Abstract
Asthma is a chronic inflammatory disease, which is characterized by activation of CD4+ T helper 2 cells orchestrating an allergic airway response. Whereas the role of Wnt family members in regulating T cell maintenance and maturation is established, their contribution to T cell activation in allergic asthma is not known. We hypothesized that Wnt10b plays a role in the modulation of the allergic airway response and affects T cell activation and polarization. Using an in vivo house dust mite asthma model, Wnt10b-deficient (Wnt10b−/−) mice were allergen-sensitized and inflammation, as well as T cell activation, was studied in vivo and in vitro. Wnt10b−/− mice exhibited an augmented inflammatory phenotype with an increase in eosinophils in the bronchoalveolar lavage and IL-4 and IL-13 in the lungs when compared with wild-type mice. In vitro studies confirmed an increased T helper type 2 polarization and increased T cell activation of Wnt10b−/− cells. Accordingly, the percentage of naive T cells was elevated by the addition of recombinant Wnt10b protein. Finally, Wnt10b−/− mice exhibited an increase in the percentage of effector T cells in the lungs after house dust mite sensitization, which indicated a heightened activation state, measured by an increased percentage of CD69hiCD11ahi cells. These findings suggest that Wnt10b plays an important role in regulating asthmatic airway inflammation through modification of the T cell response and is a prospective target in the disease process.
Keywords: Wnt pathway, Wnt10b, asthma, allergy, T helper type 2
Clinical Relevance
Whereas a role for Wnt signaling in T cell maturation and differentiation is established, the contribution of Wnt ligands to T cell activation in allergic asthma is not known. In the presented study, Wnt10b-deficient mice exhibit an augmented inflammatory phenotype in a murine model of allergic asthma. T cells lacking Wnt10b show a heightened activation state in vivo and in vitro, which can be reversed by addition of recombinant Wnt10b. This identifies Wnt10b as a novel molecule in the regulation of asthmatic inflammation and hints to new therapeutic approaches.
Allergic asthma is the most common chronic disease in children (1). It is characterized by an allergen-driven T helper (Th) 2 response that leads to sensitization and activation of effector cells in the lung. Each re-exposure to allergen is followed by repeated inflammation with the release of cytokines, chemokines, and histamine, and eventually results in structural remodeling of the lung. CD4+ Th2 cells play a crucial role in the pathogenesis of asthma, as their activation orchestrates the recruitment of effector cells, such as eosinophils and mast cells, into the lung. Whereas the mechanisms of recruitment and Th2 polarization are partly known, molecules that modulate the initial T cell response are less well described.
Wnt10b is a member of the Wnt signaling molecule family. Recent studies identified the up-regulation of Wnt10b after T cell receptor (TCR) activation with anti-CD3 or anti-CD3/-CD28 and after antigen presentation in vitro (2). Wnt10b functions equally in a paracrine and autocrine fashion, and is expressed by T cells and epithelial cells in the thymus and various T cell lines (3, 4). Functionally, Wnt10b was shown to be critical for hematopoietic stem cell proliferation and expansion after injury (5, 6). In the present study, Wnt10b is demonstrated to be also elevated in T cells in a mouse model of allergic asthma, and the role in asthma pathogenesis is explored.
The Wnt signaling pathway mediates central roles in progenitor and stem cell maintenance, as well as T cell development and differentiation. One of the earliest-described functions of the Wnt signaling pathway is the development and regulation of cellular immunity (7). The transcription factors T cell factor 1 (TCF-1) and lymphoid enhancer-binding factor 1 (LEF-1) are crucial for T cell function, and require coactivation by the downstream canonical Wnt signaling mediator, β-catenin (8). However, in lung disease, Wnt signaling is described mainly in proliferative processes, such as lung fibrosis and lung cancer, and there is limited knowledge regarding the contribution of the pathway to inflammatory diseases of the lung (9, 10). In asthma, up-regulation of the noncanonical Wnt ligand, Wnt5a, in the airway smooth muscle cell remodeling process has been documented (11). In a gene association study, Sharma and colleagues (12) discovered single-nucleotide polymorphisms for several members of the Wnt family in two childhood asthma cohorts, although the functional consequences of these modulations are unknown. A first in vivo study showed the attenuation of the allergic asthmatic response in a mouse model overexpressing Wnt1 in lung epithelial cells (13).
The effects of Wnt signaling on T cell activation and differentiation have mostly been examined through the intracellular signaling cascade. β-catenin signaling has been shown to be essential for the development and maintenance of memory CD8+ T cells and for promoting regulatory T cell survival (14, 15). Moreover, treatment of CD8+ cells with TWS119, a synthetic small molecule, which activates Wnt signaling, arrested effector T cell differentiation (16). However, little is known about the pathological role that single Wnt ligands play in the function of T cells in disease. Here, we show that Wnt10b is up-regulated in the lungs and T cells of house dust mite (HDM)–sensitized mice compared with control animals. The objective of the present study was, therefore, to define the role the Wnt ligand, Wnt10b, plays in the immunological processes in allergic asthma.
In the present study, Wnt10b deficiency is found to lead to augmented T cell activation, increased Th2 polarization shown by elevated concentrations of IL-4 and IL-13, and increased effector memory T cells in the lung in a murine model of allergic asthma. Furthermore, the addition of recombinant Wnt10b increased the percentage of naive CD4+ and CD8+ cells in vitro, providing insight into the function of Wnt10b in allergic asthma.
Material and Methods
Wnt10b-Flexible Accelerated STOP Tetracyclin-Knockin Operator Mice
Our laboratory created Wnt10b–flexible accelerated STOP tetracyclin operator (FAST) mice using the Flexible Accelerated STOP Tetracyclin Operator-knockin system previously described (17). Wnt10b-deficient (Wnt10b−/−) mice were bred by using the whole-body knockout of the Wnt10b-FAST mouse (termed Wnt10b−/−), which was generated by our laboratory, as described in Figure E1 in the online supplement. Wnt10b−/− animals have a normal lifespan, and do not exhibit any observable abnormalities in lifespan or body weight over the course of Embryonic Day (E) 14 to 1.5 years (Figure E1). Histological examination of the developing lungs from E14 to 2 months did not reveal overt pathological defects, such as changes in branching or alveolarization (Figure E1). At 2 months of age, there was no difference in mean linear intercept between Wnt10b−/− and wild-type mice (data not shown). For the experiments, the mice were backcrossed into C57B6/J background for four generations and only littermate controls from heterozygous mating pairs were used. All animal experiments were approved by the Institutional Animal Care and Use Committee of Columbia University (New York, NY).
HDM Challenge
Mice were administered HDM extract (Greer Laboratories, Lenoir, NC) intranasally, as previously described (40 μg/25 μl PBS, five times/wk for 3 weeks) (18). At 24 hours after the last antigen challenge, mice were killed for the experimental procedures.
In Situ Hybridization
In situ hybridization was performed as previously described (19). A full-length (1.8-kb) murine Wnt10b vector was used to prepare antisense and sense digoxigenin (DIG)-labeled probes. Immunohistochemistry was performed with anti–DIG-alkaline phosphatase and visualized with nitro-blue tetrazolium (NTB)/5-bromo-4-chloro-3-indolyl phosphate (BCIP) substrate (Roche, Basel, Switzerland). Digoxigenin-labeled actin was used as a positive control (Roche).
Bronchoalveolar Lavage and Differential Cell Count
The lungs were lavaged twice with 1 ml sterile PBS, centrifuged, and resuspended. Total cell concentrations were counted with a hemocytometer, and cytospin preparations were stained with Quick-Diff (Imeb Inc., San Marcos, CA), and cells counted for differential counts with morphological criteria. Remaining cells were pelleted and used for RNA isolation.
Lung Fixation, Histology, and Immunofluorescence
Lungs were either flash frozen for further analysis or pressure perfused with formalin, paraffin embedded, and sliced into 5-μm-thick sections. Hematoxylin and eosin staining was performed after deparaffinization. An inflammatory score was measured semiquantitatively by randomly obtaining pictures (Stage-Pro, Prior, Image Pro Plus, Rockland, MA) and blinded scoring on a scale from 0 to 4, as reported previously (7). Slides for immunofluorescence were deparaffinized, dehydrated, and high-pH antigen retrieval was performed. Antibodies for eosinophil major basic protein (Santa Cruz Biotechnology, Dallas, TX) were applied at antibody-dependent concentrations and incubated overnight at 4°C. Alexa488–anti-rabbit secondary antibody (Invitrogen, Life Technology, Carlsbad, CA) was used as secondary antibody.
Cytokine ELISA
Lung tissue (60 mg) was homogenized in 600 μl RIPA buffer (Santa Cruz). IL-4, IL-5, IL-13, and IL-17 ELISAs (eBioscience, San Diego, CA) were performed with 50 μl lung homogenate, per the manufacturer’s instructions.
mRNA Extraction and RT-PCR
Lung tissue was homogenized and bronchoalveolar lavage (BAL) fluid cell pellets and CD90.2-magnetically enriched splenic T cells were resuspended in RLT-buffer. RNA was extracted using the RNeasy Mini Kit (Qiagen, Germantown, MD). Taqman Gene expression assays (Invitrogen) were performed for the following probes: Wnt10b; Arginase 1; CCL2; GATA3; IL-4; and T-box expressed in T cells (T-bet). glyceraldehyde-3-phosphate dehydrogenase and β-actin were used as endogenous controls; for tissue from HDM-sensitized mice, β2-microglobulin was used as control (20). Results are represented as fold change.
Flow Cytometry
In vivo labeling with CD90.2 antibody was performed as described previously (21). Lungs of HDM-sensitized mice and controls were lavaged, and lungs dissected and digested in RPMI1640 medium with collagenase D, DNase, and trypsin for 1 hour at 37°C. Spleen, mesenteric lymph nodes, and thymus were dissected and manually disrupted. Fluorescence-conjugated antibodies were purchased from eBioscience and staining was performed according to manufacturer’s protocol uszing the BD LSRII flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ).
Lymphocyte Activation and Proliferation Assay
Splenic T cells were incubated with carboxyfluorescein diacetate succinimidyl ester (CFSE) for 8 min at 37° before seeding. They were cultured in the presence of CD3/CD28 activator beads (Invitrogen), β-Mercaptoethanol (Gibco, Life Technologies), and IL-2 (R&D Systems, Minneapolis, MN). Recombinant murine Wnt10b (R&D Systems) was added at 200 ng/ml. Proliferation was assessed using the CFSE dilution assay. For Th1/Th2 polarization experiments and RNA expression analysis, T cells were isolated using the MACS magnetic beads system (Miltenyi Biotec, San Diego, CA) with positive selection of CD90.2-positive cells, and IL-12 or IL-4 (R&D Systems) were added.
T Cell Isolation, Culture, and Low-Density PCR-Based Array
For the PCR-based array, murine T cells were isolated from spleens of C57bl6/J mice by magnetic bead–based cell separation using CD4 beads. The cells were activated by CD3/CD28 beads and, to analyze the effect of Wnt10b on mouse T cells, the cells were treated with or without purified Wnt10b protein (1 μg/ml) and incubated for 24 hours. After incubation, RNA was isolated and downstream target genes in the Wnt signaling pathway analyzed using real-time RT-PCR–based array (PAMM-243Z; Qiagen Inc., Valencia, CA), according to the manufacturer’s protocol. The data were obtained by normalization of three independent experiments.
Results
Wnt10b Expression in the Lungs of HDM-Sensitized Mice
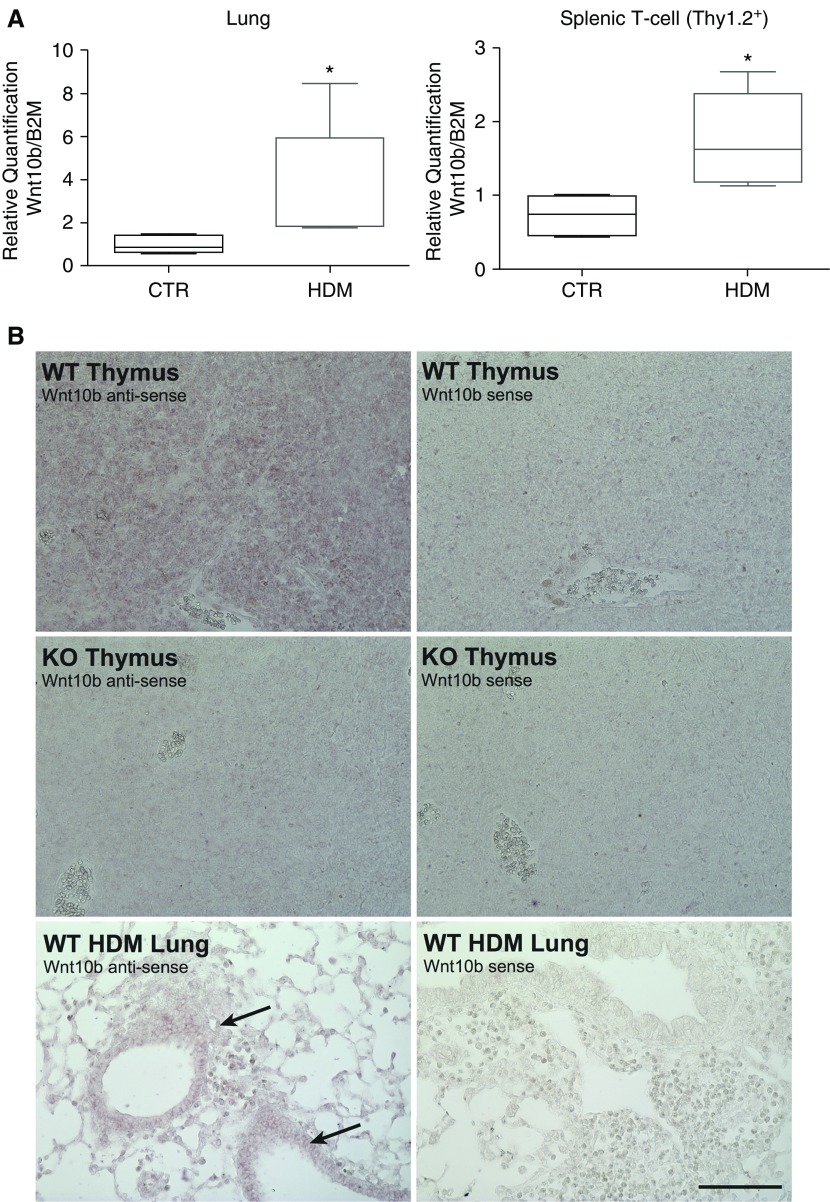
To explore the contribution of the Wnt pathway in inflammatory processes in asthma, an acute HDM allergen challenge model was examined (18). Analysis of Wnt signaling molecules in whole-lung homogenates of acutely sensitized mice exhibited increased levels of Wnt10b not only in the lung, but also in T cells (CD90.2-enriched splenic cells; Figure 1). In situ hybridization demonstrated that Wnt10b expression localized within the lung to the epithelium and inflammatory infiltrates in HDM-challenged mice. Highly positive staining was also identified in the thymus, as previously reported (Figure 1). Additional in vitro experiments with CD4-positive splenic T cells could confirm the involvement of Wnt10b in the canonical signaling pathway by activation of a specific pattern of canonical Wnt signaling targets (Figure E3).
Figure 1.
Increased Wnt10b expression in the lung and T cells of house dust mite (HDM)–challenged mice. (A) Increased mRNA expression of Wnt10b in whole-lung homogenates and Thy1.2/CD90.2-enriched T cells of HDM-challenged mice (n = 4–5/group, *P < 0.05 Mann-Whitney U test, data are presented as median with upper/lower quartile). (B) In situ hybridization with full-length Wnt10b shows efficient knockout (KO) in the thymus and localizes expression in the HDM-challenged lung to the epithelium and inflammatory cells (arrows; representative images; scale bar, 100 μm). B2M, β2-microglobulin; CTR, control; WT, wild type.
Ablation of Wnt10b Leads to Increased Inflammation after Acute HDM Challenge
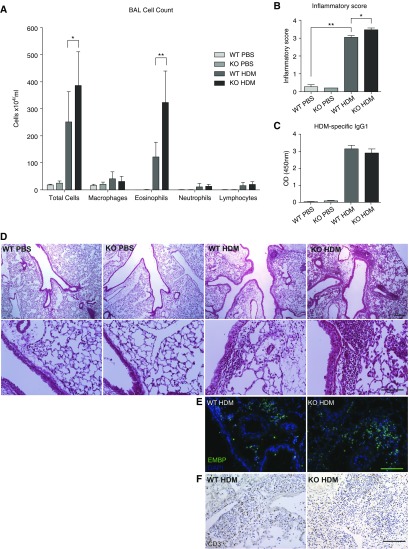
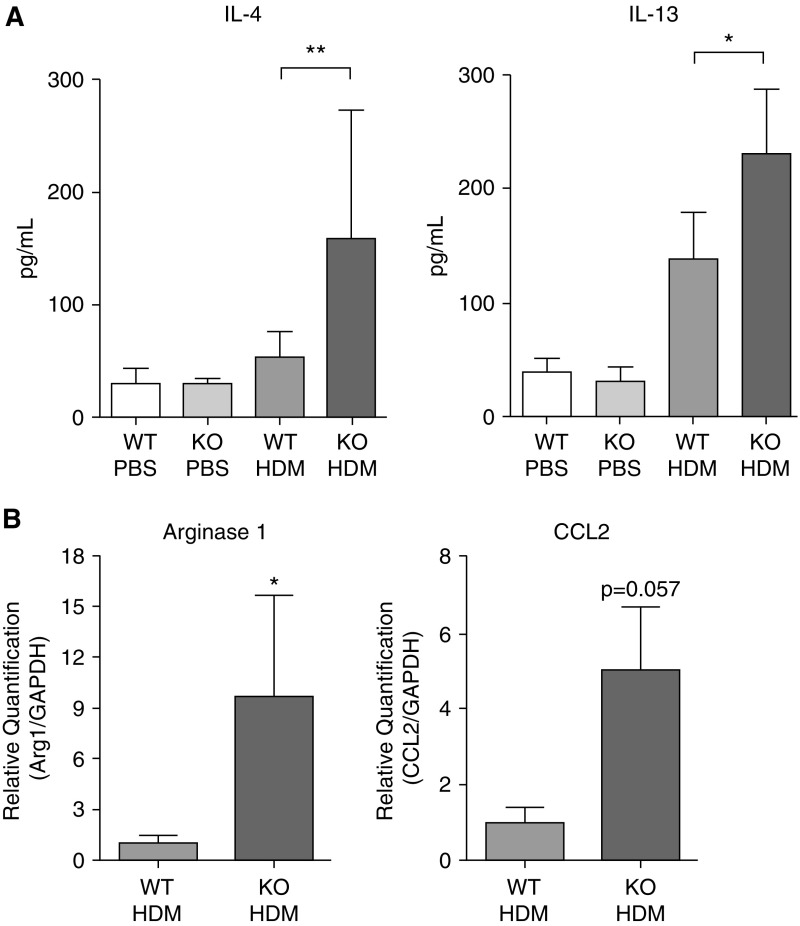
To better define the role of Wnt10b in allergic asthma, Wnt10b−/− mice were exposed to HDM and the inflammatory response compared with that in wild-type mice exposed to HDM. Without HDM stimulation, Wnt10b−/− mice exhibited no difference in T cell populations in thymus, spleen, mesenteric lymph nodes, and lung (Figure E2). After HDM challenge, Wnt10b−/− mice exhibited increased inflammation compared to wild-type littermates with a Th2 predominant phenotype (Figure 2). There was no difference in HDM antigen sensitization between genotypes, as shown by HDM-specific IgG1 concentration in the serum of challenged mice (Figure 2C). Inflammatory cell counts in the BAL fluid were increased with a predominance of eosinophils (Figure 2A). Consistent with the increased inflammation in the lavage fluid, hematoxylin and eosin–stained tissue sections revealed increased inflammation within the lung tissue of Wnt10b−/− animals when compared with their wild-type littermates (Figure 2D). To assess the peribronchial and perivascular inflammation after HDM treatment, an inflammation score was applied, and revealed significantly increased inflammation within the area surrounding the vessels and airways (Figure 2B). Immunofluorescent staining with eosinophil major basic protein confirmed increased eosinophil influx into the lung, whereas there was no apparent difference in T cell infiltration (Figures 2E and 2F). Specific markers of a Th2 response were also elevated, with a significantly increased concentration of IL-4 and IL-13 in lung homogenates (Figure 3A). RNA expression levels in the BAL cells demonstrated a significant increase in Arginase 1 (Arg1), a marker for alternatively activated macrophages and increased CCL2, a chemoattractant involved in Th2 polarization (Figure 3B).
Figure 2.
Wnt10b-deficient (Wnt10b−/−) mice exhibit increased inflammation compared with littermate WT mice after HDM challenge. (A) Bronchoalveolar lavage (BAL) cell counts show a significantly increased total number of cells and an increased number of eosinophils in Wnt10b−/− mice compared with littermate WT (n = 4–10/group, *P < 0.05 and **P < 0.01, respectively, t test; data compiled from two independent experiments, data are presented as mean ± SD). (B) Wnt10b−/− mice exhibit increased inflammatory infiltrates compared with their littermate controls after HDM challenge, shown by inflammatory index for severity of perivascular and peribronchial infiltrates (n = 3–4/group, 10 images/n; P < 0.05, t test). (C) No difference in sensitization measured by HDM-specific IgG1 was detected (n = 4–5/group, P < 0.05, t test). (D) Representative images of inflammatory infiltrates (upper panel scale bar, 250 μm; lower panel scale bar, 100 μm). (E) Immunofluorescent staining for eosinophil major basic protein also shows increased eosinophil infiltration in the lung parenchyma of Wnt10b−/− mice (representative images; scale bar, 100 μm). (F) Immunohistochemistry for CD3 does not show a clear difference in T cell infiltration in the lungs of Wnt10b−/− mice and controls (representative images; scale bar, 100 μm). OD, optical density.
Figure 3.
Ablation of Wnt10b leads to an augmented T helper (Th) 2 response after HDM challenge. (A) ELISA for IL-4 and IL-13 show significant increase in Wnt10b−/− mice after HDM challenge compared with littermate controls (n = 3–6/group, **P < 0.01 and *P < 0.05 respectively, Mann-Whitney U test, data are presented as mean ± SD). (B) mRNA expression increase of the Th2-inducing chemokine, chemokine (C-C motif) ligand 2 (CCL2), and the Th2/alternatively activated macrophage marker, arginase 1 (Arg1), in BAL cells of Wnt10b−/− mice after HDM challenge (n = 3–6/group; P = 0.057 and *P < 0.05 respectively, Mann-Whitney U test, data are presented as mean ± SD). GAPDH, gyceraldehyde-3-phosphate dehydrogenase.
Ablation of Wnt10b Leads to Increased Th2 Polarization In Vitro
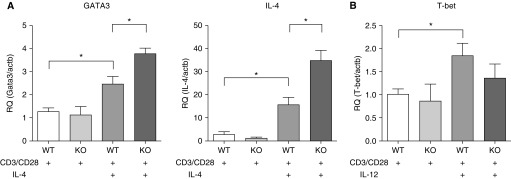
To identify whether the exaggerated Th2 response after HDM exposure in Wnt10b−/− mice was driven by an augmented Th2 response by T cells lacking Wnt10b, splenic cells were positively sorted with anti-CD90.2 beads, and these enriched T cells cultured under Th1 (addition of IL-12 to the culture) or Th2 (addition of IL-4 to the culture) conditions. Th2 differentiation was measured by the induction of GATA3, one of the main transcription factors, which potentiates Th2 responses by activating IL-4 and other cytokine transcription (22). There was no difference in GATA3 and IL-4 expression between Wnt10b−/− and wild-type primary T cells cultured under neutral conditions (Figure 4). Cultured under Th2-fostering conditions, though, Wnt10b−/− T cells exhibited an augmented Th2 response, measured by increased GATA3 and IL-4 when compared with wild-type T cells. There was no difference in T-bet expression between Wnt10b−/− and wild-type T cells under Th1-fostering conditions (Figure 4). These data suggest that Wnt10b deficiency exaggerates the Th2 response under Th2-promoting conditions.
Figure 4.
CD90.2-enriched splenic T cells of Wnt10b−/− mice exhibit increased GATA3 and IL-4 expression after Th2 polarization. (A) Primary T cells from Wnt10b−/− mice display elevated expression of GATA3 and IL-4 compared with WT cells cultured for 5 days under Th2-promoting conditions. (B) There was no significant difference for T-box expressed in T cells (T-bet) expression and Th1-promoting conditions (n = 3–5/group; *P < 0.05, t test, compiled data from two to three independent experiments, data are presented as mean ± SD). RQ, relative quantification.
Decreased Percentage of Naive Cells in Activated, Wnt10b−/− Splenic T Cells
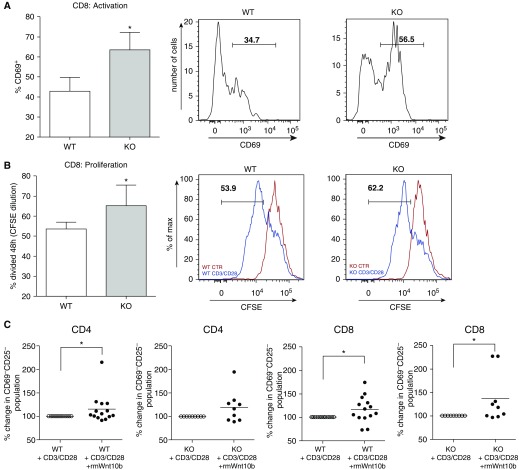
As there was no inert bias toward Th2 differentiation under activation conditions, but only after incubation in a Th2 environment, it was hypothesized that the ablation of Wnt10b might heighten the activation status of T cells and therefore render the cells more susceptible to Th2 differentiation. As such, T cell activation was measured after TCR stimulation by determining the percentage of CD69+ cells. After 72 hours in culture, CD8+ cells lacking Wnt10b expression exhibited a significantly higher percentage of CD69+ cells (Figure 5A). T cell activation by incubation with CD3/CD28 activator beads leads to T cell expansion through ligation of the TCR. Therefore, it was subsequently tested whether a heightened activation state in the Wnt10b−/− mice led to increased proliferation. A CFSE dilution assay was performed, and a higher proportion of divided cells were found in culture when compared with the splenic, CD8+ T cells of wild-type mice, indicating increased proliferation in the Wnt10b−/− mice (Figure 5B).
Figure 5.
T cell receptor activation in Wnt10b−/− T cells leads to a diminished population of naive, CD69−CD25− cells, which can be reversed by addition of recombinant Wnt10b. (A) Wnt10b−/−, CD8+ primary splenic T cells exhibit an increased population of activated, CD69+ cells after 72-hour incubation with CD3/CD28 activator beads (n = 3/group, *P < 0.05, t test, data representative of two independent experiments, data are presented as mean ± SD; representative histograms for CD69+ cells isolated from WT and Wnt10b−/− mice). (B) Wnt10b−/− CD8+ T cells show increased proliferation 48 hours after in vitro CD3/CD28 activation, measured by carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution. This effect did not reach significance in CD4+ cells (n = 6/group; *P < 0.05, t test, compiled data from two independent experiments, data are presented as mean ± SD; representative histograms with unactivated cells [red] and CD3/CD28 beads incubated cells [blue] for WT and Wnt10b−/−, respectively). (C) Addition of one or two doses of recombinant Wnt10b (200 ng/ml) increases the proportion of naive CD4+ and CD8+ cells in the total population, not only in Wnt10b−/− mice, but also in WT controls (n = 9–14/group; depicted as percent change of activated to activated and treated animal; *P < 0.05, Wilcoxon paired-rank test, compiled data from four independent experiments, data are presented as individual values with mean).
Because HDM exposure in vivo leads to up-regulation of Wnt10b, and loss of Wnt10b renders T cells more activated in vitro, we hypothesized that the up-regulation of Wnt10b in T cells likely functions physiologically by counteracting excessive activation and maintaining cells in a resting state. Therefore, the proportion of naive T cells (CD69−CD25−) after in vitro treatment with CD3/CD28 activator beads and recombinant Wnt10b protein (200 ng/ml) was measured and displayed as a percentage change compared with its respective control. An increase in the percentage of T cells remaining naive after additional treatment with Wnt10b in vitro was shown for CD4+ and CD8+ cells isolated from wild-type mice and CD8+ cells from Wnt10b−/− mice (Figure 5C), supporting our hypothesis.
HDM Challenge Enhances T Cell Activation in Lungs of Wnt10b Knockout Mice
To investigate whether the increase in Th2 response in the Wnt10b−/− mice was due to the effects of Wnt10b on T cell activation, T cell activation markers were measured in the lung tissue after HDM allergen challenge.
First, it was confirmed that there was no significant difference in single CD4+, CD8+, or double-positive CD4+CD8+ T cells in unsensitized Wnt10b−/− and wild-type mice in the thymus. There was also no significant difference in the percentage of CD4+ or CD8+ cells in the spleen, mesenteric lymph nodes, or lung (Figure E2). Therefore, the baseline activation state in the Wnt10b−/− mouse was equivalent to wild type, suggesting that antigen stimulation was necessary to elicit the T cell activation.
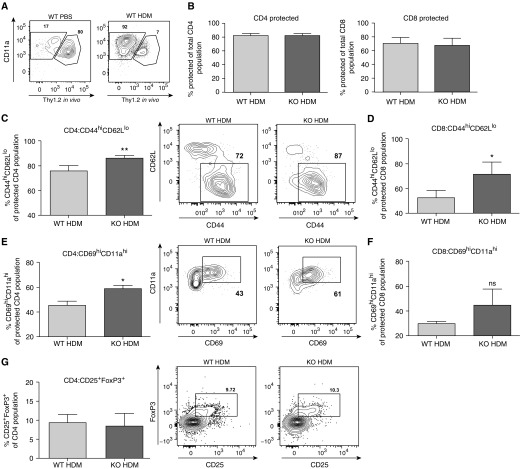
After HDM challenge, in vivo labeling with a fluorescently labeled CD90.2 antibody shortly before killing differentiates between cells within the vasculature (labeled) and cells that are recruited/infiltrated into the lung parenchyma and airways (protected from in vivo labeling) (21). Although the infiltrated T cell population was significantly increased after HDM sensitization, there was no difference in the proportion of T cells within the lungs of Wnt10b−/− mice after 3 weeks (Figures 6A and 6B). However, Wnt10b−/− mice had an increased percentage of effector cells, as shown by CD44hiCD62Llo CD4+ and CD8+ cells in the protected population in the lungs (Figures 6C and 6D). In addition, the infiltrated, protected CD4+ population showed a heightened activation state, measured by an increased percentage of CD69hiCD11ahi cells (Figures 6E and 6F). Importantly, there was no difference in T regulatory cells (CD25+Foxp3+ cells) in the lungs of Wnt10b−/− mice and their wild-type controls (Figure 6G) after stimulation.
Figure 6.
Increased percentage of effector T cells (CD62LloCD44hi) and CD69hiCD11ahi T cells in the lungs of Wnt10b−/−, HDM-challenged mice. (A) Cells protected from in vivo CD90.2 labeling (“protected population”) increases significantly from PBS- to HDM-challenged WT mice; most T cells in lung homogenates have infiltrated into the lungs (representative plots for WT PBS and WT HDM lungs). (B) The percentage of protected cells did not differ between Wnt10b−/− and control groups for CD4+ as well as CD8+ cells (WT HDM versus KO HDM; n = 3/group, data are presented as mean ± SD). (C) The protected population in the lung shows an augmented population of CD44hiCD62Llo for CD4+ cells (n = 3–4/group; **P < 0.01, t test, data representative of three independent experiments, data are presented as mean ± SD; representative plots shown for WT HDM and KO HDM). (D) CD8+ T cells also show a significantly increased population of CD44hiCD62Llo cells (n = 3–4/group; *P < 0.05, t test, data representative of three independent experiments, data are presented as mean ± SD). (E) CD4+ T cells within the lung parenchyma (protected population) exhibit heightened activation, as shown by increased percentage of CD69hiCD11ahi expression (n = 3–4/group; *P < 0.05, t test, data representative of three independent experiments, data are presented as mean ± SD; representative plots shown for WT HDM and KO HDM). (F) For CD69hiCD11ahi expression of CD8+ T cells, a trend toward an increase in the Wnt10b−/−, HDM-challenged population was detected. (G) No significant difference in percentage of CD4+CD25+Foxp3+ regulatory T cells in the lungs of HDM-challenged, Wnt10b−/−, and control mice was detected. (n = 5/group, data are presented as mean ± SD; representative plots shown for WT HDM and KO HDM). ns, not significant.
Discussion
The present studies demonstrate that the ablation of Wnt10b in a murine model of HDM-allergic airway disease amplifies the inflammatory response and leads to a higher percentage of antigen-experienced effector cells (CD44hiCD62Llo) within the lungs of Wnt10b−/− mice. Moreover, upon TCR stimulation, primary T cells from Wnt10b−/− mice exhibit increased activation markers. Adding exogenous recombinant Wnt10b protein in vitro to replicate its in vivo function correspondingly increased the proportion of naive T cells. Purified primary T cells deficient in Wnt10b express increased GATA3 levels upon Th2 polarization compared with wild-type cells. These findings suggest that Wnt10b contributes to the regulation of the immune response in allergic airway disease and may extend to a role in other inflammatory conditions.
Prior studies documenting the crucial importance of downstream targets and transcription factors of the Wnt signaling pathway for T cell maintenance, differentiation, and activation have been performed through genetic and pharmacological modulation of the signaling pathway (16, 23). The present report is the first to describe a single Wnt ligand that has been manipulated in a murine model of asthma. Eliciting the physiological role of a Wnt ligand in the whole-animal allergic airway disease model allows examination of its impact on immune functions within the context of the disease. Multiple Wnt ligands have been reported to be involved in developmental immune function regulation, notably, Wnt3a, Wnt4, Wnt5a, and Wnt10b (24–26). Wnt3a-deficient mice exhibit reduced-size thymi and, in culture, a shift from double-positive to CD8+ cells. Wnt4-deficient mice exhibit decreased thymic cellularity, and Wnt5a promotes apoptosis of double-positive thymic cells. In addition, Wnt5a-deficient, CD4+ thymocytes proliferate faster after CD3/CD28 stimulation, emulating the present finding in Wnt10b−/− T cells. An in vitro study with airway smooth muscle cells identified the noncanonical ligand, Wnt5a, to function in airway remodeling processes in smooth muscle cells through the transforming growth factor-β signaling pathway (11).
Thus far, the only canonical Wnt signaling molecule, which has been shown to play a role in asthmatic airway inflammation in an in vivo model is Wnt1. Reuter and colleagues (13) were able to demonstrate an attenuated inflammatory response in the lungs of allergen-sensitized mice exhibiting ectopic overexpression of Wnt1 in CCSP-expressing cells. Whereas in that study the overexpression of a single canonical Wnt ligand dampened the inflammatory response, our study reveals an exaggerated response after the ablation of a different canonical Wnt ligand, Wnt10b, emphasizing the importance of exploring the function of these molecules individually.
Wnt10b is mainly known as a canonical Wnt ligand, interacting with the Lrp5/6 coreceptors, complexed with Frizzled8 to activate β-catenin translocation into the nucleus. Here, a specific, canonical activation pattern for Wnt10b could be confirmed, although Wnt-triggered activation of downstream targets remains most likely cell specific and redundant. The identification of Wnt10b’s important role in immunity presents a promising approach to modulate effector functions of T cells more selectively through use of an extracellular signaling molecule.
The present data suggest that Wnt10b acts in an autocrine fashion, as its effect on activation and proliferation of T cells was recapitulated in vitro and no inherent activation bias was seen at baseline in the unsensitized mouse (Figure E2). Others have already shown that Wnt10b is expressed in CD4+ and CD8+ T cells in the thymus, under parathyroid hormone stimulus and upon TCR activation in vitro (2, 3, 27). In the present study, we demonstrate that Wnt10b expression is augmented in T cells after HDM sensitization, and the Wnt10b−/− mouse studies establish an important role in the allergic response. However, because a whole-body knockout is used, it is possible that different cell types contribute to the secretion pattern of Wnt10b in asthma. Nevertheless, the strength of these findings, demonstrating an effect of Wnt10b in a multi–cell-type disease, continues to be pertinent to the understanding of asthma.
In this study, we describe a moderate increase in the proliferation of CD8+ splenic cells after in vitro activation. Previously, Terauchi and colleagues (27) reported, contrary to our findings, that Wnt10b deficiency did not lead to increased activation and proliferation of T cells after CD3/CD28 activation. However, the measurements of Terauchi and colleagues were performed at an earlier time point—24 hours after activation—at which we also do not see a significant difference in activation. The increased activation seen in this present study was observed at 72 hours, and differences between the two studies can be attributed to the moderate effect and potential differences in experimental setup. Supporting our findings is that the deficiency of another Wnt ligand, Wnt5a, has also been reported to increase proliferation of CD4+ cells (25). Moreover, in accordance with our report, Ding and colleagues (15) observed that increased canonical Wnt pathway activation through stabilization of β-catenin in CD4+CD25− naive T cells led to decreased proliferation of the cells after 72 hours and attenuated inflammatory bowel disease in vivo. This attenuation of the T cell response is independent of the function of regulatory T cells, and is most likely an independent regulatory mechanism preventing excessive activation. Accordingly, in our model, the loss of the canonical Wnt ligand, Wnt10b, led to an increased activated T cell population in vitro and an amplified immune response in vivo. Moreover, in our study, no difference in the percentage of regulatory T cells in the lungs of Wnt10b−/− mice and controls after HDM sensitization could be detected, hinting to a more direct effect of Wnt10b on T cell activation.
Wnt signaling is necessary to allow for proper Th2 differentiation. The up-regulation of GATA3, a critical switch for the initial production of IL-4, is blunted by T cell factor 7 (Tcf7)-deficiency (23). On the contrary, our results show that ablation of Wnt10b leads to an exaggerated Th2 response with elevated IL-4 levels in the lung in vivo and increased GATA3 and IL-4 expression in vitro. Interestingly, under neutral, proliferating conditions, no difference was detected between GATA3 expression in the Wnt10b−/− and wild-type T cells with increased expression identified only under Th2-polarizing conditions. Therefore, there does not seem to be a bias toward a Th2 polarization in the proliferating, Wnt10b−/− T cell without an initiating stimulus. However, it is not clear if the increased activation status of the Wnt10b−/− cells is partially responsible for the higher GATA3 and IL-4 expressions, as a Th1 polarization did not lead to augmented T-bet levels in the Wnt10b−/− cells, suggesting an effect of Wnt10b specifically on Th2 polarization. A comparison of the knockout of one Wnt ligand to the modulation of transcription factors or common downstream targets of a variety of Wnt ligands will remain flawed, as it underestimates the complex function of Wnt ligands in vivo, where cell specificity, ligand–receptor interaction, and coexistence of inhibitors are crucial.
The present study documents an important role for the Wnt signaling molecule, Wnt10b, in T cell activation and regulation of cellular immunity in a murine model of asthma. Future studies aimed at the modulation of specific Wnt ligands in the lung have the potential to identify a novel therapeutic approach to control the inflammatory response in allergic asthma.
Acknowledgments
Acknowledgments
The authors thank Tina Zelonina and Tomoe Shiomi for their excellent technical assistance.
Footnotes
This work was supported by German Research Foundation fellowship TR 1087/2-1 (J.T.), a Parker B. Francis fellowship (D.L.T.), and by National Institutes of Health grants R01-HL086936 (J.M.D’A.) and T32-HL007343 (J.T.).
Author Contributions: J.T. acquired, analyzed, and interpreted the data, contributed to study design, and drafted the manuscript; T.S. and D.L.T. contributed to acquiring, analyzing, and interpreting the data; P.L.S., M.P.G., K.F.T., and M.X. contributed to acquiring and interpreting of the data. D.L.F. contributed to design and interpretation of the study. J.M.D’A. designed the study, interpreted the data, and contributed to drafting of the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2014-0425OC on October 5, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Centers for Disease Control and Prevention (CDC) Vital signs: asthma prevalence, disease characteristics, and self-management education: United States, 2001–2009. MMWR Morb Mortal Wkly Rep. 2011;60:547–552. [PubMed] [Google Scholar]
- 2.Roser-Page S, Vikulina T, Zayzafoon M, Weitzmann MN. CTLA-4Ig–induced T cell anergy promotes Wnt-10b production and bone formation in a mouse model. Arthritis Rheumatol. 2014;66:990–999. doi: 10.1002/art.38319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardiman G, Albright S, Tsunoda J, McClanahan T, Lee F. The mouse Wnt-10B gene isolated from helper T cells is widely expressed and a possible oncogene in BR6 mouse mammary tumorigenesis. Gene. 1996;172:199–205. doi: 10.1016/0378-1119(96)00109-6. [DOI] [PubMed] [Google Scholar]
- 4.Balciunaite G, Keller MP, Balciunaite E, Piali L, Zuklys S, Mathieu YD, Gill J, Boyd R, Sussman DJ, Holländer GA. Wnt glycoproteins regulate the expression of FoxN1, the gene defective in nude mice. Nat Immunol. 2002;3:1102–1108. doi: 10.1038/ni850. [DOI] [PubMed] [Google Scholar]
- 5.Li JY, Adams J, Calvi LM, Lane TF, Weitzmann MN, Pacifici R. Ovariectomy expands murine short-term hemopoietic stem cell function through T cell expressed CD40L and Wnt10B. Blood. 2013;122:2346–2357. doi: 10.1182/blood-2013-03-487801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Congdon KL, Voermans C, Ferguson EC, DiMascio LN, Uqoezwa M, Zhao C, Reya T. Activation of Wnt signaling in hematopoietic regeneration. Stem Cells. 2008;26:1202–1210. doi: 10.1634/stemcells.2007-0768. [DOI] [PubMed] [Google Scholar]
- 7.Myou S, Leff AR, Myo S, Boetticher E, Tong J, Meliton AY, Liu J, Munoz NM, Zhu X. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. J Exp Med. 2003;198:1573–1582. doi: 10.1084/jem.20030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molenaar M, van de Wetering M, Oosterwegel M, Peterson-Maduro J, Godsave S, Korinek V, Roose J, Destrée O, Clevers H. XTcf-3 transcription factor mediates beta-catenin–induced axis formation in Xenopus embryos. Cell. 1996;86:391–399. doi: 10.1016/s0092-8674(00)80112-9. [DOI] [PubMed] [Google Scholar]
- 9.Chilosi M, Poletti V, Zamò A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, et al. Aberrant Wnt/β-catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003;162:1495–1502. doi: 10.1016/s0002-9440(10)64282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tennis M, Van Scoyk M, Winn RA. Role of the wnt signaling pathway and lung cancer. J Thorac Oncol. 2007;2:889–892. doi: 10.1097/JTO.0b013e318153fdb1. [DOI] [PubMed] [Google Scholar]
- 11.Kumawat K, Menzen MH, Bos IS, Baarsma HA, Borger P, Roth M, Tamm M, Halayko AJ, Simoons M, Prins A, et al. Noncanonical wnt-5a signaling regulates TGF-β–induced extracellular matrix production by airway smooth muscle cells FASEB J 2013271631–43 [DOI] [PubMed] [Google Scholar]
- 12.Sharma S, Tantisira K, Carey V, Murphy AJ, Lasky-Su J, Celedón JC, Lazarus R, Klanderman B, Rogers A, Soto-Quirós M, et al. A role for Wnt signaling genes in the pathogenesis of impaired lung function in asthma. Am J Respir Crit Care Med. 2010;181:328–336. doi: 10.1164/rccm.200907-1009OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reuter S, Martin H, Beckert H, Bros M, Montermann E, Belz C, Heinz A, Ohngemach S, Sahin U, Stassen M, et al. The Wnt/β-catenin pathway attenuates experimental allergic airway disease. J Immunol. 2014;193:485–495. doi: 10.4049/jimmunol.1400013. [DOI] [PubMed] [Google Scholar]
- 14.Zhao DM, Yu S, Zhou X, Haring JS, Held W, Badovinac VP, Harty JT, Xue HH. Constitutive activation of Wnt signaling favors generation of memory CD8 T cells. J Immunol. 2010;184:1191–1199. doi: 10.4049/jimmunol.0901199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding Y, Shen S, Lino AC, Curotto de Lafaille MA, Lafaille JJ. Beta-catenin stabilization extends regulatory T cell survival and induces anergy in nonregulatory T cells. Nat Med. 2008;14:162–169. doi: 10.1038/nm1707. [DOI] [PubMed] [Google Scholar]
- 16.Gattinoni L, Zhong XS, Palmer DC, Ji Y, Hinrichs CS, Yu Z, Wrzesinski C, Boni A, Cassard L, Garvin LM, et al. Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat Med. 2009;15:808–813. doi: 10.1038/nm.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka KF, Ahmari SE, Leonardo ED, Richardson-Jones JW, Budreck EC, Scheiffele P, Sugio S, Inamura N, Ikenaka K, Hen R. Flexible accelerated STOP tetracycline operator-knockin (FAST): a versatile and efficient new gene modulating system. Biol Psychiatry. 2010;67:770–773. doi: 10.1016/j.biopsych.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldklang M, Golovatch P, Zelonina T, Trischler J, Rabinowitz D, Lemaître V, D’Armiento J. Activation of the TLR4 signaling pathway and abnormal cholesterol efflux lead to emphysema in ApoE-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1200–L1208. doi: 10.1152/ajplung.00454.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohtsuka T, Shiomi T, Shimoda M, Kodama T, Amour A, Murphy G, Ohuchi E, Kobayashi K, Okada Y. ADAM28 is overexpressed in human non–small cell lung carcinomas and correlates with cell proliferation and lymph node metastasis. Int J Cancer. 2006;118:263–273. doi: 10.1002/ijc.21324. [DOI] [PubMed] [Google Scholar]
- 20.Kelly MM, Leigh R, Bonniaud P, Ellis R, Wattie J, Smith MJ, Martin G, Panju M, Inman MD, Gauldie J. Epithelial expression of profibrotic mediators in a model of allergen-induced airway remodeling. Am J Respir Cell Mol Biol. 2005;32:99–107. doi: 10.1165/rcmb.2004-0190OC. [DOI] [PubMed] [Google Scholar]
- 21.Turner DL, Bickham KL, Thome JJ, Kim CY, D’Ovidio F, Wherry EJ, Farber DL. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014;7:501–510. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang DH, Cohn L, Ray P, Bottomly K, Ray A. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J Biol Chem. 1997;272:21597–21603. doi: 10.1074/jbc.272.34.21597. [DOI] [PubMed] [Google Scholar]
- 23.Yu Q, Sharma A, Oh SY, Moon HG, Hossain MZ, Salay TM, Leeds KE, Du H, Wu B, Waterman ML, et al. T cell factor 1 initiates the T helper type 2 fate by inducing the transcription factor GATA-3 and repressing interferon-gamma. Nat Immunol. 2009;10:992–999. doi: 10.1038/ni.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louis I, Heinonen KM, Chagraoui J, Vainio S, Sauvageau G, Perreault C. The signaling protein Wnt4 enhances thymopoiesis and expands multipotent hematopoietic progenitors through beta-catenin–independent signaling. Immunity. 2008;29:57–67. doi: 10.1016/j.immuni.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 25.Liang H, Coles AH, Zhu Z, Zayas J, Jurecic R, Kang J, Jones SN. Noncanonical Wnt signaling promotes apoptosis in thymocyte development. J Exp Med. 2007;204:3077–3084. doi: 10.1084/jem.20062692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luis TC, Weerkamp F, Naber BA, Baert MR, de Haas EF, Nikolic T, Heuvelmans S, De Krijger RR, van Dongen JJ, Staal FJ. Wnt3a deficiency irreversibly impairs hematopoietic stem cell self-renewal and leads to defects in progenitor cell differentiation. Blood. 2009;113:546–554. doi: 10.1182/blood-2008-06-163774. [DOI] [PubMed] [Google Scholar]
- 27.Terauchi M, Li JY, Bedi B, Baek KH, Tawfeek H, Galley S, Gilbert L, Nanes MS, Zayzafoon M, Guldberg R, et al. T lymphocytes amplify the anabolic activity of parathyroid hormone through Wnt10b signaling. Cell Metab. 2009;10:229–240. doi: 10.1016/j.cmet.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]