Abstract
Background:
Melaleuca leucadendron (Myrtaceae) is a kind of fruit used as Indonesian medicinal component and recorded in Jamu (tonic made of medical herbs) prescription records for the diabetes treatment. Its methanol extract exhibited a strong inhibitory activity with the half maximal inhibitory concentration (IC50) value of 2.05 μg/mL, while it is the same value with positive control RK-682.
Objective:
To isolate the chemical constituents of M. leucadendron and to evaluate their activity against protein tyrosine phosphatase 1B (PTP1B). Further, determine their toxicity potential against T-cell protein tyrosine phosphatase (TCPTP).
Materials and Methods:
Methanol extract was fractionated using silica column chromatography, and the obtained fraction was purified using Sephadex 20-LH. The structure of isolated compounds was identified based on 1H and 13Nuclear Magnetic Resonance Spectrometry. Furthermore, the compounds were examined against PTP1B and TCPTP.
Results:
Methanol extract of M. leucadendron (Myrtaceae) afforded two triterpenes: Betulinic acid and ursolic acid in high quantities. Both compounds exhibited a strong inhibitory activity against PTP1B inhibition with IC50 value of 1.5 and 2.3 μg/mL, respectively (positive control RK-682, IC50 = 2.05 μg/mL). Their activity toward TCPTP, on the other hand, were at 2.4 and 3.1 μg/mL, respectively. Based on this purification work, betulinic acid and ursolic acid presented 7.6% and 2.4%, respectively, as markedly M. leucadendron most potential for betulinic acid source among Indonesian plants. The result should have demonstrated that the antidiabetes of M. dendron could be through the inhibition of PTP1B.
SUMMARY
Melaleuca leucadendron is a good source for ursolic acid.
Confirming traditional use for type II diabetes via PTP1B inhibition.
Keywords: Betulinic acid, diabetes-obesity, Melaleuca leucadendron, protein tyrosine phosphatase 1B, ursolic acid
INTRODUCTION
In 2010, the rate of diabetes toll reached 6.6%, and it is predicted that by 2030 it will reach 7.4% of worldwide population.[1] Regarding obesity, WHO has reported that more than 1 billion adult population worldwide suffer from overweight, and around 300 million of it is clinically obese.[2,3] Protein tyrosine phosphatase 1B (PTP1B) is an enzyme present in some important insulin-targeted tissues such as liver, muscle, and fat. It plays a key role in the insulin signal transduction, in this case, as a negative regulator. Many studies have revealed that PTP1B inhibition is a validated target for curing type 2 diabetes (T2D) or reducing fat content on obese patients. Thus, inhibiting PTP1B will have a dual effect both as antidiabetes and as antiobesity. Nowadays, developing a small molecule as PTP1B inhibitor is getting of interest in a drug discovery for the T2D and obesity through a rational synthesis and natural resources.[4,5,6]
In our previous study in finding PTP1B inhibitors from the components of Indonesian herbal medicine formulation, known as Jamu medicine, 28 medicinal plants have been submitted to be tested against PTP1B.[7] Of the samples examined, the fruits of Melaleuca leucadendron (Myrtaceae) has exhibited the most potent inhibitory activity with the half maximal inhibitory concentration (IC50) value of 2.05 μg/mL, as well as the same value with positive control RK-682. In Indonesia, this fruit locally called, “Merica Bolong” or “Buah Kayu Putih” is recorded as the component of diabetes jamu formulation.[8,9] However, the information on its chemical constituent related to diabetes or obesity-related activity is limited. In this report, the isolation of its active chemical constituents against PTP1B has been described. However, since inhibiting PTP1B could implicate the immune system for sharing 74% with T-cell protein tyrosine phosphatase (TCPTP),[10] we further examined their inhibitory potential against this protein.
MATERIALS AND METHODS
General experimental procedures
Nuclear magnetic resonance (NMR) spectra were recorded on a JEOL JNM-LA400 spectrometer with tetramethylsilane as an internal standard. Meanwhile, medium pressured liquid chromatography (MPLC) was performed using Yamazen MPLC 560 pump system. Column chromatography was performed with silica gel (40–50 μm, Kanto Chemical Co., Inc., Japan) or Sephadex LH-20 (Pharmacia, Sweden). Analysis and preparation on thin layer chromatography (TLC) were conducted on precoated silica gel 60F254 or RP-18 plates (Merck, 0.25 or 0.50 mm thickness). For TLC visualization, it was conducted based on 254 and 366 nm ultraviolet (UV) rays and sprayed with Cerium (IV) sulphate before heating at 110°C around 5 min.
Plant material
M. leucadendron (Myrtaceae) was obtained from Genteng Market in Surabaya Indonesia in August 2006 and authenticated by one of the authors (Dr. Subehan). Its voucher specimen (TMPW 22276) is preserved in our previous institution the Museum of Materia Medica, Analytical Research Center for Ethnomedicines, Institute of Natural Medicine, University of Toyama, Toyama, Japan.
Extraction and isolation
One hundred and eight grams of powdered fruit of M. leucadendron was extracted with MeOH (3 × 400 mL, reflux, 2 h), and the MeOH solution was filtered and evaporated under reduced pressure to provide a MeOH extract (8.6 g). Following this, the MeOH extract was successively dissolved with ethyl acetate (EtOAc) and CHCl3 (3 × 250 mL each) to yield an EtOAc-soluble fraction (3.1 g), a CHCl3-soluble fraction (2.1 g), and a residue (2.2 g), respectively. The EtOAc-soluble fraction was rechromatographed by MPLC on a YMC silica gel column (4 cm × 30 cm, flow rate 20 mL/min) using a step gradient of CHCl3 − n-hexane (5:5, 5:4, 5:3, 5:2, 5:1, each 1.5 L, subfraction collection was 100 mL to afford 32 subfractions). Subfractions 12–15 were collected and concentrated under reduced pressure purposely to result in 640 mg of the fraction. The fraction was subsequently purified on Sephadex LH-20 (30 cm × 3 cm, collecting the volume of 50 mL) to result in 310 mg ursolic acid (2). While fraction 16–30 was rich in carbohydrates.
CHCl3-soluble fraction was separated on the same MPLC and eluted with CHCl3-MeOH (5:1, 5:2, 5:3, 5:4, 5:5, each 1.5 L) solvent systems to afford 25 subfractions. Subfractions 9–16 were collected to obtain 980 mg dry fraction. The fraction was purified on the Sephadex LH-20 column with MeOH as a solvent to afford betulinic acid (568 mg) and a mixture of ursolic acid and betulinic acid (258 mg). Subfractions 1–8 had so low quantity to separate, while subfractions 17–25 was rich in fatty acids.
Betulinic acid (1) - 1H NMR (CDCl3, 400 MHz) δ4.7 (1H, dd, J = 10.2; 5.5, H-3) 3.0 (t, J = 10.2; 5.6, H-19), 0.82 (s, H-23), 0.91 (s, H-24), 0.81 (s, H-25), 0.95 (s, H-26), and 0.93 (s, H-27); 13C NMR (CDCl3, 100 MHz) δ37.8 (C-1), 27.9 (C-2), 79.01 (C-3), 38.71 (C-4), 55.5 (C-5), 18.32 (C-6), 34.3 (C-7), 40.93 (C-8), 50.51 (C-9), 37.2 (C-10), 20.8 (C-11), 25.22 (C-12), 38.4 (C-13), 42.4 (C-14), 30.6 (C-15), 31.1 (C-16), 56.3 (C-17), 46.8 (C-18), 49.2 (C-19), 140.4 (C-20), 29.6 (C-21), 34.09 (C-22), 27.99 (C-23), 15.3 (C-24), 16.0 (C-25), 16.1 (C-26), 14.77 (C-27), 180.3 (C-28), 109.6 (C-29), and 19.4 (C-30).
Ursolic acid (1) - 1H NMR (CDCl3, 400 MHz) δ 3.43 (1H, br, H-3), 5.42 (1H, br, H-10), 2.52 (d, J = 10.2, H-18), 1.24 (s, H-23), 1.02 (s, H-24), 0.93 (s, H-25), 1.05 (s, H-26), 1.22 (s, H-27), 0.97 (s, H-29), and 0.99 (d, J = 6.1, H-30); 13C NMR (CDCl3, 100 MHz) δ 38.4 (C-1), 28.1 (C-2), 78.1 (C-3), 38.4 (C-4), 55.8 (C-5), 18.8 (C-6), 33.6 (C-7), 40.0 (C-8), 48.3 (C-9), 37.4 (C-10), 23.6 (C-11), 125.6 (C-12), 139.7 (C-13), 42.5 (C-14), 28.7 (C-15), 24.9 (C-16), 48.0 (C-17), 53.7 (C-18), 39.5 (C-19), 39.1 (C-20), 31.1 (C-21), 37.3 (C-22), 28.8 (C-23), 15.7 (C-24), 16.6 (C-25), 17.4 (C-26), 23.9 (C-27), 180.0 (C-28), 17.5 (C-29), and 21.4 (C-30).
Protein tyrosine phosphatase 1B inhibitory activity
All compounds were dissolved in dimethyl sulfoxide (DMSO). The final concentration of tested compound was 10 μg/mL, while DMSO in each well was 1% in which this concentration showed no influence on the activity of PTP1B. The assay was performed in 96-well clear polystyrene microplate (Corning, USA) according to a published procedure with a number of slight modifications.[11] Each well contained 0.05 μg PTP1B (Enzo Life Sciences, Inc., NY, USA), 2 mM p-nitrophenyl phosphate (pNPP; Wako Pure Chemical Industries, Ltd., Osaka, Japan), and 50 mM citrate buffer containing 0.1 mM NaCl, 1 mM dithiothreitol (DTT), and 1 mM N, N, N', N'-ethylenediaminetetraacetate. The final volume of the mixture was 200 μL. The reaction was initiated by the addition of pNPP, incubated at 37°C for 30 min, and terminated with the addition of a stop solution (10 M NaOH). The amount of p-nitrophenol produced was estimated by measuring the absorbance at 405 nm using a Perkin-Elmer HTS 7000 bioassay reader. To identify the level of nonenzymatic substrate hydrolysis, the absorbance of wells only containing buffer and pNPP were measured for correction. The difference between the full enzymatic activity and the correction was arbitrarily set as 100% activity. The percent residual activity was calculated using the following formula: Residual Activity (%) = [Abs(full enzymatic) − Abs(test sample) − Abs(correction)/(Abs(full enzymatic) − Abs(correction))] × 100, where Abs(full enzymatic) was the absorbance of p-nitrophenol liberated by the enzyme in the system without a test sample in contrast to Abs(test sample). The assays were performed in triplicate for all samples. A known phosphatase inhibitor, RK-682 (purity ≥ 98%; Enzo Life Sciences, Inc.,) was used as the positive control then.
T-cell protein tyrosine phosphatase inhibitory activity
The assay was performed using a DTT concentration of 10 mM with an equal procedure as the PTP1B inhibition assay.
RESULTS AND DISCUSSION
During our preliminary study, the fruit extract of M. leucadendron exhibited the most active against PTP1B-more potent than positive control RK-682. It has also been mentioned in several Jamu medicine prescription records as a component for blood sugar control. Thus, it is interesting to reveal its chemical constituents, which could be related to its inhibitory activity. During the fractionation steps, most of the subfractions contained very polar materials, which were easily soluble in MeOH. In contrast, the chloroform fractions were rich of fatty acids. Under UV rays analyses of their TLC, it did not show the sufficient indication of the presence of certain compounds. Meanwhile, upon TLC spraying by cerium (IV) sulphate, the TLC plates exhibited some purple bands. However, due to oily matters present and deduced fatty acids based on NMR spectra, the material was collected based on TLC profiles. Compound 1 was isolated as white amorphous powder. Its 1H NMR displayed the signals of six tertiary protons (δ 0.81, 0.82, 0.91, 0.93, 0.95, 1.68) and two oxygenated secondary protons (δ 3.0, 4.66, and 4.7, respectively). The signals at δ 4.6 were exhibited a singlet signal, 3.0 was exhibited in a triplet (10.2 and 5.6 Hz), and 4.7 doublet of doublet (10.2 and 5.5), respectively. Its 13C NMR spectrum showed thirty carbons signals, mostly in the range of δ 14–56 ppm. Two vinylic carbons were present consecutively at δ 109.6 and 140.4 ppm. While the carbon at 79.01 was deduced as an oxygenated carbon. Finally, the carbon at 180 was arbitrarily as a carbonyl carbon. Upon the alert observation of their 1H and 13C NMR spectra, it was found that they had some good agreements with betulinic acid.[12] It was in a high concentration (7.6%). That could be a major compound of this material. Based on inhibitory examination, it exhibited 98.% inhibition [Figure 1]. It had IC50 value of 1.5 μg/mL. That could be betulinic acid as the strong contributor toward the activity of methanol extract.
Figure 1.
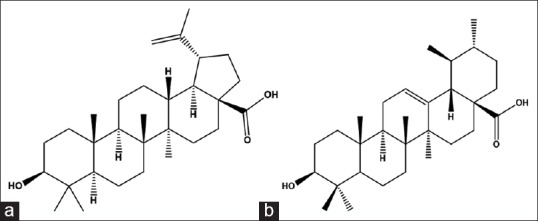
Isolated compounds from the fruits of M. dendron: Betulinic acid (a) and ursolic acid (b)
Compound 2 was present in both ethyl acetate and chloroform fractions. It has been found as a white amorphous powder. In certain fractions, it was present in a mixture with betulinic acid. Its 1H and 13C NMR spectra were similar to that of one except the position of two olefinic carbons δ 125.6 and 139. 7 ppm. This spectrum showed a good agreement with that of ursolic acid.[13]
Both compounds exhibited some strong activities at 97% and 99% inhibition in a concentration of 10 μg/mL [Figure 2]. Furthermore, IC50 examination demonstrated a similar activity with positive control RK-682 with IC50 values of 1.5 and 2.3 μg/mL, respectively [Table 1].
Figure 2.
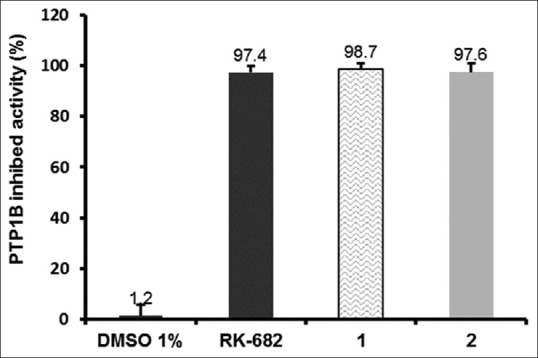
Percent inhibited actavity after treatment by 1 and 2 at 10 μg/mL. Data were expressed as the mean ± standard deviation of triplicate measurements. RK-682 was used as a positive control
Table 1.
Half maximal inhibitory concentration values of 1 and 2 against protein tyrosine phosphatase 1B and T-cell protein tyrosine phosphatase

In this study, betulinic acid was isolated in 7.6% while ursolic acid was in 2.8%. Hence, betulinic acid and ursolic acid could be the corresponding constituents related to the strong activity of its extract inhibition. Interestingly, the recent study on diabetes-related scaffold, betulinic and ursolic acids have been becoming a skeleton lead. Their synthetic derivatives have demonstrated that the compound family has exhibited as potent agonist toward energy homeostasis TGR5 and insulin receptors and more potent compared to bile acids.[14] Intriguingly, a triterpene skeleton has been developed for current PTP1B inhibitor which results in trodusquemine, possessing a steroidal main building block. Therefore, further development triterpenoid has the interesting potential for PTP1B inhibitor modification. Hence, providing sufficient betulinic acid and ursolic acid for the development will be necessary. It is worthy noted that M. leucadendron fruit contains betulinic acid concentration at 7.6% enabling it to be the most readily potential source. Since trodusquemine has been developed from a natural product, steroidal skeleton; thus, it can be stated that this finding is a clear evidence of natural product role, for example, secondary metabolite, in a drug development for numerous ailments including T2D and obesity. Although both compounds demonstrated more or less the same activity toward TCPTP (IC50 2.4 and 3.1 μg/mL), traditional usage of M. leucadendron fruits as antidiabetes could be related to both betulinic and ursolic acid against PTP1B activity.
Previously, Usia et al.[15] reported several Indonesian medicinal plants such as Zingiber aromaticum, Catharanthus roseus, and Melastoma malabratica containing the appreciable concentration of ursolic acid. That compound also present in South Asian plants such as Ziziphus jujuba,[16] Ziziphora clinopodioides,[17] and Leucas aspera.[18] Thus, the notable ursolic concentration in M. leucadendron can be as a potential ursolic as well as betulinic acid source for pharmaceutics or industrial purposes. On the basis of this work, the potent activity of compounds 1 and 2 as well as the MeOH extract should clearly demonstrate that M. leucadendron can be a potent medicinal plant for treating T2D and/or obesity.
CONCLUSION
That results demonstrated that the Indonesian medicinal plant M. leucadendron may be functionalized for the treatment and/or prevention of T2D and/or obesity. High quantities of betulinic and ursolic acid in this medicinal plant could be employed as chemical markers for its industrial processes.
Financial support and sponsorship
Universitas Muhammadiyah Surakarta (Muhammadiyah University of Surakarta) with grant number Ref.001/DM-I/FF/2014.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Azis Saifudin
Azis Saifudin, PhD is a lecturer of pharmacognosy and natural products chemistry fields at the Faculty of Pharmacy, Universitas Muhammadiyah Surakarta Indonesia. His research interests are mainly about bioassay-guided isolation, cell cultures, drug target, efficacy and safety of herbal materials, and stereochemistry. He has published his researches out put in some high impacted journals. He obtained his bachelor of pharmacy from Gadjah Mada University (2001), master's from Leiden University, the Netherlands (2008) and PhD in the field of natural products chemistry from University of Toyama Japan (2013).
REFERENCES
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes (Lond) 2008;32:1431–7. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 3.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: Systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–67. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang LJ, Jiang B, Wu N, Wang SY, Shi DY. Small molecules as potent protein tyrosine phosphatase 1B (PTP1B) inhibitors documented in patents from 2009 to 2013. Mini Rev Med Chem. 2015;15:104–22. doi: 10.2174/1389557515666150203144339. [DOI] [PubMed] [Google Scholar]
- 5.Chen J, Gao LX, Gong JX, Jiang CS, Yao LG, Li JY, et al. Design and synthesis of novel 1,2-dithiolan-4-yl benzoate derivatives as PTP1B inhibitors. Bioorg Med Chem Lett. 2015;25:2211–6. doi: 10.1016/j.bmcl.2015.03.060. [DOI] [PubMed] [Google Scholar]
- 6.Krishnan N, Koveal D, Miller DH, Xue B, Akshinthala SD, Kragelj J, et al. Targeting the disordered C terminus of PTP1B with an allosteric inhibitor. Nat Chem Biol. 2014;10:558–66. doi: 10.1038/nchembio.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saifudin A, Kadota S, Tezuka Y. Protein tyrosine phosphatase 1B inhibitory activity of Indonesian herbal medicines and constituents of Cinnamomum burmannii and Zingiber aromaticum. J Nat Med. 2013;67:264–70. doi: 10.1007/s11418-012-0674-7. [DOI] [PubMed] [Google Scholar]
- 8.Gunawan H, Prabowo A, Triwara B, Rahayu NK. 2nd ed. Semarang, Indonesia: Indonesian Pharmacist Association; 2006. Jamu Formulation Records; pp. 3–26. [Google Scholar]
- 9.Gunawan H, Prabowo A, Triwara B, Rahayu NK. 3rd ed. Semarang, Indonesia: Indonesian Pharmacist Association; 2008. Jamu Formulation Records; pp. 2–21. [Google Scholar]
- 10.You-Ten KE, Muise ES, Itié A, Michaliszyn E, Wagner J, Jothy S, et al. Impaired bone marrow microenvironment and immune function in T cell protein tyrosine phosphatase-deficient mice. J Exp Med. 1997;186:683–93. doi: 10.1084/jem.186.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumgartner RR, Steinmann D, Heiss EH, Atanasov AG, Ganzera M, Stuppner H, et al. Bioactivity-guided isolation of 1,2,3,4,6-Penta-O-galloyl-D-glucopyranose from Paeonia lactiflora roots as a PTP1B inhibitor. J Nat Prod. 2010;73:1578–81. doi: 10.1021/np100258e. [DOI] [PubMed] [Google Scholar]
- 12.Tsasi G, Samara P, Tsitsilonis O, Jürgenliemk G, Skaltsa H. Isolation, identification and cytotoxic activity of triterpenes and flavonoids from green walnut (Juglans regia L.) pericarps. Rec Nat Prod. 2016;10:83–92. [Google Scholar]
- 13.Martins D, Carrion LL, Ramos DF, Salomé KS, da Silva PE, Barison A, et al. Triterpenes and the antimycobacterial activity of Duroia macrophylla Huber (Rubiaceae) Biomed Res Int 2013. 2013 doi: 10.1155/2013/605831. 605831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Genet C, Strehle A, Schmidt C, Boudjelal G, Lobstein A, Schoonjans K, et al. Structure-activity relationship study of betulinic acid, a novel and selective TGR5 agonist, and its synthetic derivatives: Potential impact in diabetes. J Med Chem. 2010;53:178–90. doi: 10.1021/jm900872z. [DOI] [PubMed] [Google Scholar]
- 15.Usia T, Iwata H, Hiratsuka A, Watabe T, Kadota S, Tezuka Y. CYP3A4 and CYP2D6 inhibitory activities of Indonesian medicinal plants. Phytomedicine. 2006;13:67–73. doi: 10.1016/j.phymed.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 16.Mahajan RT, Chopda MZ. Phyto-pharmacology of Ziziphus jujuba Mill – A plant review. Pharmacogn Rev. 2009;3:320. [Google Scholar]
- 17.Tian S, Shi Y, Yu Q, Upur H. Determination of oleanolic acid and ursolic acid contents in Ziziphora clinopodioides Lam. by HPLC method. Pharmacogn Mag. 2010;6:116–9. doi: 10.4103/0973-1296.62898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prajapati MS, Patel JB, Modi K, Shah MB. Leucas aspera: A review. Pharmacogn Rev. 2010;4:85–7. doi: 10.4103/0973-7847.65330. [DOI] [PMC free article] [PubMed] [Google Scholar]


