Abstract
Background:
Treatment of cutaneous leishmaniasis (CL) is occasionally highly resistant to pentavalent antimonials, the gold standard in pharmacotherapy of CL. Since there is no effective vaccine, the discovery of natural antileishmanial products as complementary therapeutic agents could be used to improve the current regimens.
Objective:
In this study in vitro and in vivo antileishmanial activities of osthole, a natural coumarin known to possess antibacterial and parasiticidal activities are evaluated.
Materials and Methods:
Leishmania major infected J774.A1 macrophages were treated with increasing concentrations of osthole. CL lesions of BALB/c mice were treated topically with 0.2% osthole.
Results:
Osthole exhibited dose-dependent leishmanicidal activity against intracellular amastigotes with IC50 value of 14.95 μg/ml. Treatment of CL lesions in BALB/c mice with osthole significantly declined lesion progression compared to untreated mice (P < 0.05), however did not result in recovery.
Conclusion:
Osthole demonstrated remarkable leishmanicidal activity in vitro. Higher concentrations of osthole may demonstrate the therapeutic property in vivo.
SUMMARY
In vitro and in vivo antileishmanial activities of osthole, a pernylated coumarin extracted from Prangos asperula Boiss., are studied against Leishmania major.

Keywords: Cutaneous leishmaniasis, Leishmania major, Leishmanicidal, Osthole
INTRODUCTION
Leishmaniasis is a poverty-associated disease with diverse clinical manifestations caused by several species of protozoan parasites of the genus Leishmania. According to the World Health Organization epidemiological report, tegumentary leishmaniasis (cutaneous leishmaniasis [CL] and mucocutaneous leishmaniasis) appears around 1.5 million cases per annum in 82 countries;[1] and exhibits dermatological symptoms in exposed parts of the skin leaving permanent deformative scars. Parasiticidal pentavalent antimonial compound (meglumine antimoniate, Glucantime®) remains the first-line medication in pharmacotherapy of CL with limitations such as toxicity, low tolerability due to painful parenteral administration, and inefficacy against resistant species.[2] Since there is no effective vaccine, improvement of the current regimens could be used to control CL.[3] Hence, the discovery of natural products with anti-Leishmania activity as complementary therapeutic agents is of high importance.[4]
Osthole (Osthol) a natural coumarin known as a component of Traditional Chinese Medicine has received considerable interest as a result of its various pharmacological activities. Osthole is reported to occur in a number of plants native to China, Mediterranean, and some Prangos spp. found in the Middle-East.[5,6,7] Prangos species have been traditionally used as antihelmintic, antifungal, and antibacterial remedies in the Middle-East and Mediterranean regions.[8,9]
Pharmacological activities of osthole are being markedly under investigation, provoking it a promising natural lead compound for novel drug discovery.[10] Osthole is reported to possess parasiticidal activities against Trichomonas vaginalis, a protozoan parasite[11] and Dactylogyrus intermedius, a helminth parasite.[12]
The aim of this study is to evaluate antileishmanial activities of osthole, extracted from Prangos asperula Boiss, in vitro using Leishmania major infected murine macrophage model and therapeutic property in vivo using infected BALB/c mice.
MATERIALS AND METHODS
Extraction and purification of osthole from Prangos asperula Boiss.
P. asperula Boiss. was collected in April - June 2013 from Dena mountains, environs of Yasouj (West of Iran), at an altitude of 2500 m above the sea level. The plant was identified by Azizollah Jafari, Department of Botany, Yasouj University. Osthole was isolated via n-hexane soxhlet extraction of 100 g air dried fruits. The extract was cooled at 4–5°C following evaporation of the solvent to form a semi pure yellow mass, later washed with chilled n-hexane for several times to enhance the purification. Eventually, the sample was recrystallized until pure osthole crystalline was formed.[13] A voucher specimen of the plant is retained at the herbarium of Department of Pharmacognosy, School of Pharmacy, Isfahan University of Medical Sciences, Iran (1126).
Parasite culture
L. major (MRHO/IR/75/ER) has been isolated from the previously infected BALB/c mice spleens and kept cryopreserved at −80°C. The promastigotes were warmed up to 25°C and incubated for 3–5 days in N.N.N medium; subsequently, passaged at 25°C in RPMI 1640 medium (PAA, Australia), supplemented with 10% inactivated fetal bovine serum (Sigma-Aldrich, US), 100 U/ml penicillin, and 100 μg/ml streptomycin.[14]
Macrophage culture
Murine macrophage cell lines (J774 A.1) were purchased from Pasteur Institute, Tehran, Iran. The cryopreserved cell lines were melted, immediately washed with phosphate-buffered saline (PBS), and cultured in RPMI 1640 supplemented with 20% fetal bovine serum and 100 μg/ml streptomycin, subsequently, incubated in 5% CO2 at 37°C. By the time the macrophages developed pseudopodia and adhered to the bottom of the plates, they were harvested utilizing a cell scraper and washed by PBS (centrifuged at 1500 rpm for 5 min), and seeded in to 6 well culture plates at concentration of 2 × 106 macrophages/well.[15]
Evaluation of anti-amastigote activity
Promastigotes in the stationary phase of growth were centrifuged 3000 rpm for 10 min at room temperature, then resuspended and washed with medium. To infect the macrophages, parasites at the ratio of seven promastigotes per macrophage were introduced to the cultures in which the fresh medium had been replaced previously. The infected cultures were incubated at 33°C for 6 h, and to complete the phagocytosis process at 37°C for 24 h. Subsequently, they were washed with medium to remove the unphagocytosed parasites. Each well was daily observed using an invert microscope to determine the number of amastigotes and intensity of the infection. By the time 80% of the macrophages were infected, osthole with serial concentrations (5–50 μg/ml) was added (osthole was dissolved in dimethyl sulfoxide [DMSO] and diluted by complete medium). DMSO treated and untreated infected macrophages were used as negative controls and 100 μg/ml meglumine antimoniate, MA-treated (Glucantime®, Aventis, France) infected macrophages was used as positive control.
The cells were fixed with methanol and stained with Giemsa 12, 24, 48, and 72 h posttreatment. Anti-amastigote activity was evaluated by numeration of the amastigotes in 100 macrophages. Each assay was performed in triplicate. The IC50 value was determined using a logarithmic dose – response regression curve.[16]
Animals
The in vivo procedures of this experimental study were in compliance with guidelines of Isfahan University of Medical Sciences (Isfahan, Iran) to keep and use the laboratory animals in accordance with the Animal Ethics Committee.
The animals used in this research provided by the Center for Research and Training in Skin Diseases and Leprosy, Tehran, Iran, were 6–8 week-old-mice, 20 ± 5 g weight female BALB/c type.
The animals were housed in standard cages with access to water and standard food at 21°C ± 2°C, and 40–50% humidity conditions in a colony room under 12:12 h light/dark cycle.[17]
Infecting BALB/c mice by injection of Leishmania major
CL lesions were induced at the base of tail via subcutaneous injection of 107 viable promastigotes in 0.1 ml PBS.[18]
Treatment of infected animals
Four weeks later when CL lesions were developed, mice with mean lesion size ≈2.3 ± 0.2 mm were selected and divided into four groups of eight (A, B, C, and D). Mice of group (A) were treated topically with 0.2% osthole[19] (osthole dissolved in 1 ml DMSO diluted by xanthan gel up to 10 ml), group (B) received 100 mg/kg Glucantime® intralesionally, and group (C) was treated with 10% (v/v) DMSO-xanthan gel. The treatment was performed daily for 14 consecutive days, and mice of group (D) were left untreated.[19]
Measurement of lesion size
Before, during, and 14 days after the treatment period the horizontal and vertical diameters (mm) of lesions were measured using calipers every 4 days. Mean lesion size in each animal was determined according to the formula S = (D + d)/2.[17]
Statistical analysis
In this study, all the results are represented in means ± standard deviation. The statistical data analysis was done utilizing independent samples t-test for the in vitro study and paired samples t-test for the in vivo study. P < 0.05 is considered statistically significant.
RESULTS
In vitro study
The results obtained from numeration of intracellular amastigotes demonstrated that osthole decreased the viability of amastigotes in J774.A1 macrophages in a dose-dependent manner with IC50 value of 14.95 μg/ml after 72 h [Figure 1]. Anti-amastigote activity against L. major was found similar to Glucantime® at concentrations ≥ 20 μg/ml (P > 0.05). No significant difference in viability of amastigotes and macrophages in negative controls were observed (P > 0.05). Osthole affected cell viability of J774.A1 macrophages at concentrations ≥30 μg/ml.
Figure 1.
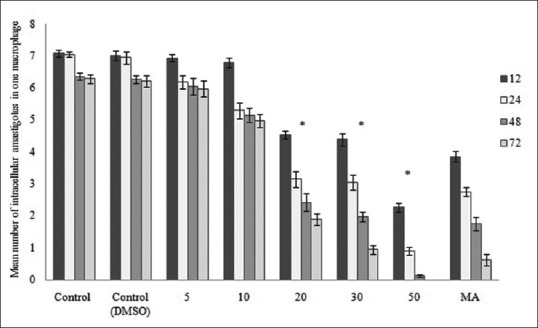
Mean number of intracellular amastigotes in negative controls, increasing concentrations of osthole, and positive control in 12, 24, 48, and 72 h. Number of amastigotes is significantly decreased at concentrations ≥20 μg/ml (P < 0.05)
In vivo study
BALB/c mice infected with promastigotes of L. major developed progressive lesions 4 weeks after the injection of inoculum. Mice of groups C and D (DMSO-xanthan treated and untreated) developed larger lesions, (5.19 ± 0.19 mm) and (5.11 ± 0.28 mm), respectively. Mean lesion size of group A (osthole treated) was found to be significantly less compared to groups C and D (P < 0.05; 3.9 ± 0.32 mm). Unlike group B (Glucantime® treated) subsidence of lesion size did not occur in group A [Figure 2].
Figure 2.
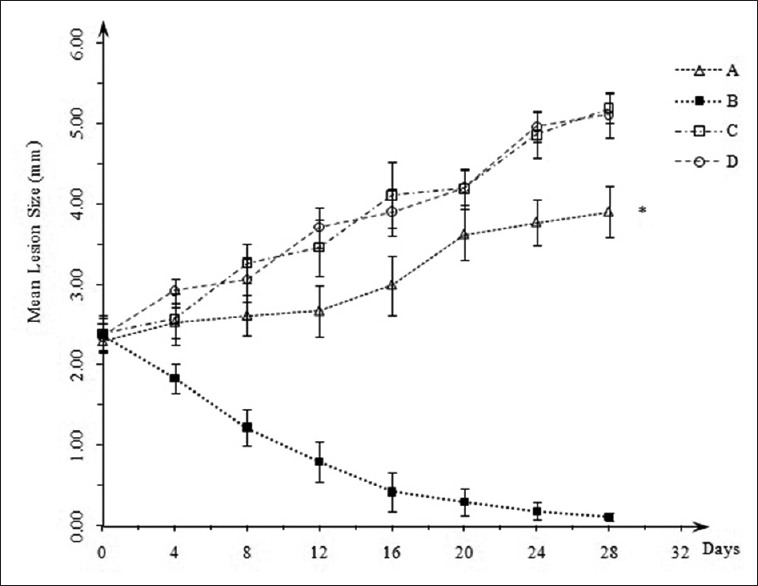
Mean lesion size of osthole treated (A) Glucantime® treated (B), dimethyl sulfoxide-xanthan treated (C) and untreated (D) mice. Lesion size of group (A) is significantly less than groups (C) and (D) (P < 0.05)
DISCUSSION
Phytochemicals are a major resource for discovery of novel parasiticidal agents.[20] Osthole, a natural prenylated coumarin, is extensively studied for its significant pharmacological activities including antithrombotic,[21] antidiabetic,[22] antiosteoporotic,[23] anticancer,[24] neuroprotective,[25] and hepatoprotective;[26] still there is few data on its parasiticidal effect. This experimental study represents the first description of anti-Leishmania activity of osthole.
Anti-Leishmania activity of several coumarins has been addressed recently. Coumarins extracted from Galipea panamensis displayed activity against amastigotes of Leishmania panamensis;[27] (-) heliettin and 3-(1'-dimethylallyl)-decursinol, isolated coumarins of Helietta apiculata demonstrated activity against promastigote form of Leishmania amazonensis, and reduced the parasite load in lesions of BALB/c mice similar to Glucantime®.[28] Our results are in line with previous studies, which demonstrated the activity of prenylated coumarins, umbelliprenin, and auraptene from Ferula szowitsiana against L. major.[29] (-) mammea A/BB, a prenylated coumarin, isolated from the leaves of Calophyllum brasiliense was shown to be active against both promastigote and amastigote forms of L. amazonensis and Leishmania braziliensis[16,30] and later it was shown that topical and intramuscular treatment with (-) mammea A/BB significantly decreased CL lesions of BALB/c mice similar to Glucantime®.[19]
Significant leishmanicidal activity of osthole in vitro inspired the researchers to conduct the in vivo study. Results obtained from the in vivo study did not demonstrate recovery of CL lesions in BALB/c mice treated with osthole; however, progression of lesions was significantly declined compared to untreated mice. Unlike untreated mice, secondary bacterial infection was not observed in mice treated with osthole, which confirms its antibacterial activity. Osthole is reported to possess antibacterial activity against Gram-positive and Gram-negative bacteria.[31]
Microarray gene expression profile of Mycobacterium tuberculosis displayed that genes encoding fumarate reductase were considerably inhibited when exposed to osthole.[32] Therefore, it appears that leishmanicidal activity of osthole could be contributed by the relevant downregulation of fumarate reductase, which is an important enzyme in the parasite respiratory chain.
It should be noted that osthole is poorly soluble in most commonly used solvents, and DMSO was found appropriate to dissolve osthole. Cytotoxic effects of DMSO reported previously,[33,34] restricted the researchers to apply concentrations of osthole higher than 0.2% in vivo; nevertheless, it was not considered as an obstacle in the in vitro study due to over 200-fold dilution by the medium. Data obtained from the measurement of CL lesions in concentrations <0.2% are not discussed due to insignificant efficacy. However, results are indicative of significant decrease in lesion progression when osthole is used at 0.2%.
CONCLUSIONS
In vitro study exhibits the considerable leishmanicidal activity of osthole against L. major. According to the in vivo study, osthole delays progression of CL lesions, nevertheless does not show therapeutic property when administered topically at 0.2%. Further studies on higher concentrations may demonstrate higher efficacy.
Financial support and sponsorship
Isfahan University of Medical Sciences.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHORS
Elaheh Kordzadeh Kermani: School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences.
Seyed Ebrahim Sajjadi: School of Pharmacy and Pharmaceutical Sciences, Isfahan University of Medical Sciences.
Seyed Hossein Hejazi: School of Medicine, Isfahan University of Medical Sciences.
Reza Arjmand: School of Medicine, Ahvaz Jundishapur University of Medical Sciences.
Sedigheh Saberi: School of Medicine, Isfahan University of Medical Sciences.
Abbas Ali Eskandarian: School of Medicine, Isfahan University of Medical Sciences.
REFERENCES
- 1.Geneva: World Health Organization; 2010. World Health Organization. Control of the Leishmaniases. World Health Organization Technical Report Series 949; pp. xii–xiii. [PubMed] [Google Scholar]
- 2.Minodier P, Parola P. Cutaneous leishmaniasis treatment. Travel Med Infect Dis. 2007;5:150–8. doi: 10.1016/j.tmaid.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Berman J. Clinical status of agents being developed for leishmaniasis. Expert Opin Investig Drugs. 2005;14:1337–46. doi: 10.1517/13543784.14.11.1337. [DOI] [PubMed] [Google Scholar]
- 4.Rocha LG, Almeida JR, Macêdo RO, Barbosa-Filho JM. A review of natural products with antileishmanial activity. Phytomedicine. 2005;12:514–35. doi: 10.1016/j.phymed.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 5.Abyshev AZ. Coumarins from the fruits of Prangos ferulacea. Chem Nat Compd. 1968;4:130–1. [Google Scholar]
- 6.Kuznetsova GA, Danchul TY, Sokolova EA, Kuzmina LV. Coumarin from the roots and epigeal mass of Prangos acaulis. Chem Nat Compd. 1979;15:752. [Google Scholar]
- 7.Sajjadi SE, Zeinvand H, Shokoohinia Y. Isolation and identification of osthol from the fruits and essential oil composition of the leaves of Prangos asperula Boiss. Res Pharm Sci. 2009;4:19–23. [Google Scholar]
- 8.Baser KH, Demirci B, Demirci F, Bedir E, Weyerstahl P, Marschall H, et al. A new bisabolene derivative from the essential oil of Prangos uechtritzii fruits. Planta Med. 2000;66:674–7. doi: 10.1055/s-2000-8627. [DOI] [PubMed] [Google Scholar]
- 9.Ulubelen A, Topcu G, Tan N, Olçal S, Johansson C, Uçer M, et al. Biological activities of a Turkish medicinal plant, Prangos platychlaena. J Ethnopharmacol. 1995;45:193–7. doi: 10.1016/0378-8741(94)01215-l. [DOI] [PubMed] [Google Scholar]
- 10.You L, Feng S, An R, Wang X. Osthole: A promising lead compound for drug discovery from a traditional Chinese medicine (TCM) Nat Prod Commun. 2009;4:297–302. [PubMed] [Google Scholar]
- 11.Xia ML, Dun LF, XU JT. An in vitro test of killing effect of osthol against Trichomonas vaginalis. China Trop Med. 2008;3:013. [Google Scholar]
- 12.Wang KY, Yao L, Du YH, Xie JB, Huang JL, Yin ZQ. Anthelmintic activity of the crude extracts, fractions, and osthole from radix Angelicae pubescentis against Dactylogyrus intermedius in goldfish (Carassius auratus) in vivo. Parasitol Res. 2011;108:195–200. doi: 10.1007/s00436-010-2058-9. [DOI] [PubMed] [Google Scholar]
- 13.Mehregan I, Sajjadi SE. Chemical composition of the essential oil of Prangos asperula Boiss. Subsp. Haussknechtii (BOISS.) Herrnst. Etheyn fruits. Daru J Pharm Sci. 2003;11:79–81. [Google Scholar]
- 14.Gamboa-León MR, Aranda-González I, Mut-Martín M, García-Miss MR, Dumonteil E. In vivo and in vitro control of Leishmania mexicana due to garlic-induced NO production. Scand J Immunol. 2007;66:508–14. doi: 10.1111/j.1365-3083.2007.02000.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Goncalves R, Mosser DM. The isolation and characterization of murine macrophages. Curr Protoc Immunol. 2008;83:1–14. doi: 10.1002/0471142735.im1401s83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brenzan MA, Nakamura CV, Dias Filho BP, Ueda-Nakamura T, Young MC, Cortez DA. Anti-leishmanial activity of crude extract and coumarin from Calophyllum brasiliense leaves against Leishmania amazonensis. Parasitol Res. 2007;101:715–22. doi: 10.1007/s00436-007-0542-7. [DOI] [PubMed] [Google Scholar]
- 17.Ezatpour B, Saedi Dezaki E, Mahmoudvand H, Azadpour M, Ezzatkhah F. In vitro and in vivo antileishmanial effects of Pistacia khinjuk against Leishmania tropica and Leishmania major. Evid Based Complement Alternat Med 2015. 2015 doi: 10.1155/2015/149707. 149707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan M, Bulinski CJ, Chang KP, Fong D. A microplate assay for Leishmania amazonensis promastigotes expressing multimeric green fluorescent protein. Parasitol Res. 2003;89:266–71. doi: 10.1007/s00436-002-0706-4. [DOI] [PubMed] [Google Scholar]
- 19.Tiuman TS, Brenzan MA, Ueda-Nakamura T, Filho BP, Cortez DA, Nakamura CV. Intramuscular and topical treatment of cutaneous leishmaniasis lesions in mice infected with Leishmania amazonensis using coumarin (-) mammea A/BB. Phytomedicine. 2012;19:1196–9. doi: 10.1016/j.phymed.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Ndjonka D, Rapado LN, Silber AM, Liebau E, Wrenger C. Natural products as a source for treating neglected parasitic diseases. Int J Mol Sci. 2013;14:3395–439. doi: 10.3390/ijms14023395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko FN, Wu TS, Liou MJ, Huang TF, Teng CM. Inhibition of platelet thromboxane formation and phosphoinositides breakdown by osthole from Angelica pubescens. Thromb Haemost. 1989;62:996–9. [PubMed] [Google Scholar]
- 22.Liang HJ, Suk FM, Wang CK, Hung LF, Liu DZ, Chen NQ, et al. Osthole, a potential antidiabetic agent, alleviates hyperglycemia in db/db mice. Chem Biol Interact. 2009;181:309–15. doi: 10.1016/j.cbi.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Tang DZ, Hou W, Zhou Q, Zhang M, Holz J, Sheu TJ, et al. Osthole stimulates osteoblast differentiation and bone formation by activation of beta-catenin-BMP signaling. J Bone Miner Res. 2010;25:1234–45. doi: 10.1002/jbmr.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang D, Gu T, Wang T, Tang Q, Ma C. Effects of osthole on migration and invasion in breast cancer cells. Biosci Biotechnol Biochem. 2010;74:1430–4. doi: 10.1271/bbb.100110. [DOI] [PubMed] [Google Scholar]
- 25.Chao X, Zhou J, Chen T, Liu W, Dong W, Qu Y, et al. Neuroprotective effect of osthole against acute ischemic stroke on middle cerebral ischemia occlusion in rats. Brain Res. 2010;1363:206–11. doi: 10.1016/j.brainres.2010.09.052. [DOI] [PubMed] [Google Scholar]
- 26.Zhang J, Xue J, Wang H, Zhang Y, Xie M. Osthole improves alcohol-induced fatty liver in mice by reduction of hepatic oxidative stress. Phytother Res. 2011;25:638–43. doi: 10.1002/ptr.3315. [DOI] [PubMed] [Google Scholar]
- 27.Arango V, Robledo S, Séon-Méniel B, Figadère B, Cardona W, Sáez J, et al. Coumarins from Galipea panamensis and their activity against Leishmania panamensis. J Nat Prod. 2010;73:1012–4. doi: 10.1021/np100146y. [DOI] [PubMed] [Google Scholar]
- 28.Ferreira ME, de Arias AR, Yaluff G, de Bilbao NV, Nakayama H, Torres S, et al. Antileishmanial activity of furoquinolines and coumarins from Helietta apiculata. Phytomedicine. 2010;17:375–8. doi: 10.1016/j.phymed.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Iranshahi M, Arfa P, Ramezani M, Jaafari MR, Sadeghian H, Bassarello C, et al. Sesquiterpene coumarins from Ferula szowitsiana and in vitro antileishmanial activity of 7-prenyloxycoumarins against promastigotes. Phytochemistry. 2007;68:554–61. doi: 10.1016/j.phytochem.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Brenzan MA, Ferreira IC, Lonardoni MV, Honda PA, Filho ER, Nakamura CV, et al. Activity of extracts and coumarins from the leaves of Calophyllum brasiliense on Leishmania braziliensis. Pharm Biol. 2008;46:380–6. [Google Scholar]
- 31.Rosselli S, Maggio A, Bellone G, Formisano C, Basile A, Cicala C, et al. Antibacterial and anticoagulant activities of coumarins isolated from the flowers of Magydaris tomentosa. Planta Med. 2007;73:116–20. doi: 10.1055/s-2006-951772. [DOI] [PubMed] [Google Scholar]
- 32.Wei J, Guo N, Liang J, Yuan P, Shi Q, Tang X, et al. DNA microarray gene expression profile of Mycobacterium tuberculosis when exposed to osthole. Pol J Microbiol. 2013;62:23–30. [PubMed] [Google Scholar]
- 33.Qi W, Ding D, Salvi RJ. Cytotoxic effects of dimethyl sulphoxide (DMSO) on cochlear organotypic cultures. Hear Res. 2008;236:52–60. doi: 10.1016/j.heares.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Da Violante G, Zerrouk N, Richard I, Provot G, Chaumeil JC, Arnaud P. Evaluation of the cytotoxicity effect of dimethyl sulfoxide (DMSO) on Caco2/TC7 colon tumor cell cultures. Biol Pharm Bull. 2002;25:1600–3. doi: 10.1248/bpb.25.1600. [DOI] [PubMed] [Google Scholar]


