Abstract
“Raw materials,” or materials capable of serving both as building blocks and as signals, which are often but not always natural materials, are taking center stage in biomaterials for contemporary regenerative medicine. In osteochondral tissue engineering, a field leveraging the underlying bone to facilitate cartilage regeneration, common raw materials include chondroitin sulfate (CS) for cartilage and β-tricalcium phosphate (TCP) for bone. Building on our previous work with gradient scaffolds based on microspheres, here we delved deeper into the characterization of individual components. In the current study, the release of CS and TCP from poly(D,L-lactic-co-glycolic acid) (PLGA) microsphere-based scaffolds was evaluated over a time period of 4 weeks. Raw material encapsulated groups were compared to ‘blank’ groups and evaluated for surface topology, molecular weight, and mechanical performance as a function of time. The CS group may have led to increased surface porosity, and the addition of CS improved the mechanical performance of the scaffold. The finding that CS was completely released into the surrounding media by 4 weeks has a significant impact on future in vivo studies, given rapid bioavailability. The addition of TCP seemed to contribute to the rough external appearance of the scaffold. The current study provides an introduction to degradation patterns of homogenous raw material encapsulated scaffolds, providing characterization data to advance the field of microsphere-based scaffolds in tissue engineering.
Keywords: PLGA, degradation, osteochondral, raw materials
Introduction
Biphasic and gradient scaffold fabrication are attractive approaches for interface tissue engineering applications [1], demonstrating that microspheres can offer spatial patterning with different microsphere layers possessing different degradation kinetics [3-6]. New methods to incorporate raw materials in biodegradable scaffolds are constantly being explored as they enhance regeneration [2-4]. Raw material encapsulation in turn alters degradation rate and the associated polymer composition that eventually leads to a potential to fabricate desirable degradation rate for improved regeneration. Polymer selection, encapsulation of bioactive factors, and alterations in porosity are some of the key techniques employed to tune the degradation rate of biodegradable scaffolds to best coincide with the rate of tissue regeneration [5-7].
With respect to in vivo osteochondral applications, it would be highly desirable to have a gradient pattern, where for example the cartilage section of the scaffolds have a faster degradation rate to allow faster access for released chondroinductive signals, and correspondingly a slightly slower degrading section for the bone region to provide better mechanical support. Particularly, the changes in the degradation rate in the first month impacts the initial cellular differentiation and sets the stage for downstream regeneration processes. In view of the imminent translational applications, we chose the polymer formulation that was employed in a Coulter Foundation-funded sheep study [8] and input from regulatory consultants.
The goal of the current study was to fabricate microsphere-based scaffolds with different raw materials, and to observe the material properties over time using scanning electron microscopy (SEM), size exclusion chromatography (SEC), and measuring retention of raw materials through biochemical assays [9]. Polymeric scaffolds were fabricated using biodegradable PLGA microspheres that encapsulated TCP or CS as the raw materials [4]. These unique microspheres were fabricated and characterized for degradation in an acellular environment over a period of 4 weeks. Throughout the study, the raw material encapsulated groups were compared to their respective control groups, which were composed solely of the polymer.
Materials and methods
Materials
Faster degrading poly(D,L-lactic-co-glycolic-acid) (PLGA) (50:50 lactic acid:glycolic acid, ester end group, Mw = 106 kDa) of intrinsic viscosity 0.6-0.8 dL/g, and slower degrading PLGA (75:25 lactic acid:glycolic acid, ester end group, Mw = 112 kDa) of intrinsic viscosity 0.6-0.8 dL/g were purchased from Evonik Industries (Birmingham, AL). The raw materials were chondroitin sulfate A sodium salt (CS) and β-tricalcium phosphate (TCP), which were obtained from Sigma (St. Louis, MO). All reagents and organic solvents utilized were of cell culture or ACS grade.
Microsphere fabrication
Four different types of microspheres were fabricated. The microspheres for the study groups were CS microspheres (50:50 PLGA+CS+NaHCO3, 77.5:20:2.5 by weight) and TCP microspheres (75:25 PLGA+TCP, 90:10 by weight). The microspheres for the control groups were “CS control” (50:50 PLGA+NaHCO3, 97.5:2.5 by weight) and a “TCP control” (75:25 PLGA) microspheres. The CS microspheres were fabricated by adding 20% w/v CS and 2.5% w/v NaHCO3 to 77.5% w/v PLGA (50:50 lactic acid:glycolic acid, ester end group) with intrinsic viscosity 0.6-0.8 dL/g dissolved in dichloromethane (DCM) (20% w/v) (Sigma-Aldrich). NaHCO3 was dissolved in 0.5 mL deionized water (DI), to which CS powder was added; it was then vortexed for 15 minutes to obtain a uniform viscous solution that was subsequently mixed with PLGA, dissolved in DCM (20% w/v), and sonicated for 2 minutes. TCP group microspheres were fabricated by adding TCP (10% w/v) to PLGA (75:25; 90% w/v) in DCM (20% w/v). The TCP powder was added to PLGA, dissolved in DCM, and sonicated for 2 minutes.
Monodisperse microspheres [10] were prepared according to our previously reported technology [8, 11-14]. Briefly, the polymer stream was broken into uniform polymer droplets by exposing the stream to acoustic excitation. An annular carrier non-solvent stream (0.5%-2% w/v polyvinyl alcohol (PVA) in DI) surrounding the droplets was produced using a nozzle coaxial to the needle. The polymer/carrier streams flowed into a beaker containing the non-solvent. The polymer droplets were stirred via magnetic stir bar and plated for 3-4 h to allow solvent evaporation. Afterward, the polymer droplets were filtered and rinsed in DI to remove residual PVA, further lyophilized for 48 h, and stored at −20°C until further use.
Scaffold assembly
The microspheres were assembled into a mold (3.5 mm in diameter and 4.5 mm in height) and fabricated into scaffolds following an hour sintering with an 95% ethanol-5% acetone solvent process as previously reported [5]. All scaffolds in this study were 3.5 mm in diameter and 4 mm in height. For all individual analyses, a sample size of n = 4 or 5 was used.
Scanning electron microscopy (SEM)
At 1, 2, 3, and 4 weeks, the PLGA scaffolds were collected, freeze-dried overnight, sliced into small sections, and set up for sputter coating. The sectioned and dried scaffolds were mounted on 10 mm aluminum SEM stubs (Ted Pella Inc., Redding, CA) and sputter coated with gold at 30 mA for 46 seconds in a chamber purged with argon gas. After sputter coating, the samples were imaged using a LEO 1550 field emission scanning electron microscope under an acceleration of 5 kV and high vacuum.
Description of experimental groups
For the entire study, four different groups were investigated: (i) homogenous microsphere scaffolds composed of only 50:50 PLGA+NaHCO3 (ester functional group with intrinsic viscosity 0.6-0.8 dL/g), acting as "CS control" (ii) “CS group” microsphere scaffolds composed of (50:50 PLGA+CS+NaHCO3) (iii) microsphere scaffolds made of 75:25 PLGA, acting as "TCP control" and (iv) "TCP group" microsphere scaffolds composed of 75:25 PLGA encapsulated with 20% w/v TCP. Please refer to Table 1 for the entire list of experimental groups and their abbreviations.
Table 1.
List of the experimental groups and their abbreviations used in the manuscript
| Group name | Abbreviation | Weight (per 100 g) |
|---|---|---|
|
| ||
| 1. 50:50 PLGA + CS + NaHCO3 | CS group | 77.5:20:2.5 |
| 2. 50:50 PLGA | CS control | 100 |
| 3. 75:25 PLGA + β-TCP | TCP group | 90:10 |
| 4. 75:25 PLGA | TCP control | 100 |
Sample collection
The microsphere-based scaffolds of all the experimental groups were put in 24 well-plates and incubated in sterile phosphate buffered saline (PBS) (ThermoFisher) that was stored at 37°C and 5% CO2 jacket controlled incubator with access to fresh air. The PBS was changed every other day and samples were collected at corresponding time points.
Dry weight analyses
At weeks 0, 1, 2, 3, and 4, the scaffolds and release media were collected and filtered. The recovered microspheres were freeze-dried, weighed [dry weight (t)], and stored at 4°C for further analysis.
The relative dry weight of the scaffold at time t was calculated as follows:
Where dry weight (t = 0) denotes the initial dry weight of the microsphere scaffolds (before exposure to PBS).
Molecular weight determination
The molecular weight of the polymeric scaffolds (n = 4) at specific time points (weeks 0, 1, 2, 3, and 4) was determined using a Shimadzu LC-20AB HPLC pump equipped with a Shimadzu RID-10A refractive index detector (Shimadzu Scientific Instruments, Columbia, MD). Briefly, lyophilized scaffolds were dissolved in chloroform to form a final concentration of 0.5% polymer and passed through a 0.22 μm syringe filter (BD Biosciences, San Jose, CA) to remove any insoluble components. After a brief centrifugation at 5,000 rpm for 10 minutes, the supernatant was collected for further analysis. Chloroform was used as the mobile phase with a flow rate of 1 mL/min and a column temperature of 40 °C. The molecular weight of the polymers was determined relative to the molecular weight of the polystyrene standards (Viscotek, Malvern, UK), and weight average molecular weight was calculated from the HPLC plots.
Chondroitin sulfate in CS group scaffolds
As previously reported, the amount of CS retained within the scaffold was measured by dissolving the polymeric constructs in 1 mL of DCM. To each polymeric construct, 0.5 mL of DI was added to extract the CS into the aqueous phase. The quantity of CS retained in the scaffold (n=4) was measured by the Blyscan dimethylmethylene blue (DMMB) assay (Biocolor, Newtownabbey, Northern Ireland).
Calcium in TCP group scaffolds
TCP group scaffolds were dissolved in 1 mL of DCM (dichloromethane) followed by the addition of 0.5 mL of DI to precipitate the calcium ions into the aqueous phase. After a brief centrifugation at 5,000 rpm for 10 minutes, the calcium content was measured via the QuantiChrom™ calcium assay kit (DICA-500; Hayward, CA) according to the manufacturer’s instructions. The final values were read using a UV microplate reader (Thermo Electron Corporation, Waltham, MA), measured at 620 nm.
Mechanical Testing
Unconfined compression was performed using a uniaxial testing apparatus (Instron Model 5848, Canton, MA, 50 N load cell) according to our previously reported method [5]. Briefly, following a tare load (0.05 N), scaffolds were compressed to 15% strain at a rate of 1% per second under PBS at 37 °C, similar to our previous testing [11]. The elastic moduli were obtained from the linear regions of the stress–strain curves. Stress was defined as the ratio of the load to the initial cross sectional area, and strain was defined as the ratio of the change in the length to the original length. Mechanical testing was performed for all of the scaffolds at weeks 0 (i.e., 24 h), 1, 2, 3, and 4.
Statistical Analyses
Statistical analyses were performed using a two-way analysis of variance (ANOVA) with Minitab 17.0 software (Minitab Incorporated, State College, PA), in conjunction with a Tukey’s post hoc comparison test for repeated measurements. The statistical significance threshold was set at α = 0.05 for all tests (with p < 0.05). All quantitative results were expressed as the mean ± standard deviation.
Results
SEM characterization of scaffolds
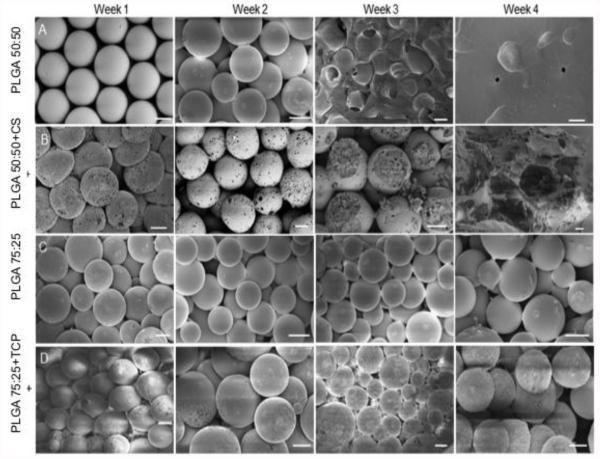
The fabricated microspheres were uniform in size and overall were porous in nature (Fig. 1). The PLGA-only scaffolds exhibited a smooth surface topology; an effect observed due to the ethanol acetone sintering [15], while the CS group displayed minute pores on the microsphere surface. TCP group exhibited rough patches on the surface compared to its control group. Over the period of 4 weeks, both the CS group and CS control group displayed macroporous degradation, with the CS control group losing complete structural integrity by week 3 and becoming paste-like by week 4. The CS group microspheres, on the other hand, retained their macroporous shape until week 4. The TCP control microspheres retained their original shape and showed little to no degradation, macroscopically, even at week 4. Conversely, the TCP group showed increased surface area roughness and did not degrade macroscopically until week 4.
Figure 1.
SEM micrographs of scaffold taken at weeks 1, 2, 3 and 4. In general, PLGA 50:50 (CS control) degraded faster than PLGA 75:25 and the CS group microspheres displayed interconnected pores and large cracks right from week 1. TCP group microspheres have a “glazed” surface due to the crystalline TCP. Scale bars = 200 μm.
Dry weight and Molecular weight analysis
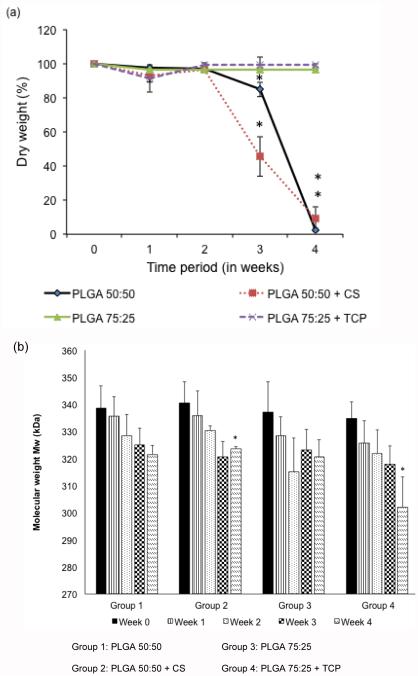
Figure 2a represents the percentage dry weight of all of the microsphere-based scaffolds over a period of 4 weeks. The CS control group did not have any significant change in weight from week 0 to week 2, but decreased by 13.4% from week 0 to week 3 (p < 0.05). By week 4, the CS control group had a significant 99.6% decrease from week 0 (p < 0.05). The CS group also did not show any significant difference in dry weight content from week 0 to week 2, but decreased by 52% from week 0 to week 3, and by 97.1% from week 0 to week 4 (p < 0.05). In comparing the CS group to its control, at week 3, we observed that the CS control had a 1.9-fold higher dry weight than the CS group (p < 0.05). However, there were no statistically significant difference between these two groups at other time points. There were no statistically significant changes in dry weight over time, or between the groups, for the TCP group and its control.
Figure 2.
Dry weight and molecular weight analysis of the scaffolds. (a) Relative dry mass (n=5), of all the groups over a 4 week period, (b) weight-average molecular weight change Mw (n=4), of polymeric scaffolds in the aqueous medium over time. Raw materials (CS & TCP) encapsulation led to faster decrease in Mw than the control groups. All groups exhibited more than 10% decrease in Mw by the end of week 4. Error bars represent mean ± SD. (*) denotes statistically significant difference (p < 0.05) in value from week 0.
Figure 2b represents the weight-average molecular weight (Mw) of all of the microsphere-based scaffolds over the period of 4 weeks. The CS control group was measured to have a starting Mw of 339 ± 9 kDa and there was no significant change in Mw over the period of 4 weeks. The CS group had a starting Mw of 342 ± 11 kDa, and there was a 9.2% decrease in Mw from week 0 to week 4 (p < 0.05). The TCP control group did not have any statistically significant change in Mw over time, however the TCP group had a 12.3% decrease in Mw from week 0 to week 4 (p < 0.05). In comparing the CS and TCP group, the CS group had a 10.5% higher Mw than the TCP group only at week 4 (p < 0.05).
Retention of chondroitin sulfate in the CS group scaffolds
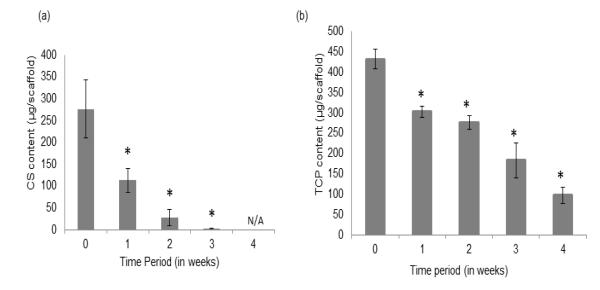
The total chondroitin sulfate retained by the CS group scaffolds from week 0 (24 hours) to week 4 is represented in Figure 3a. The GAG value measured from the scaffolds represents the CS entrapped within the CS group polymer matrix at the different time points. At week 0, the CS scaffolds had an average CS amount of 273 ± 78 μg, which decreased by 59.3% at week 1 (p < 0.05). There was a further 74.7% and 64.2% decrease from week 1 to week 2 and from week 2 to week 3 (p < 0.05), respectively. By week 4, there was no detectable CS retained in any of the scaffolds.
Figure 3.
Retention of (a) CS and (b) TCP, in the CS group and TCP group scaffolds, respectively (n = 5). The CS microsphere-based scaffolds degraded at a much faster than the TCP group microspheres. Error bars represent mean ± SD. (*) denotes statistically significant difference (p < 0.05) from week 0.
Retention of calcium in the TCP group scaffolds
The total calcium retained by the TCP group scaffolds from week 0 (24 hours) to week 4 is represented in Figure 3b. At 24 hours, the TCP group had an average calcium content of 432 ± 18 μg in the scaffolds, which decreased by 31% at week 1 (p < 0.05). The calcium content in the scaffolds decreased by 36.3% from week 0 to week 2 and 59.4% from week 0 to week 3 (p < 0.05). Finally, there was a 77.3% calcium content decrease in the TCP scaffolds from week 0 to week 4 (p < 0.05).
Mechanical testing
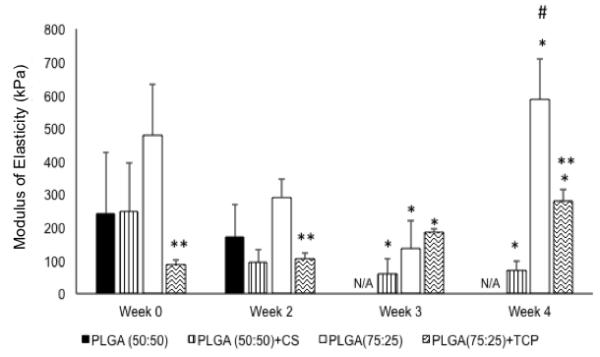
There was no significant change in the elastic modulus of the CS control group from week 0 to week 2. However, beyond week 2, these samples became too unstable to be loaded into the instrument’s sample holder. The CS group on the other hand had a significant 75.1% decrease in elastic moduli only from week 0 to week 3 and 77.2% decrease from week 0 to week 4 (p < 0.05). There was no significant change in moduli from week 0 to week 2. There was no sample at week 4 to make any comparisons. There were no significant differences in the moduli between CS control and CS group at the time points where the CS control had available data. The TCP control group had a statistically significant 73.6% decrease in elastic modulus from week 0 to week 3 (p < 0.05) and no significant change from week 0 to week 2, or from week 0 to week 4. However, there was a 2.1-fold higher modulus for the TCP control at week 4 compared to week 2 (p < 0.05). With regard to the TCP group, there was a 43.9% increase in the elastic modulus from week 0 to week 3 and a 2.8-fold higher modulus at week 4 compared to week 0 (p < 0.05). In comparing the TCP control and the TCP group, we observed that there was a 5.3-fold higher compressive elastic modulus for the TCP control group compared to the TCP group (p < 0.05) at week 0. In addition, there were 3.1-fold and 1.9-fold higher moduli for the TCP control group compared to the TCP group at week 2 and week 4, respectively (p < 0.05).
Discussion
The current study demonstrated, for the first time, the degradation pattern of nanocomposite microsphere-based scaffolds. The gradient capability that we have demonstrated with microsphere-based scaffolds allows for CS and TCP to be encapsulated in opposing gradients within a single biomaterial construct [8, 14]. We have a preliminary understanding of the mechanics and the raw material retention within these scaffolds over the crucial first month, from which we can infer gradients not only in initial material composition, but also in mechanics and degradation and release rate.
Owing to the hydrophilic nature of CS, the CS scaffolds had a significant reduction in mass compared to the control group at week 3 (p < 0.05). SEM results on the other hand showed that the CS control scaffolds experienced complete degradation in shape by week 3 but the CS scaffolds retained morphology and exhibited integrity even at week 4. Comparing these two data we can infer that although CS contributed to mass loss by the third week, it helped maintain the structural integrity as evidenced by the SEM. The select presence of CS on the surface of the scaffolds present a specific pattern preferring the degradation around the CS clusters as opposed to the entire surface degradation. The presence of small-sized polar compounds might not only help focus degradation on a particular spot but also allow for controlled cellular growth and subsequent exposure to CS for facilitated differentiation.
We are the first group to vigorously investigate the potential of TCP as a viable raw material for microsphere-encapsulation for osteochondral applications. Although other groups have used hydroxyapatite-sintered microspheres and carbon-nanotube reinforced scaffolds, no studies have fully characterized homogenous TCP-encapsulated, microsphere-based scaffold degradation in comparison to the polymer alone [16-18].
The SEM images depicted an overall porous nature of the microsphere-based scaffolds with interconnected pores and were in agreement with our previous findings with low molecular weight PLGA. [15] The CS microspheres had a porous surface that could be the effect of CS embedding and subsequent removal during the scaffold fabrication step, which was consistent with our previous publications [10, 19]. Although the current study did not measure porosity, information can be obtained from our previous manuscripts that used similar formulations.[10] The TCP group on the other hand did not possess a porous surface, but had a rough external surface instead. The nanoporosity at the surface of the microspheres, may contribute more to scaffold performance in terms of cell response to the texture and CS content at the surface than by any perceived overall increase in the total porosity of the constructs, as the nanoporosity does not contribute to the true void volume available to cells upon infiltration for migration. The next iteration of studies that employ gradient microsphere-designs will benefit from surface roughness and direct porosity measurements.
.The dry weight data were in accordance with the SEM analysis. While both the low molecular weight PLGA lost its dry weight completely by week 4, the TCP scaffolds did not have any significant change in mass even after week 4. Thus, using a higher molecular weight PLGA that had a higher percentage of lactic acid not only delayed degradation on the surface, but also maintained its overall mass percentage.
The GAG and calcium release assay that evaluated the raw material content in the scaffolds were consistent with the SEM observations. The starting values of GAG and calcium at week 0 do not represent their loading value. This may be attributed to loss during the lyophilization and the solvent sintering step. About 60% of the CS was released at week 1 into the surrounding media. The relatively high bioavailability of CS within the first week and complete release within one month may be highly desirable for initiating a bioactive chondrogenic response in vivo during the crucial period of regeneration. There was a gradual but significant decrease in calcium content at all time points compared to week 0 samples, but both the SEM and the dry weight data did not show any significant degradation visually or with respect to mass. TCP is crystalline and along with the rough surface texture observed on the surface, we can corroborate the evidence that TCP is dissolved in the surrounding polar media, thus leading to an increase in surface roughness but not significant enough to cause a mass loss.
Mechanical testing results demonstrated the compressive moduli of microsphere-based scaffolds to be of similar order of magnitude relative to articular cartilage (0.1–0.9 MPa) and within an order of magnitude of the moduli for cancellous bone (0.01–2 GPa) [10, 20-22]. Week 0 refers to the samples that were soaked in PBS for approximately 24 h. The large error bars of the samples at week 0 may be attributed to the uneven presentation of CS or TCP encapsulated in the microspheres. Please note that the SEM data also corroborates with the uneven surface of the CS and TCP scaffolds. However, other studies [23, 24] have shown that pre-wetting the scaffolds for extended periods of time leads to a homogenous distribution due to polymer plasticization. The elastic modulus of the TCP group at week 0 was in agreement with the moduli of calcium phosphates as observed by Ly et al. [25], thus confirming that the addition of TCP enhances the elastic moduli. Although the starting modulus of the PLGA groups in the current study are comparable to our previous studies, over a period of 4 weeks, there was a an order of magnitude increase in modulus for the gradient groups, which may be the combined effect of two types of PLGA and the stiffness contributed by cellular differentiation [10, 26]. In another previous study from our team, which employed raw material-encapsulated scaffolds compressed at strain rate of 1 mm/min, we observed that the elastic moduli was generally lower than the moduli reported in the current study [19]. However, the previous study employed rat bone marrow mesenchymal stem cells (rBMSCs) and gradient scaffolds with a low-molecular weight PLGA. The comparison of mechanical moduli with [26] has been clarified and included in the manuscript. Previous studies from our group have looked at the modulus of elasticity upon the addition of CaCO3 and observed that the highest recorded modulus was < 400 kPa. TCP used in the current study is a β crystalline polymorph that does not degrade quickly. CaCO3 on the other hand overall did not contribute to the increase in the modulus and is easily subjected to erosion leading to a quicker decline in the mechanical properties. In the current study, while the moduli of the TCP control scaffolds did not show any significant change over time, the TCP group had a 91.5% increase from week 2 to week 4 (p < 0.05) an observation consistent with our previous paper [27]. Both the CS control and CS groups showed a decline in elastic modulus that may be explained by the low molecular weight nature and the high glycolic content of the polymer [28]. PLGA microspheres are known to degrade via bulk erosion where the rate-limiting step is the diffusion of water molecules into the microsphere core. CS microspheres, because of their porous nature and the hydrophilic nature of CS, may have allowed faster diffusion of the water molecules into their core, thereby initiating the polymer degradation more quickly than in the other three groups, as evidenced by a significantly greater mass loss by 3 weeks. However, the structural integrity of the CS constructs was superior to the CS control through the 4 week duration. Additionally, swelling caused by penetration of water inside of the microspheres may have also played a role in the drop in elastic modulus of CS scaffolds [19]. Moreover, the polymer composition (75:25 PLGA) and microsphere morphology (absence of minute pores on surface) may have allowed the TCP scaffolds to further retain their mechanical properties. The imminent next step would be to test the impact of cells on known mechanical properties of the scaffolds and independently correlate the effect of cells versus the effect of raw materials and thus results from such studies would be valuable in designing scaffolds for future large animal models.
Findings from the current study necessitate the need to refine the technology by parameters like adjusting raw material and polymer concentration that in turn would modify the degradation rate. Since the polymer degradation and the raw material released plays a major role for in vivo studies, there is a pressing need to reach a suitable selection of polymer and raw material concentration for specific applications.
The scaffold selected to treat small animals like rats and rabbit may not match the need of a large animal model. Moreover other factors like bone stiffness, cartilage thickness must also be considered for enhanced scaffold design. The current study attempted to characterize homogenous microsphere-based scaffolds to provide a basis for analyzing degradation patterns that in turn would enable multilayered and gradient scaffold fabrication. In addition to just altering the degradation kinetics, raw materials can also provide beneficial signals to the surrounding cells guiding the differentiation and neo-tissue regeneration.
Conclusion
The current study evaluated the preliminary degradation properties of raw material encapsulated microsphere-based scaffolds over a period of 4 weeks. Overall, the results demonstrated that the incorporation of CS with 50:50 PLGA may have increased surface porosity and enhanced the structural stability over 4 weeks compared to the polymer without CS. More importantly, about 60% of the CS encapsulated was released at week 1 and 100% of the CS was released by 4 weeks. The current finding might have significant implications for the design of future in vivo microsphere-based scaffolds for chondrogenesis. The TCP incorporation with 75:25 PLGA also favored a better mechanical response over time and retention of an overall rough surface topology even at 4 weeks compared to the TCP control group. Such a complementary nature of the encapsulated raw material adds value to the polymer selection, thus aiding the fabrication of gradient or multilayered scaffolds with informed decision about degradation. Future studies will benefit from expanded analyses to further elucidate the effects of the raw materials on the degradation and mechanics of the scaffolding materials over time.
As an example, for in vivo interface tissue engineering applications that need different degradation rates to match the properties of the native tissue, the current study provides a means to evaluate the individual parameters involved in assessing the degradation pattern under an in vitro setting. The degradation pattern according to this platform can be tailored by polymer selection, nature of raw materials that are encapsulated and the concentration of both to manufacture the final scaffold. Additionally, the current study also employed a set of raw materials (CS & TCP) combined with two specific types of PLGA, and emphasized that the raw material content, along with the PLGA composition, are important parameters for controlling degradation and mechanics over time.
Figure 4.
Compressive elastic modulus at different time points. CS group scaffolds lost shape by week 3 and hence there was no solid material for measurement at week 3 and week 4. Error bars represent mean ± SD. (*) indicates statistically significant difference (p < 0.05) from week 0. (**) indicates statistically significant difference (p < 0.05) from the corresponding control group. (#) indicates statistically significant highest value of the parameter (p< 0.05) along the particular group.
Acknowledgments
The National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01 AR056347 supported research reported in this publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would also like to recognize support from the Kansas Bioscience Authority Rising Star Award. The authors would like to thank Staphany Lin for her kind assistance with dry weight analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Dormer NH, Berkland CJ, Detamore MS. Emerging techniques in stratified designs and continuous gradients for tissue engineering of interfaces. Annals of biomedical engineering. 2010;38:2121–41. doi: 10.1007/s10439-010-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cheung HK, Han TTY, Marecak DM, Watkins JF, Amsden BG, Flynn LE. Composite hydrogel scaffolds incorporating decellularized adipose tissue for soft tissue engineering with adipose-derived stem cells. Biomaterials. 2014;35:1914–23. doi: 10.1016/j.biomaterials.2013.11.067. [DOI] [PubMed] [Google Scholar]
- [3].Levett PA, Melchels FP, Schrobback K, Hutmacher DW, Malda J, Klein TJ. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta biomaterialia. 2014;10:214–23. doi: 10.1016/j.actbio.2013.10.005. [DOI] [PubMed] [Google Scholar]
- [4].Mohan N, Gupta V, Sridharan B, Sutherland A, Detamore MS. The potential of encapsulating "raw materials" in 3D osteochondral gradient scaffolds. Biotechnology and bioengineering. 2014;111:829–41. doi: 10.1002/bit.25145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mohan N, Dormer NH, Caldwell KL, Key VH, Berkland CJ, Detamore MS. Continuous gradients of material composition and growth factors for effective regeneration of the osteochondral interface. Tissue engineering Part A. 2011;17:2845–55. doi: 10.1089/ten.tea.2011.0135. [DOI] [PubMed] [Google Scholar]
- [6].Clark A, Milbrandt TA, Hilt JZ, Puleo DA. Tailoring properties of microsphere-based poly (lactic-co-glycolic acid) scaffolds. Journal of Biomedical Materials Research Part A. 2014;102:348–57. doi: 10.1002/jbm.a.34706. [DOI] [PubMed] [Google Scholar]
- [7].Sutherland AJ, Converse GL, Hopkins RA, Detamore MS. The Bioactivity of Cartilage Extracellular Matrix in Articular Cartilage Regeneration. Advanced healthcare materials. 2014 doi: 10.1002/adhm.201400165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mohan N, Gupta V, Sridharan B, Mellott AJ, Easley JT, Palmer RH, et al. Microsphere-based gradient implants for osteochondral regeneration: a long term study in sheep. Regenerative medicine. 2015 doi: 10.2217/rme.15.38. Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dormer NH, Gupta V, Scurto AM, Berkland CJ, Detamore MS. Effect of different sintering methods on bioactivity and release of proteins from PLGA microspheres. Materials science & engineering C, Materials for biological applications. 2013;33:4343–51. doi: 10.1016/j.msec.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gupta V, Mohan N, Detamore MS. Microsphere-Based Scaffolds Carrying Opposing Gradients of Chondroitin Sulfate and Tricalcium Phosphate. Frontiers in Bioengineering and Biotechnology. doi: 10.3389/fbioe.2015.00096. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Singh M, Dormer N, Salash JR, Christian JM, Moore DS, Berkland C, et al. Three-dimensional macroscopic scaffolds with a gradient in stiffness for functional regeneration of interfacial tissues. Journal of biomedical materials research Part A. 2010;94:870–6. doi: 10.1002/jbm.a.32765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Singh M, Morris CP, Ellis RJ, Detamore MS, Berkland C. Microsphere-based seamless scaffolds containing macroscopic gradients of encapsulated factors for tissue engineering. Tissue engineering Part C, Methods. 2008;14:299–309. doi: 10.1089/ten.tec.2008.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Dormer NH, Singh M, Wang L, Berkland CJ, Detamore MS. Osteochondral interface tissue engineering using macroscopic gradients of bioactive signals. Annals of biomedical engineering. 2010;38:2167–82. doi: 10.1007/s10439-010-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gupta V, Mohan N, Berkland CJ, Detamore MS. Microsphere-based scaffolds carrying opposing gradients of chondroitin sulfate and tricalcium phosphate. Frontiers in Bioengineering and Biotechnology. 2015;3:1–15. doi: 10.3389/fbioe.2015.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Singh M, Morris CP, Ellis RJ, Detamore MS, Berkland C. Microsphere-based seamless scaffolds containing macroscopic gradients of encapsulated factors for tissue engineering. Tissue Engineering Part C: Methods. 2008;14:299–309. doi: 10.1089/ten.tec.2008.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mikael PE, Amini AR, Basu J, Arellano-Jimenez MJ, Laurencin CT, Sanders MM, et al. Functionalized carbon nanotube reinforced scaffolds for bone regenerative engineering: fabrication, in vitro and in vivo evaluation. Biomedical Materials. 2014;9:035001. doi: 10.1088/1748-6041/9/3/035001. [DOI] [PubMed] [Google Scholar]
- [17].Cushnie EK, Khan YM, Laurencin CT. Amorphous hydroxyapatite-sintered polymeric scaffolds for bone tissue regeneration: Physical characterization studies. Journal of Biomedical Materials Research Part A. 2008;84:54–62. doi: 10.1002/jbm.a.31380. [DOI] [PubMed] [Google Scholar]
- [18].Lv Q, Nair L, Laurencin CT. Fabrication, characterization, and in vitro evaluation of poly (lactic acid glycolic acid)/nano-hydroxyapatite composite microsphere-based scaffolds for bone tissue engineering in rotating bioreactors. Journal of Biomedical Materials Research Part A. 2009;91:679–91. doi: 10.1002/jbm.a.32302. [DOI] [PubMed] [Google Scholar]
- [19].Mohan N, Gupta V, Sridharan B, Sutherland A, Detamore MS. The potential of encapsulating “raw materials” in 3D osteochondral gradient scaffolds. Biotechnology and bioengineering. 2014;111:829–41. doi: 10.1002/bit.25145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Keaveny TM, Hayes WC. A 20-year perspective on the mechanical properties of trabecular bone. Journal of biomechanical engineering. 1993;115:534–42. doi: 10.1115/1.2895536. [DOI] [PubMed] [Google Scholar]
- [21].Angele P, Yoo J, Smith C, Mansour J, Jepsen K, Nerlich M, et al. Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. Journal of Orthopaedic Research. 2003;21:451–7. doi: 10.1016/S0736-0266(02)00230-9. [DOI] [PubMed] [Google Scholar]
- [22].Bentley G, Biant L, Carrington R, Akmal M, Goldberg A, Williams A, et al. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. Journal of Bone & Joint Surgery, British Volume. 2003;85:223–30. doi: 10.1302/0301-620x.85b2.13543. [DOI] [PubMed] [Google Scholar]
- [23].Yang J, Shi G, Bei J, Wang S, Cao Y, Shang Q, et al. Fabrication and surface modification of macroporous poly (L-lactic acid) and poly (L-lactic-co-glycolic acid)(70/30) cell scaffolds for human skin fibroblast cell culture. Journal of biomedical materials research. 2002;62:438–46. doi: 10.1002/jbm.10318. [DOI] [PubMed] [Google Scholar]
- [24].Yang F, Murugan R, Ramakrishna S, Wang X, Ma Y-X, Wang S. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials. 2004;25:1891–900. doi: 10.1016/j.biomaterials.2003.08.062. [DOI] [PubMed] [Google Scholar]
- [25].Dai L-Y, Jiang L-S. Single-level instrumented posterolateral fusion of lumbar spine with β-tricalcium phosphate versus autograft: a prospective, randomized study with 3-year follow-up. Spine. 2008;33:1299–304. doi: 10.1097/BRS.0b013e3181732a8e. [DOI] [PubMed] [Google Scholar]
- [26].Dormer NH, Singh M, Wang L, Berkland CJ, Detamore MS. Osteochondral interface tissue engineering using macroscopic gradients of bioactive signals. Annals of biomedical engineering. 2010;38:2167–82. doi: 10.1007/s10439-010-0028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Singh M, Dormer N, Salash JR, Christian JM, Moore DS, Berkland C, et al. Three-dimensional macroscopic scaffolds with a gradient in stiffness for functional regeneration of interfacial tissues. Journal of Biomedical Materials Research Part A. 2010;94:870–6. doi: 10.1002/jbm.a.32765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Alexis F. Factors affecting the degradation and drug-release mechanism of poly (lactic acid) and poly [(lactic acid)-co-(glycolic acid)] Polymer International. 2005;54:36–46. [Google Scholar]