To the Editor:
Although most research on cystic fibrosis (CF) disease pathogenesis has focused on epithelia (1), recent work shows that CF transmembrane conductance regulator (CFTR) is expressed on human and murine monocytes and macrophages (2, 3) and that CFTR deficiency could alter these cells’ functions (2, 4, 5). Although these findings raise the possibility that immune dysfunction contributes to CF disease pathogenesis, questions remain. First, although some studies identified defects in CF monocytes and macrophages (2, 5, 6), other studies have not (7). Second, abnormalities in human cells could be a primary consequence of CFTR dysfunction or secondary effects caused by the disease environment. Third, although studies in mice may be less affected by secondary disease manifestations, they may be complicated by species differences. Finally, prior studies have investigated specific myeloid cell functions and may have missed unsuspected consequences of CFTR dysfunction.
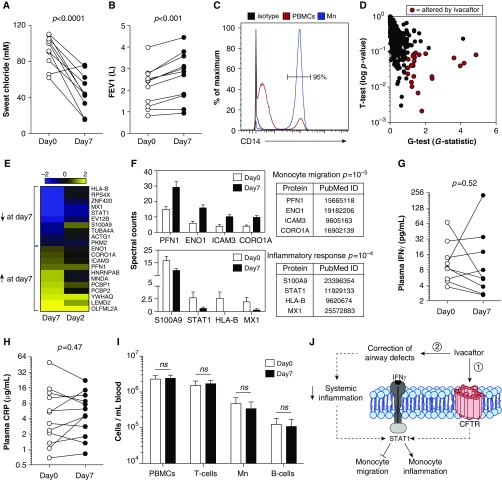
We exploited an opportunity to investigate how CFTR channel activity affects monocyte functioning by studying peripheral blood monocytes from patients starting therapy with the CFTR potentiator ivacaftor. Ivacaftor increases CFTR-G551D channel opening and improves sweat chloride and lung function (Figures 1A and 1B) (8). We measured the plasma membrane (PM)-associated proteome (9) of monocytes from 12 patients with CFTR-G551D mutations (Table 1) before and 2 and 7 days after treatment with ivacaftor. We focused on the PM-associated proteome because these proteins mediate immune functions, serve as biomarkers, and may suggest disease mechanisms (9). The study design reduced variability because each patient served as his/her own control, and studying monocytes (Figure 1C) avoided in vitro culture to produce macrophages and ensured that drug exposure occurred in vivo.
Figure 1.
Ivacaftor alters the monocyte plasma membrane (PM) proteome but does not change systemic inflammation. All measurements were performed at Day 0, before initiation of ivacaftor, and on Day 7 of therapy. Patients were prescribed ivacaftor 150 mg by mouth, every 12 hours. Effects of ivacaftor on (A) sweat chloride levels. Normal, <40 mM; borderline, 41–60 mM; abnormal (consistent with cystic fibrosis transmembrane conductance regulator [CFTR] dysfunction), >60 mM, and (B) FEV1 (liters). (C) Flow cytometry demonstrates enrichment of monocytes (Mn, CD14+ cells) after magnetic bead negative selection. (D) The graph depicts all 731 proteins detected by PM proteomics, with each dot representing one protein. Red dots indicate the 21 proteins that were statistically significantly changed in abundance between Day 0 and Day 7. (E) Heat map of PM proteins significantly regulated after 7 days of ivacaftor treatment. Protein levels are presented as fold changes (log 2 scale) from Day 7 to Day 0 and Day 2 to Day 0. Yellow, up-regulated; blue, down-regulated. (F) Bioinformatics analysis of the 21 proteins altered by ivacaftor identified that proteins involved in the inflammatory response and monocyte migration were altered by ivacaftor treatment much more frequently than expected on the basis of their representation in the genome. PM-associated levels for these proteins (spectral counts) and references implicating their involvement in the functional categories are provided. (G and H) Plasma IFNγ and C-reactive protein (CRP) levels before and after ivacaftor therapy. (I) Peripheral blood mononuclear cells (PBMCs) were analyzed by flow cytometry to determine proportions of leukocyte subsets; CD3+, T cells; CD19+, B cells; CD14+, monocytes. (J) A hypothetical model for how ivacaftor may elicit PM proteomic changes in monocytes. Model 1 proposes that exaggerated IFNγ responses are produced by dysfunctional CFTR and that ivacaftor therapy attenuates IFNγ responses by increasing monocyte CFTR channel activity. Model 2 proposes that changes in the lung caused by ivacaftor lead to systemic changes that affect monocyte IFNγ responses. CFTR is depicted in the plasma membrane for simplicity of illustration; however, CFTR may be present in other membranes within monocytes. Dashed arrows indicate possible intermediate steps, whereas solid arrows indicate direct actions. FEV1, forced expiratory volume in 1 sec; ns, not significant.
Table 1.
Patient Demographics
| Patient* | Age (yr) | Sex | Genotype G551D | BMI (kg/m2) | FEV1(L), Percentage Predicted | Sputum Microbiology |
|---|---|---|---|---|---|---|
| 1 | 23 | F | ΔF508 | 29.7 | 3.08 (87%) | PA |
| 2 | 25 | F | ΔF508 | 25.5 | 2.71 (80%) | PA, MSSA |
| 3 | 23 | F | ΔF508 | 21.8 | 2.74 (88%) | SA, AF |
| 4 | 30 | F | ΔF508 | 20.4 | 1.05 (38%) | PA |
| 5 | 27 | F | ΔF508 | 20.2 | 1.71 (58%) | PA, MRSA |
| 6 | 22 | F | 3659delC | 18.4 | 1.22 (38%) | PA, CA, AF |
| 7 | 33 | F | ΔF508 | 17.1 | 2.57 (89%) | PA |
| 8 | 57 | F | P67L | 19.5 | 0.94 (39%) | MSSA, CA |
| 9 | 35 | F | ΔF508 | 20.6 | 2.17 (78%) | PA |
| 10 | 29 | M | G551D | 23.2 | 4.16 (106%) | PA |
| 11 | 30 | M | ΔF508 | 23.4 | 1.60 (39%) | MSSA, BM |
| 12 | 33 | M | R117H | 22.1 | 2.71 (71%) | BC, CA |
Definition of abbreviations: AF, Aspergillus fumigatus; BC, Burkholderia cepacia; BM, Burkholderia multivorans; BMI, body mass index; CA, Candida albicans; CF, cystic fibrosis; F, female; FEV1, forced expiratory volume in 1 sec; M, male; MSSA, methicillin-sensitive Staphylococcus aureus; MRSA, methicillin-resistant Staphyloccus aureus; PA, Pseudomonas aeruginosa; SA, Staphyloccus aureus.
All patients received routine CF care at the adult CF clinic at St. Vincent’s Hospital, Dublin, Ireland, and this study was approved by the St. Vincent’s Hospital’s Institutional Review Board.
As our primary goal was to better understand whether CFTR dysfunction affects monocyte functions, we wished to identify proteins altered as a direct consequence of restored CFTR activity. We reasoned that proteins that changed rapidly after ivacaftor initiation were likely candidates, as late changes may be more affected by secondary effects. However, because ivacaftor requires 3–5 days to reach steady-state levels (10), studying very early time points could miss emergent changes. We addressed this problem by first identifying proteins that changed significantly at Day 7 and then testing this group of proteins for changes at Day 2, using Gene Set Enrichment Analysis (GSEA) (11). GSEA determines whether a priori-defined proteins (i.e., proteins changed at Day 7) show significant and concordant changes at a point where effects may be more modest (i.e., Day 2).
Using stringent statistical criteria that combined the t and G tests (false discovery rate, <0.05), we identified 21 PM-associated proteins whose abundance significantly changed at Day 7 (Figure 1D and see Table E1 in the online supplement). Importantly, GSEA found that many proteins that changed significantly at Day 7 showed a similar regulatory pattern, but smaller-magnitude changes, at Day 2 (P = 0.02–0.0007; Figures 1E and E1).
We used gene ontology analysis to determine whether ivacaftor affected particular functional pathways and found that ivacaftor disproportionately increased proteins associated with cell migration (P = 10−5; Figure 1F). Ivacaftor increased ENO1 and PFN1, which enhance monocyte migration across epithelia and endothelia, and ICAM3 and CORO1A, which participate in leukocyte migration. This is notable because CFTR has not previously been linked to cell migration.
Gene ontology analysis also revealed that ivacaftor disproportionately reduced monocyte proteins involved in inflammation, including S100A9, MX1, and HLA-B (P = 10−4; Figure 1F). S100A9 is a biomarker of CF inflammation (12), HLA-B mediates antigen presentation, and MX1 mediates antiviral responses.
Remarkably, all of the inflammation-related proteins reduced by ivacaftor are IFNγ induced. IFNγ can also arrest monocyte chemotaxis (13). Thus, both the decreases in inflammation-related proteins and the increases in monocyte-migration proteins we observed could be linked to dampened IFNγ responses.
The simplest mechanism to explain decreased IFNγ responses in monocytes would be that ivacaftor decreased circulating IFNγ levels. We thus measured plasma IFNγ levels and found them unchanged (Figure 1G). These findings led us to hypothesize that CF monocytes may have increased sensitivity to ambient IFN and that ivacaftor decreases sensitivity. This hypothesis predicts that ivacaftor will decrease the levels of PM-associated STAT1, as STAT1 is recruited to the PM during IFNγ signaling (14). As shown in Figure 1F, ivacaftor markedly decreased PM-associated STAT1. Together, these results suggest that CF monocytes may have increased IFNγ sensitivity, and that increased sensitivity may be mediated by increased STAT1 activation. However, IFNγ responses can also be regulated by STAT1-independent mechanisms, and additional confirmatory work is needed.
One mechanism by which CFTR dysfunction could increase monocyte IFNγ sensitivity and increase STAT1 PM recruitment is decreased IFNγ receptor endocytosis (14). Previous work indicates that CF macrophages have decreased LPS receptor endocytosis, and as a consequence, have exaggerated LPS responses (4). Thus, defective receptor endocytosis in CF myeloid cells could be a common mechanism that produces both increased LPS and IFNγ sensitivity.
A key question for future research is whether dampened IFNγ sensitivity is a direct effect of ivacaftor on monocyte CFTR channel activity or a secondary effect of decreased systemic inflammation (see model, Figure 1J). Our study cannot distinguish between these possibilities. Arguing for a direct effect, we found no ivacaftor-mediated decrease in blood leukocyte counts, IFNγ levels, or C-reactive protein (12) (Figures 1G–1I). Also arguing for a direct effect, GSEA found that many proteins significantly changed at Day 7 showed concordant changes at Day 2 (Figures 1E and E1). Arguing against a direct effect, lung function is known to increase 3 days after ivacaftor treatment (8). Thus, it is possible that the proteomic changes are secondary to ivacaftor-mediated improvements in general health.
In summary, the ivacaftor-induced proteomic changes we observed suggest unsuspected impairments in CF monocyte migration and IFNγ responses. If confirmed by future studies, our data could have implications for disease. Increased monocyte IFNγ sensitivity could hyperactivate IFNγ-induced inflammatory pathways and block monocyte migration to the lungs. Chronic monocyte activation caused by increased IFNγ sensitivity could also produce a tolerant state (15) that impairs the ability of monocytes to respond to acute fluctuations in IFNγ levels. Future studies to explore both mechanisms and consequences of altered IFNγ signaling in CF monocytes may suggest new approaches to develop therapeutics for modulating inflammation in CF.
Footnotes
This work was supported by an independent medical grant from Vertex Pharmaceuticals and the National Institutes of Health (P30DK089507).
Author Contributions: Concept, design, acquisition of data, analysis, and interpretation: K.B.H., K.Q.S., G.C., B.G., J.L.L., C.G.G., S.C.D., M.J.W., P.K.S., E.F.M., and L.B.; and drafting the manuscript for important intellectual content: K.B.H., M.J.W., P.K.S., and L.B.
This letter has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Stoltz DA, Meyerholz DK, Welsh MJ. Origins of cystic fibrosis lung disease. N Engl J Med. 2015;372:351–362. doi: 10.1056/NEJMra1300109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di A, Brown ME, Deriy LV, Li C, Szeto FL, Chen Y, Huang P, Tong J, Naren AP, Bindokas V, et al. CFTR regulates phagosome acidification in macrophages and alters bactericidal activity. Nat Cell Biol. 2006;8:933–944. doi: 10.1038/ncb1456. [DOI] [PubMed] [Google Scholar]
- 3.Sorio C, Buffelli M, Angiari C, Ettorre M, Johansson J, Vezzalini M, Viviani L, Ricciardi M, Verzè G, Assael BM, et al. Defective CFTR expression and function are detectable in blood monocytes: development of a new blood test for cystic fibrosis. PLoS One. 2011;6:e22212. doi: 10.1371/journal.pone.0022212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruscia EM, Zhang PX, Satoh A, Caputo C, Medzhitov R, Shenoy A, Egan ME, Krause DS. Abnormal trafficking and degradation of TLR4 underlie the elevated inflammatory response in cystic fibrosis. J Immunol. 2011;186:6990–6998. doi: 10.4049/jimmunol.1100396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Porto P, Cifani N, Guarnieri S, Di Domenico EG, Mariggiò MA, Spadaro F, Guglietta S, Anile M, Venuta F, Quattrucci S, et al. Dysfunctional CFTR alters the bactericidal activity of human macrophages against Pseudomonas aeruginosa. PLoS One. 2011;6:e19970. doi: 10.1371/journal.pone.0019970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonfield TL, Hodges CA, Cotton CU, Drumm ML. Absence of the cystic fibrosis transmembrane regulator (Cftr) from myeloid-derived cells slows resolution of inflammation and infection. J Leukoc Biol. 2012;92:1111–1122. doi: 10.1189/jlb.0412188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haggie PM, Verkman AS. Cystic fibrosis transmembrane conductance regulator-independent phagosomal acidification in macrophages. J Biol Chem. 2007;282:31422–31428. doi: 10.1074/jbc.M705296200. [DOI] [PubMed] [Google Scholar]
- 8.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW, et al. VX08-770-102 Study Group. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS, et al. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–625. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cambridge, MA: Vertex Pharmaceuticals; 2012. Ivacaftor (kalydeco) prescribing information. [Google Scholar]
- 11.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horsley AR, Davies JC, Gray RD, Macleod KA, Donovan J, Aziz ZA, Bell NJ, Rainer M, Mt-Isa S, Voase N, et al. Changes in physiological, functional and structural markers of cystic fibrosis lung disease with treatment of a pulmonary exacerbation. Thorax. 2013;68:532–539. doi: 10.1136/thoraxjnl-2012-202538. [DOI] [PubMed] [Google Scholar]
- 13.Hu Y, Hu X, Boumsell L, Ivashkiv LB. IFN-γ and STAT1 arrest monocyte migration and modulate RAC/CDC42 pathways. J Immunol. 2008;180:8057–8065. doi: 10.4049/jimmunol.180.12.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 15.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]