein, ASC, and pro–caspase-1) (7, 9). The activated inflammasome complex catalyzes caspase-1 activation by proteolytic cleavage of pro–caspase-1, which, in turn, catalyzes the proteolytic cleavage and maturation of proinflammatory cytokines (e.g., IL-1β, IL-18) (Figure 2). IL-1β regulates fundamental inflammatory responses, including the expression of inflammatory mediators (e.g., inducible nitric oxide synthase) and adhesion molecules, whereas IL-18 has been implicated in T helper 1 responses, including the regulation of IFN-γ production from T cells and natural killer cells (11–12).
Figure 2.
Activation of the NLRP3 inflammasome. The NLRP3 inflammasome responds to activating signals through a two-step activation model. Initiation is typically triggered by ligand binding (e.g., LPS) to Toll-like receptors (TLR; e.g., TLR4) and related receptors. This results in activation of NF-κB, which translocates to the nucleus and activates the transcription of inflammasome components, including NLRP3, and the pro forms of inflammasome-related cytokines (i.e., pro–IL-1β and pro–IL-18). Inflammasome priming is also under the regulation of kinase cascades (e.g., transforming growth factor-β activated kinase-1 [TAK1], inhibitor of κ-B kinase [IKK] kinase, Lyc/Yes Novel tyrosine kinase [Lyn], spleen tyrosine kinase [Syk]). NLRP3 is post-translationally regulated by breast cancer, early onset protein 1 and 2 (BRCA1–BRCA2)–containing complex subunit (BRCC) 3–mediated deubiquitination. A second signal is required for assembly of the inflammasome from its individual components (e.g., NLRP3, ASC, pro–caspase-1). The canonical signal is provided by P2X purinoreceptor 7 (P2X7) stimulation by ligand (e.g., extracellular ATP [eATP]), which triggers potassium ion efflux. Additional contributory signals may include the destabilization of lysosomes after ingestion and phagocytosis of crystalline substrates, such as monosodium urate crystals (MSU), and the disruption of mitochondria, leading to the release of mitochondrial damage-associated molecular patterns, such as cardiolipin and mitochondrial DNA (mtDNA). Activation of inflammasome-associated caspase-1 results in the activation (cleavage) of proinflammatory cytokines (e.g., IL-1β, IL-18) before their secretion. mtROS, mitochondrial reactive oxygen species; ROS, reactive oxygen species.
The inflammasome priming signal is initiated by engagement of TLRs with PAMPs, which activates NF-κB to promote the transcription of both pro–IL-1β and NLRP3 (13). Recent studies have identified the critical requirement for the mitogen-activated protein kinase, extracellular signal–regulated kinase-1, but not extracellular signal–regulated kinase-2, in NF-κB–dependent inflammasome priming (14). Previous studies also describe the requirement for the spleen tyrosine (Y) kinase (Syk), and the protooncogene tyrosine kinase (srk)-kinase, Lyc/Yes Novel tyrosine kinase (Lyn) kinase, Lyn, in pro–IL-1β synthesis and NLRP3 inflammasome priming in response to fungal pathogens (15), and the malarial-derived metabolic product, hemozoin (16). The tyrosine kinase, Syk, regulates pro–IL-1β synthesis downstream of the immunoreceptor, tyrosine-based activation motif–coupled fungal pattern recognition receptors, and also induces mtROS and K+ efflux required for NLRP3 inflammasome activation (15). The Src kinase, Lyn, activates Syk by phosphorylation, which directly modifies ASC, facilitating NLRP3 inflammasome activation and IL-1β production (16). Although NLRP3 expression is regulated by NF-κB activation through ligand-dependent signaling during the priming step, NLRP3 is also regulated by post-transcriptional and post-translational mechanisms. MicroRNAs (e.g., microRNA-223) can negatively regulate NLRP3 expression in myeloid cells (17–18). Binding of microRNA-223 to the 3′ untranslated region of NLRP3 messenger RNA promotes its degradation, leading to reduced NLRP3 protein expression. NLRP3 protein stability is post-translationally regulated by deubiquitination, which is mediated by the Lys-63–specific deubiquitinase, breast cancer, early onset protein 1 and 2 (BRCA1–BRCA2)–containing complex subunit 3 (BRCC3) (19), to facilitate rapid NLRP3 inflammasome formation. Post-translational inhibition of NLRP3 by nitric oxide–dependent S-nitrosylation has also been described (20).
Many diverse agonists, including exogenous stimuli, such as microbial components and toxins, and crystalline or particulate substrates (e.g., silica, asbestos) can trigger inflammasome activation (9, 21–22). Endogenous NLRP3 inflammasome activators include monosodium urate formed from phase transition of accumulated uric acid (UA) (21). Extracellular ATP (eATP), released during tissue injury, activates P2X7, triggering cellular K+ efflux and inflammasome activation (22). The intracellular milieu surrounding NLRP3 inflammasome activation may consist of several scenarios. These include: (1) the perturbation of intracellular ion content through ligand-dependent ion channel opening or exposure to pore-forming bacterial toxins (9, 23); (2) the phagocytosis of crystalline substrates leading to disruption of lysosomes (24); and (3) signal generation from destabilization of mitochondria (25–26). Recently, it has been suggested that the cytoskeleton also regulates NLRP3 inflammasome activation. Vimentin, one of the intermediate filaments involved in the stability of the intracellular architecture, modulates NLRP3 inflammasome activation by serving as a platform for protein–protein interactions between NLRP3 and ASC (27). In contrast, the noncanonical absent in melanoma 2 inflammasome responds to activation by accumulation of cytosolic double-stranded DNA, and functions primarily as a sensor for this DAMP (6).
Regulation of the NLRP3 Inflammasome by Mitochondrial Dysfunction
Signals originating from dysfunctional mitochondria may represent a unifying mechanism underlying NLRP3 inflammasome activation by cellular stress. Recent studies from this laboratory and others demonstrate that mitochondrial dysfunction results in increased NLRP3 inflammasome activation (25–26). In bone marrow–derived macrophages (BMDMs) stimulation with LPS and ATP compromised mitochondrial integrity, caused membrane depolarization (−ΔΨm), increased mtROS generation and mitochondrial DNA (mtDNA) release into the cytosol, and resulted in increased NLRP3 inflammasome activation and secretion of IL-1β and IL-18 (25). Scavenging of mtROS using mitochondrial-targeted antioxidants or reduction of cytosolic DNA by DNase transfection, dampened inflammasome activation and caspase-1–dependent IL-1β secretion (25). The translocation of mtDNA from the mitochondria to the cytosol was NLRP3 dependent, as it was diminished in NLRP3-deficient (Nlrp3−/−) BMDM (25). Additional studies have proposed that oxidized mtDNA (28) or the mitochondrial membrane lipid, cardiolipin, activate NLRP3 by direct interactions (29). These studies are unified by the concept of mitochondrial dysfunction leading to the release of mitochondrial DAMPs as a proximal trigger for inflammasome activation.
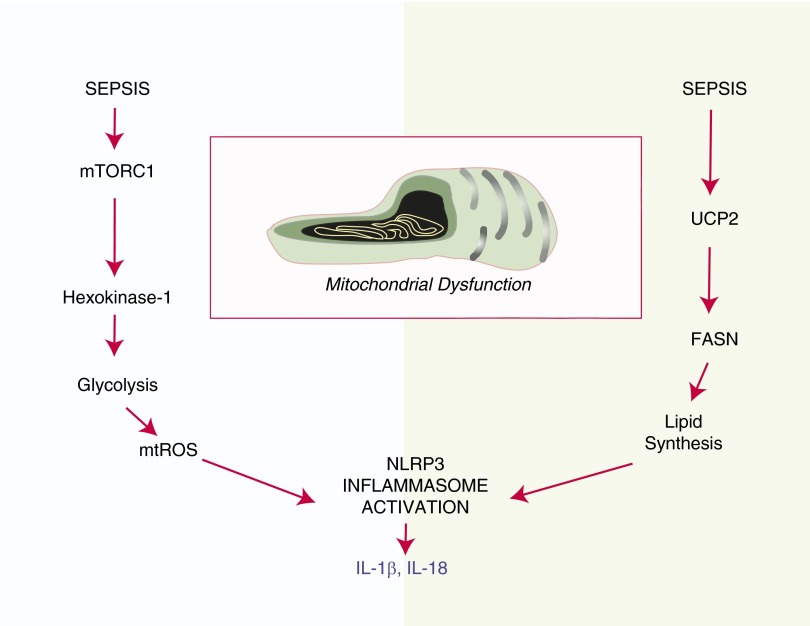
Regulation of the inflammasome by metabolic signals.
The NLRP3 inflammasome can recognize molecular patterns associated with host-derived metabolites, including glucose and saturated fatty acids (FA) (30–31). Although many studies suggest the possibility of the role of metabolic status in NLRP3 inflammasome regulation, the mechanisms correlating metabolic alteration, mitochondrial dysfunction and NLRP3 inflammasome regulation remain unclear. Our recent findings support a hypothesis that inflammasome activation in response to proinflammatory stimuli is integrated with the regulation and perturbation of cellular metabolic pathways, namely glucose metabolism and FA synthesis (Figure 3). We recently evaluated the involvement of cellular metabolic pathways in the regulation of NLRP3 inflammasome activation in activated macrophages (32–33). We revealed a role for glycolysis in NLRP3 inflammasome activation. The mammalian target of rapamycin complex 1–induced glycolysis regulates NLRP3 inflammasome activation in macrophages. Down-regulation of glycolysis through inhibition of Raptor/mammalian target of rapamycin complex 1 or of hexokinase-1, using either genetic interference or chemical inhibitors (e.g., 2-deoxyglucose), inhibited both inflammasome priming and caspase-1 activation in macrophages in response to LPS and ATP (32).
Figure 3.
Metabolic regulation of inflammasome activation. NLRP3 inflammasome activation in disease may be coupled to the perturbation of cellular metabolism. Recent studies indicate that both glucose metabolism and fatty acid (FA) synthesis may be linked to inflammasome activation (32, 33). Proinflammatory conditions activate mammalian target of rapamycin complex (mTORC) 1, leading to up-regulation of glycolysis through translational activation of hexokinase-1. Inhibition of glycolysis using 2-deoxyglucose suppressed mtROS production and NLRP3 inflammasome activation in macrophages (32). The mitochondrial inner membrane protein, uncoupling protein (UCP)-2, was recently identified as a regulator of NLRP3 inflammasome activation through the stimulation of FA synthesis (33). UCP2−/− mice were protected from experimental sepsis, and macrophages derived from these mice were compromised for inflammasome activation. NLRP3 inflammasome activation was dependent on FA synthase (FASN), the rate-limiting step in FA synthesis.
We also investigated the role of glucose-dependent FA synthesis in NLRP3 inflammasome regulation in response to the novel endogenous NLRP3 inflammasome modulator, mitochondrial uncoupling protein-2 (UCP2) (33). UCP2 is a mitochondrial inner membrane protein that regulates the electron transport chain and mitochondrial export of metabolic precursors. We observed that UCP2 deficient mice were compromised for inflammasome cytokine (e.g., IL-1β) production in a model of fecal peritonitis. In BMDM, UCP2 deficiency suppressed NLRP3-mediated NLRP3 expression and caspase-1 activation in response to LPS and ATP, associated with the down-regulation of glucose-dependent FA synthesis. In UCP2-deficient macrophages, inhibition of FA synthesis resulted from the down-regulation of FA synthase. FA synthase inhibition by small interfering RNA and treatment with chemical inhibitors, C75 and cerulenin, inhibited inflammasome priming and NLRP3-mediated caspase-1 activation in macrophages (33). Taken together, these studies suggest profound metabolic components (both glucose and FA) in macrophage inflammasome activation profiles, which may be significant in disease.
Regulation of the inflammasome by the autophagy program.
Autophagy, an evolutionarily conserved lysosome-dependent degradation process, enables the recycling of cellular components (34). During autophagy, cytosolic constituents, including mitochondria and proteins, are engulfed by autophagosomes and subsequently delivered to the lysosomes for degradation. Recent studies indicate an inhibitory role for autophagy in inflammasome activation. Autophagic gene Atg16-deficient mice (35) and BMDM isolated from microtubule associated protein-1, light chain-3B–deficient mice (25) displayed increased caspase-1–dependent production of IL-1β and IL-18 in response to LPS and other pathogen-derived signals. The proposed mechanism for the augmented caspase-1 response implicates the failure of autophagy-deficient cells to clear damaged mitochondria. In the absence of mitochondrial autophagy, damaged mitochondria accumulate and generate excessive mtROS and release DAMPs, such as mtDNA into the cytoplasm, events that trigger activation of the NLRP3 inflammasome (25, 35).
Inflammasomes in Airways Diseases
Chronic Obstructive Pulmonary Disease
Chronic obstructive pulmonary disease (COPD) involves clinical phenotypes of chronic bronchitis and airway obstruction exacerbated by secondary infections, and emphysema (36). Exposure to cigarette smoke (CS) is a primary risk factor for COPD. The mechanisms underlying COPD pathogenesis remain elusive, but include protease/antiprotease imbalance, inflammation, oxidative stress, and apoptosis. CS targets the lung epithelium, but can activate other resident lung cell types, including inflammatory cells (36).
Animal model studies have demonstrated that CS exposure can induce significant recruitment of neutrophils in the bronchoalveolar space and pulmonary parenchyma. These events were found to be reduced in TLR4-, myeloid differentiation primary response gene 88, and IL-1R1–deficient mice (37). In vitro, CS-stimulated macrophages were shown to increase the production of pro-ILβ, whereas IL-1β secretion required additional stimulation by the inflammasome activator ATP. These studies were among the first to imply a possible contributory role for inflammasome signaling and IL-1β responses in CS-induced airways inflammation (37). Targeted overexpression of the inflammasome-regulated cytokine, IL-18, in murine lungs resulted in widespread pulmonary inflammation, airway remodeling, and emphysematous lesions, suggesting a role for IL-18 in COPD pathogenesis (38).
Evidence for NLRP3 involvement in acute inflammation associated with CS exposure has been further studied in mouse models (39–40). Nlrp3−/− mice displayed reduced caspase-1 activation, IL-18/IL-1β production, and neutrophil influx in the bronchoalveolar lavage (BAL) relative to wild-type mice after acute CS exposure (39). Genetic deletion of the P2X7 receptor also reduced CS-induced caspase-1 activation, IL-1β release, and airway neutrophilia during acute CS exposure (40). Contrasting studies reported that pulmonary inflammation in response to acute CS exposure required IL-1β/α production and IL-1R, but was independent of NLRP3 and caspase-1, due to lack of phenotype in Nlrp3−/− and Casp1−/− mice, respectively (41).
CS inhalation in healthy human subjects can induce proinflammatory cytokines (e.g., IL-1β, TNF) in the lung (42). IL-1α/β were significantly increased in lung tissue and induced sputum of patients with COPD compared with never-smokers (41). Furthermore, the levels of IL-18 were found to be elevated in induced sputum of patients with COPD compared with healthy smokers and nonsmokers, and which correlated to decline of lung function (43). The levels of eATP were elevated in the BAL of patients with COPD (44). Furthermore, serum UA correlated with 30-day mortality in patients with COPD exacerbations (45).
Recently, inflammasome components were also evaluated in the bronchial mucosa and BAL of patients with stable COPD of different severity relative to control healthy smokers and nonsmokers. This study reported lack of NLRP3 inflammasome activation in COPD, with no differences in caspase-1 activation, IL-1β, or IL-18 levels in bronchial biopsies or in BAL between the groups (46). It remains conceivable of a role of the inflammasome system in toxic reactions to CS exposure, as well as a component of immune reactions during secondary infections associated with COPD. Due to contrasting, as well as negative, findings of recent studies, a precise role for NLRP3 inflammasome in COPD pathogenesis remains inconclusive.
Asthma
Allergic asthma is an inflammatory airway disease that is initiated by maladaptive responses of T helper type (Th) 2 cells to common allergen stimulation, which cause airway eosinophilia, mucus hypersecretion, and airway obstruction (47). Increased levels of IL-1β have been reported in the serum, induced sputum or BAL of humans with asthma (5, 48). Elevated eATP was measured in the BAL of humans and mice after allergen challenge (49). The involvement of NLRP3 inflammasome in allergic airway inflammation has been studied in mouse models of allergic asthma. Mitochondrial dysfunction, resulting in increased mtROS generation, has been suggested to be associated with the pathogenesis of allergic airway disease. Kim and colleagues (50) recently demonstrated that suppression of mtROS generation by the novel mitochondrial antioxidant compound NecroX-5 attenuated allergic airway inflammation associated with inhibition of NLRP3 inflammasome activation and reduced IL-1β production. Nlrp3−/−, Asc−/−, or Casp1−/− mice displayed reduced TH2 cytokine production and airway eosinophilia after ovalbumin challenge (51). Reduced Th2-type responses were also observed for mice deficient in IL-1R1, IL-1β, and IL-1α, suggesting the crucial involvement of IL-1R1 signaling in allergic inflammation (51). However, contradictory studies reported no difference in airway inflammation or clinical outcomes in Nlrp3−/− mice subjected to allergen challenge (52). Furthermore, Kool and colleagues suggested that NLRP3 does not significantly contribute to either OVA-mediated allergic airway inflammation in mice (53). In this study, UA was identified as a Th2 cell adjuvant acting independently of NLRP3 or IL-1β (53).
Thus, the role of NLRP3 in the pathogenesis of allergic airway disease remains controversial. Recent studies, however, have identified NLRP3 as an important transcriptional regulator in Th2 differentiation. NLRP3 expression in CD4+ T cells promoted a Th2 transcriptional program that did not require ASC or caspase-1. Th2-related cytokines (e.g., IL-5 and IL-4) were reduced in lung homogenates from ovalbumin-treated Nlrp3−/− mice (54).
Infectious Pulmonary Diseases
Respiratory ailments add significantly to the global burden of disease. Consistent with a general role for inflammasomes described in innate immune responses to systemic infections, several studies demonstrate the involvement of NLRP3 inflammasome in the pathogenesis of common respiratory infections (48). Influenza A virus (IAV), an RNA virus, is a widespread respiratory pathogen. IAV infection triggers NLRP3 priming through TLR7 sensing of its single-stranded viral RNA, whereas the IAV M2 protein triggers NLRP3 inflammasome assembly through K+ efflux (55). The NLRP3 inflammasome provides protection in mouse models of IAV infection, as demonstrated by increased susceptibility and reduced inflammatory responses of Nlrp3−/−, Casp1−/−, and Asc−/− mice (56–57). NLRP3 was essential for protection from respiratory infection with Streptococcus pneumoniae, of which the pneumolysin virulence factor was identified as a novel inflammasome activator (58). In Chlamydia pneumoniae infection, mitochondrial dysfunction involving loss of mitochondrial membrane potential, but not mtROS, was implicated in the mechanisms for NLRP3 inflammasome activation (59). The 6kDa early secreted antigenic target from Mycobacterium tuberculosis (MTB) activated the NLRP3 inflammasome and induced a strong IL-1β response (60). However, Nlrp3−/− mice were not overtly susceptible to MTB infection, due to myeloid-specific and NLRP3 inflammasome–independent compensatory increases in IL-1β production (61). In contrast, Asc−/− mice displayed a susceptibility phenotype to MTB infection (62).
Fibrotic Lung Disease
Idiopathic pulmonary fibrosis (IPF) is a fatal disorder of the lower respiratory tract with unknown etiology, involving inflammation and fibrosis of the interstitium, leading to destruction of the alveolar architecture (63). In a model of bleomycin-induced fibrosis, Nlrp3−/− mice displayed reduced neutrophil influx and reduced IL-1β levels in the lung. Similarly, lung neutrophil infiltration in response to bleomycin was attenuated in mice genetically deficient in ASC or caspase-1, or in mice treated with the caspase-1 inhibitor, Z-yvad-fmk (64). Lung injury and inflammation in this model were associated with increased production of UA and eATP in the BAL (64–65). In human IPF studies, elevated eATP levels were also found in the BAL (65). Statin use was recently identified as a potential risk factor for interstitial lung disease in humans. Interestingly, when tested in the mouse, statins aggravated bleomycin-induced lung injury, and amplified caspase-1 activation (66). Taken together, these studies suggest NLRP3 involvement in the pathogenesis of experimental pulmonary fibrosis, and provide intriguing possibilities for therapeutic targets in IPF.
Cystic Fibrosis
Cystic fibrosis (CF) is a fatal autosomal recessive disease caused by mutation in the CF transmembrane conductance regulator (CFTR) gene. CF is characterized by mucous obstruction of the airways, resulting in recurrent pulmonary infections. The most common CFTR gene mutation is a deletion of phenylalanine at position 508 (CFTRF508 del) (67). CF mice displayed elevated caspase-1 activation and ASC expression in the lung. The activation of the NLRP3 inflammasome in CF lung was associated with elevated levels of the signaling lipid-derived mediator, ceramide (68). In CF epithelial cells bearing the CFTR mutation, Pseudomonas aeruginosa infection promoted mitochondrial dysfunction and increased mitochondrial Ca2+ uptake, which were proposed as a mechanism underlying increased NLRP3-dependent caspase-1 activation (69).
Pulmonary Hypertension
Pulmonary arterial hypertension (PAH) is a serious disease affecting the pulmonary vasculature, characterized by sustained elevation of pulmonary arterial pressure (>25 mm Hg at rest). In PAH, pulmonary arterial remodeling occludes the vessel lumen, leading to right ventricular failure (70). In mice, hypoxia exposure causes pulmonary hypertension, including increased right ventricular systolic pressure and pulmonary vascular remodeling. These changes were also associated with activation of the NLRP3 inflammasome and caspase-1, as well as increased IL-1β production, and were reversed with a superoxide dismutase mimetic (71). Asc−/− mice were resistant to hypoxia-induced pulmonary hypertension, as evidenced by reduced right ventricular systolic pressure and reduced pulmonary vascular remodeling in response to hypoxia. However, Nlrp3−/− mice did not display a phenotype in this model, indicating the possible involvement of alternate inflammasome complexes involving ASC (72).
Acute Lung Injury
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) describe clinical syndromes of acute respiratory failure with substantial morbidity and mortality. Supplemental oxygen inhalation is frequently used to treat severe respiratory failure; however, prolonged exposure to elevated oxygen concentrations (hyperoxia) causes ALI, which, in humans, can lead to ARDS. Nlrp3−/− mice displayed increased mortality under hyperoxic conditions relative to wild-type and Il1β−/− mice. Under hyperoxia, Nlrp3−/− mice displayed reduced lung inflammatory responses, including inflammatory cell infiltration and cytokine expression, without differences in lung IL-1β production relative to wild-type mice (73).
Dolinay and colleagues (74) analyzed the role of the inflammasome in the development of ALI using a murine model of mechanical ventilation (MV)–induced lung injury. MV resulted in the significant regulation of several inflammasome-related genes, including IL-1α (Il1a), caspase-activator domain-10 (Card10), and IL-1 receptor-1 and -2 (Il1r1 and -2). The expression of the Asc gene, the inflammasome adaptor, was up-regulated after MV. Genetic deletion of IL-18 or caspase-1 reduced lung injury and inflammation in response to MV, associated with reduced alveolar–capillary barrier dysfunction and edema (74). These studies indicate a role for the inflammasome system and its regulated cytokines in the propagation of experimental ALI.
Clinical Implications of the Inflammasome and Related DAMPs
In human studies, using plasma specimens from critically ill patients, the expression of inflammasome-related caspase-1, IL-18, and IL1β messenger RNA transcripts was significantly higher in patients with sepsis/ARDS when compared with patients with systemic inflammatory response syndrome (74). Importantly, circulating IL-18 and mtDNA, a mitochondrial DAMP associated with NLRP3 inflammasome activation, were significantly elevated in plasma from critically ill human patients with illnesses such as sepsis and sepsis-induced ARDS, and significantly associated with intensive care unit mortality (74–75). Interestingly, mtDNA was superior to clinical Acute Physiology and Chronic Health Evaluation score classification and the common clinical test of lactate levels in predicting mortality in these patient cohorts (75). These data suggest that inclusion of mtDNA could improve risk prediction in the medical intensive care unit. These observations underscore the hypothesis that mitochondrial dysfunction and inflammasome system components may contribute to the pathogenesis of human diseases, such as sepsis and ALI/ARDS.
Conclusions
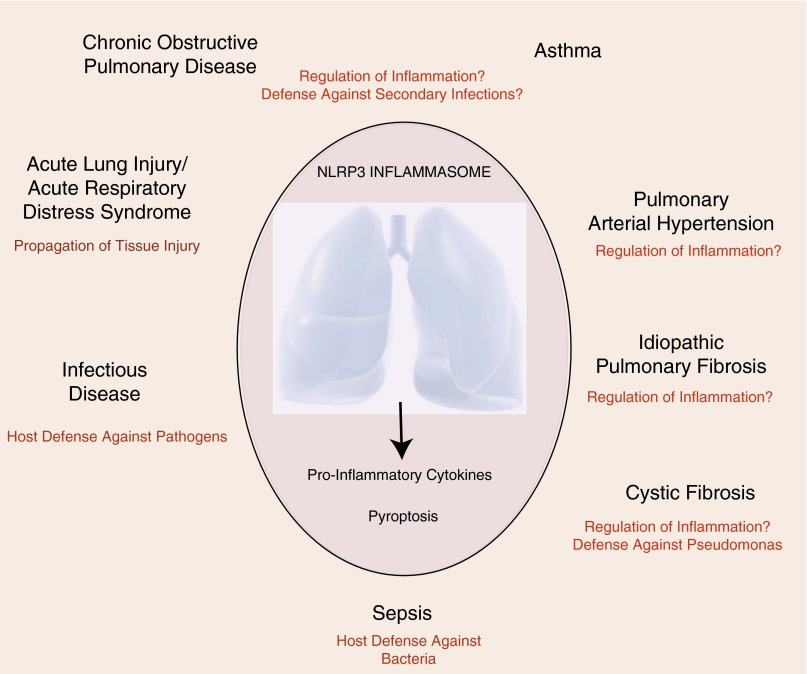
The NLRP3 inflammasome has emerged as an important regulator of the innate immune system and associated inflammatory responses that may have significant impact in the progression of human diseases, including pulmonary diseases (Figure 4). The role of NLRP3 inflammasome as a primary surveillance mechanism in infectious disease is well known. NLRP3 inflammasome represents a single component of complex innate immune responses to pathogens, particulates, and noxious stimuli (48), and, as shown in several respiratory disease models, may not be the sole regulator of IL-1β production in host responses (61, 73). Although consistent with the hypothetical role of NLRP3 in inflammatory responses, the studies that link the role of NLRP3 inflammasome in pathogenesis of specific respiratory disease, including allergic immune responses and COPD, remain unclear. Paradoxical or detrimental roles of the NLRP3 inflammasome may reflect pleiotropic effects not limited to innate immune defenses, but also involving the propagation of chronic inflammatory responses or alteration of cell death pathways, which may exacerbate injury. Emerging studies begin to elucidate the possible role of NLRP3 in lung diseases, such as IPF, PAH, and CF, which may also involve aberrant regulation of inflammatory responses, although more research is needed in these areas.
Figure 4.
Potential involvement of the NLRP3 inflammasome in lung disease. The NLRP3 inflammasome exerts incompletely understood functions in lung diseases. In diseases involving pathogen infection, bacterial sepsis, or secondary infections, NLRP3 is predicted to function as an effector of innate immune responses. The role of NLRP3 in asthma and chronic obstructive pulmonary disease remains controversial. Recent studies have implied protective roles of NLRP3 in regulating the inflammatory response in cystic fibrosis, pulmonary hypertension, and idiopathic pulmonary fibrosis. In acute lung injury/acute respiratory distress syndrome, the NLRP3 inflammasome and its regulated cytokines have been implicated in tissue injury.
Inflammasome system components are expressed in several lung cell types. Among them, alveolar macrophages and dendritic cells represent major sources of inflammasome-associated cytokines, including IL-1β and IL-18, in the lung. The potential contribution of the inflammasome in structural cells, such as epithelial cells and fibroblasts, in the process of injury and repair responses in various lung diseases with tissue remodeling needs to be further investigated. Recent studies demonstrate that lung epithelial cells contribute to protection from bacteria-induced lung injury by internalization of cardiolipin through a unique cardiolipin transporter, Atp8b, the P-type ATPase transmembrane lipid pump, expressed in type II epithelial cells (76). Given that cardiolipin has been implicated in NLRP3 inflammasome activation in macrophages (29), the relationship of this novel mechanism to potential innate immune responses in epithelial cells, such as inflammasome activation, and IL-1β production requires further investigation.
NLRP3 may also have noncanonical inflammasome-independent roles, such as the transcriptional regulation of T cell differentiation (54). In the hyperoxia-induced ALI model, NLRP3 promotes apoptosis of alveolar epithelial cells by signal transducer and activator of transcription 3 signaling activation (73). Based on the unique architecture of lung and the diverse roles of NLRP3, understanding cell type–specific roles of the inflammasome remains challenging. A further challenge will be to dissect the role of secondary effectors, such as the inflammasome cytokines, IL-1β and IL-18, or DAMPs, such as mtDNA, eATP, and UA, as either biomarkers of disease progression or effectors of disease pathogenesis. Improved understanding of the function of inflammasome effector molecules in lung pathologies may also provide novel diagnostic or therapeutic targets for regulation of the NLRP3 inflammasome in disease.
Footnotes
This work was supported by National Institutes of Health grants P01 HL108801, R01 HL079904, R01 HL055330 (A.M.K.C.), and RO1 HL060234 (S.W.R.).
Author Contributions: S.L. generated the first draft; S.L., S.W.R., and A.M.K.C. wrote the manuscript; S.W.R. and A.M.K.C. edited the manuscript; S.L. and S.W.R. generated figures; G.-Y.S., S.W.R., and A.M.K.C. supervised the project.
Originally Published in Press as DOI: 10.1165/rcmb.2015-0231TR on September 29, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol. 2015;16:27–35. doi: 10.1038/ni.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA, et al. The NLR gene family: a standard nomenclature. Immunity. 2008;28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann N Y Acad Sci. 2014;1319:82–95. doi: 10.1111/nyas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21:677–687. doi: 10.1038/nm.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brusselle GG, Provoost S, Bracke KR, Kuchmiy A, Lamkanfi M. Inflammasomes in respiratory disease: from bench to bedside. Chest. 2014;145:1121–1133. doi: 10.1378/chest.13-1885. [DOI] [PubMed] [Google Scholar]
- 6.Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, Latz E, Fitzgerald KA. AIM2 recognizes cytosolic dsDNA and forms a caspase-1–activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 8.Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1–dependent necrosis. Mol Microbiol. 2000;38:31–40. doi: 10.1046/j.1365-2958.2000.02103.x. [DOI] [PubMed] [Google Scholar]
- 9.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Jorgensen I, Miao EA. Pyroptotic cell death defends against intracellular pathogens. Immunol Rev. 2015;265:130–142. doi: 10.1111/imr.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 12.Novick D, Kim S, Kaplanski G, Dinarello CA. Interleukin-18, more than a Th1 cytokine. Semin Immunol. 2013;25:439–448. doi: 10.1016/j.smim.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 13.Gong YN, Wang X, Wang J, Yang Z, Li S, Yang J, Liu L, Lei X, Shao F. Chemical probing reveals insights into the signaling mechanism of inflammasome activation. Cell Res. 2010;20:1289–1305. doi: 10.1038/cr.2010.135. [DOI] [PubMed] [Google Scholar]
- 14.Ghonime MG, Shamaa OR, Das S, Eldomany RA, Fernandes-Alnemri T, Alnemri ES, Gavrilin MA, Wewers MD. Inflammasome priming by lipopolysaccharide is dependent upon ERK signaling and proteasome function. J Immunol. 2014;192:3881–3888. doi: 10.4049/jimmunol.1301974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross O, Poeck H, Bscheider M, Dostert C, Hannesschläger N, Endres S, Hartmann G, Tardivel A, Schweighoffer E, Tybulewicz V, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defence. Nature. 2009;459:433–436. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 16.Shio MT, Eisenbarth SC, Savaria M, Vinet AF, Bellemare MJ, Harder KW, Sutterwala FS, Bohle DS, Descoteaux A, Flavell RA, et al. Malarial hemozoin activates the NLRP3 inflammasome through Lyn and Syk kinases. PLoS Pathog. 2009;5:e1000559. doi: 10.1371/journal.ppat.1000559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauernfeind F, Rieger A, Schildberg FA, Knolle PA, Schmid-Burgk JL, Hornung V. NLRP3 inflammasome activity is negatively controlled by miR-223. J Immunol. 2012;189:4175–4181. doi: 10.4049/jimmunol.1201516. [DOI] [PubMed] [Google Scholar]
- 18.Haneklaus M, Gerlic M, Kurowska-Stolarska M, Rainey AA, Pich D, McInnes IB, Hammerschmidt W, O’Neill LA, Masters SL. Cutting edge: miR-223 and EBV miR-BART15 regulate the NLRP3 inflammasome and IL-1β production. J Immunol. 2012;189:3795–3799. doi: 10.4049/jimmunol.1200312. [DOI] [PubMed] [Google Scholar]
- 19.Py BF, Kim MS, Vakifahmetoglu-Norberg H, Yuan J. Deubiquitination of NLRP3 by BRCC3 critically regulates inflammasome activity. Mol Cell. 2013;49:331–338. doi: 10.1016/j.molcel.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 20.Hernandez-Cuellar E, Tsuchiya K, Hara H, Fang R, Sakai S, Kawamura I, Akira S, Mitsuyama M. Cutting edge: nitric oxide inhibits the NLRP3 inflammasome. J Immunol. 2012;189:5113–5117. doi: 10.4049/jimmunol.1202479. [DOI] [PubMed] [Google Scholar]
- 21.Ghaemi-Oskouie F, Shi Y. The role of uric acid as an endogenous danger signal in immunity and inflammation. Curr Rheumatol Rep. 2011;13:160–166. doi: 10.1007/s11926-011-0162-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 23.Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G. K⁺ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38:1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9:847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakahira K, Haspel JA, Rathinam VA, Lee SJ, Dolinay T, Lam HC, Englert JA, Rabinovitch M, Cernadas M, Kim HP, et al. Autophagy proteins regulate innate immune responses by inhibiting the release of mitochondrial DNA mediated by the NALP3 inflammasome. Nat Immunol. 2011;12:222–230. doi: 10.1038/ni.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 27.dos Santos G, Rogel MR, Baker MA, Troken JR, Urich D, Morales-Nebreda L, Sennello JA, Kutuzov MA, Sitikov A, Davis JM, et al. Vimentin regulates activation of the NLRP3 inflammasome. Nat Commun. 2015;6:6574. doi: 10.1038/ncomms7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V, Han R, Qiao L, et al. Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity. 2013;39:311–323. doi: 10.1016/j.immuni.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tschopp J, Schroder K. NLRP3 inflammasome activation: the convergence of multiple signalling pathways on ROS production? Nat Rev Immunol. 2010;10:210–215. doi: 10.1038/nri2725. [DOI] [PubMed] [Google Scholar]
- 32.Moon JS, Hisata S, Park MA, DeNicola GM, Ryter SW, Nakahira K, Choi AM. mTORC1-induced HK1-dependent glycolysis regulates NLRP3 inflammasome activation. Cell Reports. 2015;12:102–115. doi: 10.1016/j.celrep.2015.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Moon JS, Lee S, Park MA, Siempos II, Haslip M, Lee PJ, Yun M, Kim CK, Howrylak J, Ryter SW, et al. UCP2-induced fatty acid synthase promotes NLRP3 inflammasome activation during sepsis. J Clin Invest. 2015;125:665–680. doi: 10.1172/JCI78253. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Choi AM, Ryter SW, Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 35.Saitoh T, Fujita N, Jang MH, Uematsu S, Yang BG, Satoh T, Omori H, Noda T, Yamamoto N, Komatsu M, et al. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1β production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]
- 36.Barnes PJ. Cellular and molecular mechanisms of chronic obstructive pulmonary disease. Clin Chest Med. 2014;35:71–86. doi: 10.1016/j.ccm.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Doz E, Noulin N, Boichot E, Guénon I, Fick L, Le Bert M, Lagente V, Ryffel B, Schnyder B, Quesniaux VF, et al. Cigarette smoke-induced pulmonary inflammation is TLR4/MyD88 and IL-1R1/MyD88 signaling dependent. J Immunol. 2008;180:1169–1178. doi: 10.4049/jimmunol.180.2.1169. [DOI] [PubMed] [Google Scholar]
- 38.Kang MJ, Choi JM, Kim BH, Lee CM, Cho WK, Choe G, Kim DH, Lee CG, Elias JA. IL-18 induces emphysema and airway and vascular remodeling via IFN-γ, IL-17A, and IL-13. Am J Respir Crit Care Med. 2012;185:1205–1217. doi: 10.1164/rccm.201108-1545OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eltom S, Belvisi MG, Stevenson CS, Maher SA, Dubuis E, Fitzgerald KA, Birrell MA. Role of the inflammasome-caspase1/11-IL-1/18 axis in cigarette smoke driven airway inflammation: an insight into the pathogenesis of COPD. PLoS One. 2014;9:e112829. doi: 10.1371/journal.pone.0112829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eltom S, Stevenson CS, Rastrick J, Dale N, Raemdonck K, Wong S, Catley MC, Belvisi MG, Birrell MA. P2X7 receptor and caspase 1 activation are central to airway inflammation observed after exposure to tobacco smoke. PLoS One. 2011;6:e24097. doi: 10.1371/journal.pone.0024097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pauwels NS, Bracke KR, Dupont LL, Van Pottelberge GR, Provoost S, Vanden Berghe T, Vandenabeele P, Lambrecht BN, Joos GF, Brusselle GG. Role of IL-1α and the Nlrp3/caspase-1/IL-1β axis in cigarette smoke–induced pulmonary inflammation and COPD. Eur Respir J. 2011;38:1019–1028. doi: 10.1183/09031936.00158110. [DOI] [PubMed] [Google Scholar]
- 42.Kuschner WG, D’Alessandro A, Wong H, Blanc PD. Dose-dependent cigarette smoking–related inflammatory responses in healthy adults. Eur Respir J. 1996;9:1989–1994. doi: 10.1183/09031936.96.09101989. [DOI] [PubMed] [Google Scholar]
- 43.Rovina N, Dima E, Gerassimou C, Kollintza A, Gratziou C, Roussos C. Interleukin-18 in induced sputum: association with lung function in chronic obstructive pulmonary disease. Respir Med. 2009;103:1056–1062. doi: 10.1016/j.rmed.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 44.Mortaz E, Braber S, Nazary M, Givi ME, Nijkamp FP, Folkerts G. ATP in the pathogenesis of lung emphysema. Eur J Pharmacol. 2009;619:92–96. doi: 10.1016/j.ejphar.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 45.Bartziokas K, Papaioannou AI, Loukides S, Papadopoulos A, Haniotou A, Papiris S, Kostikas K. Serum uric acid as a predictor of mortality and future exacerbations of COPD. Eur Respir J. 2014;43:43–53. doi: 10.1183/09031936.00209212. [DOI] [PubMed] [Google Scholar]
- 46.Di Stefano A, Caramori G, Barczyk A, Vicari C, Brun P, Zanini A, Cappello F, Garofano E, Padovani A, Contoli M, et al. Innate immunity but not NLRP3 inflammasome activation correlates with severity of stable COPD. Thorax. 2014;69:516–524. doi: 10.1136/thoraxjnl-2012-203062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol. 2008;8:183–192. doi: 10.1038/nri2254. [DOI] [PubMed] [Google Scholar]
- 48.dos Santos G, Kutuzov MA, Ridge KM. The inflammasome in lung diseases. Am J Physiol Lung Cell Mol Physiol. 2012;303:L627–L633. doi: 10.1152/ajplung.00225.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med. 2007;13:913–919. doi: 10.1038/nm1617. [DOI] [PubMed] [Google Scholar]
- 50.Kim SR, Kim DI, Kim SH, Lee H, Lee KS, Cho SH, Lee YC. NLRP3 inflammasome activation by mitochondrial ROS in bronchial epithelial cells is required for allergic inflammation. Cell Death Dis. 2014;5:e1498. doi: 10.1038/cddis.2014.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Besnard AG, Guillou N, Tschopp J, Erard F, Couillin I, Iwakura Y, Quesniaux V, Ryffel B, Togbe D. NLRP3 inflammasome is required in murine asthma in the absence of aluminum adjuvant. Allergy. 2011;66:1047–1057. doi: 10.1111/j.1398-9995.2011.02586.x. [DOI] [PubMed] [Google Scholar]
- 52.Allen IC, Jania CM, Wilson JE, Tekeppe EM, Hua X, Brickey WJ, Kwan M, Koller BH, Tilley SL, Ting JP. Analysis of NLRP3 in the development of allergic airway disease in mice. J Immunol. 2012;188:2884–2893. doi: 10.4049/jimmunol.1102488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kool M, Willart MA, van Nimwegen M, Bergen I, Pouliot P, Virchow JC, Rogers N, Osorio F, Reis e Sousa C, Hammad H, et al. An unexpected role for uric acid as an inducer of T helper 2 cell immunity to inhaled antigens and inflammatory mediator of allergic asthma Immunity 201134527–540.[Published erratum appears in Immunity 34:627.] [DOI] [PubMed] [Google Scholar]
- 54.Bruchard M, Rebé C, Derangère V, Togbé D, Ryffel B, Boidot R, Humblin E, Hamman A, Chalmin F, Berger H, et al. The receptor NLRP3 is a transcriptional regulator of TH2 differentiation. Nat Immunol. 2015;16:859–870. doi: 10.1038/ni.3202. [DOI] [PubMed] [Google Scholar]
- 55.Thomas PG, Dash P, Aldridge JR, Jr, Ellebedy AH, Reynolds C, Funk AJ, Martin WJ, Lamkanfi M, Webby RJ, Boyd KL, et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. 2009;30:566–575. doi: 10.1016/j.immuni.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ichinohe T, Lee HK, Ogura Y, Flavell R, Iwasaki A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 2009;206:79–87. doi: 10.1084/jem.20081667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, Guthrie EH, Pickles RJ, Ting JP. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. 2009;30:556–565. doi: 10.1016/j.immuni.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McNeela EA, Burke A, Neill DR, Baxter C, Fernandes VE, Ferreira D, Smeaton S, El-Rachkidy R, McLoughlin RM, Mori A, et al. Pneumolysin activates the NLRP3 inflammasome and promotes proinflammatory cytokines independently of TLR4. PLoS Pathog. 2010;6:e1001191. doi: 10.1371/journal.ppat.1001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimada K, Crother TR, Karlin J, Chen S, Chiba N, Ramanujan VK, Vergnes L, Ojcius DM, Arditi M. Caspase-1 dependent IL-1β secretion is critical for host defense in a mouse model of Chlamydia pneumoniae lung infection. PLoS One. 2011;6:e21477. doi: 10.1371/journal.pone.0021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mishra BB, Moura-Alves P, Sonawane A, Hacohen N, Griffiths G, Moita LF, Anes E. Mycobacterium tuberculosis protein ESAT-6 is a potent activator of the NLRP3/ASC inflammasome. Cell Microbiol. 2010;12:1046–1063. doi: 10.1111/j.1462-5822.2010.01450.x. [DOI] [PubMed] [Google Scholar]
- 61.Dorhoi A, Nouailles G, Jörg S, Hagens K, Heinemann E, Pradl L, Oberbeck-Müller D, Duque-Correa MA, Reece ST, Ruland J, et al. Activation of the NLRP3 inflammasome by Mycobacterium tuberculosis is uncoupled from susceptibility to active tuberculosis. Eur J Immunol. 2012;42:374–384. doi: 10.1002/eji.201141548. [DOI] [PubMed] [Google Scholar]
- 62.McElvania Tekippe E, Allen IC, Hulseberg PD, Sullivan JT, McCann JR, Sandor M, Braunstein M, Ting JP. Granuloma formation and host defense in chronic Mycobacterium tuberculosis infection requires PYCARD/ASC but not NLRP3 or caspase-1. PLoS One. 2010;5:e12320. doi: 10.1371/journal.pone.0012320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Selman M, King TE, Pardo A American Thoracic Society; European Respiratory Society; American College of Chest Physicians. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 64.Gasse P, Riteau N, Charron S, Girre S, Fick L, Pétrilli V, Tschopp J, Lagente V, Quesniaux VF, Ryffel B, et al. Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. 2009;179:903–913. doi: 10.1164/rccm.200808-1274OC. [DOI] [PubMed] [Google Scholar]
- 65.Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, Kanellopoulos J, Quesniaux VF, Marchand-Adam S, Crestani B, et al. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med. 2010;182:774–783. doi: 10.1164/rccm.201003-0359OC. [DOI] [PubMed] [Google Scholar]
- 66.Xu JF, Washko GR, Nakahira K, Hatabu H, Patel AS, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, et al. COPDGene Investigators. Statins and pulmonary fibrosis: the potential role of NLRP3 inflammasome activation. Am J Respir Crit Care Med. 2012;185:547–556. doi: 10.1164/rccm.201108-1574OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 68.Grassmé H, Carpinteiro A, Edwards MJ, Gulbins E, Becker KA. Regulation of the inflammasome by ceramide in cystic fibrosis lungs. Cell Physiol Biochem. 2014;34:45–55. doi: 10.1159/000362983. [DOI] [PubMed] [Google Scholar]
- 69.Rimessi A, Bezzerri V, Patergnani S, Marchi S, Cabrini G, Pinton P. Mitochondrial Ca2+-dependent NLRP3 activation exacerbates the Pseudomonas aeruginosa–driven inflammatory response in cystic fibrosis. Nat Commun. 2015;6:6201. doi: 10.1038/ncomms7201. [DOI] [PubMed] [Google Scholar]
- 70.Farber HW, Loscalzo J. Pulmonary arterial hypertension. N Engl J Med. 2004;351:1655–1665. doi: 10.1056/NEJMra035488. [DOI] [PubMed] [Google Scholar]
- 71.Villegas LR, Kluck D, Field C, Oberley-Deegan RE, Woods C, Yeager ME, El Kasmi KC, Savani RC, Bowler RP, Nozik-Grayck E. Superoxide dismutase mimetic, MnTE-2-PyP, attenuates chronic hypoxia-induced pulmonary hypertension, pulmonary vascular remodeling, and activation of the NALP3 inflammasome. Antioxid Redox Signal. 2013;18:1753–1764. doi: 10.1089/ars.2012.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cero FT, Hillestad V, Sjaastad I, Yndestad A, Aukrust P, Ranheim T, Lunde IG, Olsen MB, Lien E, Zhang L, et al. Absence of the inflammasome adaptor ASC reduces hypoxia-induced pulmonary hypertension in mice. Am J Physiol Lung Cell Mol Physiol. 2015;309:L378–L387. doi: 10.1152/ajplung.00342.2014. [DOI] [PubMed] [Google Scholar]
- 73.Mizushina Y, Shirasuna K, Usui F, Karasawa T, Kawashima A, Kimura H, Kobayashi M, Komada T, Inoue Y, Mato N, et al. NLRP3 protein deficiency exacerbates hyperoxia-induced lethality through Stat3 protein signaling independent of interleukin-1β. J Biol Chem. 2015;290:5065–5077. doi: 10.1074/jbc.M114.603217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dolinay T, Kim YS, Howrylak J, Hunninghake GM, An CH, Fredenburgh L, Massaro AF, Rogers A, Gazourian L, Nakahira K, et al. Inflammasome-regulated cytokines are critical mediators of acute lung injury. Am J Respir Crit Care Med. 2012;185:1225–1234. doi: 10.1164/rccm.201201-0003OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakahira K, Kyung SY, Rogers AJ, Gazourian L, Youn S, Massaro AF, Quintana C, Osorio JC, Wang Z, Zhao Y, et al. Circulating mitochondrial DNA in patients in the ICU as a marker of mortality: derivation and validation. PLoS Med. 2013;10:e1001577–, discussion e1001577. doi: 10.1371/journal.pmed.1001577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ray NB, Durairaj L, Chen BB, McVerry BJ, Ryan AJ, Donahoe M, Waltenbaugh AK, O’Donnell CP, Henderson FC, Etscheidt CA, et al. Dynamic regulation of cardiolipin by the lipid pump Atp8b1 determines the severity of lung injury in experimental pneumonia. Nat Med. 2010;16:1120–1127. doi: 10.1038/nm.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]