Abstract
Background:
Coronary artery disease (CAD) is a major cause of death and disability in developed countries. Chronic stable angina is the initial manifestation of CAD in approximately 50% of the patients. Recent evidence suggests that vitamin D is crucial for cardiovascular health. The prevalence of vitamin D deficiency in our region is 83%. A low level of vitamin D is associated with chronic stable angina.
Aim:
This study was aimed at supporting or refuting this hypothesis in our population.
Materials and Methods:
The study was a prospective case-control study. We studied 100 cases of chronic stable angina and compared them with 100 matched controls. Vitamin D deficiency was defined as <20 ng/mL, vitamin D insufficiency as 20-30 ng/mL and normal vitamin D level as 31-150 ng/mL.
Results:
The prevalence of vitamin D deficiency among cases and controls was 75% and 10%, respectively. 75% of the cases were vitamin D-deficient (<20 ng/mL); 12% were vitamin D-insufficient (20-30 ng/mL), and 13% had normal vitamin D levels (31-150 ng/mL). None had a toxic level of vitamin D. Among the controls, 10% were vitamin D-deficient, 33% were vitamin D-insufficient, and 57% had normal vitamin D levels. The mean vitamin level among cases and controls was 15.53 ng/mL and 40.95 ng/mL, respectively, with the difference being statistically significant (P ≤ 0.0001). There was no statistically significant relation between the disease severities, i.e., on coronary angiography (CAG) with vitamin D level. Among the cases, we found that an increasing age was inversely related to vitamin D levels (P = 0.027).
Conclusion:
Our study indicates a correlation between vitamin D deficiency and chronic stable angina. Low levels may be an independent, potentially modifiable cardiovascular risk factor.
Keywords: Chronic stable angina, coronary artery disease (CAD), North India, prevention of coronary artery disease, vitamin D
Introduction
Coronary artery disease (CAD) is one of the leading causes of death and disability in developed countries, responsible for about one in every five deaths.[1] It is fast becoming a pandemic within the developing world as well where it involves a relatively younger population.[2] Chronic stable angina is the initial manifestation of CAD in approximately 50% of the patients.[3]
Vitamin D is a fat-soluble vitamin. It exists in many forms but two forms are very important: 25-hydroxycholecalciferal and 1, 25 dihydroxycholecalciferal.[4] In the skin provitamin D is photo-isomerized to vitamin D3. Calcitriol mediates its biological effects by binding to the vitamin D receptor (VDR), located in the nuclei of target cells in most organs.[5] The binding of calcitriol to the VDR allows the VDR to act as a transcription factor that modulates the gene expression of transport proteins involved in a multitude of different tasks.[6] Vitamin D status is best determined by the serum 25(OH) D as opposed to 1,25(HO)2 D for several reasons including: Its long circulating half-life (~3 weeks versus ~8 h) and the concentration of 25(OH) D that is thousand times higher in circulation compared to 1, 25 (OH)2 D. Thus, 1, 25 (OH)2 D levels could be elevated in severe vitamin D deficiency to maintain normal serum calcium levels.[7]
Risk factors for vitamin D deficiency include age >65 years, dark skin, obesity, kidney or liver disorders, diseases affecting fat absorption, bariatric surgeries, and environmental variables such as institutionalization, decreased outdoor physical activity, and frailty.[8]
This vitamin has received intense attention in the recent years from being strictly nutritional toward transcriptional, translational, and posttranslational levels. Recent research suggests its role in chronic diseases such as hypertension,[9] diabetes mellitus (DM),[10] cancer,[11] autoimmune diseases, some infections such as influenza,[12] tuberculosis,[13] human immunodeficiency virus (HIV);[14] multiple sclerosis,[15] and aging.[16] Its role in decreasing mortality in elderly women has been established.[17]
Receptors for vitamin D have been found in cardiac cells and vascular endothelial cells, giving these the potential to have a wide range of vascular effects.[18] Evidence supports a potential role of vitamin D in the development of cardiovascular diseases including angina, myocardial infarction (MI), and transient ischemic attacks.[7,19] The proposed mechanisms include endothelial dysfunction in vitamin D deficiency;[20] effects on the renin-angiotensin system,[21] on glycemic control,[22] and on inflammatory cytokines;[23] regulation of parathyroid hormone levels and calcium deposition in vascular smooth muscle.[24]
Different regions of the world get different hours of sunlight, which has a direct bearing on vitamin D synthesis in the body.[25] Coronary heart disease (CHD) mortality and morbidity in many countries increases by 30-50% in winter compared to summer. The hypothesis that exposure to sunlight is protective against CHD could explain the winter increase in CHD mortality and morbidity. Data show a direct relationship between latitude and CHD mortality and morbidity, and the inverse association between altitude and CHD mortality since ultraviolet radiation decreases with latitude but increases with altitude.[26]
Our study has been conducted in Kashmir valley, which is located at a high latitude in North India. It is surrounded on all sides by tall mountains of the Himalayas. The winters are very harsh and long. People use traditional long-sleeved thick clothes to cover themselves. The prevalence of vitamin D deficiency in our region is 83%[27] as compared to 35-50% in the USA and other parts of the world.[28] Very few studies have been conducted in this part of the world to find any association between vitamin D deficiency and stable CAD.
Materials and Methods
The study was a 2-year hospital-based prospective case-control study conducted in Sher-i-Kashmir Institute of Medical Sciences (SKIMS), Srinagar, Kashmir, India. One hundred patients with angiographically documented chronic stable angina and 100 matched controls were taken for the study. The cases and matched controls were selected from a pool of about 160 patients and 300 healthy volunteers, respectively. The study was approved by the Institute Ethical Committee of SKIMS. Written informed consent was given by all the patients and controls in the study.
The patients with chronic stable angina of any age group or sex were initially included in the study. Among them, those who had angiographically documented CAD were studied further. The patients having any acute illness, acute coronary syndrome, history of malignant neoplasm within the past 5 years, on vitamin D and calcium supplements, and with any parathyroid disease, etc., were excluded.
Diagnostic methods used
Proper history was taken and detailed physical examination was done on all the study subjects.
12 LEAD ECG: 12 leads resting electrocardiography was performed in all patients with angina or angina equivalent. Although the findings were normal in approximately half of the patients with stable angina,[29] we looked in particular for ST-T wave changes, left ventricular (LV) hypertrophy, or the presence of pathological Q waves, which might favor the diagnosis of CAD.[24]
Chest x-ray (CXR) posterior-anterior (P/A) view was done in each patient.
Treadmill test (TMT) was done in all patients suspected to have chronic stable angina except for those who were not fit for exercise or those who had electrocardiographic abnormalities that compromised interpretation.[30] ruce protocol was used in all the patients.
Coronary angiography (CAG) was done in all the patients having chronic stable angina with positive TMT and having no contraindication for angiography. It was done to confirm the diagnosis of CAD and to see the severity of the disease process. Angiogram was categorized into 1, 2, 3, or main vessel CAD. Other laboratory tests, which include complete lipid profile, liver function test (LFT), kidney function test (KFT), complete blood count (CBC), calcium, phosphorus, etc., were also done.
Sampling technique
Early morning fasting whole blood venous samples of about 3 mL was taken. They were protected from light, centrifuged, and stored at –20°C.
Vitamin D measurement
Vitamin D was assayed using solid phase enzyme-linked immunoassay (ELISA) based on the principle of competitive binding. Vitamin D deficiency is defined as <20 ng/mL, vitamin D insufficiency as 20-30 ng/mL, and normal vitamin D level as 31-150 ng/mL.
Statistical analysis
Data analysis was done by using standard statistical technique using chi-square test, Student's t-test, likelihood ratio, Levene's test for equality, analysis of variance (ANOVA), Mann-Whitney U test, and Fisher's exact test. All the data were analyzed on Statistical Package for the Social Sciences (IBM SPSS Statistics 23) software. Statistical significance was taken as a P value of less than 0.05.
Results
Demography
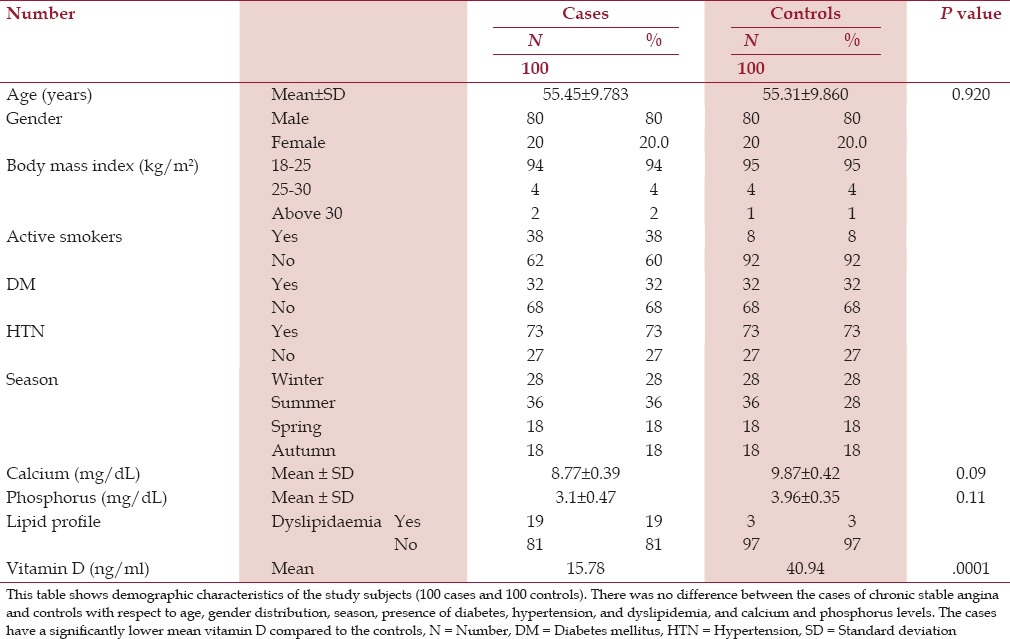
Table 1 shows the demographic characters of the cases and controls. Our study comprised 80% males and 20% females each from the cases and controls. Among the cases, the mean age was 55.45 years and among the controls, the mean age 55.31 years, with the difference being statistically insignificant (P = 0.920). Most of our cases and controls had normal body mass index (BMI). We studied 28% of the cases in winter, 36% in summer, 18% in spring, and 18% in autumn, together with the same percentage of controls in the respective seasons.
Table 1.
Demographic characteristics of the studied subjects
Vitamin D, age, and gender
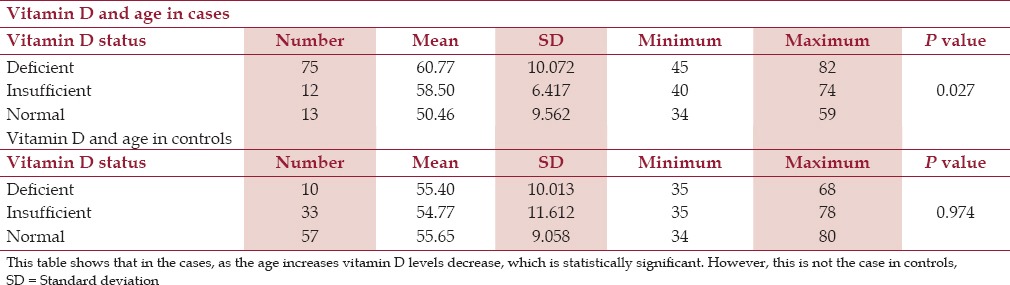
Among the female cases, 70% were vitamin D-deficient, 15% were vitamin D-insufficient, and 15% had normal levels of vitamin D. Among the male cases, 76.2% were vitamin D-deficient, 11.2% were vitamin D-insufficient, and 12.5% had normal levels of vitamin D. This may suggest that males are more vitamin D-deficient than females. But there were more number of males than females (4:1) and the difference was statistically insignificant (P = 0.840). The mean age among vitamin-deficient cases was 60.77 years compared to the mean age of 58.50 years in the vitamin D-insufficient group and 50.46 years in the normal vitamin D group [Table 2]. This showed that with age, vitamin D level decreased among cases and was statistically significant (P = 0.027). However, vitamin D-deficient subjects had a mean age that was less than that of normal individuals and only slightly higher than vitamin D-insufficient cases with P value of 0.974. Therefore, among the controls no relation of vitamin D with age was seen [Table 2].
Table 2.
Vitamin D and age among cases and controls
Vitamin D and season
The mean value of vitamin D was lowest in spring (12.58 ng/mL) followed by winter (14.81 ng/dL), summer (16.68 ng/dL), and autumn (18.718 ng/mL). This difference is statistically insignificant (P = 0.547)
Vitamin D and smoking
Among the cases who were smokers, the mean value of vitamin D was 11.024 ng/mL and among nonsmoker cases, the mean value was 17.37 ng/mL. However the difference was statistically insignificant (P = 0.134).
Symptom distribution
All our cases were symptomatic. Nineteen percent of the patients had angina on effort (AOE)/angina equivalent New York Heart Association (NYHA) class I, 52% class II, and 29% class III symptoms.
Vitamin D and angina on effort/angina equivalent (New York Heart Association, class)
Among AOE/angina equivalent NYHA class I cases, 73.7% were vitamin D-deficient (≤20 ng/mL); 21.1% were vitamin D-insufficient (21-30 ng/mL), and 5.3% had normal levels of vitamin D (31-150 ng/mL). Among AOE/angina equivalent NYHA class II, 78.7% were vitamin D-deficient, 7.7% were vitamin D-insufficient, and 13.3% had normal levels of vitamin D. Among AOE/angina equivalent NYHA class III, 69% were vitamin D-deficient, 13.8% were vitamin D-insufficient, and 17.2% had normal levels of vitamin D. The results were statistically insignificant (P = 0.446).
Vitamin D and lipid profile
Nineteen percent of the cases had dyslipidemia compared to 3% of the controls. Among 19 cases with abnormal lipid profile, 17 (89.47%) were vitamin D-deficient and 1 (5%) each had insufficient and normal vitamin D levels, respectively. Among 81 cases with normal lipid profile, 58 (71.6%) were vitamin D-deficient, 11 (13.5%) were vitamin D-insufficient, and 12 (14.8%) had normal vitamin D levels. The difference was statistically insignificant (P = 0.269).
Serum calcium and phosphorus levels among cases and controls
There was no statistically significant difference between the cases and controls [Table 1].
Electrocardiography findings
Among the cases, 50% had normal electrocardiography (ECG), 2% had sinus tachycardia, 3% had ST elevated, 7% had ST depressed, 9% had T-wave inversion, 6% cases were suggestive of LVH, 2% were suggestive of (S/O) right ventricular hypertrophy (RVH), 1% had Q waves, 4% left bundle branch block (LBBB), 12% had left axis deviation, 17% had right axis deviation, and 9% had right bundle branch block (RBBB). The ECGs of all the controls were normal.
Chest x-ray findings
Among the cases, 49% had normal x-ray of the chest, 47% had cardiomegaly, 2% had mild pleural effusion, and 2% prominent aortic knuckle. Among the controls, 81% had normal x-ray of the chest, 15% had cardiomegaly, 3% had prominent aortic knuckle, and 1% had mild pleural effusion.
Echocardiography findings
Seventy five percent of the cases had normal echocardiography (echo), 1% had regional wall motion abnormality (RWMA), 20% had left ventricular hypertrophy (LVH), 1% had valvular heart disease, and in 1% no echo report was available.
Treadmill test results
We took a total of 160 patients suspected to have chronic stable angina for TMT. Among them, 104 (65%) and 56 (35%) tested positive and negative, respectively, on TMT using Bruce protocol.
Coronary angiography findings
Fort five percent of the cases had single vessel disease (SVD), 42% had double vessel disease (DVD), and 13% had triple vessel disease (TVD)/multivessel disease. No patient had left main disease.
Vitamin D status among cases and controls
Seventy five percent of the cases were vitamin D-deficient, 12% were vitamin D-insufficient, and 13% had normal vitamin D levels. Among the controls, 10% were vitamin D-deficient, 33% were vitamin D-insufficient, and 57% had normal vitamin D levels. So, the prevalence of vitamin D deficiency among the cases and controls was 75% and 10%, respectively. The overall prevalence among the study population was 42.5%.
Mean levels of vitamin D among cases and controls
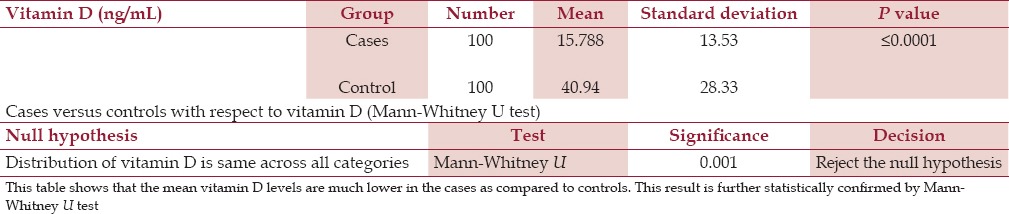
The mean vitamin level among the cases and controls was 15.53 ng/mL and 40.954 ng/mL, respectively [Figure 1]. This difference was statistically significant with P ≤ 0.0001. We rechecked it through the nonparametric test and Mann-Whitney U test in view of high standard deviation. The result was again statistically significant (P = 0.001) [Table 3]. Comparing these results, the cases were vitamin D-deficient compared to the controls, which was statistically significant (P = 0.0001). This suggested that there was a correlation between vitamin D deficiency and chronic stable angina.
Figure 1.
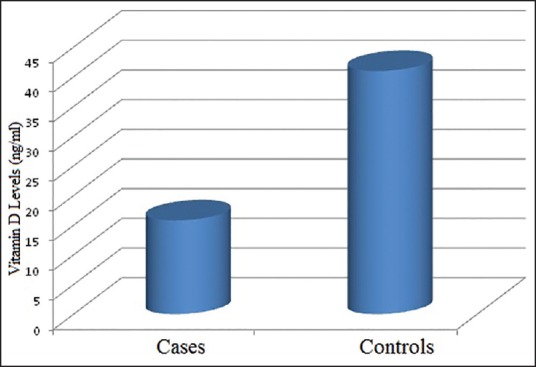
This figure shows the mean vitamin D level along the y-axis comparing the cases and controls represented on the x-axis. As can be seen, the mean vitamin D level in the cases is significantly lower than the controls and the normal vitamin D levels
Table 3.
Cases versus controls with respect to mean vitamin D levels
Coronary angiography categories and vitamin D
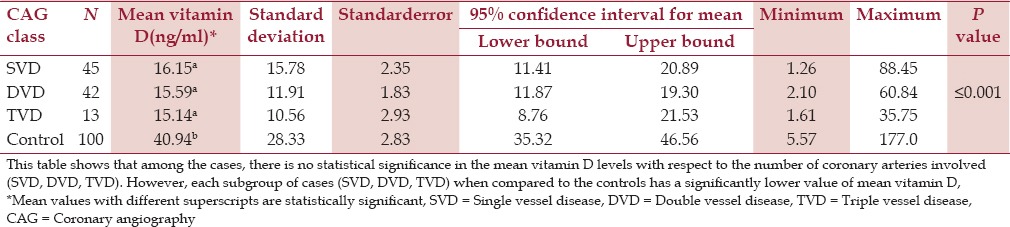
DVD was the majority finding among the cases who were deficient followed by SVD and TVD/multivessel disease. SVD was the majority finding among the cases who had insufficient or normal values of vitamin D. The mean value of vitamin D among those with SVD, DVD, and TVD/multivessel disease was 16.15 ng/mL, 15.59 ng/mL, and 15.14 ng/mL, respectively. This means that vitamin D level is least among TVD/multivessel disease followed by DVD and SVD. However, it is statistically insignificant with a P value of 0.966.
Coronary angiography categories versus controls with respect to vitamin D
The cases with SVD on CAG, when compared to the controls had low values of vitamin D, which were statistically significant (P = 0.0001). Similarly, vitamin D levels of the cases with DVD and TVD/multivessel disease on CAG had low vitamin D levels when compared to the controls. The difference between both the groups was again statistically significant (P = 0.001) [Table 4].
Table 4.
CAG categories versus controls with respect to vitamin D
Discussion
Our study suggests a significant correlation between vitamin D deficiency and chronic stable angina. In India, results similar to ours were seen by Sanjeev Kumar et al.[26] who studied 100 patients undergoing CAG. They found that the mean 25(OH) D level was 14.8 ± 9.1 ng/mL; vitamin D deficiency was present in 80% and only 7% had optimal 25(OH) D levels.
The high prevalence of vitamin D deficiency in our patient population was reflective of the generalized high prevalence rates of hypovitaminosis D in our region.[27] The high prevalence rates in our region can be explained by the prolonged winter period, inadequate sun exposure, coverage of the whole body with traditional clothes, generalized malnutrition, faulty food habits, less outdoor physical activity, and lack of vitamin D food fortification programs.
DVD was the majority finding among the cases who were vitamin D-deficient followed by SVD and TVD. However, SVD was the majority finding among cases having insufficient or normal values of vitamin D. Our study suggests no statistical significance between the severity of CAD and vitamin D deficiency. Similar results were obtained by Sanjeev et al.[31] from their study.
Our study suggests no statistical significance for vitamin D deficiency and gender. Some studies have shown that female patients of chronic stable angina are more vitamin D-deficient than males. In a study conducted by Martins et al.,[32] it was found that the 25(OH) D levels were lower in women compared to men. However, some have shown the contrary also. A study conducted by Hagenau et al.[31] showed that differences by gender were significant only in the infant age group (i.e., vitamin D status was worse among females) and were not prominent at an older age.
We observed that vitamin D level decreases among cases with age. The higher the age, the lesser is the vitamin D level. Similar results have been seen in many other studies. Lavie et al.[8] in his review mention aging as an important cause of vitamin D deficiency. Mac Laughlin et al.[33] in their study noticed that aging decreases the capacity of the human skin to produce vitamin D3. They found that a 70-year-old individual makes four times less vitamin D from the sun than a 20-year-old individual.
Our study does not suggest any seasonal trend in vitamin D deficiency. This in contrast to the studies by Ku et al.[34] and Lee et al.,[35] which support the greater prevalence of vitamin D deficiency in winter compared to other seasons. The reason behind our result could have been the overall decreased sun exposure in all seasons in our region or due to our region being in the temperate zone or there could be no relation between the two. A study conducted by Ann Burgaz et al.[36] found no significant difference between 25(OH) D concentrations with respect to sun exposure factors such as the number of hours spent outside in daylight during summer (P = 0.69) or winter (P = 0.99) and a preference for the sun or shadows (P = 0.59). Similar views were written by Hagenau et al.[31] in their review. Thus, it is again controversial and further studies are needed for the final conclusion.
Our study suggested an inverse relation between smoking and vitamin D level, which was supported by other studies as well. In a study conducted by Brutal et al.,[37] it was observed that dietary vitamin D intake, nonsmoking, and physical activity (in men) were significantly associated with higher concentrations of serum 25-hydroxyvitamin D.
We found no relation between the class of AOE and the level of vitamin D. We did not come across any study where the association of vitamin and class of angina on effort or angina equivalent was studied directly. So our study could be the first study in this direction and further work needs to be done to validate or refute the same.
Conclusion
The study showed a significant correlation between vitamin D deficiency with CAD. As ours is a developing region with limited resources, preventive measures need to be employed at the primary health care level regarding education on the importance of vitamin D. Public health programs aimed at fortification of food materials with vitamin D need to be employed in our region as has been done in the West. Overall, the cost of prevention would be much lesser than the socioeconomic effect that the cardiovascular diseases have on our society.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics — 2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–6. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Omran AR. Changing patterns of health and disease during the process of national development. In: Albrecht GL, Higgins PC, editors. Health, Illness and Medicine. Vol. 157. Chicago: Rand McNally; 1979. pp. 10–3. [Google Scholar]
- 3.Kannel WB, Feinleib M. Natural history of angina pectoris in the Framingham study. Prognosis and survival. Am J Cardiol. 1972;29:154–63. doi: 10.1016/0002-9149(72)90624-8. [DOI] [PubMed] [Google Scholar]
- 4.Brandi ML. Indications on the use of vitamin D and vitamin D metabolites in clinical phenotypes. Clin Cases Miner Bone Metab. 2010;7:243–50. [PMC free article] [PubMed] [Google Scholar]
- 5.Ross AC, Taylor CL, Yaktine AL, et al., editors. Dietary Reference Intakes for Calcium and Vitamin D. Washington (DC): National Academies Press (US); 2011. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. Available from: http://www.ncbi.nlm.nih.gov/books/NBK56070/ doi: 10.17226/13050. [PubMed] [Google Scholar]
- 6.Bouillon R, Van Cromphaut S, Carmeliet G. Intestinal calcium absorption: Molecular vitamin D mediated mechanisms. J Cell Biochem. 2003;88:332–9. doi: 10.1002/jcb.10360. [DOI] [PubMed] [Google Scholar]
- 7.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, et al. Vitamin D deficiency and risk for cardiovascular disease: A meta-analysis. Circulation. 2008;117:503–11. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavie CJ, Lee JH, Milani RV. Vitamin D and cardiovascular disease will it live up to its hype? J Am Coll Cardiol. 2011;58:1547–56. doi: 10.1016/j.jacc.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Snijder MB, Lips P, Seidell JC, Visser M, Deeg DJ, Dekker JM, et al. Vitamin D status and parathyroid hormone levels in relation to blood pressure: A population-based study in older men and women. J Intern Med. 2007;261:558–65. doi: 10.1111/j.1365-2796.2007.01778.x. [DOI] [PubMed] [Google Scholar]
- 10.Mattila C, Knekt P, Männistö S, Rissanen H, Laaksonen MA, Montonen J, et al. Serum 25-hydroxyvitamin D concentration and subsequent risk of type 2 diabetes. Diabetes Care. 2007;30:2569–70. doi: 10.2337/dc07-0292. [DOI] [PubMed] [Google Scholar]
- 11.Buttigliero C, Monagheddu C, Petroni P, Saini A, Dogliotti L, Ciccone G, et al. Prognostic role of vitamin D status and efficacy of vitamin D supplementation in cancer patients: A systematic review. Oncologist. 2011;16:1215–27. doi: 10.1634/theoncologist.2011-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134:1129–40. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nnoaham KE, Clarke A. Low serum vitamin D levels and tuberculosis: A systematic review and meta-analysis. Int J Epidemiol. 2008;37:113–9. doi: 10.1093/ije/dym247. [DOI] [PubMed] [Google Scholar]
- 14.Spector SA. Vitamin D and HIV: Letting the sun shine in. Top Antivir Med. 2011;19:6–10. [PMC free article] [PubMed] [Google Scholar]
- 15.Dörr J, Ohlraun S, Skarabis H, Paul F. Efficacy of vitamin D supplementation in multiple sclerosis (EVIDIMS trial): Study protocol for a randomized controlled trial. Trials. 2012;13:15. doi: 10.1186/1745-6215-13-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuohimaa P. Vitamin D and aging. J Steroid Biochem Mol Biol. 2009;114:78–84. doi: 10.1016/j.jsbmb.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 17.Zittermann A, Gummert JF, Börgermann J. Vitamin D deficiency and mortality. Curr Opin Clin Nutr Metab Care. 2009;12:634–9. doi: 10.1097/MCO.0b013e3283310767. [DOI] [PubMed] [Google Scholar]
- 18.Wu-wong JR, Nakane M, Ma J, Ruan X, Kroeger PE. Effects of Vitamin D analogs on gene expression profiling in human coronary artery smooth muscle cells. Atherosclerosis. 2006;186:20–8. doi: 10.1016/j.atherosclerosis.2005.06.046. [DOI] [PubMed] [Google Scholar]
- 19.Kendrick J, Targher G, Smits G, Chonchol M. 25-hydroxyvitamin D deficiency is independently associated with cardiovascular disease in the Third National Health and Nutrition Examination Survey. Atherosclerosis. 2009;205:255–60. doi: 10.1016/j.atherosclerosis.2008.10.033. [DOI] [PubMed] [Google Scholar]
- 20.Sugden JA, Davies JI, Witham MD, Morris AD, Struthers AD. Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabet Med. 2008;25:320–5. doi: 10.1111/j.1464-5491.2007.02360.x. [DOI] [PubMed] [Google Scholar]
- 21.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–38. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pittas AG, Harris SS, Stark PC, Dawson-Hughes B. The effects of calcium and vitamin D supplementation on blood glucose and markers of inflammation in nondiabetic adults. Diabetes care. 2007;30:980–6. doi: 10.2337/dc06-1994. [DOI] [PubMed] [Google Scholar]
- 23.Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: A double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. 2006;83:754–9. doi: 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 24.Rihal CS, Davis KB, Kennedy JW, Gersh BJ. The utility of clinical, electrocardiographic, and roentgenographic variables in the prediction of left ventricular function. Am J Cardiol. 1995;75:220–3. doi: 10.1016/0002-9149(95)80023-l. [DOI] [PubMed] [Google Scholar]
- 25.Alshishtawy MM. Vitamin D deficiency: This clandestine endemic disease is veiled no more. Sultan Qaboos Univ Med J. 2012;12:140–52. doi: 10.12816/0003106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Syal SK, Kapoor A, Bhatia E, Sinha A, Kumar S, Tewari S, et al. Vitamin D deficiency, coronary artery disease, and endothelial dysfunction: Observations from a coronary angiographic study in Indian patients. J Invasive Cardiol. 2012;24:385–9. [PubMed] [Google Scholar]
- 27.Zargar AH, Ahmad S, Masoodi SR, Wani AI, Bashir MI, Laway BA, et al. Vitamin D status in apparently healthy adults in Kashmir Valley of Indian subcontinent. Postgrad Med J. 2007;83:713–6. doi: 10.1136/pgmj.2007.059113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Norman AW, Bouillon R, Whiting SJ, Vieth R, Lips P. 13 th workshop consensus for vitamin D nutritional guidelines. J Steroid Biochem Mol Biol. 2007;103:204–5. doi: 10.1016/j.jsbmb.2006.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Connolly DC, Elveback LR, Oxman HA. Coronary heart disease in residents of Rochester, Minnesota. IV, Prognostic value of the resting electrocardiogram at the time of initial diagnosis of angina pectoris. Mayo Clin Proc. 1984;59:247–50. doi: 10.1016/s0025-6196(12)61257-9. [DOI] [PubMed] [Google Scholar]
- 30.Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, et al. ; American College of Cardiology; American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina). ACC/AHA 2002 guideline update for the management of patients with chronic stable angina — summary article: A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients With Chronic Stable Angina) J Am Coll Cardiol. 2003;41:159–68. doi: 10.1016/s0735-1097(02)02848-6. [DOI] [PubMed] [Google Scholar]
- 31.Hagenau T, Vest R, Gissel TN, Poulsen CS, Erlandsen M, Mosekilde L, et al. Global vitamin D levels in relation to age, gender, skin pigmentation and latitude: An ecologic metaregression analysis. Int. 2009;20:133–40. doi: 10.1007/s00198-008-0626-y. OsteoporosHYPERLINK “http://www.ncbi.nlm.nih.gov/pubmed/18458986” . [DOI] [PubMed] [Google Scholar]
- 32.Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: Data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–65. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 33.MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76:1536–8. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ku YC, Liu ME, Ku CS, Liu TY, Lin SL. Relationship between vitamin D deficiency and cardiovascular disease. World J Cardiol. 2013;5:337–46. doi: 10.4330/wjc.v5.i9.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee JH, O’Keefe JH, Bell D, Hensrud DD, Holick MF. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk factor? JACC. 2008;52:1949–56. doi: 10.1016/j.jacc.2008.08.050. [DOI] [PubMed] [Google Scholar]
- 36.Burgaz A, Akesson A, Oster A, Michaëlsson K, Wolk A. Associations of diet, supplement use, and ultraviolet B radiation exposure with vitamin D status in Swedish women during winter. Am J Clin Nutr. 2007;86:51399–404. doi: 10.1093/ajcn/86.5.1399. [DOI] [PubMed] [Google Scholar]
- 37.Brot C, Jorgensen NR, Sorensen OH. The infuence of smoking on vitamin D status and calcium metabolism. Eur J Clin Nutr. 1999;53:920–6. doi: 10.1038/sj.ejcn.1600870. [DOI] [PubMed] [Google Scholar]


