Abstract
Purpose
Reovirus is a wild-type oncolytic virus that is ubiquitous in the environment; most patients are therefore preimmune. Therapeutic administration leads to an increase in neutralizing antireovirus antibody (NARA) titer. We hypothesized that if NARA limited reovirus antitumor activity, the effect might be attenuated by coadministration of cyclophosphamide.
Experimental design
In a phase I study, patients with advanced cancer received cyclophosphamide 3 days before intravenous reovirus serotype 3 Dearing (RT3D). The primary objective was to reduce the resulting rise in NARA titer. Cyclophosphamide dose was escalated from 25–1,000 mg/m2 through nine cohorts; we aimed to define a well-tolerated immunomodulatory dose.
Results
The combination was well tolerated in 36 patients, with grade 3/4 toxicities only seen at or above the maximum tolerated dose of cyclophosphamide, which was 800 mg/m2 combined with reovirus. Immunosuppressive effect, defined as maintaining NARA titer rise below a predefined threshold, was observed in only one patient. Furthermore, despite expected myelosuppression seen at higher cyclophosphamide doses, no changes in T-cell subsets, including Tregs, occurred with dose escalation. Viable virus was detected in association with peripheral blood mononuclear cells (PBMC) from 14% of patients 10 days after the last RT3D injection, despite high plasma NARA titer, demonstrating a potential mechanism for prolonged evasion of neutralization by reovirus.
Conclusions
Coadministration of cyclophosphamide with reovirus is safe, but does not attenuate host antiviral responses. Alternative immunomodulation approaches should be explored, but association with PBMCs may allow reovirus to persist and evade even high levels of neutralizing antibodies.
Introduction
Reovirus is a wild-type double-stranded RNA virus that is ubiquitous in the environment and relatively nonpathogenic in humans (1). Exposure early in life is associated with transient minor respiratory or enteric symptoms (2–4). The virus selectively replicates in cells with activated RAS, sparing normal cells. In part, this is because RAS activation inhibits the antiviral effects of double-stranded RNA-activated protein kinase (PKR), but is also due to RAS-induced enhancement of virus uncoating, infectivity, and release (5, 6). Tumor RAS mutations, or activation of up- or downstream RAS pathway signaling elements, are present in the majority of human cancers (7). There is an unmet need for novel treatments for RAS-driven tumors, because attempts to target this oncogene with small molecules have, to date, been unsuccessful.
Reovirus serotype 3 Dearing (RT3D; REOLYSIN, Oncolytics Biotech Inc.) has selective antitumor activity, both in vitro and in tumor xenograft models, and can be safely administered intravenously with evidence of efficacy in several trials (8–13). However, there is a high seropositivity rate in healthy populations following childhood reoviral exposure (14), and systemic administration of therapeutic oncolytic viruses evokes a brisk host immune response. In particular, intravenous RT3D leads to an early rise (100- to 1000-fold) in neutralizing antireovirus antibody (NARA) titer in most patients (8, 15).
In contrast with the immunosuppression usually associated with cytotoxic agents, immunomodulation has been extensively described as an effect of cyclophosphamide (16). The combination of cyclophosphamide in combination with RT3D has been investigated in murine models, and these studies have demonstrated safety and efficacy using a carefully titrated cyclophosphamide schedule, including administration 24 hours before RT3D (17). However, significant normal tissue toxicity was seen at higher doses, similar to the administration of RT3D to B-cell knockout mice (17). Thus, careful titration of any immunomodulatory effect is required, to optimize efficacy without augmenting viral replication and toxicity in normal tissues.
This phase I dose escalation study was designed to investigate the feasibility and safety of cyclophosphamide coadministration with RT3D in man, with the primary aim of abrogating the neutralizing antireovirus antibody response, to maximize virus delivery to tumor. Secondary objectives were assessment of the safety of this approach and antitumor activity. Translational studies investigated changes in cellular immune subsets, including regulatory T cells, and circulating viral persistence in association with PBMCs.
Materials and Methods
Patients
Eligible patients had advanced or metastatic solid tumors refractory to standard treatment. Patients were required to have measurable or evaluable disease; any residual toxic effects related to prior anticancer therapy having resolved to grade 1 or lower [as defined by the Common Terminology Criteria for Adverse Events (CTCAE), version 3.0]; be >18 years of age; have received no chemotherapy, radiotherapy, biologic therapy, or hormone therapy (apart from patients with breast cancer or prostate cancer) within 28 days before receiving the study treatment; and have an Eastern Cooperative Oncology Group (ECOG) performance score of 0–2. The following baseline laboratory results were required: absolute neutrophil count >1.5 × 109/L, platelets >100 × 109/L, hemoglobin >90g/L, serum creatinine <1.5× institutional upper limit of normal (ULN), total bilirubin <1.5× ULN, aspartate transaminase and alanine transaminase <2.5× ULN, and a negative pregnancy test for females of childbearing potential. Exclusion criteria included known brain metastases, concurrent immunosuppressive therapy, known HIV, hepatitis B or C infections, pregnancy or breastfeeding, clinically significant cardiac disease (New York Heart Association class III or IV), and dementia or altered mental state that would prohibit informed consent. The study was approved by the relevant local research ethics committees (EudraCT number 2007-001937-32).
Study design
This was an open-label dose-escalating phase I combination study of cyclophosphamide given together with RT3D, conducted at 3 sites in the United Kingdom. Cyclophosphamide was administered, with the aim of immunomodulation, as an intravenous bolus on day minus 3 of the first cycle, and on day 26 of the first and subsequent cycles in the absence of dose-limiting toxicity or disease progression. RT3D was administered intravenously over 5 consecutive days (days 1–5) of a 28-day cycle. Patients continued to receive further cycles of RT3D for 5 days every 28 days, in the absence of disease progression or intolerable treatment-related toxicity.
Assay of the neutralizing antireovirus antibody (NARA) titer on day 15 of the first two cycles was used as the endpoint for the primary outcome measure of effective immunomodulation. The protocol mandated withholding cyclophosphamide if the NARA response was very weak (less than a 10-fold increase over pretreatment baseline), or if levels fell, in the first two or subsequent cycles.
Screening tests at baseline included physical examination, computerized tomography, full blood count, coagulation screen, tests of renal and liver function, urate, CK-MB and troponin I, electrocardiogram (EKG), and left ventricular ejection fraction (LVEF) by echocardiogram or multiple-gated acquisition (MUGA) scan. All adverse events and laboratory variables were assessed using the CTCAE version 3.0. LVEF was reevaluated after 8 weeks, and subsequently only in the presence of elevated cardiac enzymes, EKG abnormality, or if otherwise clinically indicated. Other safety assessments were repeated at baseline, on days 8, 15, and 22, and at weekly intervals throughout the study. Tumor response was assessed according to RECIST version 1.0 (18) at intervals of 8 weeks.
Virus administration
Reovirus was supplied by Oncolytics Biotech Inc. as single use 1 mL glass vials containing a frozen viral suspension in PBS. Stock was stored at −70°C, thawed rapidly over 2 minutes, diluted to 250 mL in 0.9% sodium chloride, and infused over 1 hour through a peripheral line in a side room. Vital signs were monitored during the infusion and for at least 1 hour afterwards.
Dose escalation
All patients received RT3D at a tissue culture infective dose (TCID50) of 3 × 1010, shown to be safe when administered as a single agent (8). The cyclophosphamide dose was escalated in successive cohorts as shown in Table 1. The starting dose of 25 mg/m2 was chosen to be well below cytotoxic levels of cyclophosphamide, and this was escalated in successive cohorts to 1,000 mg/m2. Patients were enrolled in groups of three and individually assessed for immunomodulation, safety, and dose-limiting toxicities (DLT). Patients were considered evaluable for assessment of immunomodulation if they had received at least two cycles, and for safety if they had received at least one cycle or withdrew from the study due to drug-related toxicity. Patients withdrawing from the study without meeting these criteria were replaced. If one of three patients in a cohort experienced effective immunomodulation (NARA increase between 10- and 50-fold) or DLT during the first cycle, three more patients would be added to that dose group. If two or more DLTs occurred during any cohort, the previous lower dose would be defined as the maximum tolerated dose (MTD). Treatment could be continued beyond Cycle 2 in the absence of intolerable toxicity or evidence of disease progression.
Table 1.
Dose escalation schema and exposure for cyclophosphamide in combination with reovirus
| Cohort | Number of patients | Median cycles (range) | DT3R dose D1–5 (TCID50/day) | Cyclophosphamide dose (mg/m2) D-3; D26 of each cycle |
|---|---|---|---|---|
| 1 | 5 | 2 (1–8) | 3 × 1010 | 25 |
| 2 | 5 | 2 (1–2) | 3 × 1010 | 50 |
| 3 | 3 | 2 (2–4) | 3 × 1010 | 100 |
| 4 | 5 | 2 (1–3) | 3 × 1010 | 150 |
| 5 | 3 | 2 (2–5) | 3 × 1010 | 200 |
| 6 | 4 | 2 (2–3) | 3 × 1010 | 400 |
| 8 | 7 | 2 (1–6) | 3 × 1010 | 800 |
| 9 | 4 | 2 (1–5) | 3 × 1010 | 1,000 |
Assessment of immunomodulation
Effective immunomodulation was assessed in each patient by NARA assay at baseline, day 15 of Cycle 1, and day 15 of cycle 2 and subsequent cycles as previously described (15). At each dose level, immunomodulation was considered effective if the rise in NARA titer (at day 15 of Cycles 1 and 2) was at least 10-fold lower than that observed in the single-agent studies of RT3D. Thus, an increase of <50-fold over pretreatment baseline was deemed effective immunomodulation. Cyclophosphamide treatment beyond Cycle 2 was withheld if NARA titer at day 15 of Cycles 1 or 2 increased by <10-fold over pretreatment baseline. Cohorts would be expanded to 6 if this very low level of NARA induction was observed in all of the first 3 patients at a dose level. For a dose level to be labeled the minimally effective dose (MED), immunomodulation needed to be observed in ≥4/6 patients. T-cell immune subsets were immunophenotyped by flow cytometry. Cryopreserved PBMC taken at day 15 of Cycle 1 and day 15 of Cycle 2 were thawed and washed in 0.15 mol/L PBS, Dulbecco's A (Oxoid). The LIVE/DEAD Cell Stain Kit (Invitrogen) was used to differentiate viable and dead cells. After washing the PBMC in PBS, the following anti-human monoclonal antibodies were used for flow cytometry: anti-CD3-FITC, anti-CD8-APC, anti-CD4-PECy7, anti-CD25-APC-H7, and anti-CD127-PE (BD Biosciences). After staining, the cells were washed in PBS and analyzed using a MACSQuant flow cytometer with MACSQuantify software (Miltenyi Biotec). For T-cell subset and Treg analysis, the acquisition and analysis gates were restricted to the live lymphocyte population. CD3+CD4+ and CD3+CD8+ cells were calculated as a percentage of live CD3+ lymphocytes. Tregs were identified as CD4+CD25+CD127lo/neg and calculated as a percentage of live CD4+ lymphocytes.
Dose-limiting toxicity
DLT was defined during the first cycle of treatment and included absolute neutrophil count <0.5 × 109/L, or <1.0 × 109/L with sepsis, platelet count <25 × 109/L, grade 2 rash or mucositis lasting >5 days, grade 2 nausea lasting >5 days despite the use of maximal supportive therapy, and any other grade 3 or 4 treatment-related nonhematologic toxicity (except flu-like symptoms, and nausea and vomiting if appropriate prophylactic or therapeutic measures had not been administered). Flu-like symptoms were treated with acetaminophen or nonsteroidal anti-inflammatory drugs.
Analysis of viral shedding by reverse transcription-PCR
Biologic samples (blood, urine, feces, and sputum) were collected at baseline before commencing cyclophosphamide, on days 5 and 15 of Cycle 1, and on days 1, 5, and 15 of Cycle 2. On days that RT3D was administered, samples were collected before the infusion. Sample processing and reverse transcription PCR (RT-PCR) methods were as previously described (8).
Analysis of viral persistence
A 1:3 to 1:729 dilution of patient PBMC sample was incubated on L929 cells for 6 days to allow for any functional virus to replicate. Cells and supernatants were then harvested for RT-PCR. Viral RNA was extracted from samples using QIAamp Viral Mini Kit, and RT-PCR was performed using OneStep RT-PCR (both Qiagen). Reovirus-complementary DNA-targeted primers used were 5′-GGGCTGCACATTACCACTGA (forward) and 5′-CTCCTCGCAATACAACTCGT (reverse), with a detection limit set at 30 cycles. Samples were run on a 2% agarose gel and analyzed for reovirus RNA by the presence of a 300-base pair PCR product. Reovirus or media were incubated on L929 cells alongside patient PBMC as positive and negative controls (19). Cytopathic effect on L929 cells was also photographed, and L929 cells were analyzed for survival by MTT colorimetric assay for cell viability at dilutions of 1:729.
Results
A total of 36 patients were included in the dose escalation; all contributed to the immunomodulation and safety analyses. Cyclophosphamide dose levels studied and patient numbers in each cohort (Table 1) were 25 mg/m2 (n = 5), 50 mg/m2 (n = 5), 100 mg/m2 (n = 3), 150 mg/m2 (n = 5), 200 mg/m2 (n = 3), 400 mg/m2 (n = 4), 800 mg/m2 (n = 7), and 1,000 mg/m2 (n = 4). Patient characteristics are shown in Table 2. The most frequently treated tumor types were colorectal and pancreatic cancers.
Table 2.
Patient demographics: all treated patients (n = 36)
| n (%) | |
|---|---|
| Sex | |
| Male | 25 (69%) |
| Female | 11 (31%) |
| Median age, years (range) | 63 (33–78) |
| Primary cancer | |
| Colorectal | 11 (31%) |
| Pancreatic | 5 (14%) |
| Melanoma | 3 (8%) |
| Prostate | 3 (8%) |
| Cholangiocarcinoma | 2 (6%) |
| Renal | 2 (6%) |
| Other | 10 (28%) |
| ECOG PS at screening | |
| 0 | 9 (25%) |
| 1 | 27 (75%) |
| Prior therapies | |
| Surgery | 22 (61%) |
| Radiotherapy | 12 (33%) |
| Systemic therapy | 36 (100%) |
Immunomodulation analysis
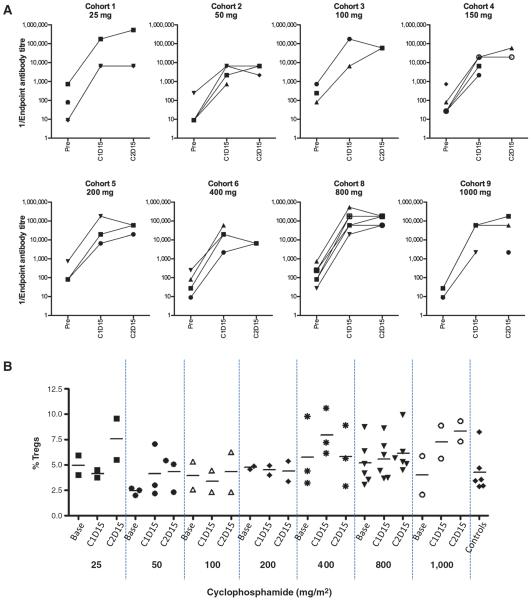
NARA was assessed for each patient at baseline in all cases, and serial on-treatment samples collected at either day 15 of Cycle 1 (C1D15), C2D15, or both, were available in 32 patients (Fig. 1a). Significant immunomodulation, defined as a <50-fold increase in NARA titer, was observed in only one patient, who received cyclophosphamide at a dose of 50 mg/m2. In this patient, NARA titer rose after exposure to reovirus by 27-fold, from a baseline that was already relatively high (Fig. 1A). In other patients, NARA rose by between 81- and over 2,000-fold. Marked immunomodulation (defined as less than a 10-fold induction over baseline), requiring subsequent cyclophosphamide doses to be withheld, was not seen in any patient.
Figure 1.
A, neutralizing antireovirus antibody (NARA) titer, and (B) regulatory T-cells (Tregs), are unchanged before and after exposure to reovirus. A, NARA titer was assessed at baseline, 10 days after patients' last infusion of reovirus in their first cycle of treatment (“C1D15”), and at C2D15. Significant immunomodulation, defined as less than 50-fold induction of NARA titer, was observed in a single patient treated with cyclophosphamide 50mg/m2 (27-fold rise; ▼ cohort 2). B, the proportion of CD4+CD25+CD127lo/neg T cells was measured for each cohort at baseline, C1D15, and C2D15. Horizontal bars represent medians. This was not significantly affected even at the highest cyclophosphamide doses, despite more prominent suppression of total white blood cell count with increasing dose (Supplementary Fig. S1).
Analysis of immune subsets similarly showed that cyclophosphamide did not induce a fall in the proportion of regulatory T cells (Tregs) even at the highest doses (Fig. 1B). This was despite more prominent suppression of neutrophil count in the later cohorts (Supplementary Fig. S1).
Safety and tolerability
All 36 (100%) patients experienced at least one adverse event (AE), irrespective of relationship to study drug. Table 3 summarizes all treatment-related AEs of any grade occurring in 3 or more patients, and all grade 3/4 treatment-related AEs. There were no grade 4 nonhematologic toxicities or treatment-related deaths.
Table 3.
Treatment-related AEs: reported by 3 or more patients, and all grade 3/4 AEs
| Reovirus titer: TCID50 3 × 1010 | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 mg/m2, n = 5 |
50 mg/m2, n = 5 |
100 mg/m2, n = 3 |
150 mg/m2, n = 5 |
200 mg/m2, n = 3 |
400 mg/m2, n = 4 |
800 mg/m2, n = 7 |
1,000 mg/m2, n = 4 |
Overall, n = 36 |
||||||||||
|
|
|
|
|
|
|
|
|
|
||||||||||
| Cyclophosphamide | All | Grade 3/4 |
All | Grade 3/4 |
All | Grade 3/4 |
All | Grade 3/4 |
All | Grade 3/4 |
All | Grade 3/4 |
All | Grade 3/4 |
All | Grade 3/4 |
All, n (%) |
Grade 3/4 |
| Fever | 4 | 0 | 4 | 1 | 2 | 0 | 2 | 0 | 3 | 0 | 2 | 0 | 3 | 0 | 1 | 0 | 21 (58.3) | 1 (2.8) |
| Fatiguea | 3 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 2 | 0 | 2 | 0 | 15 (41.6) | 0 |
| Chills | 0 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 2 | 0 | 4 | 0 | 1 | 0 | 12 (33.3) | 0 |
| Diarrhea | 2 | 0 | 0 | 0 | 2 | 0 | 2 | 0 | 1 | 0 | 1 | 0 | 2 | 0 | 2 | 1 | 12 (33.3) | 1 (2.8) |
| Nausea | 2 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 3 | 0 | 12 (33.3) | 0 |
| Influenza-like symptomsb | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 4 | 0 | 1 | 0 | 1 | 0 | 10 (27.7) | 0 |
| Vomiting | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 7 (19.4) | 0 |
| Anorexia | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 3 | 0 | 7 (19.4) | 1 (2.8) |
| Neutropenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 3 | 1 | 1 | 6 (16.7) | 4 (11.1) |
| Anemia | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 3 | 2 | 0 | 0 | 5 (13.9) | 2 (5.6) |
| Headache | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 5 (13.9) | 0 |
| Thrombocytopenia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 4 (11.1) | 0 |
| Tachycardia | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 3 (8.3) | 0 |
| Hypotension | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 3 (8.3) | 0 |
NOTE: Values shown represent the number of patients.
Fatigue and lethargy are grouped together.
Influenza-like symptoms and hyperhidrosis are grouped together.
In general, when administered in combination with cyclophosphamide, reovirus was well tolerated. The majority of toxicities were grade 1/2, the most frequently reported being fever, fatigue, and chills. Fever, chills, and influenza-like symptoms were controlled with acetaminophen or nonsteroidal anti-inflammatory drugs. Concomitant acetaminophen with reovirus was avoided in patients recruited later in this trial due to an emerging preclinical suggestion of synergistic increases in AST and ALT (20). However, several ongoing controlled trials have not shown evidence of reovirus hepatotoxicity (data on file, Oncolytics Biotech Inc.). Fatigue and diarrhea were commonly reported, but without a clear relation to dose. The commonest laboratory toxicity was neutropenia, but this did not occur at cyclophosphamide doses below 800 mg/m2.
3 DLTs occurred during the first cycle of treatment, at dose levels of 800 mg/m2 and 1,000 mg/m2. At 800 mg/m2, one of 7 patients developed grade 3 neutropenia. At 1,000 mg/m2, grade 3 diarrhea occurred beginning immediately following the day minus 3 cyclophosphamide dose, and another patient developed grade 4 neutropenia in the first cycle. All DLTs were judged by the treating investigator to be probably or definitely related to cyclophosphamide. Thus, the MTD was cyclophosphamide 800 mg/m2 combined with a RT3D TCID50 of 3 × 1010.
Viral persistence in plasma and shedding
RT3D plasma RNA was detected in 15 of 36 (42%) patients tested. These positive signals were only seen in patients who received cyclophosphamide 100 mg/m2 or above. Shedding was assessed by analysis of saliva, urine, and anal swabs; assays for viral RNA were positive on at least one occasion in 6 of 36 (17%) patients tested at multiple time points in the first 2 cycles.
Cell carriage of replication-competent virus
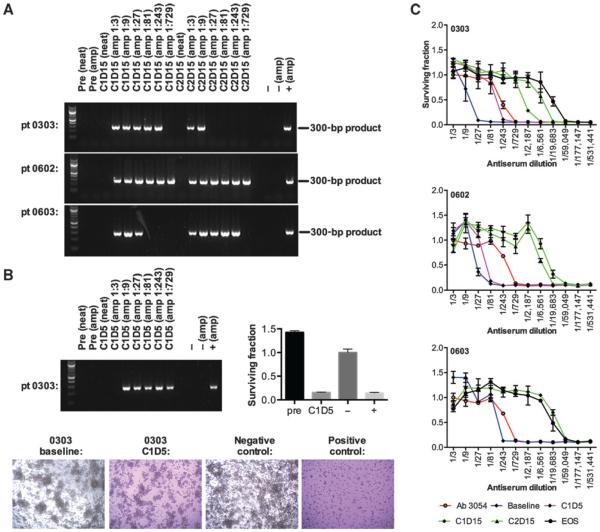
Transient carriage of reovirus by circulating white blood cells has been observed after a single cycle of treatment (21). The potential presence of viable RT3D associated with PBMCs was tested in Cycle 1 day 15 (C1D15) and C2D15 samples in 21 patients. Patient PBMC samples that were directly tested for RT3D RNA by RT-PCR were all negative. However, consistent with previous data (21), when PBMC samples were incubated on L929 cells, to allow any functional reovirus to be handed off the PBMCs and replicate in these cells, a band was detected post-treatment (at C1D15 and C2D15) in 3 of 21 (14%) of patients (0303, 0602, and 0603; Fig. 2A). Samples were available at other timepoints for patient 0303 (C1D5, C3D15 and end of study), and for patient 0603 (end of study); all of these samples were negative for RT3D associated with PBMCs, except for patient 0303 C1D5 sample (Fig. 2B). This persistence of PBMC-associated virus in some patients occurred despite induction of high NARA titer by day 15 of Cycles 1 and 2 in each of these patients (Fig. 2C).
Figure 2.
Patients carry live reovirus on their PBMCs up to 10 days after the last administration, which can replicate and kill target cells in vitro despite an increased level of circulating neutralizing antibodies. A, PBMCs were assessed directly for presence of reoviral RNA (“neat”) by RT-PCR at C1D15 and C2D15. PBMCs were also incubated on L929 target cells at the dilutions shown to allow for any functional reovirus to be handed off the PBMCs and then replicate in the target cells, before analysis for reoviral RNA by RT-PCR (“amp”). The appearance of a band demonstrates presence of RT3D, which denotes successful transfer from PBMC cells and replication of reovirus. This becomes apparent after treatment in 3 patients tested (0303, 0602, 0603), but is not detected in the amplified samples taken before treatment (“pre”). 2% DMEM or a dilution (1:10) of stock reovirus was incubated on L929 cells as negative and positive controls, respectively (−amp, +amp). B, all other available samples for these patients were analyzed, and all samples were negative for reovirus by amplification assay, except for sample C1D5 in patient 0303. Photomicrographs show cytopathic effect on L929 cells pre- and posttreatment, and MTT viability data after incubation of sample on L929 cells for patient 0303. C, neutralizing antireovirus antbody titers in patients who carried reovirus on their PBMCs. At C1D15 and C2D15 antibody titers increased as much as 729-fold compared with baseline. Error bars show SEM.
Antitumor activity
There were no partial responses. Two patients remained on study for more than 6 months, one a patient with malignant melanoma treated at 25 mg/m2, and another with pancreatic carcinoma treated at 150 mg/m2, diseases for which cyclophosphamide is not a conventionally accepted therapy.
Discussion
We conducted this trial with the aim of using immunomodulatory cyclophosphamide to improve the tumor delivery of oncolytic virotherapy. Reovirus has proved to be safe in the treatment of human cancer, and normal tissues are able to resist intracellular viral replication because of intact PKR function, which by contrast is downregulated in RAS-driven tumor cells (5). However, reovirus serotype 3 Dearing (RT3D) is a ubiquitous unmodified virus that causes upper respiratory tract infections in almost all individuals early in life. As a result, as seen with other oncolytic viruses (22, 23), neutralizing anti-reoviral antibodies (NARA) are detectable at baseline, and titers rise dramatically after the first therapeutic reexposure in the majority of patients. Modulation of NARA with subcytotoxic doses of cyclophosphamide was the primary objective of this study.
Effective and reproducible NARA modulation was not achieved, despite escalation of cyclophosphamide to cytotoxic levels associated with myelosuppression in the later cohorts. Similarly, suppression of Tregs did not occur even in later cohorts. Nevertheless, striking persistence in the circulation of viable virus was detected, in association with PBMCs, even in the presence of induced NARA. This result suggests that NARA response may be a less significant barrier to tumor delivery of oncolytic viruses than previously supposed.
Reovirus has previously been detected in human tumors after systemic administration, and clinical efficacy seen in some patients treated with single-agent virus (8). Thus, a question arises of how circulating reovirus evades immune complex formation effectively enough to reach the tumor target. There is already some evidence that carriage by PBMCs is a mechanism for persistence of circulating reovirus (19, 21). Data from our trial provide further evidence of this phenomenon and for the first time demonstrate that virus can remain associated with PBMCs for at least 2 weeks following administration, and in spite of high levels of induced NARA. In this small trial, there was no association between response and viral carriage on PBMCs.
This trial was conceived following preclinical data suggesting that cyclophosphamide may improve tumor delivery of systemically adminstered oncolytic viruses, both for reovirus (17) and for an HSV-derived agent (22). We did not replicate in patients the modulation of NARA observed with some cyclophosphamide doses and schedules in a murine model (17). Although we carefully explored a wide dose range, it may be that alternative cyclophosphamide schedules would produce a different result. For example, low-dose metronomic, rather than bolus, cyclophosphamide is associated with responses in human cancers that may be mediated via immunomodulation (24). Cyclophosphamide has been administered to patients in combination with an oncolytic adenovirus; however, that program was not a formal trial (25), but instead was conducted under a European directive hospital exemption. Patients received a variety of personalized combinations, so generalizable results were not available, and effects on NARA were not assessed (26).
Alternative strategies for control of NARA production might be considered. The anti-CD20 monoclonal antibody rituximab was ineffective in suppressing antidrug antibodies associated with an immunogenic antibody conjugate (27). Coadminstration of cyclosporin with an anticancer agent has been explored (28), but it appears to inhibit the therapeutic effect of reovirus administered to tumor-bearing animals (29).
During the course of this study, other trials exploring the safety of combining RT3D with chemotherapeutics were completed (11–13). The coadministration of gemcitabine at full therapeutic dose in a phase I trial did have an effect on the NARA response to virus. Although RT3D dose had to be reduced because of toxicity (12), experience in a larger trial demonstrated acceptable tolerability for this combination (data on file, Oncolytics Biotech Inc.). The objective of our trial was to achieve NARA suppression without resorting to myelosuppressive therapy and its associated toxicity.
Shedding of RT3D RNA was detected in 5 of 36 (14%) of patients in this trial, which was more frequent than with reovirus alone, or with combination regimens of RT3D and conventional chemotherapy. Evidence of shedding was detected in 18% of patients receiving RT3D alone (8), 8% of patients receiving docetaxel combination therapy (11), and 21% receiving combined carboplatin and paclitaxel (13). No evidence of shedding was seen in studies combining RT3D with radiotherapy (30), or with gemcitabine (12). Shedding of this unmodified virus, ubiquitous in the environment, is not a safety concern. Furthermore, detection of RT3D RNA, as in our shedding assays, is not confirmation of the excretion of viable virus.
In summary, bolus cyclophosphamide administered before RT3D therapy does not reproducibly prevent induction of reovirus-neutralizing antibodies or affect levels of Tregs. Other cyclophosphamide dosing schedules, or alternative immunomodulating agents, might be more effective. The relationships between host immunity, oncolytic viruses, and tumor are complex (31), and successful clinical manipulation of the immune component may not be readily predictable, even from effective animal models. Our data suggest that other mechanisms, in particular association with PBMCs or other blood cells (21), may allow reovirus antitumor efficacy via evasion of immune complex formation even in the presence of high levels of NARA.
Supplementary Material
Translational Relevance.
Oncolytic viruses that replicate preferentially in tumor cells have shown promise for treatment of cancer. However, systemic administration of these viruses induces host immunity, including neutralizing antibodies. These could form immune complexes with virus, potentially impairing its delivery to tumor. This phase I trial explored the combination of reovirus and intravenous cyclophosphamide, with the aim of attenuating induction of neutralizing antibodies. Significant immunomodulation was not achieved, but for the first time carriage of virus associated with PBMCs was detected in several patients as long as 2 weeks following intravenous administration. This finding suggests a mechanism for viral evasion of neutralizing antibody, so that immune response to oncolytic viruses may be a less significant barrier to tumor delivery than previously supposed.
Acknowledgments
The authors particularly thank the patients, their families, and the clinical research teams for their contribution to this study. The trial protocol was developed in part by J. Spicer at the Methods in Clinical Cancer Research Workshop, Flims, Switzerland.
Grant Support The authors acknowledge financial support from the U.K. Department of Health via the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) awards to Guy's & St Thomas' NHS Foundation Trust in partnership with King's College London and King's College Hospital NHS Foundation Trust (andNIHR Clinical Research Facility), and to The Institute of Cancer Research with Royal Marsden Hospital NHS Foundation Trust. King's College London and The Institute of Cancer Research are Experimental Cancer Medicine Centres.
H.S. Pandha reports receiving a commercial research grant from Oncolytics Biotech Inc. M. Coffey and G.M. Gill have ownership interest (including patents) in Oncolytics Biotech Inc. A.A. Melcher reports receiving a commercial research grant from Oncolytics Biotech Inc.
Footnotes
Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed by the other authors.
Authors' Contributions Conception and design: H.S. Pandha, M. Coffey, G.M. Gill, A.A. Melcher, R. Vile, K.J. Harrington, J. de Bono, J. Spicer
Development of methodology: V. Roulstone, K. Khan, M. Coffey, R. Vile, K.J. Harrington, J. de Bono, J. Spicer
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): H.S. Pandha, S. Rudman, R. Vile, K.J. Harrington, J. de Bono, J. Spicer
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): V. Roulstone, K. Khan, S. Rudman, M. Coffey, R. Vile, K.J. Harrington, J. de Bono, J. Spicer
Writing, review, and/or revision of the manuscript: V. Roulstone, K. Khan, H.S. Pandha, S. Rudman, M. Coffey, G.M. Gill, A.A. Melcher, R. Vile, K.J. Harrington, J. de Bono, J. Spicer
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): V. Roulstone, K. Khan, R. Vile, J. de Bono
Study supervision: K. Khan, H.S. Pandha, R. Vile, K.J. Harrington, J. de Bono, J. Spicer
Other (lead clinical fellow on the trial): K. Khan
Other (review and monitoring of trial results, especially safety): G.M. Gill
References
- 1.Rosen L, Evans HE, Spickard A. Reovirus infections in human volunteers. Am J Hyg. 1963;77:29–37. doi: 10.1093/oxfordjournals.aje.a120293. [DOI] [PubMed] [Google Scholar]
- 2.Sabin AB. Reoviruses. A new group of respiratory and enteric viruses formerly classified as ECHO type 10 is described. Science. 1959;130:1387–9. doi: 10.1126/science.130.3386.1387. [DOI] [PubMed] [Google Scholar]
- 3.Jackson G, Muldoon R, Cooper R. Reovirus type 1 as an etiologic agent of the common cold. J Clin Invest. 1961;40:1051. [Google Scholar]
- 4.Rubin DH, Kornstein MJ, Anderson AO. Reovirus serotype 1 intestinal infection: a novel replicative cycle with ileal disease. J Virol. 1985;53:391–8. doi: 10.1128/jvi.53.2.391-398.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17:3351–62. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marcato P, Shmulevitz M, Pan D, Stoltz D, Lee PW. Ras transformation mediates reovirus oncolysis by enhancing virus uncoating, particle infectivity, and apoptosis-dependent release. Mol Ther. 2007;15:1522–30. doi: 10.1038/sj.mt.6300179. [DOI] [PubMed] [Google Scholar]
- 7.Bos JL. Ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–9. [PubMed] [Google Scholar]
- 8.Vidal L, Pandha HS, Yap TA, White CL, Twigger K, Vile RG, et al. A phase I study of intravenous oncolytic reovirus type 3 Dearing in patients with advanced cancer. Clin Cancer Res. 2008;14:7127–37. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- 9.Gollamudi R, Ghalib MH, Desai KK, Chaudhary I, Wong B, Einstein M, et al. Intravenous administration of Reolysin, a live replication competent RNA virus is safe in patients with advanced solid tumors. Invest New Drugs. 2009;28:641–9. doi: 10.1007/s10637-009-9279-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrington KJ, Vile RG, Melcher A, Chester J, Pandha HS. Clinical trials with oncolytic reovirus: moving beyond phase I into combinations with standard therapeutics. Cytokine Growth Factor Rev. 2010;21:91–8. doi: 10.1016/j.cytogfr.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comins C, Spicer J, Protheroe A, Roulstone V, Twigger K, White CM, et al. REO-10: a phase I study of intravenous reovirus and docetaxel in patients with advanced cancer. Clin Cancer Res. 2010;16:5564–72. doi: 10.1158/1078-0432.CCR-10-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lolkema MP, Arkenau HT, Harrington K, Roxburgh P, Morrison R, Roulstone V, et al. A phase I study of the combination of intravenous reovirus type 3 Dearing and gemcitabine in patients with advanced cancer. Clin Cancer Res. 2011;17:581–8. doi: 10.1158/1078-0432.CCR-10-2159. [DOI] [PubMed] [Google Scholar]
- 13.Karapanagiotou EM, Roulstone V, Twigger K, Ball M, Tanay M, Nutting C, et al. Phase I/II trial of carboplatin and paclitaxel chemotherapy in combination with intravenous oncolytic reovirus in patients with advanced malignancies. Clin Cancer Res. 2012;18:2080–9. doi: 10.1158/1078-0432.CCR-11-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tai JH, Williams JV, Edwards KM, Wright PF, Crowe JE., Jr Dermody TS. Prevalence of reovirus-specific antibodies in young children in Nashville, Tennessee. J Infect Dis. 2005;191:1221–4. doi: 10.1086/428911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White CL, Twigger KR, Vidal L, De Bono JS, Coffey M, Heinemann L, et al. Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther. 2008;15:911–20. doi: 10.1038/gt.2008.21. [DOI] [PubMed] [Google Scholar]
- 16.Moschella F, Proietti E, Capone I, Belardelli F. Combination strategies for enhancing the efficacy of immunotherapy in cancer patients. Ann N Y Acad Sci. 2010;1194:169–78. doi: 10.1111/j.1749-6632.2010.05464.x. [DOI] [PubMed] [Google Scholar]
- 17.Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–69. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 19.Adair RA, Roulstone V, Scott KJ, Morgan R, Nuovo GJ, Fuller M, et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci Transl Med. 2012;4:138ra177. doi: 10.1126/scitranslmed.3003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maddox JF, Amuzie CJ, Li M, Newport SW, Sparkenbaugh E, Cuff CF, et al. Bacterial- and viral-induced inflammation increases sensitivity to acetaminophen hepatotoxicity. J Toxicol Environ Health A. 2010;73:58–73. doi: 10.1080/15287390903249057. [DOI] [PubMed] [Google Scholar]
- 21.Bell JC. The virus that came in from the cold. Sci Transl Med. 2012;4:138fs117. doi: 10.1126/scitranslmed.3004139. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5:881–7. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 23.Power AT, Wang J, Falls TJ, Paterson JM, Parato KA, Lichty BD, et al. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol Ther. 2007;15:123–30. doi: 10.1038/sj.mt.6300039. [DOI] [PubMed] [Google Scholar]
- 24.Penel N, Adenis A, Bocci G. Cyclophosphamide-based metronomic chemotherapy: after 10 years of experience, where do we stand and where are we going? Crit Rev Oncol Hematol. 2012;82:40–50. doi: 10.1016/j.critrevonc.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Kanerva A, Nokisalmi P, Diaconu I, Koski A, Cerullo V, Liikanen I, et al. Antiviral and antitumor T-cell immunity in patients treated with GM-CSF-coding oncolytic adenovirus. Clin Cancer Res. 2013;19:2734–44. doi: 10.1158/1078-0432.CCR-12-2546. [DOI] [PubMed] [Google Scholar]
- 26.Harrington KJ. Oncolytic virotherapy needs trials, not access programs. Clin Cancer Res. 2013;19:2595–7. doi: 10.1158/1078-0432.CCR-13-0571. [DOI] [PubMed] [Google Scholar]
- 27.Hassan R, Williams-Gould J, Watson T, Pai-Scherf L, Pastan I. Pretreatment with rituximab does not inhibit the human immune response against the immunogenic protein LMB-1. Clin Cancer Res. 2004;10:16–8. doi: 10.1158/1078-0432.ccr-1160-3. [DOI] [PubMed] [Google Scholar]
- 28.Selvaggi K, Saria EA, Schwartz R, Vlock DR, Ackerman S, Wedel N, et al. Phase I/II study of murine monoclonal antibody-ricin A chain (XOMAZYME-Mel) immunoconjugate plus cyclosporine A in patients with metastatic melanoma. J Immunother Emphasis Tumor Immunol. 1993;13:201–7. doi: 10.1097/00002371-199304000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Steele TA, Cox DC. Reovirus type 3 chemoimmunotherapy of murine lymphoma is abrogated by cyclosporine. Cancer Biother. 1995;10:307–15. doi: 10.1089/cbr.1995.10.307. [DOI] [PubMed] [Google Scholar]
- 30.Harrington KJ, Karapanagiotou EM, Roulstone V, Twigger KR, White CL, Vidal L, et al. Two-stage phase I dose-escalation study of intratumoral reovirus type 3 dearing and palliative radiotherapy in patients with advanced cancers. Clin Cancer Res. 2010;16:3067–77. doi: 10.1158/1078-0432.CCR-10-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naik JD, Twelves CJ, Selby PJ, Vile RG, Chester JD. Immune recruitment and therapeutic synergy: keys to optimizing oncolytic viral therapy? Clin Cancer Res. 2011;17:4214–24. doi: 10.1158/1078-0432.CCR-10-2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.