Abstract
Purpose
Reovirus is a naturally occurring oncolytic virus in clinical trials. Although tumor infection by reovirus can generate adaptive antitumor immunity, its therapeutic importance versus direct viral oncolysis is undefined. This study addresses the requirement for viral oncolysis and replication, and the relative importance of antitumor immunity and direct oncolysis in therapy.
Experimental Design
Nonantigen specific T cells loaded with reovirus were delivered i.v. to C57BL/6 and severe combined immunodeficient mice bearing lymph node and splenic metastases from the murine melanoma, B16ova, with assessment of viral replication, metastatic clearance by tumor colony outgrowth, and immune priming. Human cytotoxic lymphocyte priming assays were done with dendritic cells loaded with Mel888 cells before the addition of reovirus.
Results
B16ova was resistant to direct oncolysis in vitro, and failed to support reovirus replication in vitro or in vivo. Nevertheless, reovirus purged lymph node and splenic metastases in C57BL/6 mice and generated antitumor immunity. In contrast, reovirus failed to reduce tumor burden in severe combined immunodeficient mice bearing either B16ova or reovirus-sensitive B16tk metastases. In the human system, reovirus acted solely as an adjuvant when added to dendritic cells already loaded with Mel888, supporting priming of specific antitumor cytotoxic lymphocyte, in the absence of significant direct tumor oncolysis; UV-treated nonreplicating reovirus was similarly immunogenic.
Conclusion
The immune response is critical in mediating the efficacy of reovirus, and does not depend upon direct viral oncolysis or replication. The findings are of direct relevance to fulfilling the potential of this novel anticancer agent.
Oncolytic viruses are self-replicating and tumor selective with an ability to directly induce cancer cell death in vitro (1). A variety of oncolytic viruses have been investigated in phase I to III clinical trials (2). In contrast to immortalized cell lines, primary human tumor samples have been found to be relatively resistant to direct viral oncolysis (3–6). Therefore, in a clinical context, the direct oncolytic activity of these agents is likely to be more limited than suggested by experimental models. Much of the preclinical work has involved immunocompromized xenograft models, focusing upon the direct cytotoxic effect of the viral agent (7, 8). However, recent findings have suggested that virotherapy may also stimulate immune-mediated tumor responses (9). The relative importance of direct oncolysis versus immune-mediated tumor regression remains uncertain.
The generation of an effective immune response depends upon a context of “danger” within the tumor, and infectious agents represent immunologic danger signals par excellence (10). Therefore, oncolytic viruses are prime candidates to alter the immune milieu of the tumor microenvironment, via tumor cell death associated with release of tumor-associated antigens (TAA), tumor-derived cytokines, viral nucleic acid and coat proteins (which can trigger pathogen recognition receptors), and direct viral effects upon infiltrating immune cells (9). An influx of immune cells is characteristic following virotherapy (11, 12). Vesicular stomatitis virus (VSV; refs. 11, 13), reovirus (14), several herpes simplex virus strains (15–17), and an attenuated vaccinia virus (18) have all been shown to facilitate the generation of antitumor immunity.
Reovirus is a naturally occurring, nonpathogenic double-stranded RNA virus, with selective toxicity toward cells with an activated ras pathway. Activating mutations of the ras pathway are present in many human tumors (19). Ras pathway activation is thought to prevent RNA-activated protein kinase from aborting viral infection leading to tumor cell lysis (20, 21), in addition to effects upon viral uncoating, infectivity, and progeny release (22). Reovirus is currently under investigation in a range of phase I and II clinical trials (21).
We have previously shown the antitumor immunogenic potential of reovirus, in terms of its ability to activate DC (23), and to prime an adaptive antitumor immune response (14). In a murine B16tk model of lymph node (LN) metastases, a single dose of i.v. reovirus partially purged metastatic LN, in association with generation of a splenocyte immune response toward TAA. Consistent with this, we found that reovirus infection of a human melanoma cell line, Mel888, could generate an adaptive anti-Mel888 immune response in vitro (14). Critically, whether direct oncolysis was involved in tumor purging or the generation of antitumor immunity remains an open question. In contrast to the reovirus-sensitive B16tk cells, here, we show that B16ova cells do not support reovirus replication and are highly resistant to the oncolytic effects of reovirus in vitro. Using a B16ova model of LN metastasis, we addressed the relative role of direct viral oncolysis versus immune-mediated tumor clearance. Additionally, to determine whether oncolysis and viral replication are prerequisite for the generation of antitumor immunity in human systems, we adapted our previously described in vitro human priming assay (14), to preclude significant levels of direct viral oncolysis.
Materials and Methods
Reovirus
Reovirus Type 3 Dearing strain was provided by Oncolytics Biotech, Inc., and stored in the dark at neat concentrations in PBS at 4°C (maximum 3 mo) or at −80°C (long-term storage). Virus titer was determined by a standard plaque assay using L929 cells. When indicated, 480 mJ UV irradiation (Stratalinker UV 1800 Crosslinker; Stratagene) was used to ablate the replication competence of the virus, treating 100 μL volumes of viral stock in 96-well plates.
Cell culture
Murine B16ova cells (H2-Kb) were derived from B16 cells by transduction with a cDNA encoding the chicken ovalbumin (Ova) gene (24). B16tk melanoma cells were derived from B16 cells by transducing them with a cDNA encoding the herpes simplex virus thymidine kinase (tk) gene (25). Human cell lines comprised Mel888 melanoma, and the ovarian line SKOV-3 Cells were grown in DMEM (Life Technologies) supplemented with 10% (v/v) FCS (Life Technologies), 1% (v/v) l-glutamine (Life Technologies), and transgene selection antibiotic where appropriate (G418 at 5 mg/mL for B16ova cells, and puromycin at 1.25 μg/mL for B16tk cells). Cell lines were routinely tested for Mycoplasma and found to be free of infection.
Junctional adhesion molecule-1 expression
Junctional adhesion molecule-1 (JAM-1) expression determined by flow cytometry of cells labeled with Alexofluor488 rat anti-mouse CD321 (Serotec) or Alexofluor488 rat IgG1κ isotype control (BD Biosciences).
Preparation of C57BL/6 lymphoctyes
Cells were isolated from crushed spleens and LN from C57BL/6 mice, and CD8+ lymphocytes isolated where indicated, as previously described (13).
Reovirus loading of lymphocytes
Lymphocytes were pelleted, and incubated with reovirus at doses as indicated, in 100 μL PBS for 4 h at 4°C, washed thrice in PBS, and either plated for in vitro assays or used directly for in vivo transfer.
2-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
B16ova and B16tk were plated at a density of 2 × 103 cells per well in a 96-well plate. After 24 h, wells were infected with known dilutions of reovirus. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide reagent (5 mg/mL) was added at time points indicated for 5 h and the assay developed by solubilizing in dimethylsulfoxide 100% and read at 550 nm on a SPECTRAmax 384 plate reader (Molecular Devices).
Viral replication
Reovirus-infected cells and supernatants were harvested at appropriate time points and subjected to three cycles of freeze-thaw lysis. Viral titer was determined using standard plaque assays on L929 cells.
In vitro delivery of reovirus loaded lymphocytes to B16ova
Target B16ova cells were seeded at 105 cells per well in six-well plates and allowed to adhere overnight. Reovirus-loaded lymphocytes were added at a 1:1 cell ratio. Cells were harvested 48 h later, labeled with anti-CD3 (BD Pharmingen) to gate out lymphocytes, and B16ova viability was analyzed following propidium iodide (Sigma) staining.
In vivo studies
All procedures were approved by the Mayo Foundation Institutional Animal Care and Use Committee. C57BL/6 and severe combined immunodeficiency (SCID) mice were purchased from The Jackson Laboratory at age 6- to 8 wk. To establish s.c. tumors, 5 × 105 B16ova or B16tk cells were injected in 100 μL of PBS into the flanks of mice (subgroups of three mice in each experiment). Ten days later, mice were treated i.v. with PBS, 2 × 106 CD8+ lymphocytes, or CD8+ lymphocytes preloaded with reovirus 0.1 plaque-forming unit (pfu) per cell. For assessment of viral replication, tumor-draining LN were harvested at 2 and 4 d for freeze thaw lysis and plaque assay. In tumor purging experiments, tumor-draining LN and spleen were explanted 10 d posttherapy.
Colony outgrowth assay to detect metastatic B16ova and B16tk tumor cells
B16ova tumor cells stably express the neomycin-resistance gene, which allows for growth in G418 containing media at 5 mg/mL (13). B16tk tumor cells stably express the puromycin-resistance gene allowing for outgrowth in puromcyin containing media, at 1.25 μg/mL (14). Viable B16ova and B16tk tumor cells were selected for in G418 or puromycin-containing media, respectively, and colonies photographed after 7 d as previously described (13, 14).
ELISA analysis for IFN-γ secretion
Day 10 splenocytes were incubated with 5 μg/mL of appropriate peptide (synthetic H-2Kb–restricted peptides TRP-2180-188 SVYDFFVWL or ovalbumin derived SIINFEKL) or cell lysate (equivalent to 106 cells), and 48 h supernatants assayed for IFN-γ as previously described (13, 14).
Human dendritic cell generation
Peripheral blood mononuclear cells (PBMC) were obtained from buffy coats of healthy blood donors, and monocytes isolated by plastic adherence as previously described (14, 26). Immature DC were generated by culture in DC media [RPMI 1640 (Life Technologies) supplemented with 10% (v/v) fetal calf serum and 1% l-glutamine and 800U/mL granulocyte macrophage colony-stimulating factor and 500U/mL I l-4 (R&D Systems)] for 5 d.
Generation of human tumor–specific cytotoxic lymphocytes
Mel888 cells were seeded into tissue culture flasks, and allowed to adhere. Approximately 48 h postseeding, media was gently aspirated from the Mel888 cells, and immature DC were added to the Mel888 cell monolayer at a ratio of ~ 1:3 in a 50:50 mix of DC media/DMEM. After 24 h, supernatants were gently aspirated, leaving the tumor cell monolayer intact. After pelleting from supernatants, tumor-loaded DC were resuspended in CTL media [RPMI supplemented with 7.5% (v/v) human AB serum (Sigma), 1% (v/v) l-glutamine, 1% (v/v) sodium pyruvate (Life Technologies), 1% (v/v) nonessential amino acids (Life Technologies), 1% (v/v) HEPES (Life Technologies), 20 μmol/L 2β-mercaptoethanol (Sigma)]. Reovirus was then added to these cultures at 1pfu/DC, and autologous PBMC mixed at a 1:10 to 1:30 ratio. Cultures were supplemented with interleukin (IL)-7 (R&D Systems) 5 ng/mL from day 1, and IL-2 (R&D Systems) 30U/mL on day 4 only. Cultures were restimulated using the same protocol at day 7. Cells were harvested at day 14.
51Chromium cytotoxicity assay
Cytotoxicity was measured using a standard 4 h 51Chromium release assay, as previously described (14, 27).
CD107 degranulation assay
Cytotoxic lymphocyte (CTL) and tumor targets were incubated at a 1:1 ratio in the presence of anti-CD107a and b-FITC (BD Pharmingen). Brefeldin A (10 μg/mL) was added after 1 h. After a further 4 h, CTL were stained with anti–CD8-PerCP (BD Pharmingen), and analyzed by flow cytometry (FACSCalibur; Becton Dickinson; ref. 28).
Intracellular IFN-γ production
CTL and tumor targets were incubated at a 1:1 ratio; Brefeldin A (10 μg/mL) was added after 1 h. After a further 4 h, CTL were stained with anti–CD8-PerCP (BD Pharmingen) and fixed in 1% paraformaldehyde. Cells were subsequently permeabilized with 0.3% saponin, and stained with goat anti-human IFN-γ-FITC (BD Pharmingen) before flow cytometry.
Assessment of uptake of Mel888 cells by DC from a Mel888 cell monolayer
Mel888 cells were labeled with the membrane dye PKH-67 (Sigma), as per the manufacturer's protocol. Labeled Mel888 cells were adhered, and cocultured with immature DC as above. Supernatants were gently aspirated after 24 h coculture, and pelleted. DC were labeled with anti–CD11c-PE. Double positive cells and single-labeled PKH-67–positive Mel888 cells were enumerated by flow cytometry.
Assessment of MART-1–specific lymphocytes
CTL were treated with Dead Cell Discrimination kit (Miltenyi Biotec), labeled with MART-1-PE pentamer (ELAGIGITLV; Proimmune), counter-stained with CD8-FITC, and the percentage of MART-1 specific lymphocytes determined by flow cytometry as previously described (14).
Results
B16ova tumor cells are resistant to reovirus replication and cytotoxicity in vitro
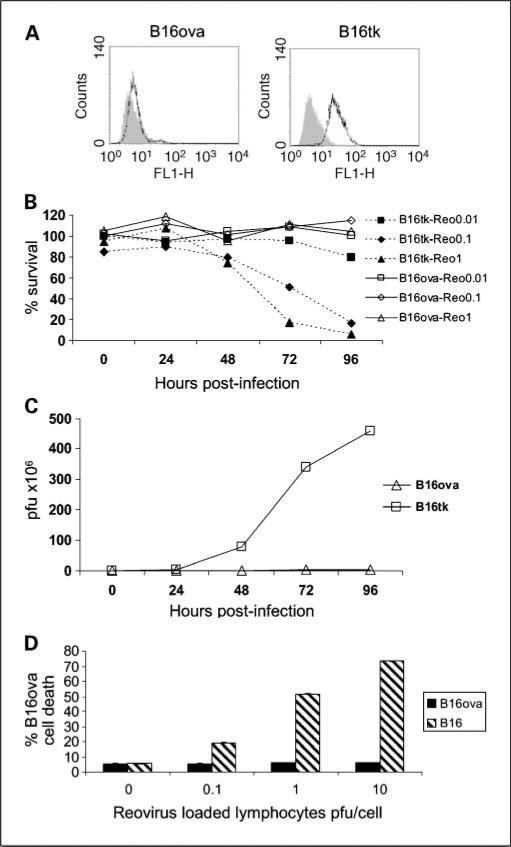
The murine melanoma B16ova model, in which B16 cells stably express the chicken ovalbumin (ova) gene as a surrogate tumor antigen (in addition to the neomycin phosphotransferase II gene), has been used to follow the generation of immune responses toward the class I H2-Kb–restricted SIINFEKL epitope of ova, and to quantify tumor load by neomycin-resistant colony outgrowth (11, 13). JAM-1 is the major receptor for reovirus (29), and is expressed at very low levels on B16ova cells in contrast to B16tk cells, encoding the HSV-thymidine kinase gene. In vitro, reovirus fails to induce oncolysis in B16ova, whereas B16tk cells are highly sensitive (Fig. 1B). Minimal levels of reovirus replication were detectable in B16ova, in contrast to the highly permissive B16tk line (Fig. 1C). Parental B16 is similarly permissive of reovirus replication and sensitive to oncolysis as B16tk, and also expresses significant levels of JAM-1 (data not shown). The mechanism(s) of resistance of B16ova to reovirus may relate to the low expression of JAM-1, although the factors determining sensitivity to reovirus are complex (22).
Fig. 1.
In vitro B16ova tumor cells are resistant to reovirus oncolysis and replication. A, JAM-1 expression was assessed by flow cytometry. Gray, isotype control; black line, JAM-1. B, B16ova and B16tk cells were treated with serial dilutions of reovirus stock, and cell survival was determined at indicated time points by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Data representative of three experiments. C, viral progeny following infection at 1 pfu per cell was determined after three cycles of freeze thaw lysis by plaque assay. B16ova cells were seeded in duplicate wells at 5 × 105 and allowed to adhere overnight. D, lymphocytes from C57BL/6 mice were incubated with reovirus at different viral titer s at 4°C for 4 h, and seeded at a 1:1 ratio into B16 or B16ova cultures. Tumor cell death was assessed at 72 h after harvesting, by propidium iodide staining after gating out CD3+ lymphocytes. Data are mean values of duplicate wells ± SE, and representative of two experiments.
A variety of different cell types can chaperone oncolytic viruses to tumors, mediating effective therapy. We have previously shown that the ability of unselected T cells to traffic to lymphoid organs can be exploited to deliver oncolytic VSV to LN and spleen bearing metastatic tumor cells (13). Similarly, reovirus-loaded lymphocytes efficiently deliver reovirus to tumor-bearing LN (30). To determine whether the delivery of reovirus loaded onto T cells alters the sensitivity of B16ova cells to reovirus, T cells preloaded with reovirus were coincubated with B16ova cell targets. B16ova cells remained resistant in vitro to reovirus, even when the virus is delivered (“handed off”) by lymphocytes (Fig. 1D).
Reovirus fails to replicate in vivo in B16ova LN metastases
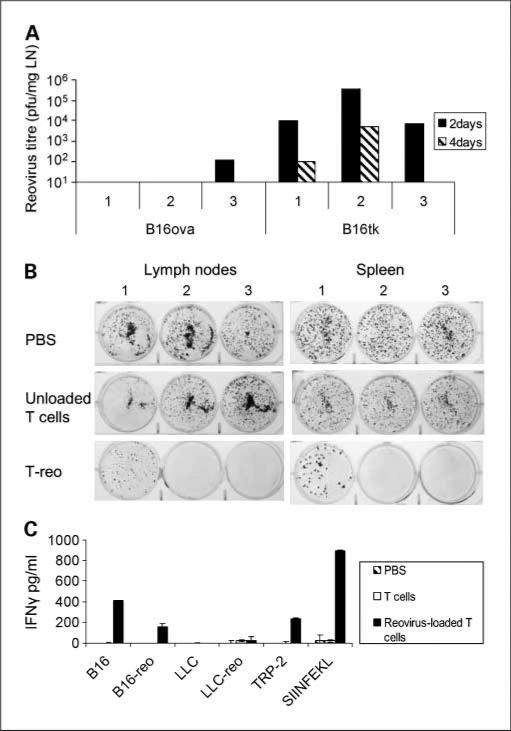
C57BL/6 mice seeded s.c. with B16ova or B16tk develop metastases in draining LN and spleen (13, 14). To confirm that B16ova remains resistant to reovirus replication in vivo, mice were seeded with either B16ova or B16tk, and treated with T cells preloaded with reovirus 0.1 pfu/T cell (T-reo) at 10 days; tumor-draining LN were harvested 2 and 4 days after treatment. Consistent with in vitro data, B16ova failed to support significant reovirus replication in vivo (Fig. 2A). By contrast, high titers of reovirus were recovered from B16tk LN metastases at 2 days, with lower levels detected at 4 days (likely reflecting a reduction in B16tk tumor burden by this time).
Fig. 2.
Reovirus loaded T cells purge B16ova LN metastases in vivo and generate antitumor immunity, in the absence of reoviral replication. A, C57BL/6 mice were seeded (three per group) at 5 × 105 B16ova or B16tk cells s.c. At 10 d, 2 × 106 T cells loaded with 0.1 pfu reovirus were adaptively transferred. Tumor-draining lymph nodes were harvested at 2 and 4 d thereafter, and viral titer determined by plaque assay. Each titer represents an individual animal. B and C, C57BL/6 mice were seeded (n = 3 per group) with 5 × 105 B16ova cells s.c. Ten days later, mice were treated with iv PBS, 2 × 106 T cells, and 2 × 106 T cells loaded with reovirus 0.1 pfu per cell (T-reo). 10 d posttherapy, tumor-draining LN and spleens were harvested, disaggregated, and 106 cells seeded in six-well plates in G418-containing media to select for B16ova cells. B, after 7 d, the number of G418-resistant colonies were photographed. C, splenocytes recovered at day 10 after treatment were pulsed with tumor cell lysates or peptides as shown, in triplicates of 7.5 × 105 cells. Forty-eight hours later, supernatants were assayed by ELISA for IFN-γ. Columns, means of triplicates; bars, SE.
Reovirus delivered on T cells purges B16ova LN metastases in vivo
We have previously shown in a B16tk model of LN metastases that a single dose of i.v. reovirus can partially purge virus-sensitive LN metastases and generate an antitumor immune response to the melanoma TAA, tyrosinase-related protein-2 (TRP-2; ref. 14). In view of the reduced sensitivity of primary human tumors to reovirus (3–6), we wished to test the efficacy of reovirus in a clinically relevant, relatively resistant model. In addition, the resistance of B16ova to reovirus replication/oncolysis provides the opportunity to selectively address the role of the immune system in mediating tumor clearance.
Ten days after seeding C57BL/6 mice s.c. with B16ova, mice received a single treatment with PBS, unloaded T cells, or T-reo. Ten days later, draining LN and spleen were analyzed. Tumor burden was assessed by the outgrowth of G418-resistant colonies from dissociated LN and spleen (Fig. 2B). T-reo partially purged both LN and splenic tumor burden.
Reovirus-loaded T-cell therapy of B16ova primes an adaptive antitumor response
To assess the antitumor versus antiviral immune response generated, splenocytes harvested at day 10 posttherapy were pulsed with cell lysates or peptides as indicated, and supernatants were assayed for IFN-γ at 48 h (Fig. 2C). T-reo therapy generated antitumor immunity, as evidenced by splenocyte reactivity toward B16, TRP-2, and SIINFEKL. This response was specific, as shown by lack of response to the control syngeneic Lewis lung carcinoma lysate. An antireovirus response was lacking after T-reo therapy, with low or absent reactivity toward reovirus-infected Lewis lung carcinoma or B16 lysates.
Taken together, these findings show that, despite its resistance to reovirus in vitro, B16ova metastases can regress after treatment in vivo in association with generation of an antitumor immune response. Because B16ova is not permissive of reovirus replication in vivo, and T-reo therapy is not associated with antiviral immunity, LN tumor purging is likely to be mediated by the antitumor immune response.
An intact immune system is required for the in vivo efficacy of T-reo against both resistant B16ova and sensitive B16tk
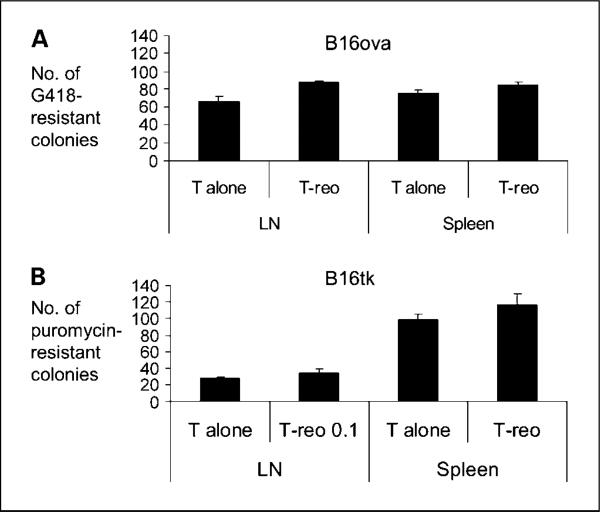
To further test the hypothesis that T-reo purging is dependent on an intact immune system, SCID mice were seeded with B16ova or B16tk tumors and treated 10 days later with unloaded T cells or T-reo. Outgrowth colonies from tumor draining LN and spleen harvested 10 days posttherapy showed no tumor purging by T-reo in comparison with unloaded T cells in the B16ova reovirus–resistant or B16tk reovirus–sensitive model (Fig. 3A and B). This is consistent with a key role for the immune system in mediating the antitumor efficacy of reovirus in tumors that are resistant and susceptible to direct viral oncolysis.
Fig. 3.
Reovirus fails to purge B16ova and B16tk metastases in SCID mice. SCID mice were seeded (three per group) with 5 × 105 B16ova or B16tk cells s.c. Ten days later, mice were treated with 2 × 106 T cells or 2 × 106 T cells preincubated with reovirus 0.1 pfu per cell. Tumor-draining LN and spleen were harvested at 10 d posttreatment. Tumor burden was assessed by colony outgrowth in G418-containing media for B16ova (A), and puromycin-containing media for B16tk (B). Colonies were counted at 7 d. Columns, mean; bars, SE.
Direct reovirus-induced oncolysis is not required for the generation of antitumor immunity in an in vitro human system
In the murine B16ova model, significant levels of direct viral oncolysis are not required for tumor response or the generation of antitumor immunity. To translate this finding toward human application, we next tested the dependency on oncolysis of priming of antitumor immunity in a human in vitro assay.
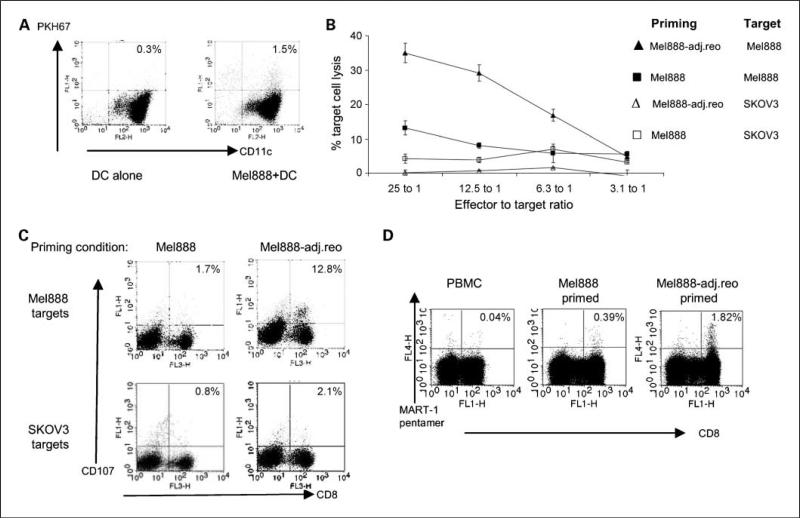
In accordance with other human melanoma lines we have screened, Mel888 cells are susceptible to reovirus-induced oncolysis (3). In CTL priming assays previously described (14), Mel888 cells were infected with reovirus before coculture with DC in suspension, and irradiation was required to prevent Mel888 outgrowth during T-cell priming. To minimize the role of direct tumor oncolysis, this protocol was modified, such that DC were added in suspension to an adherent Mel888 monolayer, before being gently aspirated after 24 hours, leaving the Mel888 monolayer intact. Figure 4A shows that although the aspirated DC had detectably phagocytosed Mel888-derived material, very few contaminating intact Mel888 cells were present.
Fig. 4.
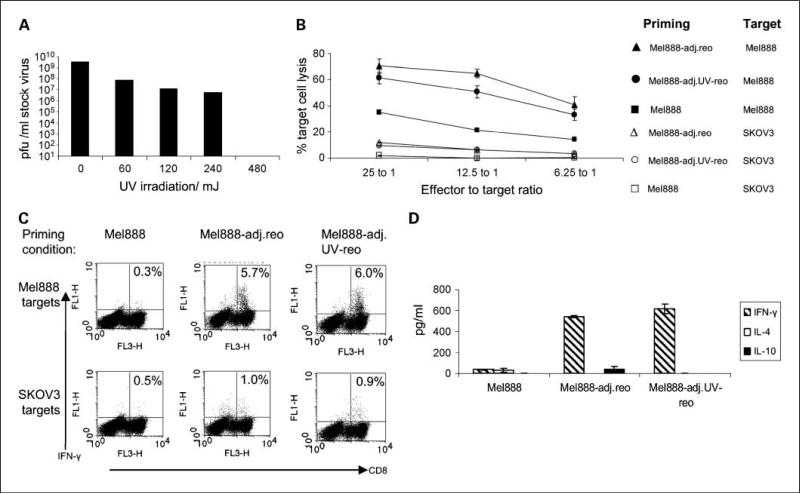
Direct reovirus-induced oncolysis is not required to prime antitumor immunity in a human in vitro system. A, PKH-67–labeled Mel888 cells were seeded and allowed to adhere. Immature DC were added to the Mel888 monolayer after 48 h at a 1:1 ratio overnight, before gentle aspiration and pelleting of supernatant. DC were labeled with anti–CD11c-PE, and flow cytometry was done to determine uptake of Mel888-derived material, and enumerate the number of intact Mel888 cells. % shown represent the % of DC double-labeling for PKH-67. Representative of two experiments. B and C, Mel888 cells were seeded and cultured with DC as in A. Reovirus was added to aspirated Mel888-preloaded DC at 1 pfu/DC (Mel888-adj.reo). DC were cocultured with autologous PBMC (1:10-30 ratio), restimulated 1 wk later. CTL activity was assayed at 14 d following culture with Mel888 or irrelevant SKOV-3 cell targets by a 51Chromium cytotoxicity assay (B), and the CD107 degranulation assay (C). Points, means of triplicate wells; bars, SE (B). % shown in C are of CD8+ T cells. Results representative of four independent experiments. D, using HLA-A2+ve donors, cross-priming of MART-1reactive CD8+ T cells was assessed by pentamer analysis. % are of CD8+ T cells. Results representative of three independent experiments.
The low number of free Mel888 cells (i.e., not loaded onto DC), is further evidenced by the observation that irradiation was not required to prevent tumor cell outgrowth. Priming assays could then be done directly adding “adjuvant” reovirus to Mel888-preloaded DC (Mel888-adj.reo), before coculture with PBMC. This allowed us to determine whether Mel888-adj.reo in the absence of additional “free” Mel888 cells undergoing viral oncolysis, were able to prime anti-Mel888 cell immunity. Consistent with our previous findings, uninfected Mel888 cells were inefficient at priming a cytotoxic response toward Mel888 target cells (Fig. 4B and C). In contrast, CTL generated when reovirus was added as an adjuvant to preloaded DC (Mel888-adj.reo) exhibited high levels of specific cytotoxicity toward Mel888 cells but not irrelevant SKOV-3 targets, as assessed by 51Chromium-labeled target killing (Fig. 4B) and lymphocyte degranulation (Fig. 4C). Mel888 cells are HLA-A2 negative (27), and following loading onto HLA-A2–positive DC, an expansion of CTL reactive to MART-1 (an HLA-A2–restricted TAA) is indicative of cross-priming. Mel888-adj.reo cross-primed an expansion of MART-1–reactive CD8 T cells (Fig. 4D), as we have previously shown for reovirus-infected Mel888 (14). Hence, addition of adjuvant reovirus to DC already loaded with tumor cells, in the absence of free tumor cells undergoing viral oncolysis, is sufficient to support priming of adaptive human antitumor immunity.
Reovirus replication is not required for the generation of human antitumor immunity
UV irradiation prevents reoviral replication (23). 480 mJ UV irradiation rendered reovirus replication incompetent, as assessed by standard plaque assays using the highly sensitive murine L929 cell line (Fig. 5A). UV-treated reovirus retains a degree of cytotoxicity toward Mel888 cells (data not shown). CTL primed by Mel888-adj.reo where reovirus was UV-treated (Mel888-adj.UVreo), exhibited high levels of specific cytotoxicity (Fig. 5B) and IFN-γ production (Fig. 5C) toward Mel888 target cells. There were no consistent differences across multiple experiments between the ability of Mel888-adj.reo and Mel888-adj.UV-reo to prime antitumor responses. Therefore reovirus replication is not required for generation of an adaptive human antitumor immune response. Consistent with this are the high levels of IFN-γ in priming cultures both with adjuvant reovirus or UV-treated reovirus, in the absence of significant levels of IL-4 or IL-10 (Fig. 5D). This cytokine pattern is indicative of a Th1-type immune response, occurring in the presence of reovirus, not dependent on viral replication.
Fig. 5.
Adjuvant replication-incompetent UV-treated reovirus also primes antitumor immunity. A, reovirus was UV irradiated in 100 μL volumes in 96-well plates, and titer assessed by an L929 plaque assay. Data are representative of two experiments. B, C, and D, reovirus treated with 480 mJ UV was added to Mel888-preloaded DC (Mel888-adj.UVreo) and assessed in priming assays done as in Fig. 4. Antitumor activity assessed by 51Chromium assay (B) and intracellular IFN-γ production (C) following coculture with Mel888 or irrelevant SKOV-3 cell targets. Points, means of triplicate wells; bars, SE. % shown in C are of CD8+ T cells. D, levels of IL-4, IL-10, and IFN-γ were determined by ELISA after 2 wk of cultures. Columns, means of triplicate wells; bars, SE. Results representative of three independent experiments.
Discussion
Oncolytic viruses are defined on the basis of direct oncolytic activity toward tumors. Primary tumor tissue is considerably less sensitive to the direct effects of oncolytic virotherapy than cell lines (3–6). In contrast to the direct cytotoxic activity of these viruses, less attention has been paid to their interaction with the immune system (7, 8). However, understanding this interaction is fundamental to fulfilling the potential of these promising novel therapeutic agents. The effect of the immune system may be detrimental, mediating rapid viral clearance via humoral or cellular immune responses. In contrast, the cellular immune response may be fundamental to the in vivo efficacy of virotherapy, via the generation of antitumor immunity (13, 15, 16), or an antiviral immune response mediating clearance of virally infected tumor cells (9, 11, 17, 31).
There are limited data regarding the immune response following virotherapy from clinical studies. Interestingly, however, when a recombinant vaccinia virus expressing GMCSF (VV-GMCSF) was injected into melanoma deposits, regression of noninjected regional dermal metastases was observed in association with an immune infiltrate in four of seven patients (32). Similarly, in a phase I study of injection of VV-GMCSF into liver tumors, evidence of response was observed in uninjected lesions in three of seven evaluable patients (33), although it was unclear whether this was due to viral dissemination or immune-mediated mechanisms (31). In a phase I study of a second-generation oncolytic HSV-expressing GMCSF, inflammation was observed in noninjected tumor deposits in 4 of 30 patients (34).
We reasoned that a tumor model exhibiting relative resistance to direct reovirus-induced oncolysis would allow focused assessment of the therapeutic potential of the immune response to reovirus, whereas mimicking more clinically relevant nonpermissive tumors. Low expression of the main reovirus receptor, JAM-1, has been found to correlate with the resistance of colorectal tumor samples to reovirus (4). B16ova expresses low levels of JAM-1 (Fig. 1A), and is resistant in vitro to reovirus (Fig. 1B and D), in contrast to parental B16 or B16tk. In view of the complex mechanisms underlying sensitivity to reovirus (22), the exact role of JAM-1 receptors in the resistance of B16ova to reovirus is the subject of ongoing studies. In addition, B16ova is poorly permissive of reovirus replication, in vitro (Fig. 1C) and in vivo (Fig. 2A). Despite in vitro resistance, LN (and splenic) metastases were largely purged by a single dose of reovirus loaded onto antigen non-specific T cells (Fig. 2B). This was associated with pronounced antitumor immunity, with little induction of antireovirus reactivity in harvested splenocytes (Fig. 2C). Antigen-nonspecific T cells were used as a highly efficient method of delivering virus (13). Experiments in SCID mice showed that in the absence of a competent immune system, purging of LN B16ova tumor was abrogated (Fig. 3A). LN metastases from the reovirus “sensitive” tumor cell line B16tk, were efficiently purged by reovirus in immunocompetent mice (14). Strikingly, despite the sensitivity of B16tk to direct reovirus-induced oncolysis, reovirus-loaded T cells still failed to purge LN metastases in SCID mice (Fig. 3B). Therefore, in these models of LN metastases, tumor purging is immune mediated and, based on the B16ova data, does not require significant levels of direct oncolysis or viral replication. Further analysis, particularly of the role of innate (as opposed to adaptive) immunity during oncolytic virotherapy is the subject of ongoing research in our laboratory.
To determine whether these murine findings apply to a human system, we adapted our in vitro priming protocol in which reovirus-infection of the human melanoma cell line Mel888, generated antitumor immunity (14). Reovirus was added, as an adjuvant, to cultures containing Mel-888–loaded DC in the absence of significant numbers of intact Mel888 cells potentially undergoing oncolysis (Fig. 4A). This system has relevance to the clinical scenario, in which tumors may undergo limited oncolysis, and where reovirus may encounter DC already loaded with uninfected tumor material. Adjuvant reovirus efficiently generated a specific anti-tumor response (Fig. 4B and C). Although this is an allogeneic system, adjuvant reovirus was importantly able to cross prime an expansion of CTL reactive to a candidate tumor-associated antigen, MART-1, from HLA-A2+ve donors (Fig. 4D). Replication incompetent UV-treated adjuvant reovirus also primed an antitumor response and generated a Th1-type cytokine profile (Fig. 5B, C, and D). Replication-competent and UV-irradiated reovirus seemed similar in their ability to facilitate antitumor priming (Fig. 5B, C, and D). Therefore neither oncolysis nor viral replication is required for reovirus-mediated priming of human adaptive antitumor immunity.
These findings show a critical role for the immune system in virotherapy, and are consistent with other oncolytic viruses (9, 11, 35). NDV administered locoregionally to liver metastases from a colorectal cancer cell line resistant to NDV in vitro, resulted in tumor growth delay, although the mechanism was not defined (35). The ability of VSV to purge LN metastases is also abrogated in SCID models (13). In a s.c. B16ova model, the efficacy of intratumoral VSV was found to be dependent on CD8+ T cells and natural killer cells, although it remained an open question whether CD8+ T cells directed to tumor or viral epitopes were required for therapy (11). In the B16ova LN metastasis model presented here, the antitumor rather than the antiviral response is associated with tumor purging (Fig. 2C).
Apparently at odds with the conclusion that the immune system is critical to antitumor efficacy are studies in immunocompetent mice demonstrating that systemic reovirus therapy is enhanced by immunosuppression (36, 37). Combining immunosuppression (cyclosporine A or combined anti-CD4 and anti-CD8 antibodies) with repeated i.v. reovirus delivery, facilitated therapy (37). Furthermore, cyclophosphamide has been shown to increase the intratumoral delivery of systemically administered reovirus (36). The beneficial effect of this interaction is likely to be an improvement in viral delivery due to reduced production of neutralizing antibodies (36). However, improved systemic viral delivery/persistence after immunosuppression does not preclude a significant therapeutic role for innate or adaptive immune-mediated antitumor responses during virotherapy. Strategies that optimize viral delivery while facilitating generation of antitumor immunity await full characterization.
In summary, we have shown that the immune response is critical for reovirus therapy. Significant levels of direct reovirus-induced oncolysis or viral replication are not required for tumor regression and antitumorimmunity. These results are significant in the clinical setting, in which primary tumors display limited sensitivity to direct viral killing. These findings provide a rationale for the design of future clinical studies aimed at facilitating the immunotherapeutic potential of reovirus.
Translational Relevance.
Oncolytic viruses are able to directly lyse cancer cells and represent a promising novel class of anticancer agents. Viruses additionally represent a potent immunologic “danger” signal, and several oncolytic viruses have been shown to facilitate the generation of antitumor immune responses in preclinical models. The relative therapeutic importance of direct viral oncolysis versus antitumor immune priming has remained uncertain. Reovirus is a naturally occurring oncolytic virus, currently in phase I and II clinical trials. This study shows that the antitumor immune response is critical to the therapeutic efficacy of reovirus, and does not dependupon direct viraloncolysis or viral replication.The immunotherapeutic activity of reovirus lays the foundation for the rationale design of treatment strategies.
Acknowledgments
Grant support: Grants from Cancer Research UK (R.J. Prestwich, F. Errington, and A.A. Melcher), and a NIH grant CA R01107032-02 (R.G. Vile), Mayo Foundation, Richard M. Schulze Family Foundation. A.A. Melcher and R.G.Vile have received research grants from Oncolytics Biotech.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Parato KA, Senger D, Forsyth PA, Bell JC. Recent progress in the battle between oncolytic viruses and tumours. Nat Rev Cancer. 2005;5:965–76. doi: 10.1038/nrc1750. [DOI] [PubMed] [Google Scholar]
- 2.Aghi M, Martuza RL. Oncolytic viral therapies - the clinical experience. Oncogene. 2005;24:7802–16. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 3.Errington F, White CL, Twigger KR, et al. Inflammatory tumour cell killing by oncolytic reovirus for the treatment of melanoma. GeneTher. 2008;15:1257–70. doi: 10.1038/gt.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Houdt WJ, Smakman N, van den Wollenberg DJ, et al. Transient infection of freshly isolated human colorectal tumor cells by reovirusT3D intermediate subviral particles. Cancer GeneTher. 2008;15:284–92. doi: 10.1038/cgt.2008.2. [DOI] [PubMed] [Google Scholar]
- 5.Nguyen TL, Abdelbary H, Arguello M, et al. Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc Natl Acad Sci U S A. 2008;105:14981–6. doi: 10.1073/pnas.0803988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tumilasci VF, Oliere S, Nguyen TL, Shamy A, Bell J, Hiscott J. Targeting the apoptotic pathway with BCL-2 inhibitors sensitizes primary chronic lymphocytic leukemia cells to vesicular stomatitis virus-induced oncolysis. J Virol. 2008;82:8487–99. doi: 10.1128/JVI.00851-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stojdl DF, Lichty B, Knowles S, et al. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat Med. 2000;6:821–5. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 8.Grote D, Russell SJ, Cornu TI, et al. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood. 2001;97:3746–54. doi: 10.1182/blood.v97.12.3746. [DOI] [PubMed] [Google Scholar]
- 9.Prestwich RJ, Harrington KJ, Pandha HS, Vile RG, Melcher AA, Errington F. Oncolytic viruses: a novel form of immunotherapy. Expert Rev Anticancer Ther. 2008;8:1581–8. doi: 10.1586/14737140.8.10.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 11.Diaz RM, Galivo F, Kottke T, et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–8. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 12.Benencia F, Courreges MC, Conejo-Garcia JR, et al. HSVoncolytictherapyupregulatesinterferon-inducible chemokines and recruits immune effector cells in ovarian cancer. MolTher. 2005;12:789–802. doi: 10.1016/j.ymthe.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 13.Qiao J, Kottke T, Willmon C, et al. Purging metastases in lymphoid organs using a combination of antigen-nonspecific adoptive T cell therapy, oncolytic virotherapy and immunotherapy. Nat Med. 2008;14:37–44. doi: 10.1038/nm1681. [DOI] [PubMed] [Google Scholar]
- 14.Prestwich RJ, Errington F, Ilett EJ, et al. Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin Cancer Res. 2008;14:7358–66. doi: 10.1158/1078-0432.CCR-08-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Toda M, Rabkin SD, Kojima H, Martuza RL. Herpes simplex virus as an in situ cancer vaccine for the induction of specific anti-tumor immunity. Hum Gene Ther. 1999;10:385–93. doi: 10.1089/10430349950018832. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Dutuor A, Tao L, Fu X, Zhang X. Virotherapy with a type 2 herpes simplex virus-derived oncolytic virus induces potent antitumor immunity against neuroblastoma. Clin Cancer Res. 2007;13:316–22. doi: 10.1158/1078-0432.CCR-06-1625. [DOI] [PubMed] [Google Scholar]
- 17.Miller CG, Fraser NW. Requirement of an integrated immune response for successful neuroattenuated HSV-1therapy in an intracranial metastatic melanoma model. MolTher. 2003;7:741–7. doi: 10.1016/S1525-0016(03)00120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greiner S, Humrich JY, Thuman P, Sauter B, Schuler G, Jenne L. The highly attenuated vaccinia virus strain modified virus Ankara induces apoptosis in melanoma cells and allows bystander dendritic cells to generate a potent anti-tumoral immunity. Clin Exp Immunol. 2006;146:344–53. doi: 10.1111/j.1365-2249.2006.03177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 20.Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–4. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 21.Comins C, Heinemann L, Harrington K, Melcher A, De Bono J, Pandha H. Reovirus: viral therapy for cancer ’as nature intended’. Clin Oncol (R Coll Radiol) 2008;20:548–54. doi: 10.1016/j.clon.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 22.Marcato P, Shmulevitz M, Pan D, Stoltz D, Lee PW. Ras transformation mediates reovirus oncolysis by enhancing virus uncoating, particle infectivity, and apoptosis-dependent release. Mol Ther. 2007;15:1522–30. doi: 10.1038/sj.mt.6300179. [DOI] [PubMed] [Google Scholar]
- 23.Errington F, Steele L, Prestwich R, et al. Reovirus activates human dendritic cells to promote innate anti-tumor immunity. J Immunol. 2008;180:6018–26. doi: 10.4049/jimmunol.180.9.6018. [DOI] [PubMed] [Google Scholar]
- 24.Linardakis E, Bateman A, Phan V, et al. Enhancing the efficacy of a weak allogeneic melanoma vaccine by viral fusogenic membrane glycoprotein-mediated tumor cell-tumor cell fusion. Cancer Res. 2002;62:5495–504. [PubMed] [Google Scholar]
- 25.Vile RG, Hart IR. Use of tissue-specific expression of the herpes simplex virus thymidine kinase gene to inhibit growth of established murine melanomas following direct intratumoral injection of DNA. Cancer Res. 1993;53:3860–4. [PubMed] [Google Scholar]
- 26.Romani N, Reider D, Heuer M, et al. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Methods. 1996;196:137–51. doi: 10.1016/0022-1759(96)00078-6. [DOI] [PubMed] [Google Scholar]
- 27.Errington F, Jones J, Merrick A, et al. Fusogenic membrane glycoprotein-mediated tumour cell fusion activates human dendritic cells for enhanced IL-12 production and T-cell priming. Gene Ther. 2006;13:138–49. doi: 10.1038/sj.gt.3302609. [DOI] [PubMed] [Google Scholar]
- 28.Betts MR, Brenchley JM, Price DA, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 29.Barton ES, Forrest JC, Connolly JL, et al. Junction adhesion molecule is a receptor for reovirus. Cell. 2001;104:441–51. doi: 10.1016/s0092-8674(01)00231-8. [DOI] [PubMed] [Google Scholar]
- 30.Ilett EJ, Prestwich RJ, Kottke T, et al. Dendritic cells and Tcells deliver oncolytic reovirus for tumour killing despite pre-existing anti-viral immunity. Gene Ther. 2009;16:689–99. doi: 10.1038/gt.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prestwich RJ, Harrington KJ, Vile RG, Melcher AA. Immunotherapeutic potential of oncolytic virotherapy. Lancet Oncol. 2008;9:610–2. doi: 10.1016/S1470-2045(08)70163-3. [DOI] [PubMed] [Google Scholar]
- 32.Mastrangelo MJ, Maguire HC, Jr., Eisenlohr LC, et al. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer GeneTher. 1999;6:409–22. doi: 10.1038/sj.cgt.7700066. [DOI] [PubMed] [Google Scholar]
- 33.Park BH, Hwang T, Liu TC, et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 2008;9:533–42. doi: 10.1016/S1470-2045(08)70107-4. [DOI] [PubMed] [Google Scholar]
- 34.Hu JC, Coffin RS, Davis CJ, et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 2006;12:6737–47. doi: 10.1158/1078-0432.CCR-06-0759. [DOI] [PubMed] [Google Scholar]
- 35.Apostolidis L, Schirrmacher V, Fournier P. Host mediated anti-tumor effect of oncolytic Newcastle disease virus after locoregional application. Int J Oncol. 2007;31:1009–19. [PubMed] [Google Scholar]
- 36.Qiao J, Wang H, Kottke T, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14:259–69. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hirasawa K, Nishikawa SG, Norman KL, et al. Systemic reovirus therapy of metastatic cancer in immune-competent mice. Cancer Res. 2003;63:348–53. [PubMed] [Google Scholar]