Abstract
Epithelial–mesenchymal cell interactions and factors that control normal lung development are key players in lung injury, repair, and fibrosis. A number of studies have investigated the roles and sources of epithelial progenitors during lung regeneration; such information, however, is limited in lung fibroblasts. Thus, understanding the origin, phenotype, and roles of fibroblast progenitors in lung development, repair, and regeneration helps address these limitations. Using a combination of platelet-derived growth factor receptor α–green fluorescent protein (PDGFRα-GFP) reporter mice, microarray, real-time polymerase chain reaction, flow cytometry, and immunofluorescence, we characterized two distinct interstitial resident fibroblasts, myo- and matrix fibroblasts, and identified a role for PDGFRα kinase activity in regulating their activation during lung regeneration. Transcriptional profiling of the two populations revealed a myo- and matrix fibroblast gene signature. Differences in proliferation, smooth muscle actin induction, and lipid content in the two subpopulations of PDGFRα-expressing fibroblasts during alveolar regeneration were observed. Although CD140α+CD29+ cells behaved as myofibroblasts, CD140α+CD34+ appeared as matrix and/or lipofibroblasts. Gain or loss of PDGFRα kinase activity using the inhibitor nilotinib and a dominant-active PDGFRα-D842V mutation revealed that PDGFRα was important for matrix fibroblast differentiation. We demonstrated that PDGFRα signaling promotes alveolar septation by regulating fibroblast activation and matrix fibroblast differentiation, whereas myofibroblast differentiation was largely PDGFRα independent. These studies provide evidence for the phenotypic and functional diversity as well as the extent of specificity of interstitial resident fibroblasts differentiation during regeneration after partial pneumonectomy.
Keywords: pneumonectomy, regeneration, myofibroblast, matrix fibroblast nilotinib
Clinical Relevance
Although tremendous progress has been made in understanding the roles and sources of epithelial progenitors in tissue repair and fibrotic mechanisms, little is known about the origin, nature of interaction, and gene regulatory programs of lung fibroblast progenitors. Despite evidence that fibroblasts from different stromal sites may be composed of distinct differentiated cell types, neither the diversity of these cells nor the nature of specificity in their differentiation has been examined systematically, because these cells have no cell lineage markers. Our study provides evidence for the existence of two phenotypically dynamic and functionally diverse sets of interstitial fibroblasts and for their role in regeneration and realveolarization after pneumonectomy. We describe, we believe for the first time, distinct mRNA signatures for myo- and matrix fibroblasts and identify a combination of cell surface markers to distinguish interstitial alveolar matrix and myofibroblasts.
In the lung parenchyma, the alveoli form epithelial–mesenchymal trophic units consisting of apposing layers of epithelial and mesenchymal cells, separated by a basement membrane and the capillary network (1, 2). During lung development, epithelial–mesenchymal cell interactions are essential for branching morphogenesis and cell differentiation (3, 4). Many of the same factors that control normal development are also key players in alveolar lung injury, repair, and fibrosis (5, 6). Regeneration and repair in the adult lung are mediated by differentiation of endogenous lung progenitor cells and proliferation of the remaining undamaged resident cells. After epithelial injury, fibroblasts become activated and induce many developmental pathways that are important for proliferation, extracellular matrix (ECM) deposition, and chemokine and cytokine secretions. Activation of fibroblasts in response to injury mediates the reparative response of the epithelium (3, 7–11), and the role of fibroblasts in compensatory lung growth and realveolarization after partial pneumonectomy (PNX) has been noted in several species (12–18). The current availability of transgenic mice provides the tools for extensive characterization of the cellular and molecular mechanisms responsible for compensatory lung regrowth after PNX.
Although tremendous progress has been made in understanding the roles and sources of epithelial progenitors in tissue repair and the fibrotic mechanisms with which fibroblasts are major players, little is known about the origin, nature of interaction, and gene regulatory programs of lung fibroblast progenitors. As such, lineage relationship and functional studies among lung fibroblasts have been identified only recently (19–23). For instance, fibroblast growth factor (FGF) 10-expressing cells of the distal lung are reported to give rise to multiple mesodermal components in the adult, including airway smooth muscle and alveolar lipofibroblasts (24). Recent lineage tracing studies also identified Gli1-expressing cells as progenitors of multiple lung fibroblasts, including alveolar myofibroblasts and other mesoderm-derived fibroblasts (25). Despite the evidence that fibroblasts from different stromal sites may be composed of distinct cell types, neither the diversity of these cells nor the nature of specificity in their differentiation has been examined systematically because these cells have no cell lineage markers. On the other hand, understanding the phenotypic characteristics and the roles of mesodermally derived lung fibroblasts in postnatal alveolarization and post-PNX realveolarization is profoundly important.
Previous reports have indicated that platelet-derived growth factor receptor α (PDGFRα)-expressing lung fibroblasts are resident fibroblasts located in the epithelial-mesenchymal tropic unit of the alveolus and play a role in alveolarization and realveolarization (26–30). In addition, PDGFA knockout mice have shown impaired interstitial fibroblast function and reduced elastin deposition in the alveoli (31). We have also shown previously that a subpopulation of interstitial resident fibroblasts (iReFs) changed dynamically to myofibroblasts and are important in the formation of new alveolar septa after partial PNX (30), highlighting a dual role for the interstitial lung fibroblasts in lung wound repair and regeneration. However, the transcriptional signature, phenotypic identity, and biological dynamic activities of these fibroblasts are understudied.
We report the phenotypic and functional diversity of interstitial lung fibroblasts in mice by characterizing two platelet-derived growth factor receptor α green fluorescent protein (PDGFRα+GFP) (dim and bright) subpopulations. PDGFRα+GFPbright, neutral lipid, and CD34-expressing cells are identified as matrix-associated fibroblasts, whereas PDGFRα+GFPdim, α smooth muscle actin (SMA), and CD290-expressing cells are regarded as myofibroblasts. This study also revealed that PDGFRα kinase activity was important for matrix fibroblast differentiation, whereas the myofibroblast differentiation pathway was largely independent of this pathway. Our study provides evidence for the existence of two phenotypically dynamic and functionally diverse sets of iReFs during the course of lung regeneration and realveolarization after PNX.
Materials and Methods
Additional detail on the methods is provided in the online supplement.
Transgenic Mice
B6.129S4-Pdgfrαtm11(EGFP)Sor/J are referred to as PDGFRα-GFP (32). The endogenous Pdgfrα promoter drives expression of the H2B-eGFP fusion gene.
B6N.Cg-Tg(Pdgfrα-cre/ERT)467Dbe/J, herein designated PDGFRαCreERT (33), and Ap2CreERT mice (34), are both transgenic mice that express CreERT under the PDGFRα or AP2 promoter.
B6.129S4-Pdgfrαtm12Sor/J is herein designated PDGFRα-D842V (35). The PDGFRα(S)K conditional knockin allele has the endogenous PDGFRα sequences replaced with a loxP-flanked PGK-neo (lox-stop-lox) cassette upstream of the constitutively active PDGFRαK mutant isoform.
SFTPC -rtTA/tetOdnFGFR-Hfc are referred to as dominant-negative (dn) FGF receptor (FGFR) mutant mice. The dnFGFR transgene expression inhibits FGF signaling in the lungs of double-transgenic mice during antibiotic exposure.
Flow Cytometry Details
Lungs were inflated and digested with dispase and DNase and filtered through a 20-μm filter. Cells were washed and resuspended in phosphate-buffered saline/1 mM EDTA/25 mM HEPES/2% fetal bovine serum. Cells were blocked with antimouse CD16/CD32, stained with a cocktail of antibodies, and incubated on ice for 30 minutes. Fluorescence minus one controls and fluorescent beads were used to set the positive gates and for compensation of the overlap of fluorescence emission spectra. Doublets were excluded using side scatter pulse-width and forwards scatter pulse-height and forwards scatter pulse-width and side scatter pulse-height; dead cells were excluded using the viability dye. Immune cells (CD45+), endothelial cells (CD31+, Pecam), and epithelial cells (CD326+, EpCAM) were excluded to isolate the stromal cell fraction. Stromal cells were then analyzed for their nuclear PDGFRα-GFP and membrane PDGFRα protein (CD140α) expression.
Cell Enrichment/ RNA Preparation and Amplification
PDGFRα+ iReF and epithelial cells were enriched by magnetic cell separation using Miltenyi Biotec MACS technology: MACS (Miltenyl Biotec, Gladbach, Germany) anti-mouse CD45 MicroBeads (for negative selection), anti-Mouse CD140α Biotin (PDGFRα) (Miltenyl Biotec, Auburn, CA), anti-Mouse CD326 Biotin (EpCAM) (eBioscience, San Diego, CA), and anti-Biotin MicroBeads (No. 130-090-485; MACS).
RNA was isolated from MACS-separated PDGFRα+ (CD140α) and EpCAM+ (CD326) cells using RNeasy Mini Kit (No. 74104) and Micro Kit (No. 74004) (QIAGEN, Germantown, MD). RNA was transcribed into complementary DNA using the Verso complementary DNA synthesis kit (AB-1453; LifeTechnologies, Carlsbad, CA). Expression of selected genes was quantified by real-time polymerase chain reaction (PCR) using the 7300 Real Time PCR system from Applied Biosystems and TaqMan Gene Expression Assays (Waltham, MA).
RT2 Array:
Epidermal growth factor (EGF)/PDGF pathway-focused gene expression analysis was performed using RT2Profile PCR arrays from QIAGEN. Arrays were performed according to the manufacturer’s instructions and were analyzed with complimentary online tools.
In Vitro Culturing of PDGFRα-GFP Bright and Dim iReFs
PDGFRα+-GFPbright or -GFPdim iReFs were isolated after 3 days of sham-operated or PNX mice and were sorted using a fluorescence-activated cell sorter, and then cultured for 24 and 72 hours with or without transforming growth factor (TGF)-β1. After this, the proliferation, activation, and differentiation status (Ki67+, CD34, CD29, and α-SMA) were analyzed using a fluorescence-activated cell sorter.
Results
Expression Profiling Reveals Two Distinct PDGFRα+ Fibroblast Populations
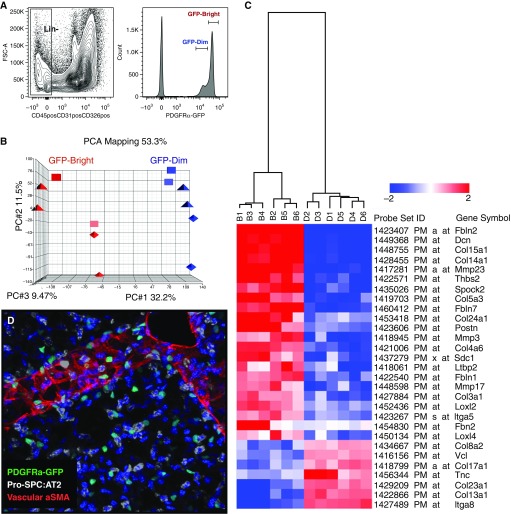
Using a transgenic mouse with a nuclear GFP reporter gene knocked into the Pdgfrα locus (PDGFRα-GFP [32]), we characterized two PDGFRα-GFP–expressing iReF populations. On the basis of the level of GFP expression, PDGFRα+GFP+ cells were sorted into GFPdim or GFPbright populations (Figure 1A) (30). The gene expression profile and principal component analysis demonstrated that PDGFRα+GFPdim and PDGFRα+GFPbright cells define two distinct fibroblast populations with 875 genes that were fourfold or more differentially expressed (Figure 1B and see Figure E1A in the online supplement). Gene ontology analysis of 613 genes overexpressed in PDGFRα+GFPbright cells identified a matrix fibroblast gene signature with the ECM as the dominant cellular component; cell adhesion, matrix organization, and proliferation as dominant biological processes; and metallopeptidase activity as the main molecular functions; 263 genes overexpressed in PDGFRα+GFPdim cells identified a myofibroblast gene signature, with the plasma membrane and cell junctions as the dominant cellular components; tube development and locomotion as dominant biological processes; and actin binding as the main molecular function (Figures 1C, E1A, and E1B).
Figure 1.
Platelet-derived growth factor receptor α–green fluorescent protein (PDGFRα+-GFP) mice reveal two distinct fibroblast phenotypes. (A) Single cells isolated from adult mouse lungs were sorted into Lin⁻ (CD45⁻, CD31⁻, CD326⁻) and PDGFRα+GFPdim or GFPbright cells. (B) RNA was subjected to microarray analysis, and PCA mapping identified a myo- and a matrix messenger RNA signature. Using all probe sets, the first three principal components cover 75% of all variations across samples and clearly separate the two subpopulations (red and blue). (C) Collagen and matrix-related expression differences between PDGFRα-GFPbright and PDGFRα-GFPdim fibroblasts. Two-dimensional hierarchical clustering of 29 genes associated with extracellular matrix synthesis and collagen expression is differentially expressed between bright (B) and dim (D) cells. Red blocks represent up-regulation, and blue blocks represent down-regulated expression. (D) Three-dimensional confocal reconstruction of adult mouse lung demonstrate that PDGFRα-GFP+ cells (green) are adjacent to alveolar type-2 cells (pro-SPC; white) and are distributed evenly throughout the alveolar compartment. α-SMA, α-smooth muscle actin; FSC, forward scatter; GFP, green fluorescent protein; Lin−, lineage negative; PC, principal component; PCA, principal component analysis; pro-SPC, proprotein for surfactant protein C.
On the basis of their gene expression signature, we refer to the PDGFRα+GFPbright cells as interstitial resident matrix fibroblasts (matrix-iReFs) and PDGFRα+GFPdim cells as interstitial resident myofibroblasts (myo-iReFs). Because matrix- and myo-iReFs shared the gene ontology term ECM, we examined differentially expressed collagen and elastic fiber organizing genes in the two iReF populations. Matrix-iReFs showed increased expression of fibrillar collagens (Col III and Col V) that form extracellular fibrils, and Fibulin1, Fibulin2, and Fibrilin2. Matrix- iReFs also showed increased Lysyl oxidase-like (Loxl) 2 and 4, which crosslink elastin and collagen fibers, as well as matrix metalloproteinase (MMP) 3, 17 and 23 expression, which degrade ECM and process bioactive molecules. On the other hand, myo-iReFs overexpressed full-length transmembrane collagens (CollXIII, XVII, XVIII, and XXIII), integrin, and vinculin (Figure 1C). Although matrix-iReFs expressed more matricellular proteins, such as thrombospondin, osteonectin, and periostin, which are nonstructural ECM proteins that modulate cell–matrix interactions and cell regulatory functions, myo-iReFs expressed tenascin-C (Figure 1C). In addition, differential expression of genes from the Wnt signaling pathway was observed between the matrix- and myo-iReFs. Thirty-two Wnt pathway genes were differentially up-regulated in the matrix-iReFs, whereas 14 genes were differentially up-regulated in the myo-iReFs (Figure E1B).
PDGFRα-expressing fibroblasts are reported to localize to the alveolar entry ring and have characteristics of myofibroblasts during pulmonary alveolar septal formation (28). In adult lungs, GFP-expressing iReFs are located in the alveolar interstitium, where α-SMA and elastic fibers accumulate during reseptation (Figure 1D).
Phenotypic Characterization of PDGFRα (CD140α) Fibroblasts
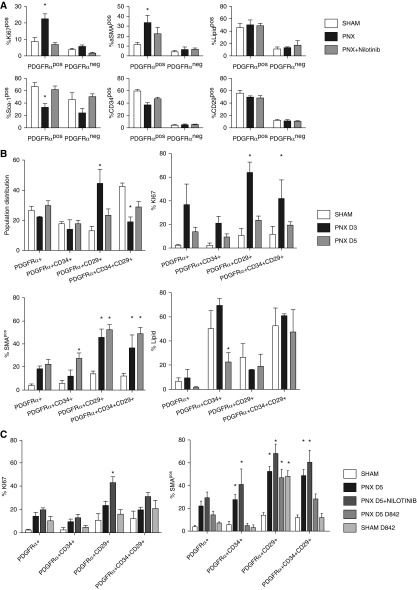
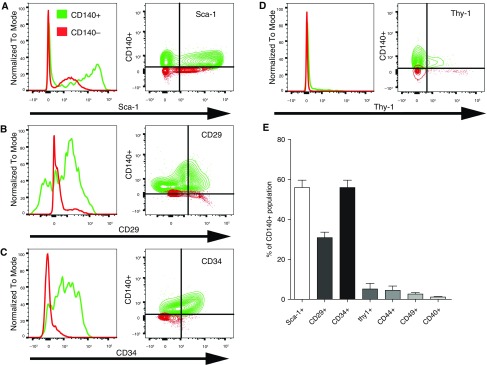
Single-cell suspensions of Lin⁻ (CD45⁻[hematopoietic], CD326⁻[epithelial], and CD31⁻[endothelial]) cells were gated for CD140α, GFP, Ki-67, and α-SMA (Figure E2A). Flow cytometry identified that not all PDGFRα-GFP–expressing cells coexpressed CD140α and that not all CD140α-expressing cells are GFP positive. Proliferation, α-SMA induction, and expression of cell surface markers after partial PNX in these PDGFRα-GFP/CD140α cell populations was analyzed (Figures E2, E3, and E4). Proliferation and α-SMA induction during the course of alveolar regeneration after partial PNX were significant in CD140α+ fibroblast populations, which are responsive to PDGFRα gain and loss of function. CD140α⁻ fibroblast populations did not induce proliferation or α-SMA after PNX (Figure 3A). Because the changes in surface marker expression between PDGFRα-GFP– and PDGFRα-expressing cells were not significant, we focused on PDGFRα (CD140α)-expressing cells in all subsequent experiments. Because various cell surface markers including Sca-1, CD34, CD29, Thy-1 (CD90), CD44, CD49, and CD40 have been associated previously with mesenchymal stem cells and lung fibroblast populations (22, 23, 36–38), we further characterized iReFs. Lin⁻ (CD45⁻CD326⁻CD31⁻) single cells were gated for CD140α positive (green) and negative (red) subpopulations. The levels of Sca-1, CD29, and CD34 expression were significantly high in CD140α+ iReFs, but expressions of Thy-1, CD44, CD49, and CD40 were negligible (Figures 2A–2E).
Figure 3.
PDGFRα+ interstitial resident fibroblasts (iReFs) proliferate and induce α-SMA during alveolar regeneration. (A) PDGFRα+ and PDGFRα− fibroblasts were analyzed for proliferation (Ki67+) and for the expression levels of α-SMA, neutral lipid, sca-1, CD34, and CD29. Freshly isolated PDGFRα-positive and -negative lung fibroblasts were compared among sham (white), pneumonectomy (PNX, black), and PNX with nilotinib treatment (PNX + nilotinib: gray). (B) Freshly isolated PDGFRα+ iReFs were stained for CD29 and CD34 to separate PDGFRα+ iReFs into four subpopulations. Each population was analyzed for proliferation (Ki67+), lipid content, and α-SMA expression 3 and 5 days after PNX. Proportion, proliferation (Ki67), α-SMA expression, and neutral lipid contents were analyzed in fibroblast populations and compared between sham (white) and PNX (PNX D3: black; and PNX D5: gray). (C) Freshly isolated PDGFRα+ iReFs were stained for CD29 and CD34 to separate PDGFRα+ iReFs into four subpopulations and were analyzed for proliferation, and α-SMA expression in the presence or absence of the PDGFR kinase inhibitor nilotinib and the dominant active PDGFRα-D842V mutation were compared between sham (white), PNX after 5 days (PNX: black), PNX with nilotinib treatment (PNX + nilotinib: dark gray) and PNX in PDGFRα-D842V mutant mice (PNX+D842V: gray, SHAM+D842V: light gray). *Significant changes compared with sham. Data are expressed as mean ± SEM. Comparisons among groups were made by analysis of variance, with P < 0.05 considered significant. Each group represents three or more animals. Neg, negative; pos, positive.
Figure 2.
Immunophenotyping of PDGFRα+ and PDGFRα⁻ fibroblasts in adult mouse lungs. Lin⁻ (CD45⁻CD326⁻CD31⁻) stromal cells were gated for CD140α+ (green) or CD140α (red) subpopulations, and expressions of (A) SCA-1, (B) CD29 (integrin β1), (C) CD34, (D) Thy1 (CD90), CD44, and CD49, and (E) CD40 were compared between the CD140α+ and CD140α⁻ fibroblasts. Histograms and bivariate contour plots of surface marker expression of PDGFR-positive and -negative cells are shown by overlay. (E) Percentage of all CD140α+ coexpressing one of the surface markers was determined by flow cytometry and was summarized in the bar graph. Data are expressed as mean ± SEM, and comparisons among groups were made by analysis of variance, with P < 0.05 considered significant (n > 3).
Phenotype and Activation of PDGFRα+ Cells after PNX
PDGFRα-expressing iReFs are implicated as playing a major role in alveolarization and alveolar regeneration (26, 27, 29, 30). Six days after partial PNX, 74% of all newly forming alveolar septae are present (39); inhibition of FGF signaling during alveolar regeneration impaired myofibroblast differentiation of interstitial PDGFRα-expressing cells 5 days after PNX and subsequent alveolar reseptation (30). We therefore examined the activation, proliferation, and differentiation status of PDGFRα+ and PDGFRα− fibroblasts after partial PNX. Compared with PDGFRα− cells, PDGFRα+ iReFs showed increased proliferation and α-SMA induction (Figure 3A, upper panel) but reduced Sca-1 and CD34 expression (Figure 3A, lower panel) after partial PNX, which was restored by nilotinib, a PDGFRα tyrosine kinase inhibitor. Neutral lipid content and CD29 expression did not change significantly in PDGFRα+ iReFs at 3 days after surgery (Figure 3A). We further characterized the activation, proliferation, and differentiation status of PDGFRα+ iReFs between 3 and 5 days after PNX. Analysis of the PDGFRα+ subpopulations CD140α+CD34⁻CD29⁻, CD140α+CD34+CD29⁻, CD140α+CD34⁻CD29+, and CD140α+CD34+CD29+ revealed a trend of a marked increase in CD29 and a reduction in CD34 expression between 3 to 5 days after surgery (Figure 3B). Proliferation among the four subpopulations of PDGFRα+ iReFs was highest for CD29+, followed by CD34+CD29+ at 3 days, which was reduced after 5 days of surgery (Figure 3B). Furthermore, the CD29+ cells were the highest in α-SMA expression, followed by CD34+CD29+ iReFs at 3 days, and the trend was further increased at 5 days after PNX (Figure 3B). Neutral lipid content was not different among subpopulations except for CD34+ iReFs after 5 days of PNX (Figure 3B). The results suggest that PDGFRα+ iReFs expressing CD34 are transitional lipofibroblasts differentiating into CD29-expressing myofibroblasts during the course of lung repair and regeneration.
PDGFRα Kinase Gain or Loss of Function during Lung Regeneration
To investigate the role of PDGFRα kinase activity in iReF phenotypes, we treated mice with nilotinib (PDGFRα inhibitor) or used knockin mice harboring an activating kinase mutation (D842V) of PDGFRα. Nilotinib treatment inhibited cell proliferation and delayed myofibroblast differentiation in PDGFRα+ subpopulations 3 days after partial PNX (Figure 3A). However, 5 days after PNX, proliferation was not inhibited by nilotinib; it was even higher for CD29+ iReF subpopulations (Figure 3C). This suggests that proliferation per se may be regulated by alternate pathways independent of PDGFRα kinase during later phases of lung repair and regeneration. In addition, proliferation was comparable between wild-type and the constitutively active (D842V) mutant iReFs, suggesting that increased kinase activity did not activate proliferation. On the other hand, α-SMA expression in all CD34+, CD29+, and CD34+CD29+ subpopulations was increased in nilotinib-treated animals (Figure 3C), suggesting that basal kinase activity is not a sole regulator of α-SMA induction 5 days after partial PNX. However, the dominant active D842V mutation markedly increased CD29 expression and α-SMA induction irrespective of PNX. The D842V-mediated α-SMA expression was minimal in CD34-expressing iReFs and its up-regulation was dependent on CD29 expression (Figure 3C), suggesting that activation of PDGFRα kinase regulates CD29 expression and subsequent α-SMA induction.
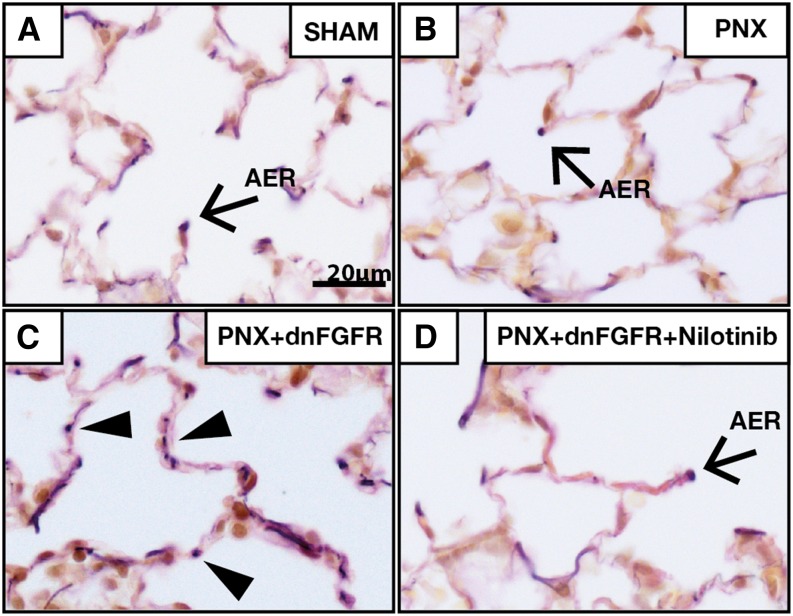
Because α-SMA expression was not affected by PDGFRα inhibition at later stages of lung regeneration, we thought other signaling mechanisms could be involved in myogenic differentiation. We have reported previously that inhibition of FGFR signaling blocked myogenic differentiation after partial PNX (30), and previous published data also suggested that PDGFRα+ fibroblasts can differentiate into elastin and ECM-producing cells (31). To investigate whether FGF signaling regulates matrix-iReF differentiation to α-SMA–expressing myo-iReFs, we analyzed elastin deposition in the lungs of wild-type and soluble dnFGFR-expressing transgenic mice (30). In both sham and wild-type operated lungs, elastin bundles were located at the tip of the septum, the “alveolar entry ring,” which prevents collapse of the alveolus (Figures 4A and 4B). In the alveolar walls of soluble dnFGFR-expressing mice, ectopic elastin bundles were found (Figure 4C), which were suppressed by nilotinib treatment (Figure 4D). These data suggest that FGFR2 signaling is important for myo-iReF but not matrix-iReF differentiation, whereas PDGFRα signaling is necessary for activation of matrix-iReFs.
Figure 4.
Nilotinib-inhibited elastin deposition in fibroblast growth factor receptor (FGFR) 2 mutant mice in vivo. Weigert’s elastin stain was performed on a 5-μm paraffin section of (A) sham, (B) PNX, (C) PNX in dominant-negative FGFR (dnFGFR) 2 mutant lungs (PNX + dnFGFR), and (D) nilotinib-treated dominant negative FGFR2 mutant lungs 21 days after PNX surgery. Elastin bundles (dark purple staining) are localized in the tips of alveolar septae (A, B, and D). (C) Ectopic elastin bundles (arrowheads) are found in the alveolar wall of dnFGFR2 mutant lungs 21 days after surgery. Scale bar, 20 μm; figures are representative of n > 3. AER, alveolar entry ring.
Proportion of PDGFRα-GFPbright Cells Shifts to PDGFRα-GFPdim CD29 and α-SMA–Expressing Fibroblasts In Vivo and In Vitro
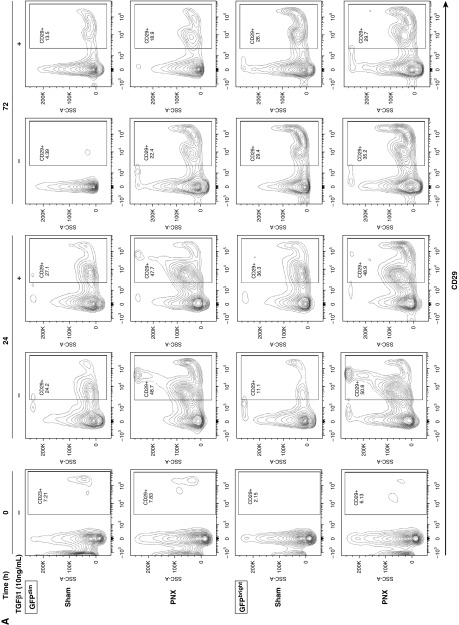
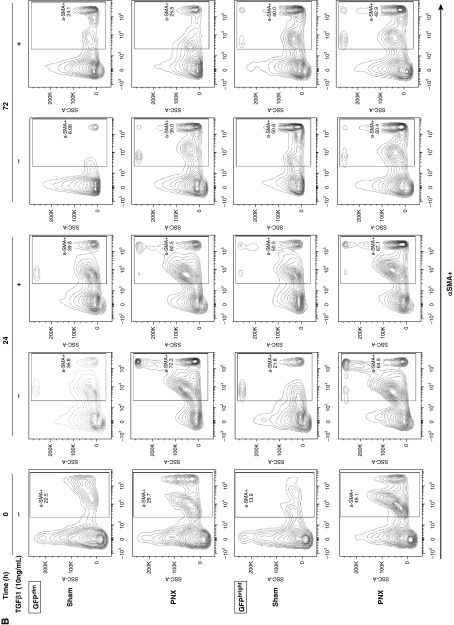
Three days after sham and PNX surgery, the proportions of PDGFRα-GFPbright and -GFPdim cells were similar. However, 5 days after surgery, the ratio of GFPdim to GFPbright increased by more than fourfold (Figure E5). To further clarify whether the PDGFRα-GFPbright and -GFPdim cells were derived from the same precursor and were at different stages of differentiation, GFPbright and GFPdim cells were cytometry sorted and were cultured separately in the presence of TGF-β1 or vehicle. The intensity of GFP expression diminished over 24 hours of in vitro culture in all PDGFRα-GFP populations. In GFPbright cells, isolated after partial PNX, in vitro culture increased α-SMA (fourfold) and CD29 (threefold) expression in the first 24 hours of culturing, and declined thereafter. TGF-β1 treatment in these PNX-GFPbright cells did not further increase α-SMA and CD29 expression. However in GFPbright cells isolated from sham-operated mice, TGF-β1 treatment increased α-SMA (threefold) and CD29 (twofold) expression. In GFPdim cells isolated from sham-operated mice, TGF-β1 treatment did not affect α-SMA or CD29 expression (Figures 5A and 5B). GFPbright cells increased proliferation for the first 24 hours and declined for the next 72 hours, suggesting that active proliferating cells are undergoing differentiation (Figure E6). These data suggest that in vitro GFPbright matrix-iReFs are differentiating to GFPdim CD29 and α-SMA–expressing myo-iReFs, either with TGF-β1 treatment or when isolated after partial PNX.
Figure 5.
PDGFRα+GFPbright cells differentiate to CD29 and α-SMA–expressing interstitial resident myofibroblast cells in vitro. PDGFRα+GFP cells were isolated from mice 3 days after surgery and sorted into GFPbright and GFPdim, and then separately cultured for 0, 24, and 72 hours in the presence or absence of TGF-β1. Proliferation (Figure E6), (A) CD29 expression (upper panel, GFPdim, and lower panel, GFPbright) and (B) α-SMA expression (upper panel, GFPdim, and lower panel, GFPbright) were analyzed. SSC, side scatter pulse; TGF-β1, transforming growth factor-β1.
PDGFRα Kinase Regulates Matrix-Associated Gene Expression
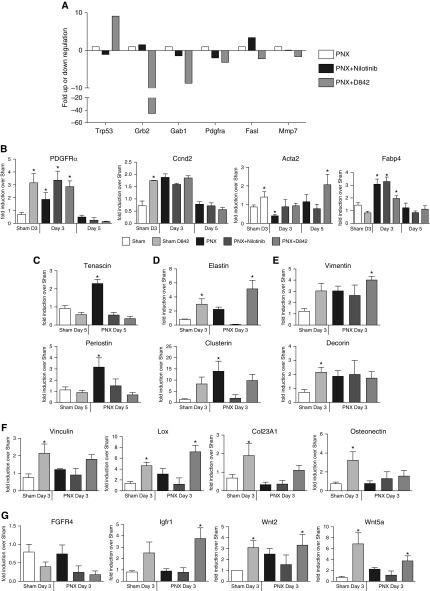
To further investigate transcriptional profile changes during alveolar regeneration in the presence or absence of nilotinib or PDGFRα-D842V mutation, we performed RT2-EGF/PDGF signaling PCR array analysis of RNA isolated from PDGFRα+ iReFs 3 or 5 days after PNX. Threefold up- or down-regulation in the presence of nilotinib or D842V mutation compared with untreated wild-type fibroblasts after PNX was considered significant. Nilotinib treatment reduced Trp53, Gab1, and Pdgfrα but increased Grb2 and Fasl messenger RNA (mRNA) expression. Although the D842V mutation increased Trp53, it reduced Grb2, Gab1, PDGFRα, Fasl, and MMP7 mRNA expression (Figure 6A). Genes regulated by nilotinib or the dominant active PDGFRα-D842V mutant (Trp53, Grb2, Gab1, Pdgfrα, Fasl, and MMP7) were used as gene training sets for the previously determined myo- and matrix gene profiles to build a gene network using ToppCluster and Cytoscape (Figure E8) (40, 41). Our data demonstrate that nilotinib inhibited ectopic elastin deposition (Figure 4D), further supporting the matrix modulating association via PDGFRα and GRB2 (Figure 6A). On the basis of our microarray expression profiles (Figure 1), we selectively interrogated representative mRNA expression levels of myo- and matrix signature genes. Increased but transient expression levels of Ccdn2, Fabp4, Pdgfrα, and Acta2 (after 5 d) were observed between 3 and 5 days after PNX, signifying proliferation and matrix/lipo- or myofibroblast differentiation. With the exception of Fabp4, the three other genes were also up-regulated by D842V mutation independent of PNX (Figure 6B).
Figure 6.
Transcription of p53 and major components of the ERK/MAPK pathway are regulated by nilotinib and the PDGFRα-D842V mutation. (A) PDGFRα+ iReFs were purified by magnetic beads from lungs of mice after 3 or 5 days of sham or PNX (white), PNX with nilotinib treatment (black), and PNX in PDGFRα-D842V mutants (gray). The RNA was extracted and complementary DNA prepared; then RT2-EGF/PDGF pathway arrays were performed in triplicates. Gene expression levels in the experimental groups were normalized to sham- and PNX-operated animals. PDGFRα+ iReFs were purified from lungs after 3 or 5 days of sham or PNX of wild-type, nilotinib-treated, or PDGFRα-D842V mutant mice. (B) Messenger RNA expression for PDGFRα, Ccnd2 (proliferation), acta2 (myo phenotype), Fabp4 (lipo phenotype). (C) Tenascin and periostin, (D) tropoelastin and clusterin, (E) vimentin and decorin, (F) Vinculin, Lox, Col23A, and osteonectin, and (G) FGFR4, IGFR1, Wnt2, and Wnt5a expressed in myo- and matrix-iRefs (n = 3–5). Data are expressed as mean ± SEM, and comparisons among groups were made by Student’s t test or analysis of variance, with P < 0.05 considered significant. IGFR, insulin growth factor receptor.
PNX-induced tenascin-C and periostin expression were attenuated when nilotinib treated and in D842V mutant mice after 5 days of surgery (Figure 6C), suggesting a significant reduction in the intermediate adhesions, which promote cell migration and wound healing (42). In addition, tropoelastin and clusterin expression were inhibited by nilotinib but increased by PDGFRα-D842V (Figure 6D). These data suggest a direct activity of PDGFRα kinase in regulating these matrix-iReF signature genes. Vimentin and decorin were markedly induced in D842V mice regardless of PNX; treatment of mice with nilotinib did not inhibit surgery-induced expression of these genes (Figure 6E). The expression levels of Vinculin and Lox were not affected by nilotinib treatment and surgery but were markedly augmented in D842V mutant mice independent of PNX (Figure 6F). Gene expression of osteonectin and Coll23A, myo-iReF signature genes, ECM-associated genes, were significantly induced in PDGFRα-D842V sham operated mice, but reduced 3 days after surgery (Figure 6F), suggesting that early phases of lung regeneration promotes matrix fibroblasts, which may later be differentiated to myofibroblasts.
Because the PDGF, FGF, insulin growth factor (IGF), and WNT signaling pathways are implicated in proliferation, growth, development, and fibroblast differentiations, we also assessed the impact of PDGFRα kinase activity on these gene expression levels. Although Fgfr4 expression was reduced slightly in nilotinib-treated or D842V mutant mice but not after PNX, Igfr1 induction was observed only in D842V mutant mice (Figure 6G), suggesting that Fgfr4 expression is influenced by both gain and loss of PDGFRα function but Igfr1 expression only during activated PDGFRα kinase activity. Wnt2 and Wnt5a are differentially expressed in matrix- and myo-iReFs. The expression levels of Wnt2 and Wnt5a were increased by both PNX and constitutively active D842V mutation (Figure 6G), suggesting that wnt2 and wnt5a expression may be regulated by PDGFRα during lung repair and regeneration.
Discussion
PDGFRα-expressing cells have been shown to localize in the alveolar entry ring, exhibiting the characteristics of myofibroblasts and supporting alveolar septal formation (27–30, 43). Previously, we reported that realveolarization after partial PNX requires alveolar septae formation and elastogenesis by α-SMA–expressing interstitial myofibroblasts that are derived from a population of interstitial PDGFRα+ fibroblasts. We have shown previously that repression of FGFR2 inhibits differentiation of the α-SMA–expressing interstitial myofibroblasts (31). Despite these insights, little is understood about the identity or phenotypic, functional, signaling, or transcriptional pathways of PDGFRα+ fibroblasts that are involved in the alveolar septal regeneration that occurs after PNX.
Using a transgenic mouse with a nuclear GFP reporter gene knocked into the Pdgfrα locus (PDGFRα-GFP) (31, 37), we identified two dynamically, phenotypically, and functionally diversified interstitial lung fibroblasts (PDGFRα+GFPbright and GFPdim) with distinct differential transcriptional signatures. Using genetically engineered mice with a combination of flow cytometry, microarray analysis, immunofluorescence, and in vitro culturing, we further characterized GFPbright fibroblasts as matrix-iReFs and PDGFRα+GFPdim cells as myofibroblast-like resident fibroblasts (myo-iReFs).
The gene expression profiles and the principal component analysis showed a clear segregation of the fibroblast phenotypes with more than 800 differentially expressed genes. In addition, immunophenotyping, flow cytometry, and in vitro culturing identified myofibroblasts as PDGFRα+CD29+ cells, whereas transitioning lipo/matrix fibroblasts could be identified by positive staining for PDGFRα+CD34+ or PDGFRα+CD34+CD29+ cells. Moreover, loss of PDGFRα function using the pharmacological inhibitor nilotinib or its gain of function using mice engineered with constitutively active PDGFRα-D842V revealed that PDGFRα kinase activity was important for matrix fibroblast differentiation, whereas myofibroblast differentiation was largely independent of this pathway. This study reports the existence of the two phenotypically dynamic and functionally diverse interstitial fibroblasts during the course of lung regeneration and realveolarization after partial PNX.
The matrix-iReF gene signature revealed increased expression of fibrillar collagens, fibril-associated collagens with interrupted triple helices collagens, matricellular proteins, fibulin1, fibulin2, Loxl2 and 4, CDK2, as well as MMP. In line with this report, fibrillar collagen is reported to increase MMP production and activation. Fibulins and fibrilins are also indicated to be essential for both elastic matrix fiber assembly and function, and Loxl2 is shown to crosslink elastin and collagen fibers and to generate a more robust ECM (44–46). In vitro, fibrillar collagen is also reported to reduce CDK2-mediated cell proliferation and to attenuate TGF-β–induced differentiation of parenchymal fibroblasts into myofibroblasts (47). These data are consistent with our findings that matrix-iRefs express fibrillar collagen that could promote activation, proliferation, and SMA expression in quiescent iReFs. These data are supported by our in vitro data that demonstrate that TGF-β activates α-SMA and CD29 in quiescent (not PNX-activated) GFPbright/matrix iReFs but not GFP-dim/myo-iReFs. Therefore, controlling the balance between matrix synthesis and matrix degradation by metalloproteinase in matrix-iReF is one of the key mechanisms that may allow proper repair without fibrosis.
Expression of the constitutively active PDGFRα-D842V mutation significantly increased ColXXIII, Vinculin and Tenascin-C, and Integrin α8. It also induced Pdgfrα, Igfr1, and osteonectin expression, but down-regulated Fgfr4 and delayed SMA expression. These data suggest that PDGFR and FGFR signaling are antagonistic to α-SMA induction in myofibroblasts, whereas PDGFR and IGFR could act synergistically toward matrix synthesis in matrix fibroblasts. In contrast, tenascin-C, in myo-iReFs, supports a state of intermediate adhesion, characterized by disruption of focal adhesions and a reorganization of actin stress fibers. PDGFRα-D842V mutation–suppressed PNX induced periostin and tenascin-C expression. These data suggest that PDGFRα signaling via the ERK1/2 pathway is important for periostin and tenascin activation and that increased phospholipase C γ (PLCγ) activity in D842V may suppress their gene expression. We have reported previously that expression of a dnFGFR2 mutation induces Wnt2 expression in lung fibroblasts (30). Our microarray analysis associated increased Wnt2 expression with myofibroblasts and Wnt5a with matrix fibroblasts. In PDGFRα-D842V mutants, Wnt2 and Wnt5a expression is increased.
In our experiments, 50% of all PDGFRα+ iReFs expressed high levels of stem cell antigen-1 (SCA-1), and McQualter and colleagues used SCA-1 in combination with negative selection for hematopoietic (CD45), endothelial (PECAM; CD31), and epithelial (EpCAM; CD326) cells to isolate lung fibroblasts that are critical to support self-renewal (22). Our data suggest that the majority of PDGFRα+CD34+ iReFs are lipofibroblasts and that reduction in this PDGFRα+CD34+ population 5 days after surgery is indicative of a transition from lipofibroblasts to myofibroblasts. Freshly isolated adipose-derived mesenchymal stem cells are reported to express CD34 (48). In the lung, these cells express CD140α, CD34, and CD29 and could differentiate into CD29- and α-SMA–expressing myofibroblasts after PNX in vivo or after 24-hour TGF-β1 stimulation in vitro (22, 38). Dominant active kinase (D842V) delayed myofibroblast differentiation in the PDGFRα+CD34+ iReFs and induced expression of the matrix-related genes Col23A1, osteonectin, Lox, and tropoelastin. These data suggest that the PDGFRα+CD34+–positive population differentiated from lipo iReFs to matrix iReFs. This is consistent with previously published data suggesting that PDGFRα activation predicts proliferation but inhibits myofibroblast differentiation and promotes fibrosis (28, 35). After PNX, CD140+CD29+ iReFs proliferated and differentiated into myofibroblasts. Neither gain nor loss of PDGFRα kinase activity changed myofibroblast differentiation in the CD140α+CD29+ iReFs. These data suggest that CD29-expressing iReFs could be activated for α-SMA induction and myofibroblast differentiation independent of PDGFRα expression, which is consistent with our previous findings, demonstrating that FGF signaling but not PDGFRα played an important role in myo-iReFs differentiation (29, 30). In addition, up-regulation of transmembrane collagens, organization of the actin cytoskeleton, modulation of the ECM, and reduction of cell adhesion observed in myo-iReFs are hallmarks of activated fibroblasts during developmental differentiation and wound healing.
Conclusions
Questions remain about the precise molecular mechanisms and driving forces required for myofibroblast differentiation to occur during post-PNX realveolarization, and the paracrine and the environmental niche of these cells. It is likely that PDGFR+ iReF may expand and differentiate early after PNX and contribute important paracrine signals that promote type II alveolar epithelial cells (AECII) proliferation and differentiation during regeneration. These studies, however, provide compelling evidence for the phenotypic and functional diversity, as well as the dynamics in time and the extent of specificity, of iReF differentiation in wound repair and regeneration after partial PNX.
Acknowledgments
Acknowledgments
The authors thank Susan Wert and Sheila Bell for critical input on the manuscript. They also thank the fluorescence-activated cell sorter core at the Cincinnati Children's Hospital Medical Center for technical assistance and the staff of the surgery suite for assistance with surgery and care of the animals.
Footnotes
Supported by National Institutes of Health grant HL 10400301 (A.-K.T.P.).
Author Contributions: Conception and design: A.-K.T.P.; analysis and interpretation: J.G., M.E., and H.A.; drafting of the manuscript for important intellectual content: M.E. and A.-K.T.P.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1165/rcmb.2015-0095OC on September 28, 2015
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Brewster CE, Howarth PH, Djukanovic R, Wilson J, Holgate ST, Roche WR. Myofibroblasts and subepithelial fibrosis in bronchial asthma. Am J Respir Cell Mol Biol. 1990;3:507–511. doi: 10.1165/ajrcmb/3.5.507. [DOI] [PubMed] [Google Scholar]
- 2.Evans MJ, Van Winkle LS, Fanucchi MV, Plopper CG. The attenuated fibroblast sheath of the respiratory tract epithelial-mesenchymal trophic unit. Am J Respir Cell Mol Biol. 1999;21:655–657. doi: 10.1165/ajrcmb.21.6.3807. [DOI] [PubMed] [Google Scholar]
- 3.Herriges M, Morrisey EE. Lung development: orchestrating the generation and regeneration of a complex organ. Development. 2014;141:502–513. doi: 10.1242/dev.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morrisey EE, Hogan BL. Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beers MF, Morrisey EE. The three R’s of lung health and disease: repair, remodeling, and regeneration. J Clin Invest. 2011;121:2065–2073. doi: 10.1172/JCI45961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi W, Xu J, Warburton D. Development, repair and fibrosis: what is common and why it matters. Respirology. 2009;14:656–665. doi: 10.1111/j.1440-1843.2009.01565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warburton D, Tefft D, Mailleux A, Bellusci S, Thiery JP, Zhao J, Buckley S, Shi W, Driscoll B. Do lung remodeling, repair, and regeneration recapitulate respiratory ontogeny? Am J Respir Crit Care Med. 2001;164:S59–S62. doi: 10.1164/ajrccm.164.supplement_2.2106064. [DOI] [PubMed] [Google Scholar]
- 8.Borok Z, Li C, Liebler J, Aghamohammadi N, Londhe VA, Minoo P. Developmental pathways and specification of intrapulmonary stem cells. Pediatr Res. 2006;59:84R–93R. doi: 10.1203/01.pdr.0000203563.37626.77. [DOI] [PubMed] [Google Scholar]
- 9.Smith RS, Smith TJ, Blieden TM, Phipps RP. Fibroblasts as sentinel cells. Synthesis of chemokines and regulation of inflammation. Am J Pathol. 1997;151:317–322. [PMC free article] [PubMed] [Google Scholar]
- 10.Knight DA, Lane CL, Stick SM. Does aberrant activation of the epithelial-mesenchymal trophic unit play a key role in asthma or is it an unimportant sideshow? Curr Opin Pharmacol. 2004;4:251–256. doi: 10.1016/j.coph.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Behzad AR, McDonough JE, Seyednejad N, Hogg JC, Walker DC. The disruption of the epithelial mesenchymal trophic unit in COPD. COPD. 2009;6:421–431. doi: 10.3109/15412550903341471. [DOI] [PubMed] [Google Scholar]
- 12.Hsia CC. Signals and mechanisms of compensatory lung growth. J Appl Physiol (1985) 2004;97:1992–1998. doi: 10.1152/japplphysiol.00530.2004. [DOI] [PubMed] [Google Scholar]
- 13.Cagle PT, Thurlbeck WM. Postpneumonectomy compensatory lung growth. Am Rev Respir Dis. 1988;138:1314–1326. doi: 10.1164/ajrccm/138.5.1314. [DOI] [PubMed] [Google Scholar]
- 14.Brown LM, Rannels SR, Rannels DE. Implications of post-pneumonectomy compensatory lung growth in pulmonary physiology and disease. Respir Res. 2001;2:340–347. doi: 10.1186/rr84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paxson JA, Gruntman A, Parkin CD, Mazan MR, Davis A, Ingenito EP, Hoffman AM. Age-dependent decline in mouse lung regeneration with loss of lung fibroblast clonogenicity and increased myofibroblastic differentiation. PLoS One. 2011;6:e23232. doi: 10.1371/journal.pone.0023232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McBride JT, Wohl ME, Strieder DJ, Jackson AC, Morton JR, Zwerdling RG, Griscom NT, Treves S, Williams AJ, Schuster S. Lung growth and airway function after lobectomy in infancy for congenital lobar emphysema. J Clin Invest. 1980;66:962–970. doi: 10.1172/JCI109965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laros CD, Westermann CJ. Dilatation, compensatory growth, or both after pneumonectomy during childhood and adolescence. A thirty-year follow-up study. J Thorac Cardiovasc Surg. 1987;93:570–576. [PubMed] [Google Scholar]
- 18.Butler JP, Loring SH, Patz S, Tsuda A, Yablonskiy DA, Mentzer SJ. Evidence for adult lung growth in humans. N Engl J Med. 2012;367:244–247. doi: 10.1056/NEJMoa1203983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogan BL, Barkauskas CE, Chapman HA, Epstein JA, Jain R, Hsia CC, Niklason L, Calle E, Le A, Randell SH, et al. Repair and regeneration of the respiratory system: complexity, plasticity, and mechanisms of lung stem cell function. Cell Stem Cell. 2014;15:123–138. doi: 10.1016/j.stem.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertoncello I, McQualter JL. Lung stem cells: do they exist? Respirology. 2013;18:587–595. doi: 10.1111/resp.12073. [DOI] [PubMed] [Google Scholar]
- 21.Volckaert T, Dill E, Campbell A, Tiozzo C, Majka S, Bellusci S, De Langhe SP. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J Clin Invest. 2011;121:4409–4419. doi: 10.1172/JCI58097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McQualter JL, Brouard N, Williams B, Baird BN, Sims-Lucas S, Yuen K, Nilsson SK, Simmons PJ, Bertoncello I. Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the sca-1 positive cell fraction. Stem Cells. 2009;27:623–633. doi: 10.1634/stemcells.2008-0866. [DOI] [PubMed] [Google Scholar]
- 23.McQualter JL, McCarty RC, Van der Velden J, O’Donoghue RJ, Asselin-Labat ML, Bozinovski S, Bertoncello I. TGF-β signaling in stromal cells acts upstream of FGF-10 to regulate epithelial stem cell growth in the adult lung. Stem Cell Res (Amst) 2013;11:1222–1233. doi: 10.1016/j.scr.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 24.El Agha E, Herold S, Al Alam D, Quantius J, MacKenzie B, Carraro G, Moiseenko A, Chao CM, Minoo P, Seeger W, et al. Fgf10-positive cells represent a progenitor cell population during lung development and postnatally. Development. 2014;141:296–306. doi: 10.1242/dev.099747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Li M, Li S, Xing Y, Yang CY, Li A, Borok Z, De Langhe S, Minoo P. Progenitors of secondary crest myofibroblasts are developmentally committed in early lung mesoderm. Stem Cells. 2015;33:999–1012. doi: 10.1002/stem.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boström H, Willetts K, Pekny M, Levéen P, Lindahl P, Hedstrand H, Pekna M, Hellström M, Gebre-Medhin S, Schalling M, et al. PDGF-A signaling is a critical event in lung alveolar myofibroblast development and alveogenesis. Cell. 1996;85:863–873. doi: 10.1016/s0092-8674(00)81270-2. [DOI] [PubMed] [Google Scholar]
- 27.McGowan SE, Grossmann RE, Kimani PW, Holmes AJ. Platelet-derived growth factor receptor-α-expressing cells localize to the alveolar entry ring and have characteristics of myofibroblasts during pulmonary alveolar septal formation. Anat Rec (Hoboken) 2008;291:1649–1661. doi: 10.1002/ar.20764. [DOI] [PubMed] [Google Scholar]
- 28.Kimani PW, Holmes AJ, Grossmann RE, McGowan SE. PDGF-Rα gene expression predicts proliferation, but PDGF-A suppresses transdifferentiation of neonatal mouse lung myofibroblasts. Respir Res. 2009;10:119. doi: 10.1186/1465-9921-10-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perl AK, Gale E. FGF signaling is required for myofibroblast differentiation during alveolar regeneration. Am J Physiol Lung Cell Mol Physiol. 2009;297:L299–L308. doi: 10.1152/ajplung.00008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Acciani T, Le Cras T, Lutzko C, Perl AK. Dynamic regulation of platelet-derived growth factor receptor α expression in alveolar fibroblasts during realveolarization. Am J Respir Cell Mol Biol. 2012;47:517–527. doi: 10.1165/rcmb.2012-0030OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindahl P, Karlsson L, Hellström M, Gebre-Medhin S, Willetts K, Heath JK, Betsholtz C. Alveogenesis failure in PDGF-A-deficient mice is coupled to lack of distal spreading of alveolar smooth muscle cell progenitors during lung development. Development. 1997;124:3943–3953. doi: 10.1242/dev.124.20.3943. [DOI] [PubMed] [Google Scholar]
- 32.Hamilton TG, Klinghoffer RA, Corrin PD, Soriano P. Evolutionary divergence of platelet-derived growth factor α receptor signaling mechanisms. Mol Cell Biol. 2003;23:4013–4025. doi: 10.1128/MCB.23.11.4013-4025.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Biase LM, Kang SH, Baxi EG, Fukaya M, Pucak ML, Mishina M, Calabresi PA, Bergles DE. NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J Neurosci. 2011;31:12650–12662. doi: 10.1523/JNEUROSCI.2455-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imai T, Jiang M, Chambon P, Metzger D. Impaired adipogenesis and lipolysis in the mouse upon selective ablation of the retinoid X receptor α mediated by a tamoxifen-inducible chimeric Cre recombinase (Cre-ERT2) in adipocytes. Proc Natl Acad Sci USA. 2001;98:224–228. doi: 10.1073/pnas.011528898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olson LE, Soriano P. Increased PDGFRα activation disrupts connective tissue development and drives systemic fibrosis. Dev Cell. 2009;16:303–313. doi: 10.1016/j.devcel.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hittinger M, Czyz ZT, Huesemann Y, Maneck M, Botteron C, Kaeufl S, Klein CA, Polzer B. Molecular profiling of single Sca-1+/CD34+,- cells--the putative murine lung stem cells. PLoS One. 2013;8:e83917. doi: 10.1371/journal.pone.0083917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu S, Xu SW, Blumbach K, Eastwood M, Denton CP, Eckes B, Krieg T, Abraham DJ, Leask A. Expression of integrin beta1 by fibroblasts is required for tissue repair in vivo. J Cell Sci. 2010;123:3674–3682. doi: 10.1242/jcs.070672. [DOI] [PubMed] [Google Scholar]
- 38.Bertoncello I, McQualter JL. Endogenous lung stem cells: what is their potential for use in regenerative medicine? Expert Rev Respir Med. 2010;4:349–362. doi: 10.1586/ers.10.21. [DOI] [PubMed] [Google Scholar]
- 39.Fehrenbach H, Voswinckel R, Michl V, Mehling T, Fehrenbach A, Seeger W, Nyengaard JR. Neoalveolarisation contributes to compensatory lung growth following pneumonectomy in mice. Eur Respir J. 2008;31:515–522. doi: 10.1183/09031936.00109407. [DOI] [PubMed] [Google Scholar]
- 40.Kaimal V, Bardes EE, Tabar SC, Jegga AG, Aronow BJ. ToppCluster: a multiple gene list feature analyzer for comparative enrichment clustering and network-based dissection of biological systems. Nucleic Acids Res. 2010;38:W96-102. doi: 10.1093/nar/gkq418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chiodoni C, Colombo MP, Sangaletti S. Matricellular proteins: from homeostasis to inflammation, cancer, and metastasis. Cancer Metastasis Rev. 2010;29:295–307. doi: 10.1007/s10555-010-9221-8. [DOI] [PubMed] [Google Scholar]
- 43.McGowan SE, McCoy DM. Fibroblasts expressing PDGF-receptor-α diminish during alveolar septal thinning in mice. Pediatr Res. 2011;70:44–49. doi: 10.1203/PDR.0b013e31821cfb5a. [DOI] [PubMed] [Google Scholar]
- 44.Nakamura T, Lozano PR, Ikeda Y, Iwanaga Y, Hinek A, Minamisawa S, Cheng CF, Kobuke K, Dalton N, Takada Y, et al. Fibulin-5/DANCE is essential for elastogenesis in vivo. Nature. 2002;415:171–175. doi: 10.1038/415171a. [DOI] [PubMed] [Google Scholar]
- 45.Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki T, Göhring W, Miosge N, Abrams WR, Rosenbloom J, Timpl R. Tropoelastin binding to fibulins, nidogen-2 and other extracellular matrix proteins. FEBS Lett. 1999;460:280–284. doi: 10.1016/s0014-5793(99)01362-9. [DOI] [PubMed] [Google Scholar]
- 47.Schuliga MJ, See I, Ong SC, Soon L, Camoretti-Mercado B, Harris T, Stewart AG. Fibrillar collagen clamps lung mesenchymal cells in a nonproliferative and noncontractile phenotype. Am J Respir Cell Mol Biol. 2009;41:731–741. doi: 10.1165/rcmb.2008-0361OC. [DOI] [PubMed] [Google Scholar]
- 48.Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, Lin CS. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–1063. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]