Abstract
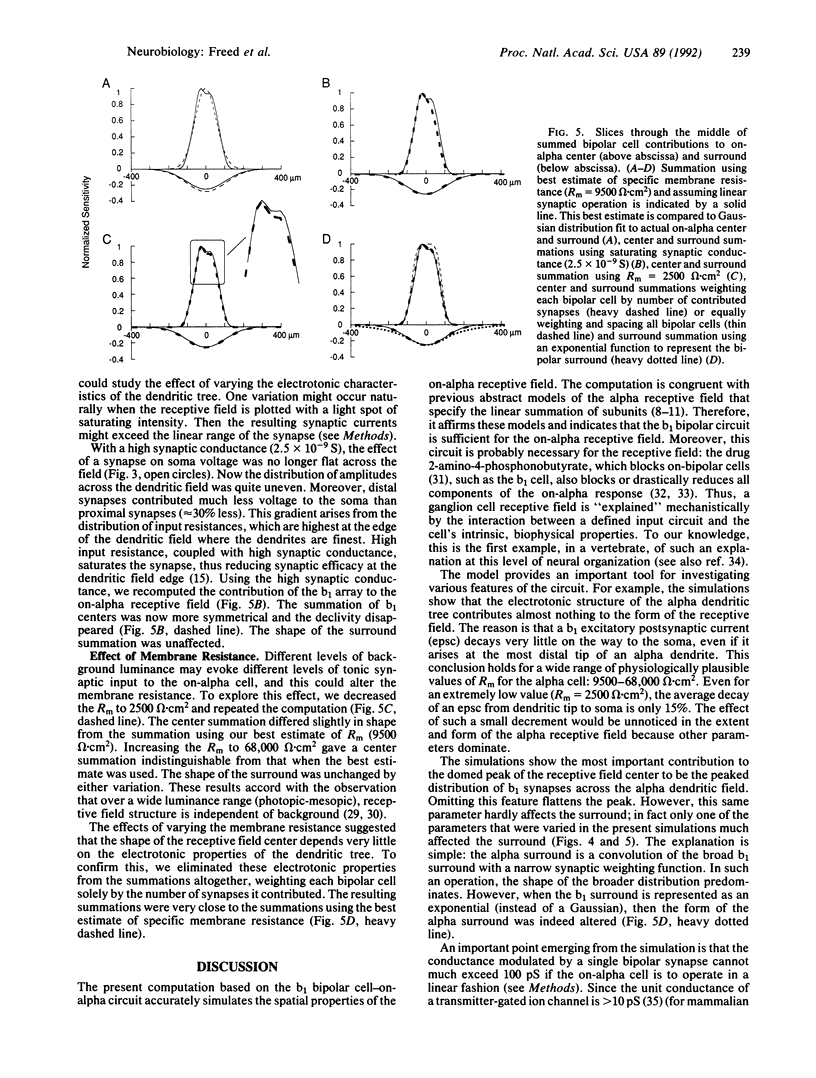
The on-alpha ganglion cell in the area centralis of the cat retina receives approximately 450 synapses from type b1 cone bipolar cells. This bipolar type forms a closely spaced array (9 microns), which contributes from 1 to 7 synapses per b1 cell throughout the on-alpha dendritic field. Here we use a compartmental model of an on-alpha cell, based on a reconstruction from electron micrographs of serial sections, to compute the contribution of the b1 array to the on-alpha receptive field. The computation shows that, for a physiologic range of specific membrane resistance (9500-68,000 omega.cm2) and a linear synapse, inputs are equally effective at all points on the on-alpha dendritic tree. This implies that the electrotonic properties of the dendritic tree contribute very little to the domed shapes of the receptive field center and surround. Rather, these shapes arise from the domed distribution of synapses across the on-alpha dendritic field. Various sources of "jitter" in the anatomical circuit, such as variation in bipolar cell spacing and fluctuations in the number of synapses per bipolar cell, are smoothed by the overall circuit design. However, the computed center retains some minor asymmetries and lumps, due to anatomical jitter, as found in actual alpha-cell receptive fields.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARLOW H. B. Summation and inhibition in the frog's retina. J Physiol. 1953 Jan;119(1):69–88. doi: 10.1113/jphysiol.1953.sp004829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISHOP P. O., KOZAK W., VAKKUR G. J. Some quantitative aspects of the cat's eye: axis and plane of reference, visual field co-ordinates and optics. J Physiol. 1962 Oct;163:466–502. doi: 10.1113/jphysiol.1962.sp006990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. P., Linsenmeier R. A. Centre components of cone-driven retinal ganglion cells: differential sensitivity to 2-amino-4-phosphonobutyric acid. J Physiol. 1989 Dec;419:77–93. doi: 10.1113/jphysiol.1989.sp017862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland B. G., Harding T. H., Tulunay-Keesey U. Visual resolution and receptive field size: examination of two kinds of cat retinal ganglion cell. Science. 1979 Sep 7;205(4410):1015–1017. doi: 10.1126/science.472720. [DOI] [PubMed] [Google Scholar]
- Cohen E., Sterling P. Microcircuitry related to the receptive field center of the on-beta ganglion cell. J Neurophysiol. 1991 Feb;65(2):352–359. doi: 10.1152/jn.1991.65.2.352. [DOI] [PubMed] [Google Scholar]
- Coleman P. A., Miller R. F. Measurement of passive membrane parameters with whole-cell recording from neurons in the intact amphibian retina. J Neurophysiol. 1989 Jan;61(1):218–230. doi: 10.1152/jn.1989.61.1.218. [DOI] [PubMed] [Google Scholar]
- Derrington A. M., Lennie P. The influence of temporal frequency and adaptation level on receptive field organization of retinal ganglion cells in cat. J Physiol. 1982 Dec;333:343–366. doi: 10.1113/jphysiol.1982.sp014457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Freeman A. W. The receptive-field spatial structure of cat retinal Y cells. J Physiol. 1987 Mar;384:49–79. doi: 10.1113/jphysiol.1987.sp016443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Hertz B. G., Lennie P. Convergence of rod and cone signals in the cat's retina. J Physiol. 1977 Jul;269(2):297–318. doi: 10.1113/jphysiol.1977.sp011903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C., Robson J. G. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966 Dec;187(3):517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferster D. X- and Y-mediated current sources in areas 17 and 18 of cat visual cortex. Vis Neurosci. 1990 Feb;4(2):135–145. doi: 10.1017/s0952523800002297. [DOI] [PubMed] [Google Scholar]
- Freed D. M. On the involvement of the locus ceruleus in Parkinson's disease. J Neuropsychiatry Clin Neurosci. 1990 Winter;2(1):114–115. doi: 10.1176/jnp.2.1.114. [DOI] [PubMed] [Google Scholar]
- Freed M. A., Sterling P. The ON-alpha ganglion cell of the cat retina and its presynaptic cell types. J Neurosci. 1988 Jul;8(7):2303–2320. doi: 10.1523/JNEUROSCI.08-07-02303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F., KATZ B. Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):424–448. doi: 10.1113/jphysiol.1952.sp004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstein S., Shapley R. M. Linear and nonlinear spatial subunits in Y cat retinal ganglion cells. J Physiol. 1976 Nov;262(2):265–284. doi: 10.1113/jphysiol.1976.sp011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyner R. W., Westerfield M., Moore J. W., Stockbridge N. A numerical method to model excitable cells. Biophys J. 1978 May;22(2):155–170. doi: 10.1016/S0006-3495(78)85481-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUFFLER S. W. Discharge patterns and functional organization of mammalian retina. J Neurophysiol. 1953 Jan;16(1):37–68. doi: 10.1152/jn.1953.16.1.37. [DOI] [PubMed] [Google Scholar]
- Koch C., Poggio T., Torre V. Retinal ganglion cells: a functional interpretation of dendritic morphology. Philos Trans R Soc Lond B Biol Sci. 1982 Jul 27;298(1090):227–263. doi: 10.1098/rstb.1982.0084. [DOI] [PubMed] [Google Scholar]
- Kolb H. The inner plexiform layer in the retina of the cat: electron microscopic observations. J Neurocytol. 1979 Jun;8(3):295–329. doi: 10.1007/BF01236124. [DOI] [PubMed] [Google Scholar]
- Linsenmeier R. A., Frishman L. J., Jakiela H. G., Enroth-Cugell C. Receptive field properties of x and y cells in the cat retina derived from contrast sensitivity measurements. Vision Res. 1982;22(9):1173–1183. doi: 10.1016/0042-6989(82)90082-7. [DOI] [PubMed] [Google Scholar]
- Lipton S. A., Tauck D. L. Voltage-dependent conductances of solitary ganglion cells dissociated from the rat retina. J Physiol. 1987 Apr;385:361–391. doi: 10.1113/jphysiol.1987.sp016497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F., Wässle H., Voigt T. Pharmacological modulation of the rod pathway in the cat retina. J Neurophysiol. 1988 Jun;59(6):1657–1672. doi: 10.1152/jn.1988.59.6.1657. [DOI] [PubMed] [Google Scholar]
- Nelson R., Kolb H. Synaptic patterns and response properties of bipolar and ganglion cells in the cat retina. Vision Res. 1983;23(10):1183–1195. doi: 10.1016/0042-6989(83)90032-9. [DOI] [PubMed] [Google Scholar]
- Peichl L., Wässle H. Size, scatter and coverage of ganglion cell receptive field centres in the cat retina. J Physiol. 1979 Jun;291:117–141. doi: 10.1113/jphysiol.1979.sp012803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkel D. H., Mulloney B. Electrotonic properties of neurons: steady-state compartmental model. J Neurophysiol. 1978 May;41(3):621–639. doi: 10.1152/jn.1978.41.3.621. [DOI] [PubMed] [Google Scholar]
- Rall W. Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol. 1967 Sep;30(5):1138–1168. doi: 10.1152/jn.1967.30.5.1138. [DOI] [PubMed] [Google Scholar]
- Redman S. Quantal analysis of synaptic potentials in neurons of the central nervous system. Physiol Rev. 1990 Jan;70(1):165–198. doi: 10.1152/physrev.1990.70.1.165. [DOI] [PubMed] [Google Scholar]
- Rodieck R. W., Stone J. Analysis of receptive fields of cat retinal ganglion cells. J Neurophysiol. 1965 Sep;28(5):832–849. doi: 10.1152/jn.1965.28.5.833. [DOI] [PubMed] [Google Scholar]
- Slaughter M. M., Miller R. F. 2-amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981 Jan 9;211(4478):182–185. doi: 10.1126/science.6255566. [DOI] [PubMed] [Google Scholar]
- Smith R. G., Sterling P. Cone receptive field in cat retina computed from microcircuitry. Vis Neurosci. 1990 Nov;5(5):453–461. doi: 10.1017/s0952523800000572. [DOI] [PubMed] [Google Scholar]
- Soodak R. E., Shapley R. M., Kaplan E. Fine structure of receptive-field centers of X and Y cells of the cat. Vis Neurosci. 1991 Jun;6(6):621–628. doi: 10.1017/s0952523800002613. [DOI] [PubMed] [Google Scholar]
- Stuart G. J., Redman S. J. Voltage dependence of Ia reciprocal inhibitory currents in cat spinal motoneurones. J Physiol. 1990 Jan;420:111–125. doi: 10.1113/jphysiol.1990.sp017903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardi N., Masarachia P. J., Sterling P. Structure of the starburst amacrine network in the cat retina and its association with alpha ganglion cells. J Comp Neurol. 1989 Oct 22;288(4):601–611. doi: 10.1002/cne.902880407. [DOI] [PubMed] [Google Scholar]
- Victor J. D., Shapley R. M. The nonlinear pathway of Y ganglion cells in the cat retina. J Gen Physiol. 1979 Dec;74(6):671–689. doi: 10.1085/jgp.74.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hateren J. H., Laughlin S. B. Membrane parameters, signal transmission, and the design of a graded potential neuron. J Comp Physiol A. 1990 Feb;166(4):437–448. doi: 10.1007/BF00192015. [DOI] [PubMed] [Google Scholar]