Abstract
The majority of current vaccines depend on a continuous “cold chain” of storage and handling between 2–8°C. Vaccines experiencing temperature excursions outside this range can suffer from reduced potency. This thermal sensitivity results in significant losses of vaccine material each year and risks the administration of vaccines with diminished protective ability, issues that are heightened in the developing world. Here, using peptide self-assemblies based on the fibril-forming peptide Q11 and containing the epitopes OVA323-339 from ovalbumin or ESAT651-70 from Mycobacterium tuberculosis, the chemical, conformational, and immunological stability of supramolecular peptide materials were investigated. It was expected that these materials would exhibit advantageous thermal stability owing to their adjuvant-free and fully synthetic construction. Neither chemical nor conformational changes were observed for either peptide when stored at 45°C for 7 days. ESAT651-70-Q11 was strongly immunogenic whether it was stored as a dry powder or as aqueous nanofibers, showing undiminished immunogenicity even when stored as long as six months at 45°C. This result was in contrast to ESAT651-70 conjugated to a protein carrier and adjuvanted with alum, which demonstrated marked thermal sensitivity in these conditions. Antibody titers and affinities were undiminished in mice for OVA323-339-Q11 if it was stored as assembled nanofibers, yet some diminishment was observed for material stored as a dry powder. The OVA study was done in a different mouse strain and with a different prime/boost regimen, and so it should not be compared directly with the study for the ESAT epitope. This work indicates that peptide self-assemblies can possess attractive thermal stability properties in the context of vaccine development.
Keywords: Vaccine, cold chain, self-assembly, tuberculosis, self-adjuvanting
Graphical abstract
Introduction
Almost all current vaccines must be maintained within a tight and refrigerated temperature range, usually between 2–8°C. Any excursions outside this range can diminish a vaccine’s efficacy or require that it be discarded. This challenge is presently addressed using the “cold chain” system of constant refrigeration from production to administration. This system, relying on refrigerated packaging, refrigerated shipping vehicles, clinics with reliable refrigerators, and well-trained staff, is highly effective when executed properly, but it is costly and prone to lapses in temperature control [1,2]. It is also at times difficult or impossible to implement, especially in the developing world or in locations without reliable electricity. Because of this, the introduction of new thermally stable vaccines that do not rely heavily on the cold chain has become an important goal [3,4].
Temperature excursions can damage vaccines in various different ways, depending on their construction and formulation. High temperatures can cause protein antigens to unfold in subunit vaccines, dissociate polysaccharides from protein carriers in conjugate vaccines, or reduce the viability and infectivity of live attenuated vaccines [3]. Different vaccines within each of these categories have shown variable sensitivities (reviewed in [3–5]), although live attenuated organisms tend to be especially problematic. For example, most measles vaccines based on live viruses can only withstand exposure to 37°C for about 7 days [4]; some can lose potency by a factor of 10 after only 8h at 37°C [6]. Liquid-formulated oral poliovirus vaccines based on live attenuated virus are only stable at 37°C for two days [3,5]. The live attenuated tuberculosis vaccine BCG loses greater than 20% effectiveness after one month at 37°C and is unstable above 45°C [3,5]. Vaccines not based on live organisms can fare better. For example, diphtheria and tetanus toxoids in liquid formulations can be stable for months at 37°C, and because they are simple polypeptides, they only lose their potency when heated above temperatures at which secondary structure is lost [5]. Liquid formulations of hepatitis B vaccines, which are composed of recombinant purified hepatitis B surface antigen adsorbed onto aluminum salts, are also relatively stable. However, at 45°C, even these relatively stable vaccines can lose potency over the course of a few weeks (for toxoids [5]) or days-to-weeks (for hepatitis B vaccines [7,8]). Further, toxoid vaccines and those based on recombinant protein antigens require adjuvants, and when they are formulated with alum adjuvant, they are sensitive to freezing and freeze-thaw cycles [3,5]. One study found more than a 60% reduction in potency of some components of alum-adjuvanted diphtheria-tetanus-pertussis (DTP) vaccines after only two freeze-thaw cycles [5]. For alum-adjuvanted vaccines, freeze-thaw cycles can induce permanent, irrecoverable changes in the association between alum particles and the antigen and lead to aggregation and precipitation, all of which diminishes efficacy [5,9]. In this way, the presence of adjuvant complicates a vaccine’s thermostability.
Deviations above the safe temperature window can happen for a number of reasons, including faulty or poorly maintained refrigeration equipment, lack of refrigeration capacity, power outages caused by any number of events such as weather or loss of fuel, or errors in handling, especially during arrival procedures [5]. Inadvertent freezing can occur as a result of improperly controlled refrigeration or shipping on ice packs. Assessments made by the World Health Organization have highlighted each of these issues for improvement [5], as the consequences of such deviations, both above and below 2–8°C can be significant. In the best cases, any temperature excursions are known and caught before the vaccine is administered, and the vaccine is discarded. This leads to significant wastage but does not put patients at risk. In worse cases, unknown temperature deviations result in diminished vaccines being administered to patients, putting them at risk for infection. Therefore, there is a significant need for vaccines that are stable to both freezing conditions and elevated temperatures, and thermostable vaccines are seeing active development [10,11].
Recent efforts include stabilizing vaccines within silk films [10], by mineralizing the surface of viral capsids [11], by lyophilization [12], by optimizing formulations and utilizing various stabilizing agents [3]. Here we report on the thermal stability characteristics of self-assembled peptide nanofiber vaccines, which are both self-adjuvanting and constructed from short, unfolded peptide epitopes. Short peptides containing fibrillizing domains such as Q11 (QQKFQFQFEQQ) [13–18] or KFE8 (FKFEFKFE) [17] can be appended with T or B cell epitopes; when they are mixed with physiologic buffers, media, or fluids, they self-assemble into nanofibers displaying the epitopes, and we have found that these nanofibers raise strong immune responses without supplemental adjuvants [13,15–17]. The modularity of the platform makes it simple to adjust the epitope content within the nanofibers, and we have observed phenotypic modulation of T cell subsets when the T and B cell epitope ratios are varied [15]. In this way, self-assembled peptide nanofibers are a promising platform for immunotherapy development owing to their adjuvant-free and tailorable properties.
In this study, we investigated the thermostability of self-assembled peptide nanofibers, hypothesizing that their purely synthetic design and lack of any adjuvant, proteins, or live organisms would make them advantageously thermostable compared to other more conventional vaccine platforms. We investigated two model epitopes, OVA323-339 and an epitope corresponding to residues 51–70 of M. tuberculosis 6kDa early secretory antigenic target (ESAT-6), synthesizing self-assembled nanofibers from both using the Q11 assembly domain. Both epitopes are able to raise B cell and T cell responses [13, 19]. Having both a B cell epitope and a T cell epitope is essential for Q11-based vaccines, as Q11 nanofibers lacking either do not raise strong antibody responses [15,17]. We investigated the thermostability of lyophilized peptides as well as aqueous suspensions of nanofibers, finding that some formulations were more thermostable than others, with the most thermostable (ESAT-Q11) being capable of withstanding elevated temperatures of 45°C for as long as six months without diminishment of effectiveness.
1. Materials and methods
1.1. Peptides, vaccine preparations, and heat treatments
The peptides Q11 (Ac-QQKFQFQFEQQ-Am), pOVA (also known as OVA323-339, H2NISQAVHAAHAEINEAGR-COOH), OVA-Q11 (H2N-ISQAVHAAHAEINEAGR-SGSGQQKFQFQFEQQ-Am), pESAT (corresponding to residues 51–70 of the 6kDa early secretory antigenic target of M. tuberculosis, H2N-YQGVQQKWDATATELNNALQ-Am), ESAT-Q11 (H2N-YQGVQQKWDATATELNNALQ-SGSG-QQKFQFQFEQQ-Am), and Cys-pESAT (H2N-CYQGVQQKWDATATELNNALQ-Am) were synthesized using standard Fmoc solid-phase chemistry, purified by HPLC, and lyophilized as previously reported [20,21]. Epitope peptides were appended to the Q11 assembly domain by a flexible Ser-Gly-Ser-Gly linker. Cys-pESAT was conjugated to keyhole limpet hemocyanin (KLH) using the Imject Maleimide Activated mcKLH Kit (cat #77666) from Thermo Scientific. Peptide identity was confirmed using a Bruker Ultraflextreme MALDI-TOF mass spectrometer, using α-cyano-4-hydroxycinnamic acid as the matrix.
To prepare immunizations, lyophilized OVA-Q11, ESAT-Q11 or pESAT peptides were dissolved in sterile water at 8 mM and stored overnight at 4°C. Then, sterile and endotoxin-free 1× PBS (HyClone SH30028.02) was added to bring the peptide to a working concentration of 2 mM, and the solution was incubated 3–5 h at room temperature before immunization. This step, mixing with PBS, induced assembly into nanofibers, as we have previously reported [15,17,18].
For adjuvanted peptide/carrier groups, 0.1mg Cys-pESAT conjugated to KLH was mixed 1:1 v/v with Imject Alum (Pierce) and vortexed for 30–60 min to allow adsorption. Endotoxin measurements were conducted on the same peptide solutions that were used for immunizations, using the Limulus Amebocyte Lysate chromogenic endpoint assay (Lonza). Endotoxin levels of all peptide solutions used for immunization were <0.1 EU/mL (<0.01 EU per 100 μL dose).
For heat treatments, peptides were either heated as lyophilized powders (before the initial water dissolution step), or after they had been formed into final nanofibers following the water and PBS dilution steps described above (2mM in PBS final peptide concentration). Peptides were heated in one or the other condition to between 45–80 °C for time periods ranging from 1 day to 6 months, as indicated in each experiment described. ESAT-KLH-Alum was heated in its final formulation.
1.2. Transmission electron microscopy
Q11, OVA-Q11, and ESAT-Q11 were dissolved in >18.1 MΩ resistivity water (Millipore MilliQ system) at 8 mM, incubated overnight at room temperature, and subsequently diluted to 2 mM in PBS. Four hours later, these samples were diluted to 0.2mM with PBS and then applied to a carbon-coated 400 mesh copper grid (FCF 400-Cu, EMS), negatively stained with 1% uranyl acetate, and analyzed with a FEI Tecnai G2 Spirit TEM.
1.3. Circular dichroism analysis
Peptide solutions were prepared as described above for immunization formulations. Control samples were stored at 4°C for the same period of time as heated samples, to account for time-dependent structural evolution [22]. The sodium chloride in the final PBS dilution step was replaced with potassium fluoride, to improve CD transparency (137 mM final KF concentration). Peptide solutions were analyzed with an AVIV 202 Circular Dichroism Spectrometer in a 0.1 cm path length quartz cell. Triplicate scans at 25°C were averaged.
1.4. Mice and immunizations
OVA-based materials were investigated in female wild-type C57BL/6 mice (Harlan Sprague Dawley), while ESAT6-based materials were investigated in female wild-type CBA/J mice (The Jackson Laboratory), owing to haplotype specificities of the two epitopes studied. Mice were housed in a centralized animal facility at the University of Chicago; all procedures were approved by the University of Chicago Institutional Animal Care and Use Committee and were in compliance with the NIH Guide for the Care and Use of Laboratory Animals. Anesthetized mice were immunized subcutaneously with the indicated solutions (2×50 μL) and boosted with a single half-dose (2×25 μL). This consisted of a primary immunization of 0.2 μmol OVA-Q11 or ESAT-Q11 and a boost of 0.1 μmol of the same peptide. Boosting occurred after 4 weeks for OVA-Q11 groups and after 10 weeks for ESAT-Q11 groups. The boost for the ESAT-Q11 group was delayed until 10 weeks to observe whether there was any decay in titers after the primary immunization. For adjuvanted alum/carrier group, the immunizations contained 0.042 μmol (100 μg) Cys-pESAT conjugated to KLH. Blood was drawn via the submandibular maxillary vein, and sera were stored at −80°C until analysis.
1.5. Antibody titer measurements by ELISA
ELISA plates (Costar cat#3690) were coated with 20 μg/mL of either OVA or ESAT-Q11 peptide in PBS overnight at 4°C and blocked with 100 μL of 1% BSA in PBST (0.5% Tween-20 in PBS) for 1 h. Serum was applied in dilutions between 1:100-1:109 for total Ig and 1:500 for Ig isotypes for 1 h at room temperature, followed by peroxidase-conjugated goat anti-mouse total Ig (H+L) (Jackson Immuno Research cat#115-035-003) (1:5000 in 1% BSA-PBST, 50 μL/well) or isotype-specific antibodies (Sigma cat#IOS2-1KT) (1:1000 in 1% BSA-PBST, 50 μL/well). Plates were developed using TMB substrate (eBioscience cat#00-4201-56) and absorbance values were read at 450 nm. Absorbance values of wells lacking antigen were subtracted to account for background. The plates were washed between each step with PBST. Additional details are described in our previous work [13,15,16].
1.6. Antibody affinity measurements
Serum samples collected after the booster immunizations were analyzed for antibody binding and desorption kinetics on a pOVA-coated or pESAT-coated surface, using a BioRad ProteOn XPR36 SPR optical biosensor in the University of Chicago Biophysics Core Facility. A ProteOn NLC sensor chip was used, in which the surface of the chip was functionalized with NeutrAvidin. To conjugate pOVA or pESAT to the surface, solutions of N-terminally biotinylated peptides (biotin-OVA or biotin-ESAT; 100 nM, 40 nM, and 10 nM in PBS with 0.05% Tween20) were passed through the vertical lanes of the chip (30 μL/min for 1 min; 100, 40, 10 RU bound respectively). When working with serum samples, PBS with 1% BSA and 0.05% Tween20 was used as the running buffer, to reduce non-specific adsorption. Each sample run consisted of flowing Running Buffer through the horizontal lanes of the chip (30 μL/min, 2 min), followed by serum solutions (diluted 10-fold in Running Buffer; 30 μL/min, 5 min) to allow antibody binding, and finally Running Buffer again (30 μL/min, 45 min) to observe desorption kinetics. The surface of the chip was regenerated twice between each sample run using 50 mM NaOH (100 μL/min, 0.5 min). Because purified antibodies were not available, only koff rates were calculated.
1.7. T cell responses by ELISPOT
Mice were sacrificed 7 days after the booster immunization, and the cells from the draining lymph nodes were collected. Single-cell suspensions of draining lymph node cells were prepared by pushing tissue through a 40-μm nylon mesh cell strainer. Complete media consisted of RPMI with L-glutamine (Mediatech cat#10-040-CV), supplemented with 10% FCS, 1x MEM non-essential amino acids (Cellgro cat#25-025-CI), 1 mM sodium pyruvate (Invitrogen cat#11360-070), and 0.055 mM 2-mercaptoethanol (Invitrogen cat#21985-023). 96-well ELISPOT plates (Millipore, cat#MSIPS4510) were pretreated with 15 μL of 35% ethanol according to the procedures in the product information. Plates were then washed and coated overnight with anti-IL-4 (BD cat#551878) or anti-IFN-γ (BD cat#551881) capture antibody (2 mg/mL). After blocking with complete medium for 1–2 hr, 1 × 106 cells were plated in each well (200 μL volume) and stimulated with 10 μg/mL pOVA or pESAT for 48 h before developing the plates. For visualization of spots, plates were incubated with biotinylated anti-IFN-γ (BD cat# 51-1818KA) or anti-IL-4 (BD cat# 51-1804KZ) detection antibodies, then with streptavidin-alkaline phosphatase (Mabtech cat#3310-10), and finally with the substrate Sigmafast BCIP/NBT (sigma cat#B5655). Plates were allowed to dry overnight before the spots were imaged and counted using an ELISPOT reader (Cellular Technology, Ltd).
1.8. Statistical analysis
Statistical comparisons between groups were made using 1- or 2-way ANOVA with Tukey post-hoc tests to determine p-values, as indicated in figure legends. Error bars represent standard deviations unless otherwise noted.
2. Results
2.1. Effect of heating on chemistry and self-assembly behavior
We first investigated the extent to which heating could alter the folding or composition of the non-epitope-bearing peptide, Q11. In this study, we focused on performance at 45 °C, because at this temperature even the more stable existing vaccines such as those based on toxoids and recombinant antigens begin to lose potency within experimentally convenient time scales. For example, DTP vaccines show diminished potency after less than one week at 45 °C [5]. Hepatitis B vaccines can show diminishment in potency after only 3 days at this temperature [5]. At 35 °C, diphtheria and tetanus toxoid vaccines show deterioration of 1% per day [23]. Most other current vaccines are considerably less stable at 45°C [5], including vaccines containing polysaccharides (e.g meningococcal vaccines or Haemophilus influenzae type b, Hib), which depolymerize within hours at ambient temperatures [5].
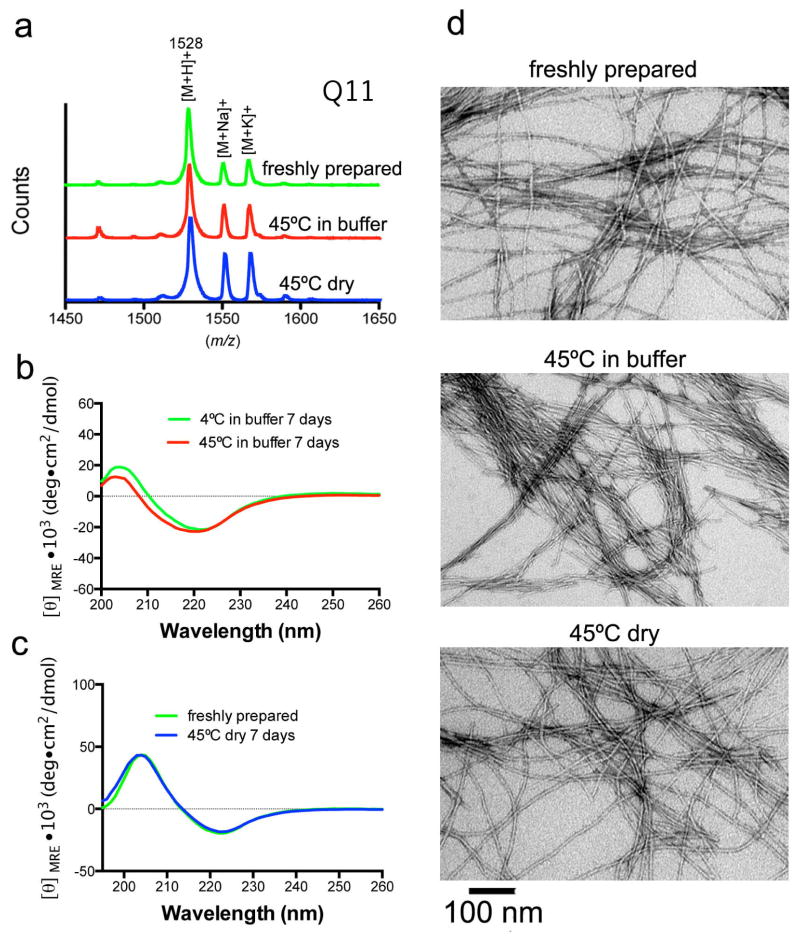
After one week of heating, Q11 was stable at 45°C, both as a dry powder and when formulated as nanofibers in buffer, as expected (Fig. 1). These temperatures are well below the thermal decomposition temperature of peptides, and MALDI mass spectrometry indicated no change in mass after heating in either condition, with peaks associated with the hydrogen, sodium, and potassium adducts apparent (Fig. 1a). Circular dichroism spectra for heated and freshly prepared peptide were nearly identical, both for dry peptides and for formed nanofibers in PBS (Fig. 1b–c), and were consistent with previously reported CD spectra for Q11 [25–27]. Nanofibers of heated Q11 appeared indistinguishable from freshly prepared nanofibers by TEM (Fig. 1d), whether the peptide was heated as a dry powder or as formed nanofibers in buffer. The CD and TEM data indicated that the elevated temperatures induced no observable changes in secondary structure or fibrillar assembly.
Fig. 1. Influence of heating on the structure and folding of Q11.
The structure and folding of Q11 were unaffected by heat treatment at 45°C for 7 days. By MALDI-MS, no significant alterations in m/z were observed after heating. Q11 [M+H]+ m/z calc’d 1528 (a). Expanded m/z ranges shown in Supplemental Fig. 1. By circular dichroism, Q11 exhibited a predominantly β-sheet structure both before and after heat treatment, whether the peptide was incubated in PBS (b) or as a dry powder (c). By TEM, nanofibers were similar, whether from freshly prepared Q11 or Q11 that had been heated in either condition (d, scale bar 100 nm for all images).
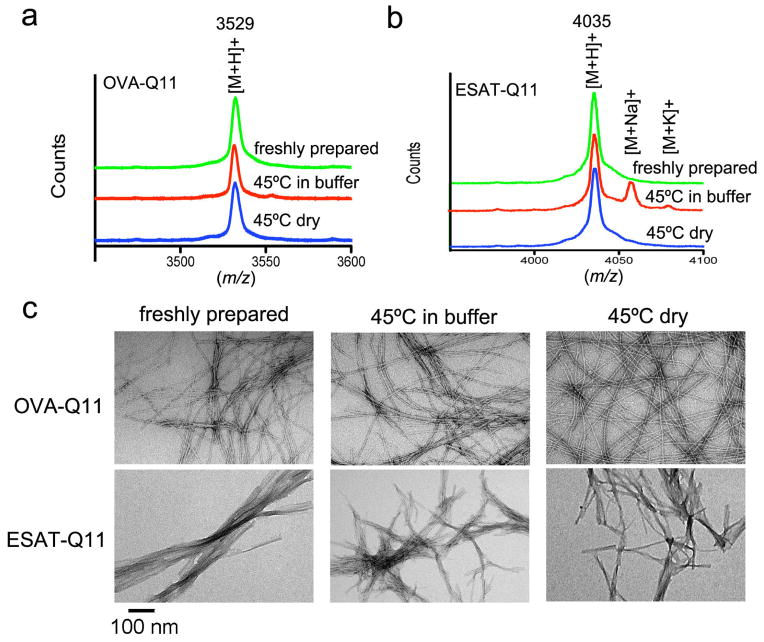
Next, epitope-bearing peptides were evaluated in the same manner, after being heated as either dry powders or in buffer. The two epitopes studied were the OT-II model epitope from chicken ovalbumin (OVA, residues 323-339) and an epitope from the M. tuberculosis 6kDa early secretory antigenic target (ESAT-6, residues 51–70). After seven days of heating at 45°C, OVA-Q11 (containing OVA323-339 appended to the N-terminus of Q11) and ESAT-Q11 (containing ESAT651-70 at the N-terminus) did not undergo observable changes in mass (Fig. 2a–b). Similarly, by TEM, the nanostructure of fibrils formed from fresh or heated peptides were indistinguishable, whether the peptides were heated as dry powders or in buffer (Fig. 2c).
Fig. 2. Influence of heating on the structure and folding of epitope-bearing Q11.
The structure and mass of epitope-bearing peptide fibers were not significantly altered by heating at 45°C for 7 days. MALDI mass spectra of OVA-Q11 (a) and ESAT-Q11 (b) were similar between unheated and heated samples (OVA-Q11 [M+H]+ m/z calc’d 3529; ESAT-Q11 [M+H+] m/z calc’d 4035). Expanded m/z ranges shown in Supplemental Fig. 1. Nanofibers were formed by OVA-Q11 and ESAT-Q11 peptides before and after heat treatment (c, TEM, scale bar 100 nm for all images).
2.2. Effect of heating on immunogenicity in mice
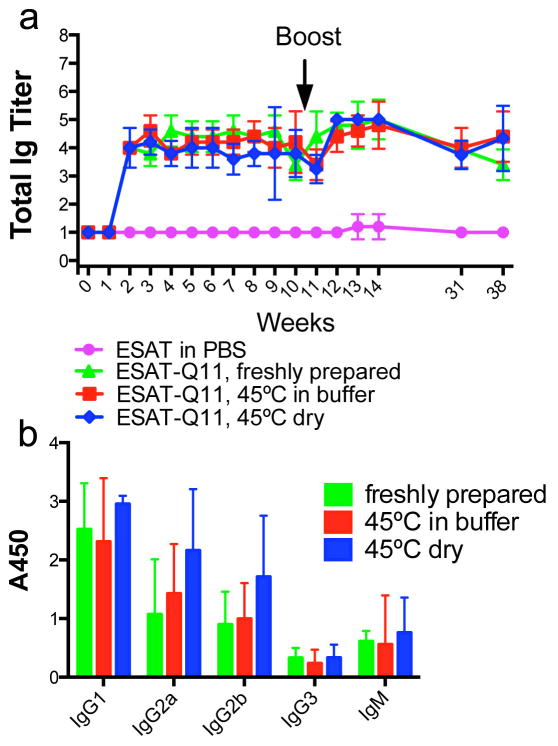
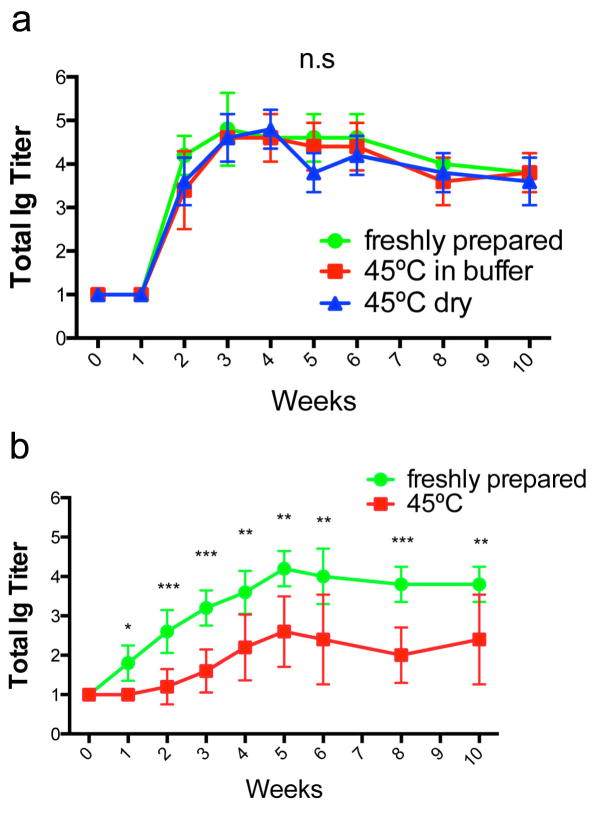
We next investigated the immunogenicity of unadjuvanted, self-assembled ESAT-Q11 in mice. CBA/J mice were immunized with 0.2 μmol of ESAT-Q11 and boosted with 0.1 μmol of the same, subjected to the same heating conditions as described above. Serum antibody responses to the ESAT651-70 epitope were measured by ELISA plates coated with ESAT-Q11. Plates coated with pESAT (epitope only, lacking the Q11 domain) showed similar titers to those coated with ESAT-Q11 (not shown). We found that the total Ig raised against ESAT-Q11 was undiminished when the peptide was heated at 45°C for 7 days, both in the dry and wet formulations, and that responses were durable, lasting at least for the duration of the study (38 weeks) (Fig. 3a). In a similar manner to other Q11-based epitope systems that have been studied [15,17,27], the epitope peptide pESAT was not immunogenic when delivered by itself in PBS, but fibrillized ESAT-Q11 raised antibodies to titers of about 4–5, even after heating. A mild booster response was observed for all groups. Also, Ig isotype profiles were similar between freshly prepared, dry heated, and wet heated preparations, with no significant skewing evident (Fig. 3b).
Fig. 3. Total Ig titers (a) and isotypes at week 12 (b) of antibodies raised against ESAT-Q11.
Antibody responses to ESAT-Q11 were unaffected by heating at 45°C for 7 days. Dry powder formulations and nanofibers prepared in buffer were both stable. Titers were measured by ELISA against ESAT-Q11 after s.c. immunizations (primary immunization at week 0; boosted after week 10). No significant differences measured by ANOVA between various ESAT-Q11 preparations at equivalent time points (p > 0.05, n=5).
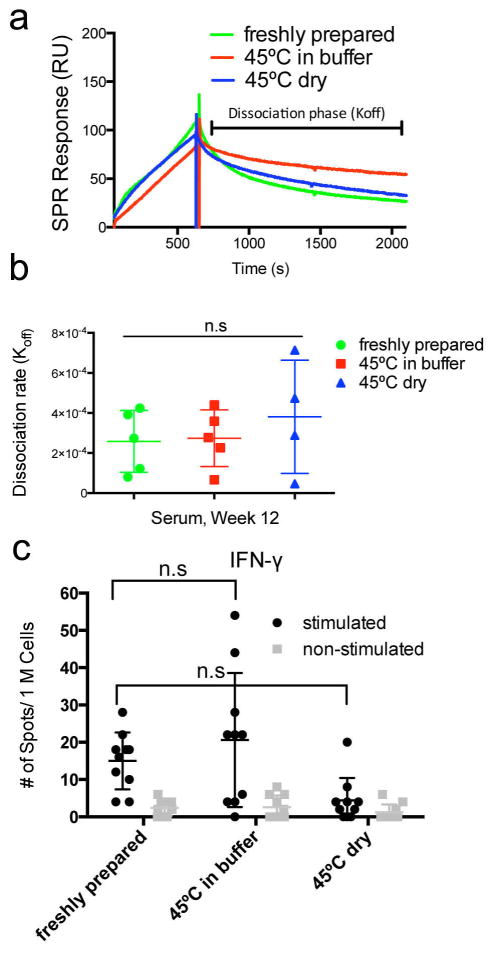
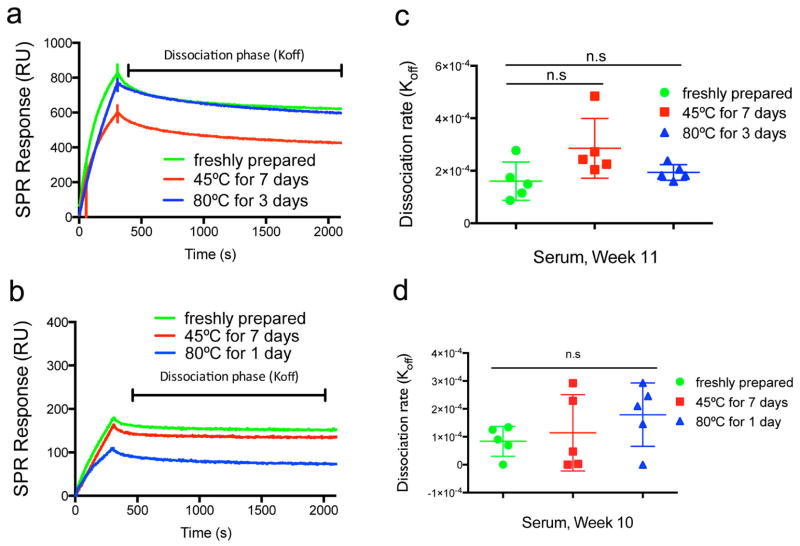
Using surface plasmon resonance (SPR), we evaluated the antibody responses raised by heated and unheated peptides. We found that the quantity of serum antibodies binding to pESAT-coated chips was similar in sera from mice immunized with peptides heated at 45°C for one week and unheated peptides (Fig. 4a). Further, the Koff values of raised antibodies, which are a measure of antibody affinity, were also not significantly altered when the immunizing peptides had been heated at 45°C for one week (Fig. 4b), although they did show considerable variability.
Fig. 4. SPR and ELISPOT analysis.
SPR measurement of Koff (a, b) and ELISPOT measurements of IFN-γ responses by pESAT-stimulated draining lymph node cells from immunized mice (c), for peptides unheated or heated at 45°C for 7 days. ESAT-Q11 raised antibodies with similar Koff values before and after heating, both as a dry powder and when formulated as nanofibers in buffer (a, representative SPR traces; b, Koff calculated). n=5 (one died from anesthesia in the 45°C dry group), n.s. = p > 0.05 by ANOVA with Tukey post-hoc tests.
T cell responses, as measured by IFN-γ secreted by draining lymph node cells stimulated with pESAT in culture, were modest but measurable for ESAT-Q11-immunized mice, consistent with previous observations for other Q11-based peptides [15]. Lower levels of IFN-γ secretion were observed for mice immunized with ESAT-Q11 heated to 45°C for one week in the dry condition. This difference was not statistically significant (Fig. 4c), but the distribution for the dry heated group has only one strongly responding mouse. Therefore, even though some diminishment in IFN-γ production seems to be present, this did not affect the antibody titers, which remained high for this heating regimen. Secretion of IL-4 in pESAT-stimulated lymph node cell cultures was not detected.
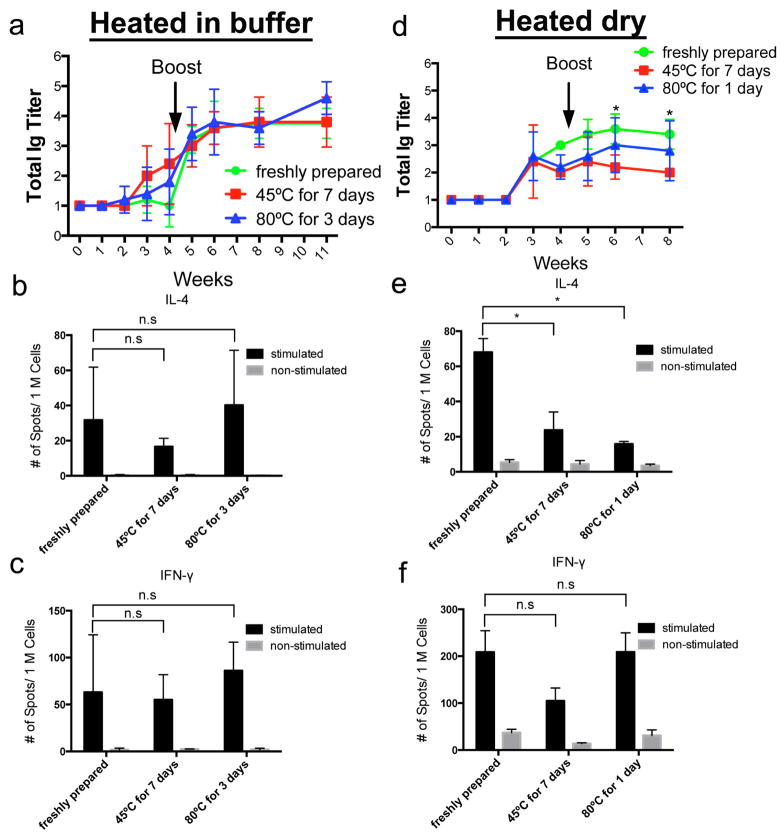
Having established that heating at 45°C for 7 days did not have any deleterious effect on the titers or affinities of the antibodies raised by ESAT-Q11, we next investigated an extreme case, heating at 45°C for six months. This constitutes a thermal insult well beyond what would be expected in the field. Surprisingly, the immunogenicity of ESAT-Q11 was unaffected by heated storage for this prolonged period, whether stored as a dry powder or as nanofibers prepared in buffer (Fig. 5a). In marked contrast, a conventional peptide-carrier conjugate using the same epitope (pESAT conjugated to keyhole limpet hemocyanin and adjuvanted with alum, ESAT-KLH) was significantly diminished by heating at 45°C for six months (Fig. 5b). Freshly prepared and alum-adjuvanted ESAT-KLH raised similar titers to unadjuvanted ESAT-Q11, but the establishment of maximal titers was delayed compared to ESAT-Q11. After heating at 45°C for six months, ESAT-KLH’s immunogenicity was considerably compromised. Our conclusion is that unlike conventional alum-adjuvanted peptide-carrier systems, ESAT-Q11’s ability to raise antibody responses is highly stable to prolonged storage at elevated temperatures.
Fig. 5. Comparison of the Q11 system with a carrier protein.
Antibody responses to ESAT-Q11 (a) and alum-adjuvanted ESAT-KLH (b, pESAT conjugated to keyhole limpet hemocyanin) after heating at 45°C for six months. Immunization doses were 0.2 μmol for ESAT-Q11 and 0.042 μmol for ESAT-KLH (n=5, n.s. p > 0.05; * p < 0.05; ** p < 0.01; *** p < 0.001 by ANOVA with Tukey post-hoc tests).
Interestingly, the ESAT-651-70 epitope has been reported as a T cell epitope [28, 29], but when appended to Q11 and fibrillized it exerts both T cell and B cell epitope functionality. Investigation with other mouse strains, non-rodent species, and with different haplotypes will be important for evaluating the haplotype specificity of this epitope. Previous work with peptide nanofibers found that the incorporation of both T and B cell epitopes has been required to raise any antibody responses. It has not mattered whether the N-terminal functional peptides have mixed T cell/B cell epitope function in the same linear sequence, such as the case with OVA323-339 [13,16,17], or if two separate epitope-Q11 peptides are co-mixed into integrated fibers, with one peptide bearing a B cell epitope and the other a T cell epitope [15]. In both cases as long as there have been both T cell and B cell epitopes present in the nanofiber, then antibody responses have been observed. Thus, we conclude that in the Q11 system, ESAT-651-70 functions both as a T cell epitope and as a B cell epitope.
2.3. Effect of heating on the immunogenicity of OVA-Q11 in mice
We next evaluated OVA-Q11, a model peptide that has been investigated previously by our group [13,16,17]. For OVA-Q11, some storage conditions led to measurable declines in immune responses in mice. In testing OVA-Q11, we applied a thermal treatment of 45°C for 7 days, as described for ESAT-Q11. For peptides heated as nanofibers formed in buffer, neither of these conditions diminished antibody responses in mice (Fig. 6a). Total Ig responses were nearly identical between heated and unheated groups. Conversely, heating in the dry condition led to diminished immunogenicity for OVA-Q11, reflected by lower total Ig titers against pOVA (Fig. 6d). At the same time, T cell responses were somewhat variable. For instance, repeated experiments for unheated OVA-Q11 returned average ELISPOT measurements with about 2-fold differences for IL-4 (Fig. b, e) and 4-fold differences for IFN-γ (Fig. 6c, f). Previous investigations by our group have also found comparatively variable T cell responses to OVA-Q11, even when antibody titers remain considerably more consistent [15, 16]. This variability in T cell response yet stability in antibody response appears to be a property of β-sheet fibrillar materials. In the present study, statistically significant differences in IL-4 secretion were only observed for dry-heated OVA-Q11, while IFN-γ secretion and IL-4 secretion for OVA-Q11 heated in buffer were not statistically different, yet the natural variability in T cell responses to β-sheet nanofibers may mask small differences. Also, although total Ig titers were diminished upon heating for OVA-Q11, in SPR measurements the average Koff values of heated and unheated samples remained similar (Fig. 7). More mouse-to-mouse variability was observed for antibodies raised in mice immunized with OVA-Q11 that had been heated in the dry condition, however (Fig. 7d). In all, OVA-Q11 was reliably immunogenic when heated as nanofibers in buffer, but less predictable when heated as a dry powder. Heated in the dry condition for 3 days at 80°C, OVA-Q11’s antibody responses were not reproducible in repeat experiments, being diminished in one experiment, then nearly unaltered in a repeat experiment (not shown). The source of this variability for the dry condition is not known.
Fig. 6. Thermostability of OVA-Q11.
Antibody (a, d) and T cell responses (b, c, e, f) to OVA-Q11 after being heated as nanofibers in buffer (a, b, c) or as a dry powder (d, e, f). Titers measured by ELISA against pOVA; T cell responses measured by ELISPOT of draining lymph node cells from immunized mice stimulated with pOVA. b, c, e, f indicate means ± s.e.m. n=5 *p < 0.05 by ANOVA with Tukey post-hoc tests.
Fig. 7. SPR analysis of responses against OVA-Q11.
OVA-Q11 raised antibodies with similar Koff values before and after heating, both when formulated as nanofibers in buffer (a, c) and as a dry powder (b, d) (a, b representative SPR traces; c, d Koff calculated). Koff values were not significantly different between the groups (n=5, p > 0.05, ANOVA with Tukey post-hoc tests).
3. Discussion
This is the first report of the thermostability characteristics of self-adjuvanting vaccines constructed from self-assembling peptides. Fibrillizing peptides such as those based on Q11 can render an epitope immunogenic, particularly if it contains both T cell and B cell epitope functionality [13, 15–17]. This activity is in part based on the particulate nature of the materials [17] and is likely also based on the significant multivalency of the nanofibers. These materials operate via T cell-dependent mechanisms [15, 17], but their full mechanism of antigen presenting cell activation has not yet been elucidated.
The work in the present paper was conceived as a step towards addressing the significant challenge of distributing vaccines to developing countries and regions that may not have an intact, reliable cold chain. Our approach has been to design vaccination materials based on the Q11 self-assembling domain, which can form nanofibers capable of raising strong immune responses directly, without the use of supplemental adjuvants. Given that adjuvant precipitation (in the case of alum-adjuvanted subunit vaccines) microbial viability (in the case of live attenuated vaccines), or antigen denaturation are primary reasons why continuous cold chains are required for existing vaccines, we sought to exploit the synthetic nature and relative thermal stability of short peptides.
We found that the Mycobacterium tuberculosis ESAT-651-70 epitope, when appended to the self-assembling Q11 domain and fibrillized into β-sheet rich nanofibers, is remarkably thermostable and capable of inducing immune responses without adjuvants after prolonged heating. Heating at 45°C for six full months did not diminish its immunogenicity. This was in marked contrast to a conventional alum-adjuvanted carrier protein system (KLH), and also compared favorably with the reported thermostability of the currently used tuberculosis vaccine, BCG. BCG preparations are filled as bacterial suspensions in vials and dried through lyophilization to increase their thermal stability, but this practice is known to decrease viability, and the lyophilized product is still thermally sensitive, losing greater than 20% of potency after one month above 20°C and being unstable for any time above 45°C [5, 30]. It remains to be determined to what degree responses raised by only the ESAT-6 epitope can confer protection against tuberculosis infection, but in principle more epitopes could be formulated into a polyvalent vaccine should protection with one epitope prove to be insufficient.
Another interesting finding was that different peptide epitopes appeared to demonstrate different thermal stabilities when appended to Q11 and assembled into nanofibers. Although ESAT-Q11 was highly thermostable in all conditions tested, OVA-Q11 showed some thermal lability as a dry powder. The source of this thermal lability is not fully known, but it could relate to the OVA epitope being somehow able to alter the organization of insoluble oligomers in the dry state, perhaps by changing the structure of any seeds that go on to propagate and form the mature fibrils. It is possible that the differences in methods used to study the ESAT epitope and the OVA epitope could have led to different sensitivities in measuring the effects of thermal treatment. Different mouse strains were used for each study owing to the haplotype specificities of each epitope, and different timings of the boost were used between the two studies. Because of this, each study should be considered as a stand-alone experiment. Making quantitative measurements of the precise differences in thermal stability between the two epitopes would require additional experiments in a mouse strain that is reactive to both epitopes and would use identical priming and boosting regimens. We observed some turbidity for both OVA-Q11 and ESAT-Q11 in heated samples, but it did not influence immunogenicity for ESAT-Q11. Another possibility is that the secondary structure of the OVA epitope may be more important for its immunogenicity or more sensitive to thermal denaturation than the ESAT epitope. A more detailed analysis of the physical basis for variable thermal stability among epitopes would be important for the continued development of this system as a viable vaccine platform.
4. Conclusions
The Q11 platform was found to be considerably thermostable, although some epitope-specific variability was observed. In the most extreme case, ESAT51-70-Q11 was found to have undiminished immunogenicity in mice after storage for as long as six months at 45°C. This contrasted with carrier protein-based preparations of the same ESAT51-70 epitope, which were significantly more thermally labile. Owing to the adjuvant-free nature of this system and its peptide construction, its thermal stability may additionally offer novel routes for addressing the limitations of the current cold chain system of global vaccine distribution.
Supplementary Material
Acknowledgments
The work described in this article was supported by the Bill and Melinda Gates Foundation (OPP1061315) and the National Institutes of Health (NIBIB, 1R01EB009801; NIAID 1R01AI118182).
Footnotes
Its contents are solely the responsibility of the authors and do not necessarily represent the official views of these agencies.
None of the authors have financial conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen X, Fernando GJP, Crichton ML, Flaim C, Yukiko SR, Fairmaid EJ, Corbett HJ, Primiero CA, Ansaldo AB, Frazer IH, Brown LE, Kendall MA. Improving the reach of vaccines to low-resource regions, with a needle-free vaccine delivery device and long-term thermostabilization. J Controlled Release. 2011;152:349–355. doi: 10.1016/j.jconrel.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 2.Das P. Revolutionary vaccine technology breaks the cold chain. Lancet Infect Dis. 2004;4:719. doi: 10.1016/s1473-3099(04)01222-8. [DOI] [PubMed] [Google Scholar]
- 3.Chen D, Kristensen D. Opportunities and challenges of developing thermostable vaccines. Expert Rev Vaccines. 2009;8:547–557. doi: 10.1586/erv.09.20. [DOI] [PubMed] [Google Scholar]
- 4.Kristensen D, Chen D, Cummings R. Vaccine stabilization: Research, commercialization, and potential impact. Vaccine. 2011;29:7122–7124. doi: 10.1016/j.vaccine.2011.05.070. [DOI] [PubMed] [Google Scholar]
- 5.Temperature sensitivity of vaccines. WHO publications; 2006. http://whqlibdoc.who.int/hq/2006/WHO_IVB_06.10_eng.pdf. [Google Scholar]
- 6.Schlehuber LD, McFadyen IJ, Shu Y, Carignan J, Duprex WP, Forsyth WR, Ho JH, Kitsos CM, Lee GY, Levinson DA, Lucier SC, Moore CB, Nguyen NT, Ramos J, Weinstock BA, Zhang J, Monagle JA, Gardner CR, Alvarez JC. Towards ambient temperature-stable vaccines: The identification of thermally stabilizing liquid formulations for measles virus using an innovative high-throughput infectivity assay. Vaccine. 2011;29:5031–5039. doi: 10.1016/j.vaccine.2011.04.079. [DOI] [PubMed] [Google Scholar]
- 7.Van Damme P, Cramm M, Safary A, Vandepapeliere P, Meheus A. Heat stability of a recombinant DNA hepatitis B vaccine. Vaccine. 1992;10:366–367. doi: 10.1016/0264-410x(92)90064-q. [DOI] [PubMed] [Google Scholar]
- 8.VanDamme Pierre, Ward John, Shouval Daniel, Wiersma Steven, Zanetti Alessandro. Chapter 16, Hepatitis B Vaccine. In: Plotkin Stanley, Orenstein Walter, Offit Paul., editors. Vaccines. 6. Elsevier; Philadelphia: 2012. pp. 205–234. [Google Scholar]
- 9.Braun LaToya Jones, Tyagi Anil, Perkins Shalimar, Carpenter John, Sylvester David, Guy Mark, Kristensen Debra, Chen Dexiang. Development of a freeze-stable formulation for vaccines containing aluminum salt adjuvants. Vaccine. 2009;27:72–79. doi: 10.1016/j.vaccine.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Zhang J, Pritchard E, Hu X, Valentin T, Panilaitis B, Omenetto FG, Kaplan DL. Stabilization of vaccines and antibiotics in silk and eliminating the cold chain. Proc Natl Acad Sci USA. 2012;109:11981–11986. doi: 10.1073/pnas.1206210109. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Wang G, Cao RY, Chen R, Mo L, Han JF, Wang X, Xu X, Jiang T, Deng YQ, Lyu K, Zhu SY, Qin ED, Tang R, Qin CF. Rational design of thermostable vaccines by engineered peptide-induced virus self-biomineralization under physiological conditions. Proc Natl Acad Sci USA. 2013;110:7619–7624. doi: 10.1073/pnas.1300233110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolff L, Flemming J, Schmitz R, Gröger K, Müller-Goymann C. Protection of aluminum hydroxide during lyophilisation as an adjuvant for freeze-dried vaccines. Colloids and Surf A. 2008;330:116–126. [Google Scholar]
- 13.Rudra JS, Tian YF, Jung JP, Collier JH. A self-assembling peptide acting as an immune adjuvant. Proc Natl Acad Sci USA. 2010;107:622–627. doi: 10.1073/pnas.0912124107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chesson CB, Huelsmann EJ, Lacek AT, Kohlhapp FJ, Webb MF, Nabatiyan A, Zloza A, Rudra JS. Antigenic peptide nanofibers elicit adjuvant-free CD8+T cell responses. Vaccine. 2014;32:1174–1180. doi: 10.1016/j.vaccine.2013.11.047. [DOI] [PubMed] [Google Scholar]
- 15.Pompano RR, Chen J, Verbus EA, Han H, Fridman A, McNeely T, Collier JH, Chong AS. Titrating T-Cell Epitopes within Self-Assembled Vaccines Optimizes CD4+ Helper T Cell and Antibody Outputs. Adv Healthc Mater. 2014;3:1898–1908. doi: 10.1002/adhm.201400137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Pompano RR, Santiago FW, Maillat L, Sciammas R, Sun T, Han H, Topham DJ, Chong AS, Collier JH. The use of self-adjuvanting nanofiber vaccines to elicit high-affinity B cell responses to peptide antigens without inflammation. Biomaterials. 2013;34:8776–8785. doi: 10.1016/j.biomaterials.2013.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudra JS, Sun T, Bird KC, Daniels MD, Gasiorowski JZ, Chong AS, Collier JH. Modulating Adaptive Immune Responses to Peptide Self-Assemblies. ACS Nano. 2012;6:1557–64. doi: 10.1021/nn204530r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudalla GA, Sun T, Gasiorowski JZ, Han H, Tian YF, Chong AS, Collier JH. Gradated assembly of multiple proteins into supramolecular nanomaterials. Nat Mater. 2014;13:829–836. doi: 10.1038/nmat3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harboe M, Malin AS, Dockrell HS, Wiker HG, Ulvund G, Holm A, Jørgensen MC, Andersen P. B-cell epitopes and quantification of the ESAT-6 protein of Mycobacterium tuberculosis. Infect Immun. 1998;66:717–723. doi: 10.1128/iai.66.2.717-723.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jung JP, Nagaraj AK, Fox EK, Rudra JS, Devgun JM, Collier JH. Co-assembling peptides as defined matrices for endothelial cells. Biomaterials. 2009;30:2400–2410. doi: 10.1016/j.biomaterials.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jung JP, Jones JL, Cronier SA, Collier JH. Modulating the mechanical properties of self-assembled peptide hydrogels via native chemical ligation. Biomaterials. 2008;29:2143–2151. doi: 10.1016/j.biomaterials.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manning MC, Illangasekare M, Woody RW. Circular-Dichroism Studies of Distorted Alpha-Helices, Twisted Beta-Sheets, and Beta-Turns. Biophys Chem. 1988;31:77–86. doi: 10.1016/0301-4622(88)80011-5. [DOI] [PubMed] [Google Scholar]
- 23.Kumar V, Sahai G, Gupta P, Kumar A. Studies on the stability of tetanus and pertussis components of DPT vaccine on exposure to different temperatures. Indian J Pathol Microbiol. 1982;25:50–54. [PubMed] [Google Scholar]
- 24.Jung JP, Gasiorowski JZ, Collier JH. Fibrillar peptide gels in biotechnology and biomedicine. Biopolymers. 2010;94:49–59. doi: 10.1002/bip.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gasiorowski JZ, Collier JH. Directed Intermixing in Multicomponent Self-Assembling Biomaterials. Biomacromolecules. 2011;12:3549–3558. doi: 10.1021/bm200763y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hudalla GA, Modica JA, Tian YF, Rudra JS, Chong AS, Sun T, Mrksich M, Collier JH. A self-adjuvanting supramolecular vaccine carrying a folded protein antigen. Adv Healthc Mater. 2014;2:1114–1119. doi: 10.1002/adhm.201200435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rudra JS, Mishra S, Chong AS, Mitchell RA, Nardin EH, Nussenzweig V, Collier JH. Self-assembled peptide nanofibers raising durable antibody responses against a malaria epitope. Biomaterials. 2012;33:6476–6484. doi: 10.1016/j.biomaterials.2012.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vordermeier HM, Brown J, Cockle PJ, Franken WPJ, Arend SM, Ottenhoff THM, Jahans K, Hewinson RG. Assessment of Cross-Reactivity between Mycobacterium bovis and M. kansasii ESAT-6 and CFP-10 at the T-Cell Epitope Level. Clin Vaccine Immunol. 2007;14:1203–1209. doi: 10.1128/CVI.00116-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen AW, Hansen PR, Holm A, Andersen P. Efficient protection against Mycobacterium tuberculosis by vaccination with a single subdominant epitope from the ESAT-6 antigen. Eur J Immunol. 2000;30:1724–1732. doi: 10.1002/1521-4141(200006)30:6<1724::AID-IMMU1724>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 30.Kim TH, Kubica GP. Long-Term Preservation and Storage of Mycobacteria. Appl Microbiol. 1972;24:311–317. doi: 10.1128/am.24.3.311-317.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.