Abstract
Background:
In Togo, malaria constitutes a major public health problem but, until now, the population still mostly relies on herbal medicine for healing. This study aimed to document medicinal plants used for malaria therapy in the Plateau region of the country.
Methodology:
Semi-structured questionnaire interviews were used to gather ethnobotanical and sociodemographic data from traditional healers of the study area.
Results:
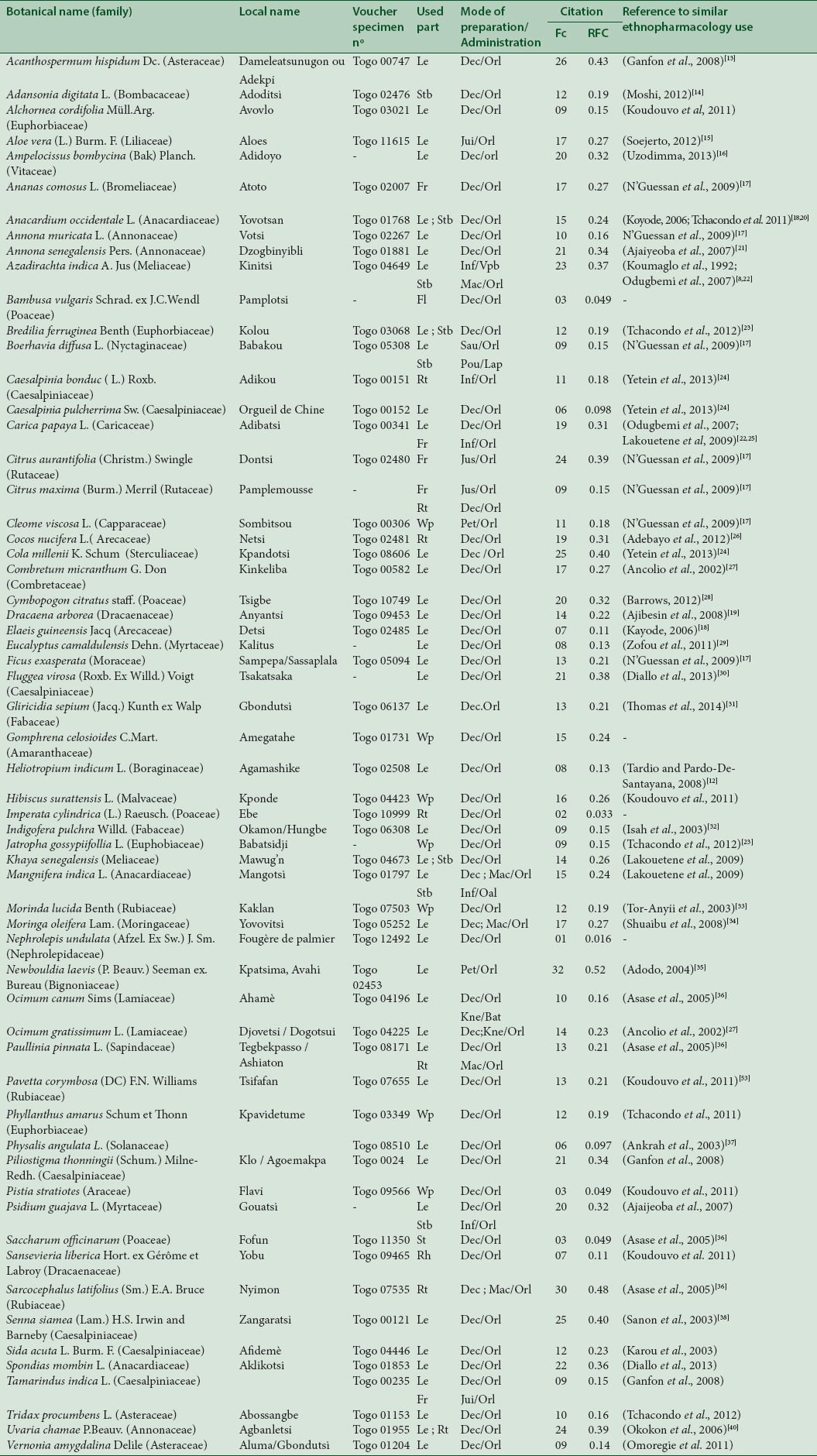
A total of 61 plants species belonging to 33 families were found to be in use for malaria therapy in the Plateau region. Caesalpiniaceae were the most represented family with 7 species, followed by Euphorbiaceae and Poaceae with 4 species each. According to the relative frequency of citation (RFC), Newbouldia laevis Seem. (RFC =0.52), Sarcocephalus latifolius (Sm.) E.A. Bruce (RFC =0.48), Acanthospermum hispidum DC. (RFC =0.43), and Senna siamea (Lam.) H.S. Irwin and Barneby (RFC =0.40) were the most cited in the treatment of malaria in the traditional medicine in the Plateau region. The parts of plants used could either be the barks, roots, leaves, or whole plants. The recipes also could be a combination of various species of plants or plant parts.
Conclusion:
This study highlights the potential sources for the development of new antimalarial drugs from indigenous medicinal plants found in the Plateau region of Togo. Such results could be a starting point for in vitro antimalarial screenings.
SUMMARY
61 plants species from 33 families are use for malaria therapy in the Plateau region of Togo
The main families are Caesalpiniaceae Euphorbiaceae and Poaceae
The most used species are Newbouldia laevis Seem. (RFC = 0.52), Sarcocephalus latifolius (Sm.) E.A. Bruce (RFC = 0.48), Acanthospermum hispidum DC. (RFC = 0.43), and Senna siamea (Lam.) H.S. Irwin and Barneby (RFC = 0.40)
Abbreviations Used: RFC: Relative frequency of citation, FC: Frequency of citation, Dec: Decoction, Orl: Oral route, Mac: Maceration, Jui: Juice, Inf: Infusion, Sau: Sauce, Kne: Kneading, Le: Leaves, Rt: Roots, Wp: Whole plant, St: Stem, Stb: Stem bark, Rh: Rhizome, Fr: Fruits, Pf: Plasmodium falciparum, IC50: Concentration of extract killing 50% parasites
Keywords: Caesalpiniaceae, malaria, medicinal plants, relative frequency of citation
INTRODUCTION
Malaria is a global disease that is predominant in the tropics and caused by blood parasites, Plasmodium falciparum, Plasmodium ovale, Plasmodium malariae, Plasmodium vivax, and Plasmodium knowlesi.[1] About 3.3 billion people worldwide are at risk of malaria. In 2013, there were about 216 million cases and approximately 528,000 malaria deaths (range: 315,000–689,000). Globally, 90% of all deaths from the disease were recorded in the World Health Organization (WHO) African Region, mainly in children under five years of age.[2]
In Togo, malaria was on average 40% of outpatient visits and 26% of hospitalizations in public health facilities in 2010 with an average hospital stay of 5 days. The hospital mortality rate of malaria was 21% in 2010 and children of 0–5 years old were the most affected in a proportion of 48%.[3] Currently, the programs against malaria integrate several areas including the prevention and the treatment. Since the declaration of WHO Alma-Ata in 1978,[4] the WHO recognizes and encourages the use of resources of medicine and traditional pharmacopeia in primary health care. Despite the scientific advances made by modern medicine, the WHO estimates that 80% of Africa's population still use traditional medicine for primary health care.[5]
An effective management of malaria requires the use of all resources available, accessible, and culturally acceptable.[2] Worldwide, the traditional pharmacopeia has played and continues to play a very important role in the discovery of new molecules of therapeutic interest and particularly in the fight against malaria. With the emergence of resistance of the parasite P. falciparum to conventional synthetic drugs, the research for new therapeutic targets by ethnopharmacological methods is an interesting approach, including the search for new antimalarial drugs.[5]
In Togo, few studies had focused on medicinal plants used in the treatment of malaria.[6,7,8,9,10,11] Koumaglo et al. evaluated the antimalarial activity of compounds isolated from Azadirachta indica and Morinda lucida.[8,9] Other studies conducted by Gbeassor et al. concerned the crude extracts of some widely used plants;[6,7] however, these screenings were not preceded by an ethnobotanical survey. Only the study of Koudouvo et al. was a complete study including the survey and the in vitro screening of the most cited plants.[10,11] This study was conducted in the maritime region of the country and until now; there is no published data on the medicinal plants used to treat malaria in the four other regions of Togo. Therefore, this study was conducted in the Plateau region of Togo, to further explore these antimalarial plants and to assess for the validity of their traditional therapeutic uses through phytochemical information and pharmacological characteristics.
METHODOLOGY
The study area
Togo is the West African country located in the tropics (6°6'N to 11°8'N) bounded on the North by Burkina Faso, to the south by the Atlantic Ocean, to the East by Benin and West by Ghana [Figure 1]. The country is divided into five economic regions from North to South: The savannah region, Kara region, central region, Plateau region, and Maritime region.
Figure 1.
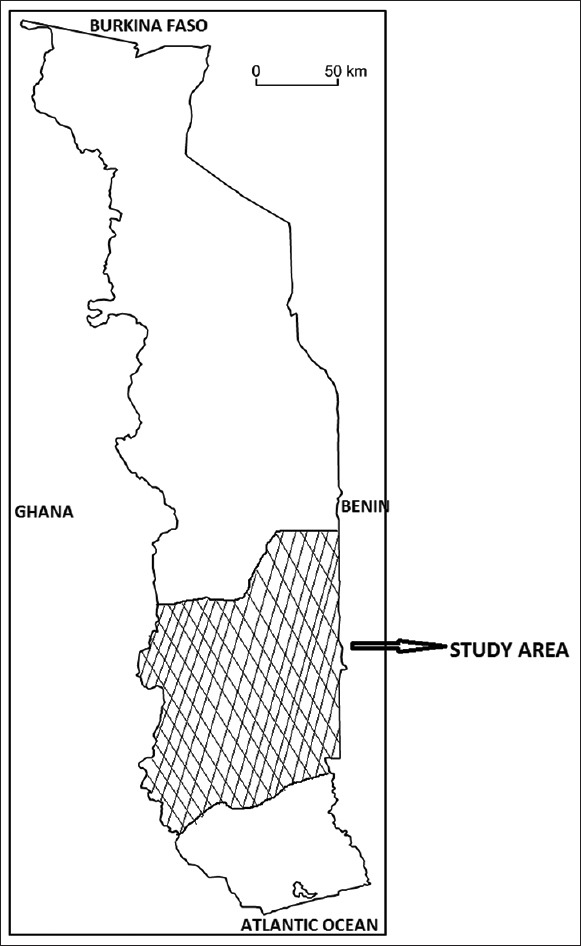
Map of Togo showing the study area, the Plateau Region
The survey was conducted in the Plateau region from May to July 2014. This region covers an area of 16.980 km2 that represent about 30% of Togo area. Its administrative center is Atakpamé. It prevails in the region a tropical humid climate. There are two rainy seasons and two dry seasons. The longest rainy season is from April to July and the lowest September-October. Annual rainfall average is about 1200–1600 mm. In South-West is a mountainous zone where there are still tropical and subtropical forests, despite expansion of coffee and cocoa plantations. Three major ethnic groups are native to the area: The Adja-Ewe, Ana-Ife, and Akposso-Akébou.
Ethnobotanical survey
The ethnobotanical survey was conducted using a full oral questioning of traditional healers.
Questions were focused on the sociodemographic profile of the traditional healer and the knowledge of medicinal plants used in to fight malaria:
Traditional healer identity, i.e., name and surname, sex, age, and educational level
Knowledge origin
Diagnosis, i.e. main symptoms
Remedy: Local names of the plants, used parts, period of harvest of plants materials, remedy formulation, administration route, dosage, and duration of the treatment.
Plant samples were collected in the field and pictures were taken to aid in the identification. Identification was made by Botany Department of Lomé University by comparison with available voucher specimens. Nomenclature of species was done using the online data base of IPNI website: Http://www.ipni.org/ipni/plantnamesearchpage.do.
Data analysis
Microsoft Excel was used to calculate the different average and to draw graphics. The importance of each plant in the treatment of malaria was assessed by the relative frequency of citation (RFC) calculated using the following formula:[12]
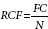
where FC was the number of people who mentioned the use of the species and N the total number of individuals.
RESULTS
Demographic data and knowledge about malaria
Investigations were conducted with 62 respondents who had knowledge of antimalarial plants including 22 women and 40 men. The knowledge of plants uses was received from parents and society, by learning from other traditional healers or in academic or professional studies [Table 1].
Table 1.
Demographic data of the informants (n=62)
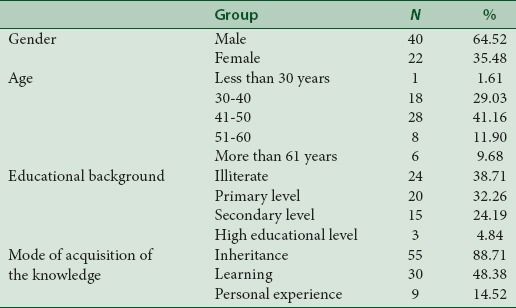
Malaria diagnosis in patients was done with some clinical signs that include fever (82.25%), headache (70.97%), shivering (51.61%), weakness (29.03%), and lack of appetite (16.13%). However, 7 respondents (11.29%) asserted that they used parasitological diagnostic of malaria before the treatment.
Plants used for the treatment of malaria
A total of 61 plant species belonging to 33 families were mentioned by respondents as curing malaria. Caesalpiniaceae family, with 7 species was the most represented, followed by Euphorbiaceae and Poaceae with 4 species each. For the rest, 19 families were represented by only one species [Figure 2]. The calculated RFC indicated that species such as Newbouldia laevis (RFC = 0.52), Sarcocephalus latifolius (RFC = 0.48), Acanthospermum hispidum Dc (RFC = 0.43), and Senna siamea (RFC = 0.40) were the most used in the treatment of malaria in traditional medicine in the Plateau Region [Table 2].
Figure 2.
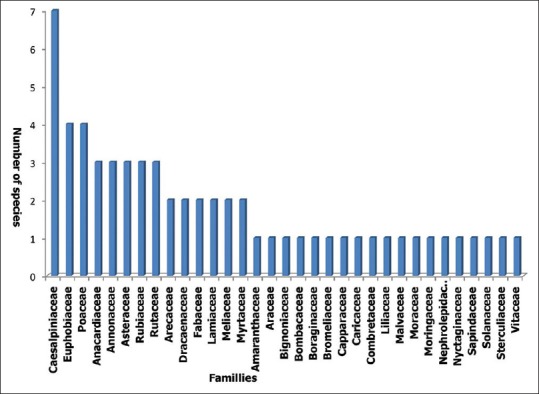
Antimalarial plant species distribution among families
Table 2.
Plant species used against malaria
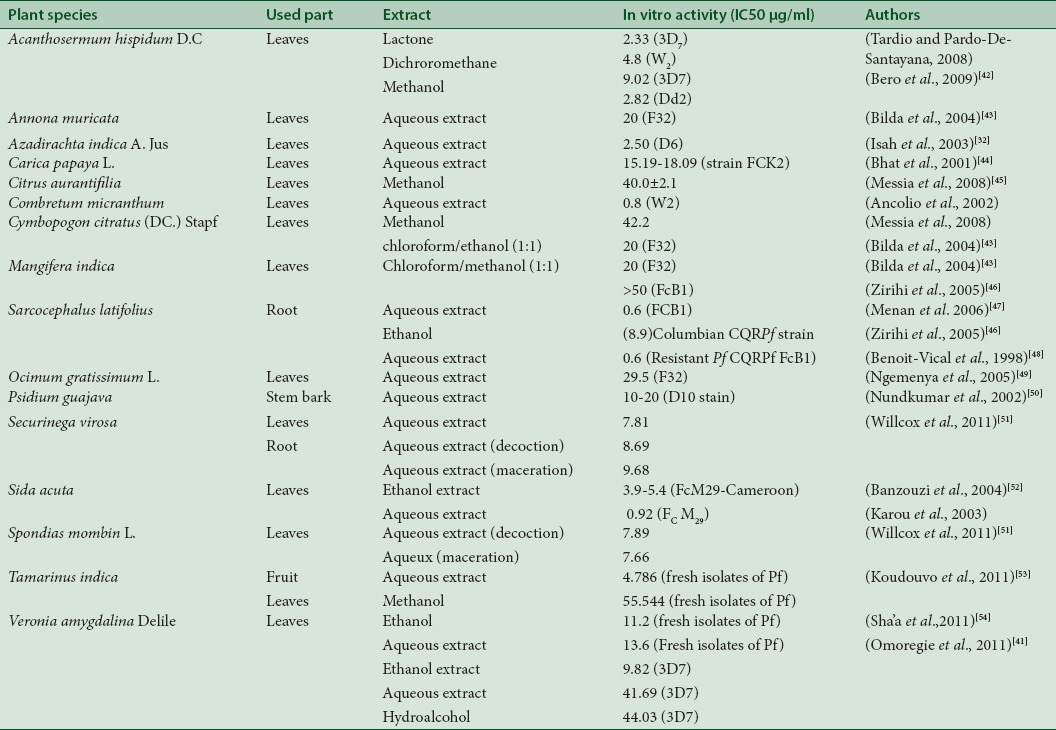
A bibliographic research was made to seek for previous citations of the recorded species in similar studies. The results revealed are presented in Table 2. According to these results, only four plants namely Bambusa vulgaris Schrad. Ex J.C. Wendl, Gomphrena celosioides C. Mart., Imperata cylindrica (L.) Raeusch. and Nephrolepis undulata (Afzel. Ex Sw.) J. Sm. were not previously cited in similar studies. In addition, the bibliographic research focused the screened species cited by the healers in the Plateau region was achieved. This allowed assessing the recorded inhibitory concentrations (ICs) of extracts through in vitro test against Plasmodium strains [Table 3]. According to this bibliographic data, Sida acuta, S. latifolius, and Combretum micrathum are the cited plants with IC50 values below 1 μg/mL.
Table 3.
Bibliographic record of ethnopharmacological work done on some of the most cited species during the ethnobotanical survey
Used parts, method of preparation and administration of drugs
In healers' habit of Plateau region, the most used parts of plants in recipes preparation are leaves (60%), roots (11%), and stem bark (11%) [Figure 3]. Recipes are prepared as decoction, infusion, maceration, and kneading in water or sauce [Figure 4]. The main route of administration is oral. However, some are taken by simple bath or steam bath. These plants are used alone or in combination in the preparation of recipes. The dosage is ranging from one to four doses per day and up to 10 days of treatment depending on recipe, severity of malaria, and patient's age.
Figure 3.
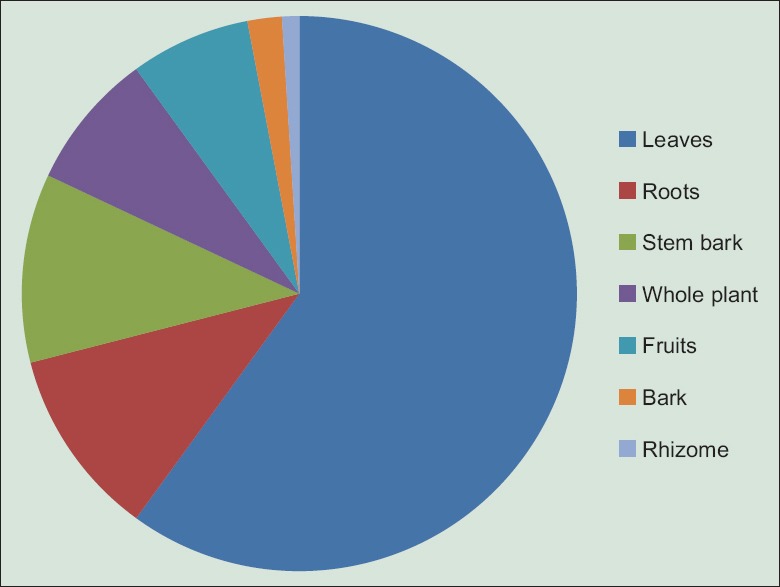
Proportion of different plants parts used in the management of malaria on Plateau region in Togo
Figure 4.
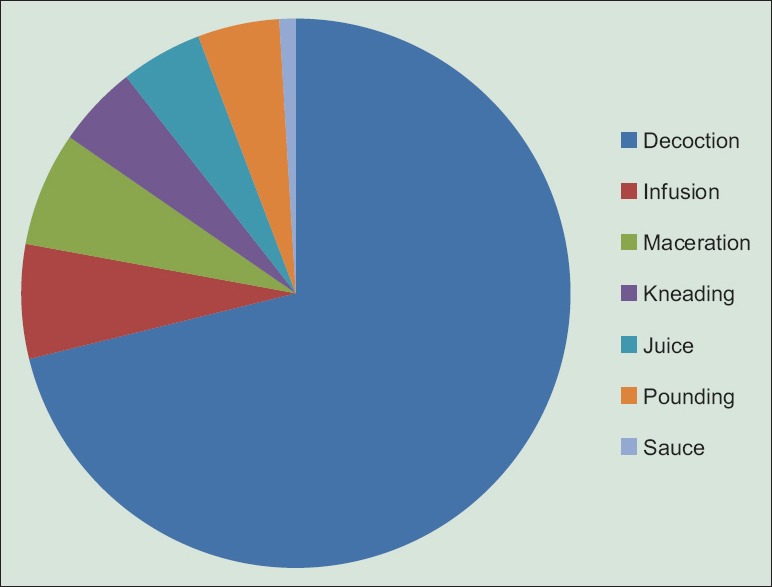
Methods of preparation of remedies in the management of malaria on Plateau Region in Togo
DISCUSSION
The issue of this investigation revealed that plants of families Caesalpiniaceae, Euphobiaceae, Poaceae, Rubiaceae, and Rutaceae were particularly well known by the people interviewed. They were widely used and contribute to malaria treatment, used either alone or in combination with other species. In a similar study conducted in the Maritime region of Togo, Koudouvo et al.[11] had identified 52 species of plants used in traditional malaria treatment belonging to 29 families.[10] Caesalpiniaceae, Euphobiaceae, Rubiaceae, and Rutaceae were generally predominant as families with the greatest number of species treating malaria in previous studies 2009.[11,25,30] These plants were similar to those used in the treatment of malaria in Benin,[24] Nigeria,[19,22,23] Ivory Coast,[17] Ghana,[36] and Guinea.[30] The Rubiaceae and Caesalpiniaceae were often cited for their antimalarial properties due to their alkaloids contents (Karou et al.,[39,59] Amoa Onguéné et al.[55]). The four most cited plants S. siamea, S. latifolius, N. laevis, and A. hispidum were also well documented in several studies: N. laevis, A. hispidum, and S. siamea by Yetein et al.[24] The species B. vulgaris, G. celosioides, I. cylindrica, and Nephrolepis undulaata were not found in the literature for their antimalarial uses and need be explored. Antimalarial properties of some recorded species in this study were also reported by previous studies focused on in vitro antiplasmodial activity of these species. For example, the aqueous extract of root of S. latifolius tested in vitro against the strains of P. falciparum FCB1 was active with IC50 = 0.6 μg/ml.
Leaves were the most used part of the plant (60%). The same result was found by Lakouetene in 2008 with 60%;[25] 68% by Yetein et al. in 2013.[24] Therefore, it was noted an intense collection of leaves, levy that did not have at all an important danger to the plant, according to Poffenberger et al.[56] According to these authors, the levy of 50% of the leaves of a plant does not significantly affect its survival, while uprooting and debarking participate in the destruction of the plant. In addition, to the preference of leaves is that they are the main photosynthetic organs and, therefore, tanks and photosynthesizes exudates containing secondary bioactive compounds that protect against external aggressions. These compounds have medicinal values for human body.[24,57]
Samples were collected in forests, fields, and home gardens that grow rare species.
Most recipes used were prepared by decoction (77%) followed distantly by infusion and maceration. In general, plant material amount and the volume of water used and preparation duration were not precisely defined. The oral route of administration was the most used in the Plateau region for taking antimalarial traditional recipes (97%). Koudouvo et al. also had obtained in the Maritime region in Togo this mode as the principal (82.05%).[11]
The drugs were taken with gourds, glass (beer or liquor), spoon, or cup. In general, the amount administered to the patient is not very accurately measured, and the dosage is very difficult to estimate. In all cases, there was a wide variation depending on the experience of each traditional therapist. These inaccuracies make difficult the standardization of the use of these plants. The direct consequence is the development of resistance of Plasmodium toward drug use.
Traditional medicine of the Plateau region sometimes had used combinations of plants to increase the efficiency of the recipe in the treatment of malaria and its symptoms such as fever, headache, vomiting, and anemia. These plants associations, mismatched, are sometimes dangerous. In Africa, for example, about 30% of the fatal accidents were caused because of mixtures that were complex remedies.[58] These products create in the long-term complications such as kidney and liver failure.
Only 7/62 respondents had used parasitological diagnostic of malaria (thick blood film, blood smear, and rapid diagnostic test) before treatment. The rest had used signs such as fever, headache, vomiting, conjunctival pallor, diarrhea, chills, and generalized tiredness. This raised the problem of definitive diagnosis before treatment because other diseases had almost same clinical signs as malaria.
Informants ranged from 29 to 75 years old. Younger informants were less represented than old ones. From 62 traditional herbalists, 1.61% was aged <30 years while 29.03% were from 30 to 40 years, and 69.36% were more than 50 years old. This is in agreement with previous results described by Traore.[30] Consequently, there is an urgent need for documentation of this invaluable knowledge since there is a persistent gap in knowledge of herbal practice between the younger and older generations. The educational level of the interviewees was low: 32.26% had some primary schooling, and 24.19% had some secondary schooling. Only 4.84% had attended a higher education institution. Many traditional medical practitioners (38.71%) were illiterate and consequently could not document their practice. Inheritance (88.70%) was the major source of knowledge acquisition. It is advocated that knowledge of treatment of the disease acquired by inheritance and training must be documented for future generation.[30]
CONCLUSION
Investigations results had identified 61 species commonly used in Togolese traditional medicine to treat malaria. Given the high prevalence of malaria and the widespread use of traditional medicine, it is capital to rationalize the use of these medicinal plants. These medicinal plants may probably contain yet undiscovered antimalarial properties, which can serve as a template for the production of cheap antimalaria drug from indigenous plants in Togo. There is a need for a multidisciplinary approach to develop potentially effective drugs while noting dangerous drugs and practices that should be discarded.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors are grateful to the West Africa monetary union for financial support.
REFERENCES
- 1.Singh S. Current scenario of control of malaria. Trop Parasitol. 2011;1:52–3. doi: 10.4103/2229-5070.86922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Malaria Report: 2014. Geneva, Switzerland: WHO Press; 2014. World Health Organization. [Google Scholar]
- 3.2006-2010. Programme National de Lutte Contre le Paludisme: Plan Stratégique National du Togo «faire reculer le paludisme». [Google Scholar]
- 4.Alma-Ata, USSR; 1978. Sep 6-12, World Health Organization. Declaration of Alma-Ata International Conference on Primary Health Care. [Google Scholar]
- 5.Geneva, Switzerland: WHO Press; 2008. World Health Organization. Factsheet No. 134. Traditional Medicine. [Google Scholar]
- 6.Gbeassor M, Kossou Y, Amegbo K, de Souza C, Koumaglo K, Denke A. Antimalarial effects of eight African medicinal plants. J Ethnopharmacol. 1989;25:115–8. doi: 10.1016/0378-8741(89)90051-2. [DOI] [PubMed] [Google Scholar]
- 7.Gbeassor M, Kedjagni AY, Koumaglo K, De Souza C, Agbo K, Aklikokou K, et al. In vitro antimalarial activity of six medicinal plants. Phytother Res. 1990;4:115–7. [Google Scholar]
- 8.Koumaglo K, Gbeassor M, Nikabu O, de Souza C, Werner W. Effects of three compounds extracted from Morinda lucida on Plasmodium falciparum. Planta Med. 1992;58:533–4. doi: 10.1055/s-2006-961543. [DOI] [PubMed] [Google Scholar]
- 9.Koumaglo K, Gbéassor M, Nikabou O, de Souza C. Activité de la Guedunine extraite de l'écorce du fruit de Azadirachta indica (Linn.) sur Plasmodium falciparum in vitro. Phytother J. 2004;4:622–36. [Google Scholar]
- 10.Koudouvo K, Karou DS, Kokou K, Essien K, Aklikokou K, Glitho IA, et al. An ethnobotanical study of antimalarial plants in Togo Maritime Region. J Ethnopharmacol. 2011;134:183–90. doi: 10.1016/j.jep.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Koudouvo K, Karou SD, Ilboudo DP, Kokou K, Essien K, Aklikokou K, et al. In vitro antiplasmodial activity of crude extracts from Togolese medicinal plants. Asian Pac J Trop Med. 2011;4:129–32. doi: 10.1016/S1995-7645(11)60052-7. [DOI] [PubMed] [Google Scholar]
- 12.Tardío J, Pardo-de-Santayana M. Cultural importance indices: A comparative analysis based on the useful wild plants of Southern Cantabria (Northern Spain)1. Econ Bot. 2008;62:24–39. [Google Scholar]
- 13.Ganfon H. In vitro evaluation of antiplasmodial activity of plant samples used in traditional medicine in Benin. Planta Med. 2008;74:1140. [Google Scholar]
- 14.Moshi MJ, Otieno DF, Weisheit A. Ethnomedicine of the Kagera Region, North Western Tanzania. Part 3: Plants used in traditional medicine in Kikuku village, Muleba District. J Ethnobiol Ethnomed. 2012;8:14. doi: 10.1186/1746-4269-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soejarto DD, Gyllenhaal C, Kadushin MR, Southavong B, Sydara K, Bouamanivong S, et al. An ethnobotanical survey of medicinal plants of Laos toward the discovery of bioactive compounds as potential candidates for pharmaceutical development. Pharm Biol. 2012;50:42–60. doi: 10.3109/13880209.2011.619700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uzodimma D. Medico-ethnobotanical inventory of Ogii, Okigwe Imo State, South Eastern Nigeria-I. Glob Adv Res J Med Plants. 2013;2:30–44. [Google Scholar]
- 17.N'Guessan K, Tra BF, Koné MW. Étude ethnopharmacologique des plantes antipaludiques utilisées en médecine traditionnelle chez les Abbey et Krobou d'Agboville (Côte-d'Ivoire) Ethnopharmacologia. 2009;44:42–50. [Google Scholar]
- 18.Kayode J. Conservation of indigenous medicinal botanicals in Ekiti State, Nigeria. J Zhejiang Univ Sci B. 2006;7:713–8. doi: 10.1631/jzus.2006.B0713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ajibesin KK, Ekpo BA, Bala DN, Essien EE, Adesanya SA. Ethnobotanical survey of Akwa Ibom State of Nigeria. J Ethnopharmacol. 2008;115:387–408. doi: 10.1016/j.jep.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 20.Tchacondo T, Karou SD, Batawila K, Agban A, Ouro-Bang'na K, Anani KT, et al. Herbal remedies and their adverse effects in Tem tribe traditional medicine in Togo. Afr J Tradit Complement Altern Med. 2011;8:45–60. doi: 10.4314/ajtcam.v8i1.60522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ajaiyeoba EO, Abiodun OO, Falade MO, Ogbole NO, Ashidi JS, Happi CT, et al. In vitro cytotoxicity studies of 20 plants used in Nigerian antimalarial ethnomedicine. Phytomedicine. 2006;13:295–8. doi: 10.1016/j.phymed.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 22.Odugbemi TO, Akinsulire OR, Aibinu IE, Fabeku PO. Medicinal plants useful for malaria therapy in Okeigbo, Ondo State, Southwest Nigeria. Afr J Tradit Complement Altern Med. 2006;4:191–8. doi: 10.4314/ajtcam.v4i2.31207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tchacondo T, Karou SD, Agban A, Bako M, Batawila K, Bawa ML, et al. Medicinal plants use in central Togo (Africa) with an emphasis on the timing. Pharmacognosy Res. 2012;4:92–103. doi: 10.4103/0974-8490.94724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yetein MH, Houessou LG, Lougbégnon TO, Teka O, Tente B. Ethnobotanical study of medicinal plants used for the treatment of malaria in plateau of Allada, Benin (West Africa) J Ethnopharmacol. 2013;146:154–63. doi: 10.1016/j.jep.2012.12.022. [DOI] [PubMed] [Google Scholar]
- 25.Lakouéténé D, Ndolngar G, Berké B, Moyen L, KoshKomba E, Zinga I, et al. Enquête ethnobotanique des plantes utilisées dans le traitement du paludisme à Bangui. Bull Soc Pharm Bord. 2009;148:123–38. [Google Scholar]
- 26.Adebayo JO, Santana AE, Krettli AU. Evaluation of the antiplasmodial and cytotoxicity potentials of husk fiber extracts from Cocos nucifera, a medicinal plant used in Nigeria to treat human malaria. Hum Exp Toxicol. 2012;31:244–9. doi: 10.1177/0960327111424298. [DOI] [PubMed] [Google Scholar]
- 27.Ancolio C, Azas N, Mahiou V, Ollivier E, Di Giorgio C, Keita A, et al. Antimalarial activity of extracts and alkaloids isolated from six plants used in traditional medicine in Mali and Sao Tome. Phytother Res. 2002;16:646–9. doi: 10.1002/ptr.1025. [DOI] [PubMed] [Google Scholar]
- 28.Jorim RY, Korape S, Legu W, Koch M, Barrows LR, Matainaho TK, et al. An ethnobotanical survey of medicinal plants used in the eastern highlands of Papua New Guinea. J Ethnobiol Ethnomed. 2012;8:47. doi: 10.1186/1746-4269-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zofou D, Tene M, Ngemenya MN, Tane P, Titanji VP. In vitro antiplasmodial activity and cytotoxicity of extracts of selected medicinal plants used by traditional healers of Western cameroon. Malar Res Treat 2011. 2011 doi: 10.4061/2011/561342. 561342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Traore MS, Baldé MA, Diallo MS, Baldé ES, Diané S, Camara A, et al. Ethnobotanical survey on medicinal plants used by Guinean traditional healers in the treatment of malaria. J Ethnopharmacol. 2013;150:1145–53. doi: 10.1016/j.jep.2013.10.048. [DOI] [PubMed] [Google Scholar]
- 31.Thomas J, Govindan S, Kurup M. Isolation and characterisation of mosquitolarvicidal compound from Gliricidia sepium Jacq. 2014;2:173–8. [Google Scholar]
- 32.Isah AB, Ibrahim YK, Iwalewa EO. Evaluation of the antimalarial properties and standardization of tablets of Azadirachta indica (Meliaceae) in mice. Phytother Res. 2003;17:807–10. doi: 10.1002/ptr.1231. [DOI] [PubMed] [Google Scholar]
- 33.Tor-Anyiin TA, Sha'ato R, Oluma HO. Ethnobotanical survey of anti-malarial medicinal plants amongst the Tiv people of Nigeria. J Herbs Spices Med Plants. 2003;10:61–74. [Google Scholar]
- 34.Shuaibu MN, Wuyep PA, Yanagi T, Hirayama K, Tanaka T, Kouno I. The use of microfluorometric method for activity-guided isolation of antiplasmodial compound from plant extracts. Parasitol Res. 2008;102:1119–27. doi: 10.1007/s00436-008-0879-6. [DOI] [PubMed] [Google Scholar]
- 35.Adodo A. Nature Power: A Christian Approach to Herbal Medicine. Generation Press. 2004. p. 304. ISBN: 978-1-4918-7834-7(sc): https://books.google.tg/books?id=VQdnAQAAQBAJ&printsec=frontcover&hl=fr&source=gbs_ge_summary_r&cad=0#v=onepage&q&f=false .
- 36.Asase A, Oteng-Yeboah AA, Odamtten GT, Simmonds MS. Ethnobotanical study of some Ghanaian anti-malarial plants. J Ethnopharmacol. 2005;99:273–9. doi: 10.1016/j.jep.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 37.Ankrah NA, Nyarko AK, Addo PG, Ofosuhene M, Dzokoto C, Marley E, et al. Evaluation of efficacy and safety of a herbal medicine used for the treatment of malaria. Phytother Res. 2003;17:697–701. doi: 10.1002/ptr.1196. [DOI] [PubMed] [Google Scholar]
- 38.Sanon S, Ollivier E, Azas N, Mahiou V, Gasquet M, Ouattara CT, et al. Ethnobotanical survey and in vitro antiplasmodial activity of plants used in traditional medicine in Burkina Faso. J Ethnopharmacol. 2003;86:143–7. doi: 10.1016/s0378-8741(02)00381-1. [DOI] [PubMed] [Google Scholar]
- 39.Karou D, Dicko MH, Sanon S, Simpore J, Traore AS. Antimalarial activity of Sida acuta Burm. f. (Malvaceae) and Pterocarpus erinaceus Poir. (Fabaceae) J Ethnopharmacol. 2003;89:291–4. doi: 10.1016/j.jep.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Okokon JE, Ita BN, Udokpoh AE. The in-vivo antimalarial activities of Uvaria chamae and Hippocratea africana. Ann Trop Med Parasitol. 2006;100:585–90. doi: 10.1179/136485906X118512. [DOI] [PubMed] [Google Scholar]
- 41.Omoregie E, Pal A, Sisodia B. In vitro Antimalarial and Cytotoxic Activities of Leaf Extracts of Vernonia amygdalina (Del) Nigerian Journal of Basic and Applied Science. 2011;19:121–6. [Google Scholar]
- 42.Bero J, Ganfon H, Jonville MC, Frédérich M, Gbaguidi F, DeMol P, et al. In vitro antiplasmodial activity of plants used in Benin in traditional medicine to treat malaria. J Ethnopharmacol. 2009;122:439–44. doi: 10.1016/j.jep.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Bidla G, Titanji V, Joko B, Ghazali G, Bolad A, Berzins K. Antiplasmodial activity of seven plants used in African folk medicine. Indian J Pharmacol. 2004;36:245. [Google Scholar]
- 44.Bhat GP, Surolia N. In vitro antimalarial activity of extracts of three plants used in the traditional medicine of India. Am J Trop Med Hyg. 2001;65:304–8. doi: 10.4269/ajtmh.2001.65.304. [DOI] [PubMed] [Google Scholar]
- 45.Mesia GK, Tona GL, Nanga TH, Cimanga RK, Apers S, Cos P, et al. Antiprotozoal and cytotoxic screening of 45 plant extracts from Democratic Republic of Congo. J Ethnopharmacol. 2008;115:409–15. doi: 10.1016/j.jep.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 46.Zirihi GN, Mambu L, Guédé-Guina F, Bodo B, Grellier P. In vitro antiplasmodial activity and cytotoxicity of 33 West African plants used for treatment of malaria. J Ethnopharmacol. 2005;98:281–5. doi: 10.1016/j.jep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Ménan H, Banzouzi JT, Hocquette A, Pélissier Y, Blache Y, Koné M, et al. Antiplasmodial activity and cytotoxicity of plants used in West African traditional medicine for the treatment of malaria. J Ethnopharmacol. 2006;105:131–6. doi: 10.1016/j.jep.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 48.Benoit-Vical F, Valentin A, Cournac V, Pélissier Y, Mallié M, Bastide JM. In vitro antiplasmodial activity of stem and root extracts of Nauclea latifolia S.M. (Rubiaceae) J Ethnopharmacol. 1998;61:173–8. doi: 10.1016/s0378-8741(98)00036-1. [DOI] [PubMed] [Google Scholar]
- 49.Titanji VP, Zofou D, Ngemenya MN. The antimalarial potential of medicinal plants used for the treatment of malaria in Cameroonian folk medicine. Afr J Tradit Complement Altern Med. 2008;5:302–21. [PMC free article] [PubMed] [Google Scholar]
- 50.Nundkumar N, Ojewole JA. Studies on the antiplasmodial properties of some South African medicinal plants used as antimalarial remedies in Zulu folk medicine. Methods Find Exp Clin Pharmacol. 2002;24:397–401. doi: 10.1358/mf.2002.24.7.696540. [DOI] [PubMed] [Google Scholar]
- 51.Willcox ML, Graz B, Falquet J, Diakite C, Giani S, Diallo D. A “reverse pharmacology” approach for developing an anti-malarial phytomedicine. Malar J. 2011;10(Suppl 1):S8. doi: 10.1186/1475-2875-10-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Banzouzi JT, Prado R, Menan H, Valentin A, Roumestan C, Mallié M, et al. Studies on medicinal plants of Ivory Coast: Investigation of Sida acuta for in vitro antiplasmodial activities and identification of an active constituent. Phytomedicine. 2004;11:338–41. doi: 10.1078/0944711041495245. [DOI] [PubMed] [Google Scholar]
- 53.Koudouvo K, Karou SD, Ilboudo DP, Kokou K, Essien K, Aklikokou K, et al. In vitro antiplasmodial activity of crude extracts from Togolese medicinal plants. Asian Pac J Trop Med. 2011;4:129–32. doi: 10.1016/S1995-7645(11)60052-7. [DOI] [PubMed] [Google Scholar]
- 54.Sha'a KK, Oguche S, Watila IM, Ikpa TF. In vitro antimalarial activity of the extracts of Vernonia amygdalina commonly used in traditional medicine in Nigeria. Sci World J. 2011;6:5–9. [Google Scholar]
- 55.Amoa Onguéné P, Ntie-Kang F, Lifongo LL, Ndom JC, Sippl W, Mbaze LM. The potential of anti-malarial compounds derived from African medicinal plants, part I: A pharmacological evaluation of alkaloids and terpenoids. Malar J. 2013;12:449. doi: 10.1186/1475-2875-12-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poffenberger M, McGean B, Khare S, Campbell J. II. New Delhi: Society for Promotion of Wastelands Development; 1992. Field Method Manual, Community Forest Economy and Use Pattern: Participatory and Rural Appraisal (PRA) Methods in South Gujarat India. [Google Scholar]
- 57.Balick MJ, Cox PA. New York: WH Freeman & Co; 1996. Plants, People, and Culture: The Science of Ethnobotany; p. 6. [Google Scholar]
- 58.el-Said F, Sofowara EA, Malcolm SA, Hofer A. An investigation into the efficacy of Ocimum gratissimum as used in Nigerian native medicine. Planta Med. 1969;17:195–200. doi: 10.1055/s-0028-1099846. [DOI] [PubMed] [Google Scholar]
- 59.Karou SD, Tchacondo T, Ilboudo DP, Simpore J. Sub-Saharan Rubiaceae: A review of their traditional uses, phytochemistry and biological activities. Pak J Biol Sci. 2011;14:149–69. doi: 10.3923/pjbs.2011.149.169. [DOI] [PubMed] [Google Scholar]


