Abstract
Socioeconomic status (SES) is strongly associated with cognition and achievement. Socioeconomic disparities in language and memory skills have been reported from elementary school through adolescence. Less is known about the extent to which such disparities emerge in infancy. Here, 179 infants from socioeconomically diverse families were recruited. Using a cohort-sequential design, 90 infants were followed at 9 and 15 months, and 89 were followed at 15 and 21 months. SES disparities in developmental trajectories of language and memory were present such that, at 21 months of age, children of highly educated parents scored approximately .8 standard deviations higher in both language and memory than children of less educated parents. The home language and literacy environment and parental warmth partially accounted for disparities in language, but not memory development.
Keywords: cognitive development, socioeconomic status, language, memory, infancy
INTRODUCTION
Childhood socioeconomic status (SES) is strongly associated with later cognitive development and academic achievement (Bradley, Corwyn, Burchinal, McA-doo, & Garcia Coll, 2001; Brooks-Gunn & Duncan, 1997; Evans, 2004; Hoff, 2003; McLoyd, 1998). By the time of school entry, children from lower SES backgrounds typically score between one-half and one full standard deviation lower than other children on most academic achievement tests (Rouse, Brooks-Gunn, & McLanahan, 2005). The income-achievement gap has widened substantially over the last 25 years, and is currently more than twice as large as the black–white achievement gap (Reardon, 2011). Such disparities in turn have long-lasting ramifications for physical and mental health (Brooks-Gunn & Duncan, 1997).
Family socioeconomic status (SES) is typically characterized by several factors, including parental educational attainment, family income, and parental occupation (McLoyd, 1998). Studies examining the association between SES and cognitive development have classically focused on important but generalized cognitive and academic milestones, such as child IQ, grade retention, and school graduation rates (Brooks-Gunn & Duncan, 1997). Such measures tell us in broad strokes that socioeconomic differences in childhood are associated with large differences in achievement.
Although achievement is clearly influenced by brain development, until recently the study of SES disparities in child development operated with virtually no input from neuroscience. We know that a construct such as “achievement” is in fact the complex product of multiple cognitive systems supported by different brain regions and networks, which undergo development from the earliest ages. A cognitive neuroscience approach is based on an understanding of the different neural structures and circuits that support the development of distinct cognitive skills. By incorporating such an approach into the study of SES disparities, we may be better able to design targeted preventions and interventions to specific neurocognitive deficits (Neville, Stevens, Pakulak, & Bell, 2013; Noble & Farah, 2013).
A number of recent studies have adopted a cognitive neuroscience framework to understanding SES differences in cognition (Hackman & Farah, 2009; Raizada & Kishiyama, 2010). By employing cognitive tasks that selectively engage one neurocognitive system while placing minimal burden on others, socioeconomic differences in children’s performance in specific neurocognitive systems can be compared. For example, when compared with children from higher SES backgrounds, 5 to 13-year-old children from lower SES backgrounds show lower performance in various aspects of language development (Farah et al., 2006; Noble, McCandliss, & Farah, 2007; Noble, Norman, & Farah, 2005) including skills which are supported by a left lateralized network in the temporal, temporo-occipital, and frontal cortices (Dehaene-Lambertz et al., 2006; McCandliss & Noble, 2003; Turkeltaub, Gareau, Flowers, Zeffiro, & Eden, 2003; Vannest, Karunanayaka, Schmithorst, Szaflarski, & Holland, 2009). More modest but consistent socio-economic disparities have been reported for other neurocognitive functions, such as declarative memory (Farah et al., 2006; Noble et al., 2007, 2005), which is largely supported by the hippocampus and other medial temporal lobe structures (McEwen & Gianaros, 2010; Richmond & Nelson, 2008). One study of children in first grade reported that, for each standard deviation increase in SES (operationalized as a composite of parental education, occupation, and income), performance on a composite of language skills increased by more than half a standard deviation, while performance on a composite of memory skills increased by approximately one-third of a standard deviation (Noble et al., 2007). However, the literature to date leaves several key questions unexamined.
Timecourse
One open question concerns timing. By early adolescence, SES disparities are greatest in language and declarative memory, relative to other neurocognitive systems such as executive functioning or visuospatial skills (Farah et al., 2006). Both language (Halle et al., 2009) and memory (Barr, Dowden, & Hayne, 1996) show individual differences in development in the first 2 years of life and these skills are predictive of later cognitive development (Bornstein & Sigman, 1986; Fagan & Singer, 1983; Halle et al., 2009; Hoff, 2003). As such, these skills serve as model systems for examining emerging socioeconomic disparities in neurocognition in infancy and early childhood.
Socioeconomic differences in various aspects of language development, including expressive language skills, vocabulary, language processing efficiency, and gesture use, have been reported in the first 2 years of life (Fernald, Marchman, & Weisleder, 2013; Halle et al., 2009; Hoff, 2003; Rowe & Goldin-Meadow, 2009). Recently, SES differences in resting EEG frontal gamma power have been reported as early as 6 months of age (Tomalski et al., 2013); such differences have previously been related to later language development (Gou, Choudhury, & Benasich, 2011). However, the extent to which these early neurophysiological differences suggest that behavioral differences in language acquisition may be detected early in infancy remains unclear. Further, little is known regarding how socioeconomic disparities in language development compare to developmental disparities in other aspects of cognition.
In contrast to language development, little is known about the emergence of early socioeconomic disparities in memory. While SES is linked to memory skill by the start of school (Noble et al., 2007) and indeed, socioeconomic disparities in memory skill extend across the lifespan (Stern, Albert, Tang, & Tsai, 1999), findings have been inconsistent concerning disparities in memory development earlier in childhood. Historically, relations between SES and infant novelty preference were inconsistent (Fagan & Singer, 1983; O’Connor, Cohen, & Parmelee, 1984; Rose & Wallace, 1985), and a recent meta-analysis reported no evidence of SES differences in infant operant conditioning (Gerhardstein, Dickerson, Miller, & Hipp, 2012). Thus, although hippocampally mediated declarative memory skill emerges over the first 2 years of life (Barr et al., 1996; Barr, Walker, Gross, & Hayne, 2013), the extent to which individual differences in memory development can be explained by SES at these early ages requires further investigation.
Mediating Factors
A second open question concerns the proximal experiences that mediate associations between SES and neurocognitive development. It is unclear whether the pathways that account for disparities in language development are similar to those that explain disparities in memory development (Farah et al., 2008).
The quantity and quality of language exposure in the home have been related to SES disparities in language development (Hart & Risley, 1995). Differences in the amount and complexity of maternal speech (Hoff, 2003; Huttenlocher, Vasilyeva, Waterfall, Vevea, & Hedges, 2007; Pan, Rowe, Singer, & Snow, 2005) and gesture (Rowe & Goldin-Meadow, 2009) help to explain SES differences in children’s vocabulary and other aspects of language development. Maternal speech input during infancy likely results in a cascade of effects on the development of neural networks specialized for processing language (Kuhl, 2010). Additionally, past studies have reported that even after accounting for primary caregiver IQ and education, the literacy environment (number of books in the home, frequency of joint book reading, etc.) still accounted for a significant proportion of the variance in child language ability (Payne, Whitehurst, & Angell, 1994). Thus, socioeconomic disparities in the quality and quantity of linguistic stimulation may lead to differences in the development of language-supporting neural networks, underlying SES disparities in language skills (Noble, Houston, Kan, & Sowell, 2012b; Sheridan, Sarsour, Jutte, D’Esposito, & Boyce, 2012).
A separate literature has described SES disparities in family stress, including uncertainty about material resources, chaotic households, and harsh parenting (Brooks-Gunn & Duncan, 1997; Evans, 2004). Both stress in general (McEwen & Gianaros, 2010), and adverse parenting specifically (Champagne et al., 2008), have direct effects on the hippocampus, which is critical for the development of memory. Exposure to stress in childhood may therefore operate on this structure to mediate SES disparities in memory (Sheridan, How, Araujo, Schamberg, & Nelson, 2013). Indeed, SES factors have been associated with hippocampal size both in children (Hanson, Chandra, Wolfe, & Pollak, 2011; Jednoróg et al., 2012; Noble et al., 2012b) and adults (Noble et al., 2012a; Staff et al., 2012). Thus, differences in family stress and parenting may lead to differences in the development of the hippocampus, underlying SES disparities in memory skills (Noble et al., 2012b).
To test these hypotheses, we longitudinally assessed a socioeconomically diverse sample of children in the first 2 years of life on measures of language and memory development. We hypothesized that SES disparities would be found in both language and memory development. We further hypothesized that the home learning environment would mediate SES differences in language, whereas parental warmth and exposure to stressful life events would mediate SES disparities in memory, recognizing that these pathways may not be mutually exclusive.
METHOD
Participants
Utilizing a cohort-sequential design, 179 children (80 males) were recruited, with 90 children enrolled at 9 months of age ±2 weeks (M = 9.45, SD = .45), and 89 children enrolled at 15 months of age ±2 weeks (M = 15.42, SD = .42). Participants in the present study were recruited from a cohort of participants in a large, epidemiologic, longitudinal study investigating the relation between prenatal exposures and birth outcomes (http://safepassagestudy.org/). For the larger study, families were recruited from the pool of patients receiving prenatal care across four sites.1 The present study took place at a single participating clinic site in an urban Midwest community.
Recruitment of participants for the present study consisted of contacting the families of all children participating at the study site who were approaching their 9 or 15 month birthdays, until 90 participants were enrolled in each age group. Participants were excluded from participating in the present study on the basis of major neurological or developmental deficits, birth before 37 weeks gestation, multiple births, or maternal age under 18 years. Data from one participant in the 15 month group were collected and subsequently excluded from all analyses, as the parents reported a congenital neurological deficit following data collection. Children in both the larger study and the present study were enrolled without regard to prenatal exposures. The present study was not powered to detect effects of these exposures; further, at the time of this writing, investigators remained blind to these exposures, as data collection in the larger study was ongoing.
Families participated in two lab visits: one at the time of enrollment and another 6 months later. Families also received a home visit when infants were 15 months of age (M = 15.56, SD = .97). Among the 90 participants enrolled at 9 months, 86 (95.6%) returned for the second lab visit at 15 months (M = 15.35, SD = .46), and 88 (97.8%) completed a home visit at 15 months. Among the 89 participants enrolled at 15 months, 80 (89.9%) returned for the second lab visit at 21 months (M = 21.08, SD = .56), and 85 (95.5%) completed a home visit at 15 months. On average, lab visits occurred within 14 days of the target age. All parents provided written informed consent for their family’s participation in this study. Research procedures were approved by the Columbia University Medical Center IRB and the Sanford Health IRB.
Measures
Socioeconomic Status
A Parental Sociodemographic Questionnaire was administered verbally during the 15 month home visit. This questionnaire included items pertaining to educational attainment (total years of education for mother and father), household composition (number of adults and children in household), and income (estimated gross annual income). An income-to-needs (ITN) ratio for each family was calculated by dividing reported annual income by the federal poverty level for a family of that size in the year the data were collected. We asked that the child’s primary caregiver provide responses for the entire questionnaire; mothers comprised 95% of respondents. In single-mother households, only maternal demographic information was obtained.2
Life Experiences Survey
A 44-item survey measuring adults’ experience of major life events over the past year (Sarason, Johnson, & Siegel, 1978) was administered at the 15 month home visit. This survey included questions regarding the incidence of both positive and negative life events and the extent to which the event had a positive or negative impact on the respondent’s life. A seven-point Likert scale ranging from −3 to 3 assessed the impact of the event. The absolute score of each event was summed to represent the total amount of change experienced by the respondent in the past year.
Home Environment
At the 15 month home visit, a trained experimenter administered the Infant-Toddler Home Observation for Measurement of the Environment (IT-HOME) (Caldwell & Bradley, 1984). The IT-HOME is a 45-item structured interview and observational checklist designed to measure the quality of home life for children from birth to age three. Scores rely on a combination of experimenter observations (e.g., “Parent spontaneously praises child at least twice”) and interview items directed toward the parent (e.g., “Parent structures child’s play period). Items are scored as 1 if the behavior or resource is displayed or confirmed by the respondent. Subscales of Support of Learning and Literacy (LL—e.g., “Parent reads stories to child at least three times weekly”) and Parental Warmth (PW—e.g., “Parent caresses/kisses/hugs child at least once during visit”) were derived by summing the scores for corresponding items. These subscales have previously been shown to be internally consistent and to predict children’s cognitive skills in multiple large datasets (Fuligni, Han, & Brooks-Gunn, 2004).
Neurocognitive Tasks
At each lab visit, children were administered the following tasks to assess infant language and memory development.
Language
The Preschool Language Scale-4 (PLS) is a standardized language measure that has been normed from birth through age six (Zimmerman & Castilleja, 2005). This measure assesses young children’s receptive and expressive language development through a series of interactive items designed to elicit increasingly complex language skills. The Auditory Comprehension subscale examines a child’s ability to understand and respond to spoken language. Individual items assess skills like direction following, vocabulary knowledge, and spatial understanding. The Expressive Communication subscale examines a child’s ability to verbally express their needs and respond to questions. Items assess language skills such as phoneme production, social communication, and sentence complexity. Test-retest reliability has been previously assessed (with short testing intervals of 3 to 28 days), and the reliability coefficients ranged from .82 to .95 for the subscales and .90 to .97 for the total language score (Zimmerman, Steiner, & Pond, 2009). Children sat with their parent and the experimenter at a small table or on the floor of a well-lit room. Parents were instructed not to reply or help their child unless specifically instructed to do so. Responses from seven children are missing due to experimenter error (n = 1 at 9 mo) and child fussiness/refusal to respond (n = 2 at 15 mo, n = 4 at 21 mo).
Memory
The Visual Paired Comparison (VPC) task (Morgan & Hayne, 2006) assesses the degree to which children remember a familiar visual stimulus by comparing looking time to the familiar stimulus versus a novel stimulus. This task has been used in past studies of short-term and long-term visual recognition memory (Pascalis & de Haan, 2003). Amnesic patients with hippocampal damage and both infant and adult monkeys with damage to the medial temporal lobe demonstrate impaired performance on this task, suggesting a hippocampally dependent form of nonverbal declarative memory (Bachevalier, Brickson, & Hagger, 1993; McKee & Squire, 1993; Pascalis, Hunkin, Holdstock, Isaac, & Mayes, 2004; Richmond, Colombo, & Hayne, 2007). Unlike the language measure, memory performance using the VPC task has not been standardized across ages, but a past study has reported stability in week-to-week reliabilities during the first year of development (coefficients between .37 and .56) (Colombo, Mitchell, & Horowitz, 1988).
In this brief task, children were seated on their parents’ laps 40 in. (101.6 cm) away from two 20 in. (50.8 cm) monitors situated 33 in. (83.8 cm) apart at their centers. A video camera was situated between the monitors to capture the participant’s gaze. Parents were told to close their eyes or look directly between the monitors so as not to influence the child’s response. First, to orient the participant toward the monitors, each screen displayed an identical spinning ball for 13 s. During the 10 s familiarization block, each screen displayed an identical blue, mailbox shaped face. This was followed by the first 10 s novelty preference block in which one of the blue faces was replaced by a circular yellow face. In the second 10 s novelty preference block, the yellow face was replaced by the familiar blue face, and the other screen displayed a square red face. Responses from 16 children are missing due to child fussiness (n = 2 at 15 mo), the child not attending to stimuli (n = 3 at 9 mo, n = 8 at 15 mo), computer error (n = 2 at 15 mo), and parent interference (n = 1 at 21 mo).
Coders reviewed the videos frame-by-frame to establish total looking time for each block. At every 200 ms interval, the coder determined whether the child was attending to the left monitor, right monitor, or neither. This enabled calculation of the ratio of novel looking time (i.e., attending to red or yellow faces) to total looking time (i.e., attending to any face). Ratios above .5 indicate greater looking time for novel stimuli relative to the familiar stimulus. Reliability checks were run on 20% of the scores. Inter-rater reliability was greater than 95%.
In Deferred Imitation (DI) tasks, infants observe a series of simple actions and are given the opportunity to imitate the actions after a delay. The deferred imitation paradigm has been a useful tool in examining age-related changes in declarative memory processing. As the DI task is based on a brief observation of the target actions and involves no practice, such tasks are considered reliable measures of declarative memory among preverbal children (Barr & Hayne, 2000; Meltzoff, 1995). Studies have demonstrated that adults with temporal lobe amnesia fail traditional tests of DI, suggesting a dependence on the hippocampus (McDonough, Mandler, McKee, & Squire, 1995).
Stimuli and task administration were based on the puppet task by Barr and colleagues (1996) and the rattle task by Herbert & Hayne (2000). Validation studies show that infants are unlikely to perform the actions without prior demonstration (Barr et al., 1996) and previous research has reported high week-to-week test-retest reliability (r =.62) with 12 month-olds (Goertz, Kolling, Frahsek, Stanisch, & Knopf, 2008). Puppet task stimuli consisted of three handheld puppets 12 in. (30.5 cm) in height. The puppets were a gray mouse, a black and white cow, and a yellow duck, each fashioned with a matching, removable felt mitten that fit over the right hand of the puppet. A jingle bell was attached to the inside of the mitten to create a noise when shaken. For the rattle task, we constructed two rattles, one green and one red. The handle for the green rattle consisted of a 5 in. (12.7 cm) green wooden stick attached to a plastic lid with a Velcro underside. This handle could be attached to or detached from a clear plastic cup 3 in. (7.6 cm) in height with a .5 in (1.3 cm) diameter opening in the Velcro top. A green wooden bead .5 in. (1.3 cm) in diameter fit through the hole. The handle for the red rattle consisted of a red wooden stick and curved piece attached to a wooden plug. This handle fit inside a blue plastic ball with a 1.75 in. (4.45 cm) circular opening. A blue wooden bead of .5 in. (1.3 cm) diameter fit through the opening. At all ages, testing took place in a small, well-lit room. Parents were asked to refrain from touching, pointing to, or speaking about the stimuli.
Nine-month-old participants were administered the puppet task only. Parents were asked to sit on a chair and hold their child on their lap. The experimenter knelt on the floor in front of the seated participant and held the mouse puppet at the child’s eye level, approximately 32 in. (81.3 cm) away from the child. After the child oriented to the puppet, the experimenter removed the mitten from the puppet’s hand, shook the mitten three times to ring the bell inside, then replaced the mitten on the puppet’s right hand. The experimenter repeated these steps twice more for a total of three demonstrations. The demonstration phase was followed by an approximately 40 min delay during which the child completed other neurocognitive tasks. Participants were then seated on the chair again for the test portion of the task. At test, the bell was removed from the puppet’s mitten. The experimenter knelt in front of the participants and held the puppet within reach of the child (approximately 12 in. (30.5 cm) away). The experimenter encouraged the child to interact with the puppet if the child did not do so readily. After the child touched the puppet, he or she was given 120 s from the time the puppet was first touched to imitate the previously demonstrated actions. Response from one child was omitted due to child inattention to the demonstration (n = 1).
Fifteen-month-old participants completed both the puppet and rattle tasks. For the puppet task, the stimuli and demonstration procedures were identical to those used at 9 months. During the test portions, children were given 90 s to imitate the target actions on the puppet. The puppet demonstration was immediately followed by the rattle demonstration. Parents were asked to sit on the floor with the child on their lap. The experimenter then placed the pieces of the green rattle in a line on the floor. After the child oriented to the rattle pieces, the experimenter picked up the bead, pushed it through the opening of the cup, attached the handle to the top of the cup, and shook the constructed rattle. The experimenter then dismantled the rattle and placed the pieces back on the floor. This demonstration was repeated twice more for a total of three demonstrations. After the 35–45 min delay, the puppet and rattle tests were initiated. For the rattle test, the experimenter placed the green rattle pieces on the floor within reach of the child (approximately 8 in. (20.32 cm) away). The child was encouraged to interact with the rattle pieces and given 60 s to imitate the previously demonstrated actions from the time they first touched any of the rattle pieces. Responses from 21 children were omitted or not collected due to child fussiness (n = 11), child inattention to demonstration (n = 3), child unwillingness to play with stimuli (n = 4), experimenter error (n = 1), or parent interference (n = 2).
Like the 15 month participants, 21 month-old participants completed both puppet and rattle tasks. Administration of the tasks was identical to administration at 15 months; however, to increase task complexity, the stimuli differed, such that the cow puppet was used for the demonstration portion of the puppet task, and the duck puppet was used at test. For the rattle task, the green rattle was used during the demonstration, and the red rattle was used during the test portions. By using different stimuli for the demonstration and test, we aimed to assess the degree to which participants generalized their memory of the actions to a novel but perceptually similar stimulus (Barr & Brito, 2013). Responses from fifteen children were omitted or not collected due to child fussiness (n = 10), unwillingness to play with stimuli (n = 2), experimenter error (n = 2), or sibling interference (n = 1).
All deferred imitation tasks were recorded by a digital video recorder. Coders reviewed the videos frame-by-frame to score participants’ attention to the demonstration and performance during testing. For both the puppet and the rattle tests, behavior was coded from the time of first touch of the experimental items. Memory was evaluated by determining the number of individual target behaviors the child imitated during the test session. For the puppet task, participants were awarded one point for exhibiting each of the following target actions: removing the mitten from the puppet’s hand, shaking the mitten, attempting to replace the mitten on either hand. For the rattle task, participants were awarded one point for each of the following target actions: placing the ball in the cup, attaching the lid to the cup, shaking the rattle with the ball inside. Nine-month-olds could score between 0 and 3 points for their performance on the puppet task. Scores at 15 and 21 months were summed across the puppet and rattle tasks; participants could score between 0 and 6 points for their imitation of the target actions. Reliability checks were run on 20% of the scores to ensure the target actions had been scored properly. Inter-rater reliability was greater than 95%.
Data Analysis
Composite scores were created for the language and memory measures at each age. To place the memory tests on a single common scale, scores were converted to z scores relative to the distribution of children within a given age group. These z scores were then averaged together to create a Memory Composite score for each child at each age, representing children’s relative position in the distribution at each age. Similarly, at each age the PLS Auditory and Expressive subscale raw scores were z transformed and averaged together to create a Language Composite on the same scale as the Memory Composite.
Mixed effects models are the most appropriate statistical method for analyzing cognitive trajectories in the present dataset. Such models allow for attrition and estimate missing data (via the EM algorithm for parameter estimation by restricted maximum likelihood —REML), and can include all data from the cohort-sequential design within a single model. In addition, these models allow for correlated variability among observations, unequal variances, and unbalanced data and allow for the addition of covariates in order to measure both individual and group differences within the same model. Scores on the language and memory composites were not significantly correlated, and therefore the two composites were run as dependent variables in separate models. As the variation in length of time between assessments was small (SD approximately 14 days at each time point) and there was no correlation between SES measures and child age, a centered index was created indicating time point (child age at each assessment) and was used as the within-person time variable. A random coefficient model (random intercept and slopes) was utilized and an unstructured covariance structure provided best fit of the data, based on AIC values, after testing other covariance matrices.
Finally, mediation analyses using the bootstrapping method with bias-corrected confidence estimates (Preacher & Hayes, 2008) were employed to test hypothesized mediating pathways (parental warmth and learning and literacy) between SES factors and language and memory development.
RESULTS
Descriptive statistics
Descriptive analyses showed an average parent education of 15 years (SD = 1.4, range = 11.5–17.0), and an average income-to-needs (ITN) of 3.6 (SD = 2.4, range = .2–19.7); see Table 1. The majority of children were Caucasian (n = 168), with the remaining children of mixed race (n = 7), Hispanic (n = 2), and American Indian/Alaskan Native (n = 2). There were no differences between the two cohorts (cohort 1 tested at 9 and 15 months and cohort 2 tested at 15 and 21 months) in any infant or parental demographic of interest (all p’s > .30), and thus data from the two cohorts were pooled together in analyses as described below.
Table 1.
Demographics
| Infant Sex | Parent ED | Parent Income | |
|---|---|---|---|
| 9mo/15mo Cohort | Males = 36 Female = 54 | M = 14.97 SD = 1.39 | M = $75,990 SD = 59,255 |
| 15mo/21mo Cohort | Males = 44 Females = 45 | M = 15.17 SD = 1.44 | M = $80,099 SD = 58,280 |
Parent ED = Average postnatal parental education. Parent Income = Average postnatal family income. There were no differences between the two cohorts (cohort 1 tested at 9 and 15 months and cohort 2 tested at 15 and 21 months) in any infant or parental demographic of interest.
As expected, there were high correlations between all SES factors and home environmental measures, as shown in Table 2. There were no significant differences in any SES factor or HOME variable by sex (all p’s > .20). In all analyses below, outliers >3 S.D. from the mean were winsorized, that is, replaced with values exactly three S.D. from the mean.
Table 2.
Correlations
| Postnatal ED |
Postnatal ITN |
HOME–Total | HOME–PW | |
|---|---|---|---|---|
| Postnatal ITN | .37*** | — | ||
| HOME–Total | .37*** | .31*** | — | |
| HOME–PW | .26*** | .16* | .67*** | — |
| HOME–LL | .29*** | .27*** | .72*** | .48*** |
Postnatal ED, average parental postnatal education; Postnatal ITN, postnatal income to needs; HOME-Total, total HOME scores; HOME-PW, HOME scores for parental warmth subscale; HOME-LL, HOME scores for learning and literacy subscales.
p < .05,
p < .001.
Table 3 shows the means and standard deviations for each age group for each neurocognitive task. Consistent with our selection of tasks that have previously shown individual differences at the ages represented here, all tasks showed a normal distribution in performance, without evidence of floor or ceiling effects at any age. Children in the two age-group cohorts performed similarly on all measures at 15 months (all p’s > .1); thus, the data were pooled at 15 months.
Table 3.
Descriptive Statistics
| VPC | DI | PLS–A | PLS–E | |
|---|---|---|---|---|
| 9-months | M = .66 | M = 1.17 (38.9%) | M = 19.40 (114.26) | M = .46 (124.49) |
| SD = .15 | SD = .944 | SD = 1.56 (9.17) | SD = 2.08 (9.77) | |
| n = 87 | n = 89 | n = 89 | n = 89 | |
| 15-months | M = .63 | M = 3.92 (65.3%) | M = 20.59 (103.52) | M = 25.50 (117.00) |
| SD = .14 | SD = 1.42 | SD = 1.37 (10.31) | SD = 1.62 (6.51) | |
| n = 162 | n = 158 | n = 171 | n = 173 | |
| 21-months | M = .61 | M = 3.48 (57.96%) | M = 25.66 (103.20) | M = 29.47 (111.25) |
| SD = .11 | SD = 1.32 | SD = 3.19 (10.84) | SD = 2.51 (6.65) | |
| n = 79 | n = 67 | n = 76 | n = 79 |
VPC, visual paired comparison; DI, deferred imitation. Range of scores for DI task at 9 months is 0–3; range of scores for DI task at 15 and 21 months is 0–6. For DI scores, percent target actions imitated is shown in the parentheses. PLS–A, Preschool Language Scale–Auditory Comprehension Subscale; PLS–E, Preschool Language Scale–Expressive Communication Subscale. For PLS Raw scores, standardized scores are shown in the parentheses.
Predictors of Language and Memory Outcomes
In general, concurrent correlations among tasks, as well as correlations within and between tasks from one time point to the next, were modest. When considering concurrent relations between measures at 9 months, DI score was correlated with VPC (r = .22; p < .05) as well as with PLS–E (r = .25; p < .05). When considering concurrent relations between measures at the older ages, PLS–A was concurrently correlated with PLS–E at both 15 months (r = .32; p < .001) and at 21 months (r = .47; p < .001). No other significant concurrent correlations between tasks were present at any age.
Table 4 shows that the stability of scores from one time point to the next was low from 9 to 15 months and modest from 15 to 21 months, suggesting that individual differences in performance over time were not well explained by performance just 6 months prior. There were no significant 9–15 month correlations between different tasks. PLS–E at 15 months was significantly correlated with PLS–A at 21 months (r = .34; p < .01). There were no other significant correlations between 15 and 21 month scores on different tasks. Table 5 shows the bivariate correlations between SES factors and the Language and Memory Composites at each age, as well as with all individual language and memory tasks at each age. Significant correlations were found between parental education and the Language Composite at 21 months of age (r =.34; p < .002) and between parental education and the Memory Composite at 21 months of age (r =.31; p < .01). Steiger’s z-test showed that these respective correlations did not significantly differ in their magnitude (t = .23; p > .10).3 No significant bivariate associations were found between ITN and any of the language or memory composites or tasks.4
Table 4.
Correlations in Task Scores From 9 to 15 Months and 15 to 21 Months
VPC, visual paired comparison; DI, deferred imitation; PLS–A, Preschool Language Scale–Auditory Comprehension Subscale, PLS– E, Preschool Language Scale–Expressive Communication Subscale.
p < .1,
p < .01,
p < .001.
Table 5.
Correlations Between SES Factors, Individual Task Scores, and Composite Scores
| Parental Education | Income-to-Needs | |
|---|---|---|
| 9-Months | ||
| Language Composite | .05 (n = 89) | −.02 (n = 86) |
| PLS–Auditory Comprehension | −.001 (n = 89) | .05 (n = 86) |
| PLS–Expressive Communication | .08 (n = 89) | −.08 (n = 86) |
| Memory Composite | −.01 (n = 86) | −.15 (n = 83) |
| Visual Paired Comparison | .04 (n = 87) | −.05 (n = 84) |
| Deferred Imitation | −.05 (n = 89) | −.17 (n = 86) |
| 15-Months | ||
| Language Composite | .12 (n = 173) | .01 (n = 168) |
| PLS–Auditory Comprehension | .18 (n = 173)* | .13 (n = 168) |
| PLS–Expressive Communication | .01 (n = 175) | −.10(n = 170) |
| Memory Composite | .03 (n = 148) | .05 (n = 143) |
| Visual Paired Comparison | .02 (n = 162) | .03 (n = 157) |
| Deferred Imitation | .01 (n = 157) | .02 (n = 152) |
| 21-Months | ||
| Language Composite | .34 (n = 76)** | .07 (n = 76) |
| PLS–Auditory Comprehension | .32 (n = 76)** | .15 (n = 76) |
| PLS–Expressive Communication | .27 (n = 79)* | −.03 (n = 79) |
| Memory Composite | .31 (n = 66)* | .14 (n = 66) |
| Visual Paired Comparison | .19 (n = 79) | .13 (n = 79) |
| Deferred Imitation | .17 (n = 67) | .05 (n = 67) |
p <.05,
p<.01.
Next we tested mixed effects regression models to examine the extent to which socioeconomic disparities existed in developmental trajectories of language and memory performance across the entire cohort from 9 to 21 months. There were no significant main effects of parent education for either the Language or Memory composites. However, there was a significant parental education* age interaction for both the Language Composite (F = 1.90, p = .04) and the Memory Composite (F = 2.52, p = .01), suggesting widening disparities with age in performance of both neurocognitive systems. Separate mixed models among the younger and older cohorts confirmed that these disparities for both language and memory emerged from 15 to 21 months of age for both language (F = 2.38, p = .01) and memory (F = 2.01, p = .04). No significant education* age interactions were present from 9 to 15 months.
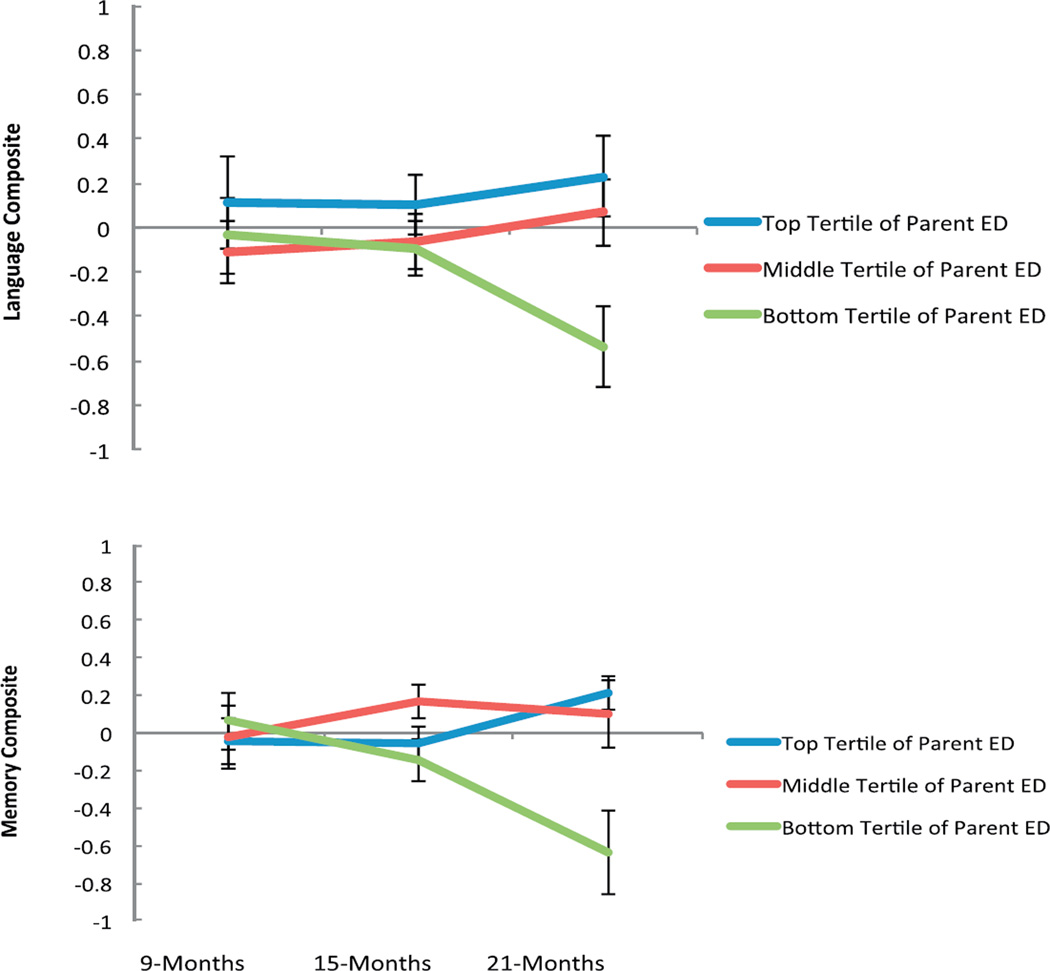
In order to visualize these interactions, for each dependent variable (Language and Memory Composites), Average Parental Education was considered in three equal tertiles following the distribution of the data: Highest parent education (16 years or higher); Middle parent education (14.5–15.5 years); and Lowest parent education (11–14 years) (See Tables 6 and 7 and Fig. 1). Of note, the z scores portrayed in Figure 1 represent children’s relative position in the distribution. Using these standardized scores, mean linear or nonlinear growth in performance across time would be represented by a flat slope at a score of 0. Although Figure 1 shows that children of the least educated parents have an average decrease in z scores for both language and memory, this does not necessarily represent a drop-off in absolute performance, but instead represents a drop in performance relative to the mean during that time period. In turn, this could reflect a decrease in absolute performance for these children, a flat growth trajectory, or simply a less steep positive growth trajectory. To clarify the underlying trajectories in language skill, we examined PLS raw scores, using the summed raw scores of the auditory and expressive language scales at each time point. As would be expected, all children showed growth in language skill over time, but the rate of growth was greatest among children of the highest educated parents. Pairwise comparisons showed significant differences between the trajectories of children of the least educated parents (slope = .97) and highest educated parents (slope = 1.28) on these raw scores (p = .004).
Table 6.
Results of the Linear Mixed-Effects Model of Language Development
| Parameter | Estimate | SE | p value |
|---|---|---|---|
| Fixed Effects | |||
| Intercept | .055 | .113 | .63 |
| ED=low | .029 | .169 | .87 |
| ED=middle | −.157 | .177 | .38 |
| ED=high | — | — | — |
| Age | .011 | .014 | .45 |
| Age*ED (low) | −.053 | .023 | .02 |
| Age*ED (middle) | −.001 | .024 | .96 |
| Age*ED (high) | — | — | — |
| Random Effects | |||
| Intercept + Age (9-mos) | .176 | .082 | |
| Intercept + Age (15-mos) | .005 | .006 | |
| Intercept + Age (21-mos) | .001 | .001 |
p values are not given for covariance parameters.
Table 7.
Results of the Linear Mixed-Effects Model of Memory Development
| Parameter | Estimate | SE | p value |
|---|---|---|---|
| Fixed Effects | |||
| Intercept | −.112 | .114 | .30 |
| ED=low | .242 | .173 | .17 |
| ED=middle | .163 | .182 | .37 |
| ED=high | — | — | — |
| Age | .022 | .014 | .13 |
| Age*ED (low) | −.078 | .023 | .001 |
| Age*ED (middle) | −.010 | .024 | .67 |
| Age*ED (high) | — | — | — |
| Random Effects | |||
| Intercept + Age (9-mos) | .143 | .109 | |
| Intercept + Age (15-mos) | −.011 | .015 | |
| Intercept + Age (21-mos) | .001 | .002 |
p values are not given for covariance parameters.
FIGURE 1.
Language and Memory Trajectories in the First Two Years Vary by Parent Education. Trajectories of language and memory development were examined longitudinally from 9 to 21 months using mixed effects models. For visualization purposes, Parental Education was considered in three equal tertiles following the distribution of the data: Highest parent education (16 years or higher); Middle parent education (14.5–15.5 years); Lowest parent education (11–14 years). Scores are depicted as Z-scores, to enable comparison across skills. For the Language Composite (top), pairwise comparisons showed significant differences between the slopes of the lowest and middle educated groups (p =.05) and of the lowest and highest educated groups (p =.02). For the Memory Composite (bottom), pairwise comparisons showed significant differences between the slopes of the lowest and middle educated groups (p = .01) and between the lowest and highest educated groups (p = .001).
A similar examination of the absolute trajectory of memory skill presents more of a challenge, as different procedures were used at different ages, dictated by the developmental appropriateness of the tasks, and the lack of any normed and standardized measures of memory skill in this age range. However, in an attempt to make raw scores as comparable as possible, we averaged the puppet and rattle DI tasks, so that, at 15 and 21 months, the total possible DI score was 3, equivalent to the total possible 9 month DI score (noting also that the DI procedures were slightly different at each age). The DI and VPC raw scores were then weighted and summed at each time point. When examined in this way, children of the most highly educated parents had a positive growth trajectory for memory (slope .014), whereas the children of the least educated parents had a trajectory that did not significantly differ from 0 (slope −.004). Pairwise comparisons showed significant differences between the slopes of these groups on these scores (p = .02).
At 21 months of age, children whose parents were in the top tertile of education in the sample, such that they held a college degree or more, had a Language Composite score that was .77 standard deviations higher than children whose parents were in the bottom tertile of educational attainment in the sample, having obtained 2 years of college or fewer (t(56) = −2.64, p = .01). Similarly, 21 month olds whose parents had at least a college degree had a Memory Composite score that was .85 standard deviations higher than children whose parents attended no more than 2 years of college, t(19.84) = −3.58, p = .002).5
When considering associations with individual tasks, average parental education was significantly correlated with PLS-auditory at 15 months (r = .18; p = .02) and 21 months (r = .32; p = .006). Comparing children whose parents received at least a college degree versus children whose parents attended no more than 2 years of college, this represents a gap of 2 months in receptive language skill at both 15 months and 21 months. This was driven by the fact that the children with the higher educated parents scored, on average, at the 17-month-level at 15 months and at the 23-month level at 21 months. Average parental education was also correlated with the PLS-expressive subtest at 21 months (r = .27; p = .02), representing a 1 month gap in expressive language skill between these two groups of children. Again, this was due to the fact that the children with the higher educated parents were scoring above age norms, with an average developmental score of 24 months. In contrast, none of the associations between parent education and the individual memory tasks reached significance.
Mediating Role of Proximal Home Factors
The next set of analyses aimed to understand the role of proximal factors in accounting for SES disparities in language and memory change over time. Using the entire sample, a “Language Change” score was computed by constructing a model in which the language composite at the second lab visit was regressed on the composite from the prior visit (i.e., 21 month scores controlling for scores at 15 months or 15 month scores controlling for scores at 9 months), additionally controlling for cohort group (older or younger), and then saving the residuals. An analogous “Memory Change” score was computed using a separate regression. Analyzing these residuals as the dependent variables for language and memory within the mediation analyses allows us to assess the change in language and memory scores across time points for all participants in relation to parental education and proximal factors. We initially hypothesized that SES associations with language development would be mediated by the Learning and Literacy (LL) subscale of the HOME, whereas SES disparities in memory development would be mediated by the Parental Warmth (PW) subscale of the HOME and exposure to stressful life events as measured by the Life Experiences Survey (LES). Because the LES was not significantly correlated with any measure of language or memory development, this variable was dropped from further consideration in mediation models.6
First we assessed whether LL mediated the relationship between parental education and language. Results indicated that parent education was significantly positively associated with Language Change (B = .136, p = .02). Next it was found that parent education was also positively significantly related to the LL subscale (B = .210, p = .002). Finally, there was a strong trend for the mediator, LL, to be significantly associated with Language Change (B = .124, p = .05). By including the indirect effect of LL, the direct effect of parent education on Language Change was reduced (B = .110; p = 0.016). Because both the direct and indirect paths were significant, mediation analyses were tested using the bootstrapping method with bias-corrected confidence estimates. The 95% confidence interval of the indirect effects was obtained with 5,000 bootstrap resamples (Preacher & Hayes, 2008). Results of the mediation analysis confirmed that LL partially mediates the relation between parent education and Language Change (B = .026; CI = .006–.059).
Next, as it was predicted that LL, and not PW, would mediate the relation between parent education and Language Change, we ran a mediation model to assess the prediction that PW would not significantly mediate the link between parent education and child language. First, as above, parent education was positively associated with Language Change (B = .136, p = .002). Next, parent education positively related to the PW subscale (B = .110; p = .013). The PW subscale also significantly predicted Language Change (B = .219; p = .007). When including the indirect effect of PW, the link between parent education and Language Change was reduced (B = .112; p = .011). Bootstrapping showed that this reduction was, contrary to predictions, significant (B = .024; CI = .002–.012).
Finally, even when controlling for both PW and LL, parent education was still positively associated with Language Change (R2 change = .049, β = .103; p = .02), suggesting that additional, unmeasured factors account for part of the variation in language trajectories that occur across parental education. Because of this, the total HOME score was entered as a mediator instead of the subscales, at which point a fully mediated model was produced. Specifically, parent education was associated with Language Change (B = .136, p = .002) and with the total HOME score (B = .809; p<.0001); the HOME score significantly predicted Language Change (B = .058; p = .004); and when including total HOME score in the model, the link between parent education and language development was non-significant (B = .089; n.s.).
It was predicted that PW, and not LL, would mediate the relation between parent education and change in memory. However, neither the PW nor the LL subscales, nor the total HOME score, were significantly associated with memory change, so no further mediation models were tested using the Memory Change scores.
As a final test of mediation, we included the LL and PW terms in the original mixed effects models of the Language and Memory Composites, respectively. When doing so, the age* education interaction term in the Language Composite model was no longer significant (p = .10), suggesting that these factors accounted for the differential effect of education on language as a function of age. However, in the Memory Composite model, the age* education interaction remained significant (p = .007), confirming our finding above that these factors did not mediate the effect of education on memory development.
Finally, there was no evidence of moderation, by either the total HOME or its subscales, on educational disparities in either language or memory.
DISCUSSION
Here we showed for the first time that socioeconomic disparities in developmental trajectories of both language and memory are present by the second year of life, and further that these disparities are similar across these distinct neurocognitive systems. Specifically, in line with past evidence (Fernald et al., 2013; Halle et al., 2009; Hoff, 2003; Rowe & Goldin-Meadow, 2009), disparities in receptive language were detectable by 15 months, and disparities in expressive language were detectable by 21 months. Additionally, a novel finding was that disparities were detectable in a composite of declarative memory skills by 21 months of age. The language and learning environment, as well as parental warmth, statistically mediated socioeconomic differences in language trajectories. Neither of these factors, nor a more global assessment of the home environment, mediated SES differences in memory trajectories.
Several points are worth noting. First, although differences in language skills were evidenced earlier than differences in memory, by 21 months, effect sizes were of similar magnitudes, with approximately .8 standard deviations separating children whose parents attained a college degree versus those whose parents attained no more than 2 years of college. This represents a large effect size and demonstrates SES disparities early in development in cognitive domains other than language.
Second, within the present sample, disparities in language skill were largely driven by above-average performance of children from the highest SES families. This is likely due to the relatively truncated socioeconomic gradient in the present sample. Most parents in the sample had attended at least some college, and very few would be classified as poor or near-poor. Indeed, the lowest tertile of parental education included parents with up to two years of postsecondary education. Perhaps reflecting these relatively high levels of parental achievement, even children of “lower educated” parents tended to have at least average language skills, relative to national norms. It is possible that with a greater socioeconomic gradient, we would have detected earlier or larger disparities. As no normed, standardized measures of memory exist for children this young, we cannot say with certainty whether disparities in memory were driven by better-than-average performance of children from higher SES families or below-average performance of children from lower SES families.
The magnitude of these effects has important implications for developmental surveillance and screening, and possibly for intervention. They suggest that the first signs of socioeconomic disparities in language and memory are identifiable by the middle of the second year of life. Early screening and detection of language or memory delay is likely to lead to earlier access to interventional services, which are more effective when implemented early (Committee on Children with Disabilities, 2001).
Associations with SES were driven by parental education levels, and not family income. Although these factors are highly correlated, research suggests that different facets of SES are associated with different proximal variables, which may have specific developmental effects (Duncan & Magnuson, 2012; Noble et al., 2012b). Further, effects of income may be nonlinear, with the steepest gradients at the lowest income levels (Brooks-Gunn & Duncan, 1997; Duncan & Magnuson, 2012). As only 4% of the present sample was living below the poverty line, associations with income may have been detected in a more socio-economically disadvantaged sample. It is also possible that income disparities in cognitive outcomes are detectable only after the developmental window studied here. Previous studies have reported that income, but not education, is associated with hippocampal structure later in childhood and adolescence (Hanson et al., 2011; Noble et al., 2012b).
Similar to previous reports of infant cognition (e.g., Feldman et al., 2005), the language and memory skills assessed here showed relatively low stability from one time point to the next. Indeed, for this reason it has long been suggested that general intelligence scales in early infancy are not suitable for predicting long-term outcomes (Lewis & Brooks-Gunn, 1981; Lewis & McGurk, 1972). One possibility is that the ability to accurately measure language and memory skills increases with age, thus raising the prospect that the socioeconomic disparities described here were in fact present (but unable to be detected) earlier than we report them. Alternatively, developmental psychology theories of experiential canalization have long held that environmental factors show the strongest correlations after the first 2 years of life (Blair & Raver, 2012; McCall, 1981), suggesting that environmental factors may influence infant memory or language at a developmental time point when memory and language skills become more contextually relevant to children’s development. While the data we report here are unable to distinguish between these two possibilities, they do suggest that early family SES may be a better predictor of risk for neurocognitive impairment than is early neurocognitive skill (Feinstein, 2003). That is, a child’s family background may place him or her at risk for reduced cognitive abilities, well before such outcomes are clinically apparent. This has important implications for close developmental surveillance, and perhaps enrollment in preventive programs, for socioeconomically disadvantaged children.
We had hypothesized that the home language and literacy environment would statistically mediate SES differences in language development (Hoff, 2003). This was partially supported, in that the association between parent education and language development was significantly reduced when considering the indirect effect of the home learning environment (acknowledging that the path from the mediator to the dependent variable narrowly missed significance).
Results showed that parental warmth also partially mediated the link between parent education and language development. When controlling for parental warmth, the learning and literacy environment no longer predicted language change. Although we had not hypothesized this, parental warmth can be an important predictor of language development, mediated by differences in interactivity and conversational complexity (Darling & Steinberg, 1993). Additionally, lower SES mothers are more likely to be depressed (Berger, Paxson, & Waldfogel, 2009), which may affect both warmth and verbal interactions (Rowe, Pan, & Ayoub, 2005).
We had hypothesized that parental warmth would mediate socioeconomic disparities in memory development (Champagne et al., 2008). Results did not support this hypothesis. One possibility is that our observational interview was not sensitive to differences in warmth that would have been evident through naturalistic observations. Another possibility is that early parenting differences may exert effects on memory that are not detectable until later in childhood (Farah et al., 2008). It is also possible that other unobserved variables account for these disparities, such as differences in prenatal substance exposures, nutrition, or environmental toxins.
This study has numerous strengths, including utilization of a cohort-sequential design to conduct a partial longitudinal study with extremely high retention rates, the ability to directly compare SES disparities in the same children across two neurocognitive systems, and convergence of evidence using prenatal and postnatal SES.
We also acknowledge several limitations. Although the socioeconomic range in the sample was reasonable, it did not include the highest or lowest extremes. Had our sample included a truly disadvantaged group, observed disparities may have been even wider, and we may have detected associations with income, in addition to those reported for education.
Because of the cohort-sequential study design, a greater number of participants were tested at 15 months than at either 9 or 21 months. One possibility is that, with a larger sample, disparities could have been detected as early as 9 months of age (Halle et al., 2009). However, the majority of significant results were detected at 21 months, when there were a similarly limited number of data points. Further, while individual differences in language and memory performance were evident as early as 9 months of age, SES factors did not account for this variation. This is consistent with the fact that SES disparities in IQ are first detectable in the latter half of the second year (Honzik, 1976).
Of course, we cannot say with certainty whether the associations reported here are indicative of causal relations. We hypothesize that socioeconomic disparities in proximal factors lead to differences in the development of specific brain and cognitive systems. It is also possible that higher-achieving children solicit richer linguistic or cognitive environments or engender more warmth from their parents (Song, Spier, & Tamis-Lemonda, 2013). Regardless, early disparities in language and memory skills may have long-lasting ramifications for achievement (Rose, Feldman, & Jankowski, 2004; Walker, Greenwood, Hart, & Carta, 1994). Thus, interventions are warranted to maximize children’s potential for full development in these domains. As large socioeconomic disparities are evident in children’s language and memory skills by the second year of life, an investment in interventions designed to improve early language and memory skills may hold promise for children’s educational attainment and ultimately outcomes for future generations.
Acknowledgments
We gratefully acknowledge Samantha Moffett and Elizabeth Victor for research assistance, Lisa Sullivan for statistical consultation and Jeanne Brooks-Gunn for helpful comments on an earlier draft. This publication was supported by NIH Grants UL1TR000040, U01HD055154, U01HD055155, U01HD045991, U01AA016501, and R37HD032773.
Footnotes
Approximately 7 in 10 pregnant women at the clinic were randomly approached for recruitment in the larger study. Women were excluded from participating in the larger study if they carried three or more fetuses during the pregnancy, planned abortion, planned to move out of the catchment area prior to estimated date of delivery, were unable to provide informed consent, or if the health care provider advised against participation.
In the event that the Parental Sociodemographic Questionnaire was not administered, we used sociodemographic information that was collected prenatally at the time of recruitment for the larger study, at 20–24 weeks of pregnancy. This questionnaire included total years of education for parents in the home, and estimated monthly income, reported in the following bins: ≤$500; $501–1,000; $1,001–2,000; $2,001–3,000; $3,001–4,000; $4,001–5,000; ≥$5,001. Annual income was estimated by multiplying the mean of each income bin by 12. An ITN ratio was calculated as described above.
Postnatal parental education levels were available for all but the six families who did not complete a 15 month home visit. Correlations between prenatal and postnatal education were quite high (r = .94; p < .001), and a within-subjects t-test showed no significant differences (t(172) = 1.57, p = .12). Therefore, for the six families missing postnatal education data, prenatal education data, obtained approximately one year prior, were substituted. Analyses were re-run excluding the six data points for which we substituted prenatal educational attainment, and results were unchanged. Additionally, results were not substantially changed when maternal education was used instead of average parental education (correlation with Language Composite at 21 months: r = .27; p < .017; correlation with Memory Composite at 21 months: r = .27; p < .028), or if prenatal rather than postnatal education was used for all participants (correlation with 21 month Language Composite: r = .30; p < .009; correlation with 21 month Memory Composite: r = .33; p < .007).
ITN was assessed at 15 months. Data were excluded for the six families who did not complete a home visit, as well as for the one additional family who provided prenatal but not postnatal income information, as we were unable to calculate an ITN in these cases. We ran additional analyses using prenatal and postnatal income instead of ITN, and results were unchanged.
In this relatively well educated sample, the bottom tertile of education included parents with up to 2 years of college. Recognizing this, we attempted to run similar analyses using more “ecologically valid” cutoffs of educational attainment. However, such categories were quite skewed, with very few parents attaining a high school education or less (n = 7), and most parents having some college (n = 95), or a college degree or more (n = 77). Results showed no significant education* age interactions in these cases.
A path analysis (AMOS Version 22) was run to assess the extent to which the LL subscale specifically mediated the association between education and language development, and the PW subscale specifically mediated the association between education and memory development. This model did not have a good overall fit (χ < .01; root mean square error of approximation [RMSEA]=.24; and comparative fit index [CFI]=.45). Therefore, to assess whether evidence supported one or more components of the hypothesized model, separate regressions were run to assess mediation.
Conflicts of interest: The authors have no conflict of interest to declare.
REFERENCES
- Bachevalier J, Brickson M, Hagger C. Limbic-dependent recognition memory in monkeys develops early in infancy. NeuroReport. 1993;4(1):77–80. doi: 10.1097/00001756-199301000-00020. [DOI] [PubMed] [Google Scholar]
- Barr R, Brito N. From specificity to flexibility: Developmental changes during infancy. In: Bauer P, Fivush R, editors. Wiley-blackwell handbook on the development of children’s memory. Chichester: John Wiley and Sons; 2013. pp. 453–479. [Google Scholar]
- Barr R, Dowden A, Hayne H. Developmental changes in deferred imitation by 6- to 24-month-old infants. Infant Behavior & Development. 1996;19(2):159–170. [Google Scholar]
- Barr R, Hayne H. Age-related changes in imitation: Implications for memory development. In: Rovee-Collier C, Lipsitt LP, Hayne H, editors. Progress in infancy research. Vol. 1. Mahwah, NJ: Erlbaum; 2000. pp. 21–67. [Google Scholar]
- Barr R, Walker J, Gross J, Hayne H. Age-related changes in spreading activation during infancy. Child Development. 2013 doi: 10.1111/cdev.12163. [DOI] [PubMed] [Google Scholar]
- Berger LM, Paxson C, Waldfogel J. Income and child development. Child and Youth Services Review. 2009;31(9):978–989. doi: 10.1016/j.childyouth.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair C, Raver CC. Child development in the context of adversity: Experiential canalization of brain and behavior. American Psychologist. 2012;67(4):309–318. doi: 10.1037/a0027493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein MH, Sigman MD. Continuity in mental development from infancy. Child Development. 1986;57(2):251–274. doi: 10.1111/j.1467-8624.1986.tb00025.x. [DOI] [PubMed] [Google Scholar]
- Bradley RH, Corwyn RF, Burchinal M, McAdoo HP, Garcia Coll C. The home environments of children in the United States part II: Relations with behavioral development through age thirteen [Article] Child Development. 2001;72(6) doi: 10.1111/1467-8624.t01-1-00383. [DOI] [PubMed] [Google Scholar]
- Brooks-Gunn J, Duncan GJ. The effects of poverty on children [Review] [63 refs] Future of Children. 1997;7(2):55–71. [PubMed] [Google Scholar]
- Caldwell B, Bradley R. Home observation for measurement of the environment. Little Rock, AR: University of Arkansas Press; 1984. [Google Scholar]
- Champagne DL, Bagot RC, van Hasselt F, Ramakers G, Meaney MJ, de Kloet ER, Krugers H. Maternal care and hippocampal plasticity: Evidence for experience-dependent structural plasticity, altered synaptic functioning, and differential responsiveness to glucocorticoids and stress. Journal of Neuro-science. 2008;28(23):6037–6045. doi: 10.1523/JNEUROSCI.0526-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo J, Mitchell DW, Horowitz FD. Infant visual attention in the paired-comparison paradigm: Test-retest and attention performance relations. Child Development. 1988;59:1198–1210. doi: 10.1111/j.1467-8624.1988.tb01489.x. [DOI] [PubMed] [Google Scholar]
- Committee on Children with Disabilities (CCD) Developmental surveillance and screening of infants and young children. Pediatrics. 2001;108(1):192–195. doi: 10.1542/peds.108.1.192. [DOI] [PubMed] [Google Scholar]
- Darling N, Steinberg L. Parenting style as context: An integrative model. Psychological Bulletin. 1993;113(3):487. [Google Scholar]
- Dehaene-Lambertz G, Hertz-Pannier L, Dubois J, Meriaux S, Roche A, Sigman M, Dehaene S. Functional organization of perisylvian activation during presentation of sentences in preverbal infants. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(38):14240–14245. doi: 10.1073/pnas.0606302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GJ, Magnuson K. Socioeconomic status and cognitive functioning: Moving from correlation to causation. Wiley Interdisciplinary Reviews: Cognitive Science. 2012;3(3):377–386. doi: 10.1002/wcs.1176. [DOI] [PubMed] [Google Scholar]
- Evans GW. The environment of childhood poverty. American Psychologist. 2004;59(2):77–92. doi: 10.1037/0003-066X.59.2.77. [DOI] [PubMed] [Google Scholar]
- Fagan JF, Singer LT. Infant recognition memory as a measure of intelligence. Advances in Infancy Research. 1983;2:31–78. [Google Scholar]
- Farah MJ, Betancourt L, Shera DM, Savage JH, Giannetta JM, Brodsky NL, Hurt H. Environmental stimulation, parental nurturance and cognitive development in humans. Developmental Science. 2008;11(5):793–801. doi: 10.1111/j.1467-7687.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Shera DM, Savage JH, Betancourt L, Giannetta JM, Brodsky NL, Hurt H. Childhood poverty: Specific associations with neurocognitive development. Brain Research. 2006;1:166–174. doi: 10.1016/j.brainres.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Feinstein L. Inequality in the early cognitive development of British children in the 1970 cohort. Economica. 2003;70(277):73–97. [Google Scholar]
- Feldman HM, Dale PS, Campbell TF, Colborn DK, Kurs-Lasky M, Rockette HE, Paradise JL. Concurrent and predictive validity of parent reports of child language at ages 2 and 3 years. Child Development. 2005;76(4):856–868. doi: 10.1111/j.1467-8624.2005.00882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernald A, Marchman VA, Weisleder A. SES differences in language processing skill and vocabulary are evident at 18 months. Developmental Science. 2013;16(2):234–248. doi: 10.1111/desc.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuligni AS, Han WJ, Brooks-Gunn J. The infant-toddler HOME in the 2nd and 3rd years of life. Parenting. 2004;4(2–3):139–159. [Google Scholar]
- Gerhardstein P, Dickerson K, Miller S, Hipp D. Early operant learning is unaffected by socio-economic status and other demographic factors: A meta-analysis. Infant Behavior & Development. 2012;35(3):472–478. doi: 10.1016/j.infbeh.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Goertz C, Kolling T, Frahsek S, Stanisch A, Knopf M. Assessing declarative memory in 12-month-old infants: A test-retest reliability study of the deferred imitation task. European Journal of Developmental Psychology. 2008;5(4):492–506. [Google Scholar]
- Gou Z, Choudhury N, Benasich AA. Resting frontal gamma power at 16, 24 and 36 months predicts individual differences in language and cognition at 4 and 5 years. Behavioural Brain Research. 2011;220(2):263–270. doi: 10.1016/j.bbr.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackman DA, Farah MJ. Socioeconomic status and the developing brain. Trends in Cognitive Sciences. 2009;13(2):65–73. doi: 10.1016/j.tics.2008.11.003. doi: http://dx.doi.org/10.1016/j. tics.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halle T, Forry N, Hair E, Perper K, Wandner L, Wessel J, Vick J. Disparities in early learning and development: Lessons from the early childhood longitudinal study-birth cohort (ECLS-B) Washington, DC: Child Trends; 2009. [Google Scholar]
- Hanson JL, Chandra A, Wolfe BL, Pollak SD. Association between income and the hippocampus. PLoS ONE. 2011;6(5):e18712. doi: 10.1371/journal.pone.0018712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart B, Risley T. Meaningful differences in the everyday experience of young american children. Baltimore, MD: Brookes; 1995. [Google Scholar]
- Hoff E. Causes and consequences of SES-related differences in parent-to-child speech. In: Bornstein MH, Bradley RH, editors. Socioeconomic Status, Parenting and Child Development. Mahwah, NJ: Lawrence Erlbaum Associates; 2003. pp. 145–160. [Google Scholar]
- Honzik MP. Value and limitations of infant tests: An overview. Origins of Intelligence: Infancy and Early Childhood. 1976:59–95. [Google Scholar]
- Huttenlocher J, Vasilyeva M, Waterfall HR, Vevea JL, Hedges LV. The varieties of speech to young children. Developmental Psychology. 2007;43(5):1062. doi: 10.1037/0012-1649.43.5.1062. [DOI] [PubMed] [Google Scholar]
- Jednorog K, Altarelli I, Monzalvo K, Fluss J, Dubois J, Billard C, Ramus F. The influence of socioeconomic status on children’s brain structure. PLoS ONE. 2012;7(8):e42486. doi: 10.1371/journal.pone.0042486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK. Brain mechanisms in early language acquisition. Neuron. 2010;67(5):713–727. doi: 10.1016/j.neuron.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M, Brooks-Gunn J. Visual attention at three months as a predictor of cognitive functioning at two years of age. Intelligence. 1981;5(2):131–140. [Google Scholar]
- Lewis M, McGurk H. The Evaluation of Infant Intelligence: Infant Intelligence Scores-true Or False? ERIC. 1972 doi: 10.1126/science.178.4066.1174. [DOI] [PubMed] [Google Scholar]
- McCall RB. Nature-nurture and the two realms of development: A proposed integration with respect to mental development. Child Development. 1981:1–12. [Google Scholar]
- McCandliss BD, Noble KG. The development of reading impairment: A cognitive neuroscience model. Mental Retardation & Developmental Disabilities Research Reviews. 2003;9(3):196–204. doi: 10.1002/mrdd.10080. [DOI] [PubMed] [Google Scholar]
- McDonough L, Mandler JM, McKee RD, Squire LR. The deferred imitation task as a nonverbal measure of declarative memory. Proceedings of the National Academies of Science. 1995;92:7580–7584. doi: 10.1073/pnas.92.16.7580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Central role of the brain in stress and adaptation: Links to socioeconomic status, health, and disease. Annals of the New York Academy of Sciences. 2010;1186(1):190–222. doi: 10.1111/j.1749-6632.2009.05331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee RD, Squire LR. On the development of declarative memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1993;19(2):397. doi: 10.1037//0278-7393.19.2.397. [DOI] [PubMed] [Google Scholar]
- McLoyd VC. Socioeconomic disadvantage and child development. American Psychologist. 1998;53(2):185–204. doi: 10.1037//0003-066x.53.2.185. [DOI] [PubMed] [Google Scholar]
- Meltzoff AN. What infant memory tells us about infantile amnesia: Long-term recall and deferred imitation. Journal of Experimental Child Psychology. 1995;59:497–515. doi: 10.1006/jecp.1995.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan K, Hayne H. Age-related changes in memory reactivation by 1- and 2-year-old human infants. Developmental Psychobiology. 2006;48(1):48–57. doi: 10.1002/dev.20110. [DOI] [PubMed] [Google Scholar]
- Neville H, Stevens C, Pakulak E, Bell TA. Commentary: Neurocognitive consequences of socioeconomic disparities. Developmental Science. 2013 doi: 10.1111/desc.12081. [DOI] [PubMed] [Google Scholar]
- Noble KG, Farah MJ. Neurocognitive consequences of socioeconomic disparities: The intersection of cognitive neuroscience and public health. Developmental Science. 2013;16(5):639–640. doi: 10.1111/desc.12076. [DOI] [PubMed] [Google Scholar]
- Noble KG, Grieve SM, Korgaonkar MS, Engelhardt LE, Griffith EY, Williams LM, Brickman AM. Hippocampal volume varies with educational attainment across the life-span. Frontiers in Human Neuroscience. 2012a;6(307) doi: 10.3389/fnhum.2012.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Kan E, Sowell ER. Neural correlates of socioeconomic status in the developing human brain. Developmental Science. 2012b;15(4):516–527. doi: 10.1111/j.1467-7687.2012.01147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, McCandliss BD, Farah MJ. Socioeconomic gradients predict individual differences in neurocognitive abilities. Developmental Science. 2007;10(4):464–480. doi: 10.1111/j.1467-7687.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- Noble KG, Norman MF, Farah MJ. Neurocognitive correlates of socioeconomic status in kindergarten children. Developmental Science. 2005;8(1):74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- O’Connor MJ, Cohen S, Parmelee AH. Infant auditory discrimination in preterm and full-term infants as a predictor of 5-year intelligence. Developmental Psychology. 1984;20(1):159. [Google Scholar]
- Pan BA, Rowe ML, Singer JD, Snow CE. Maternal correlates of growth in toddler vocabulary production in low-income families. Child Development. 2005;76(4):763–782. doi: 10.1111/j.1467-8624.2005.00876.x. [DOI] [PubMed] [Google Scholar]
- Pascalis O, de Haan M. Recognition memory and novelty preference: What model. Progress in Infancy Research. 2003;3:95–120. [Google Scholar]
- Pascalis O, Hunkin N, Holdstock J, Isaac C, Mayes A. Visual paired comparison performance is impaired in a patient with selective hippocampal lesions and releatively intact item recognition. Neuropsychologia. 2004;42:1293–1300. doi: 10.1016/j.neuropsychologia.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Payne AC, Whitehurt GJ, Angell AL. The role of home literacy environment in the development of language ability in preschool children from low-income families. Early Childhood Research Quarterly. 1994;9:427–440. [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior research methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Raizada RD, Kishiyama MM. Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to levelling the playing field. Frontiers in Human Neuroscience. 2010;4:3. doi: 10.3389/neuro.09.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon SF. The widening academic-achievement gap between the rich and the poor: New evidence and possible explanations. In: Duncan GJ, Murnane RJ, editors. Whither opportunity?: Rising inequality, schools, and children’s life chances. New York, NY: Russell Sage Foundation; 2011. [Google Scholar]
- Richmond J, Colombo M, Hayne H. Interpreting visual preferences in the visual paired-comparison task. Journal of Experimental Psychology. 2007;33(5):823–831. doi: 10.1037/0278-7393.33.5.823. [DOI] [PubMed] [Google Scholar]
- Richmond J, Nelson CA. Mechanisms of change: A cognitive neuroscience approach to declarative memory development. In: Nelson CA, Luciana M, editors. Handbook of Developmental Cognitive Neuroscience. 2nd. Cambridge, MA: Bradford; 2008. [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Infant visual recognition memory. Developmental Review. 2004;24(1):74–100. doi: 10.1037/0012-1649.39.3.563. [DOI] [PubMed] [Google Scholar]
- Rose SA, Wallace IF. Visual recognition memory: A predictor of later cognitive functioning in preterms. Child Development. 1985:843–852. [PubMed] [Google Scholar]
- Rouse C, Brooks-Gunn J, McLanahan S. Introducing the issue [In volume School Readiness: Closing Racial and Ethnic Gaps] The Future of Children. 2005;15(1):3–14. [Google Scholar]
- Rowe ML, Goldin-Meadow S. Differences in early gesture explain SES disparities in child vocabulary size at school entry. Science. 2009;323(5916):951–953. doi: 10.1126/science.1167025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe ML, Pan BA, Ayoub C. Predictors of variation in maternal talk to children: A longitudinal study of low-income families. Parenting. 2005;5(3):259–283. [Google Scholar]
- Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: Development of the life experiences survey. Journal of Consulting and Clinical Psychology. 1978;46(5):932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- Sheridan MA, Sarsour K, Jutte D, D’Esposito M, Boyce WT. The impact of social disparity on prefrontal function in childhood. PLoS One. 2012;7(4):e35744. doi: 10.1371/journal.pone.0035744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, How J, Araujo M, Schamberg MA, Nelson CA. What are the links between maternal social status, hippocampal function, and HPA axis function in children? Developmental science. 2013;16(5):665–675. doi: 10.1111/desc.12087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Spier ET, Tamis-Lemonda CS. Reciprocal influences between maternal language and children’s language and cognitive development in low-income families. Journal of Child Language. 2013:1–22. doi: 10.1017/S0305000912000700. [DOI] [PubMed] [Google Scholar]
- Staff RT, Murray AD, Ahearn TS, Mustafa N, Fox HC, Whalley LJ. Childhood socioeconomic status and adult brain size: Childhood socioeconomic status influences adult hippocampal size. Annals of Neurology. 2012;71(5):653–660. doi: 10.1002/ana.22631. [DOI] [PubMed] [Google Scholar]
- Stern Y, Albert S, Tang M-X, Tsai W-Y. Rate of memory decline in AD is related to education and occupation Cognitive reserve. Neurology. 1999;53(9):1942–1942. doi: 10.1212/wnl.53.9.1942. [DOI] [PubMed] [Google Scholar]
- Tomalski P, Moore DG, Ribeiro H, Axelsson EL, Murphy E, Karmiloff-Smith A, Kushnerenko E. Socioeconomic status and functional brain development-associations in early infancy. Developmental Science. 2013;16(5):676–687. doi: 10.1111/desc.12079. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Gareau L, Flowers DL, Zeffiro TA, Eden GF. Development of neural mechanisms for reading. Nature Neuroscience. 2003;6(7):767–773. doi: 10.1038/nn1065. [DOI] [PubMed] [Google Scholar]
- Vannest J, Karunanayaka PR, Schmithorst VJ, Sza-flarski JP, Holland SK. Language networks in children: Evidence from functional MRI studies. American Journal of Roentgenology(2009 May) 2009 doi: 10.2214/AJR.08.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D, Greenwood C, Hart B, Carta J. Prediction of school outcomes based on early language production and socioeconomic factors. Child Development. 1994;65(2 Spec No):606–621. [PubMed] [Google Scholar]
- Zimmerman IL, Castilleja NF. The role of a language scale for infant and preschool assessment. Mental Retardation & Developmental Disabilities Research Reviews. 2005;11(3):238–246. doi: 10.1002/mrdd.20078. [DOI] [PubMed] [Google Scholar]
- Zimmerman IL, Steiner VG, Pond RE. Preschool Language Scale, Fourth Edition. San Antonio, TX: The Psychological Corporation; 2009. [Google Scholar]