Abstract
Background:
Many fruits have been used as nutraceuticals because the presence of bioactive molecules that play biological activities.
Objective:
The present study was designed to compare the anti-inflammatory and antioxidant effects of methanolic extracts of Lycium barbarum (GOJI), Vaccinium macrocarpon (CRAN) and Vaccinium myrtillus (BLUE).
Materials and Methods:
Mices were treated with extracts (50 and 200 mg/kg, p.o.), twice a day through 10 days. Phytochemical analysis was performed by high-performance liquid chromatography. Antioxidant activity was determine by 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, reducing power, lipid peroxidation thiobarbituric acid reactive substances (TBARS), reduced glutathione (GSH) and catalase (CAT) activity. Anti-inflammatory activity was evaluated by paw edema followed by determination of myeloperoxidase (MPO) and TBARS.
Results:
High amount of phenolic compounds, including rutin, were identified in all berries extracts. However, quercetin was observed only in BLUE and CRAN. GOJI presents higher scavenging activity of DPPH radical and reducing power than BLUE and CRAN. The extracts improved antioxidant status in liver; BLUE showed the largest reduction (75.3%) in TBARS when compared to CRAN (70.7%) and GOJI (65.3%). Nonetheless, CAT activity was lower in BLUE group. However, hepatic concentrations of GSH were higher in animals treated with GOJI rather than CRAN and BLUE. Despite all fruits caused a remarkable reduction in paw edema and TBARS, only BLUE and CRAN were able to reduce MPO.
Conclusion:
These results suggest that quercetin, rutin, or other phenolic compound found in these berry fruits extracts could produce an anti-inflammatory response based on modulation of oxidative stress in paw edema model.
SUMMARY
Within fruits broadly consumed because of its nutraceuticals properties include, Lycium barbarum (Goji berry), Vaccinium myrtillus (Blueberry or Bilberry) and Vaccinium macrocarpon (Cranberry)
The objectives of this study were the investigation and comparison of chemical composition, antioxidant activity “in vitro” and “in vivo” and anti inflammatory property of berry fruits bought dry form.
In summary, two main findings can be addressed with this study: (1) Berry fruits presented antioxidant and anti inflammatory activities “in vitro” and “in vivo”; (2) the extracts of GOJI, CRAN, and BLUE modulate the inflammatory process by different mechanisms.
Keywords: Anti-inflammatory, antioxidant, blueberry, cranberry, Goji berry, paw edema
INTRODUCTION
Oxidative stress has been recognized as a contributing factor in several diseases such as inflammation, hypertension, cancer, diabetes, aging and neurodegenerative disorders. The beneficial effect of consuming plant foods is, among others, the presence of antioxidants, which is associated with counteracting risk to the development of several diseases mentioned above.[1]
As a consequence of this, there is a growing demand for functional foods in order to supply exogenous antioxidants. Many fruits have been used as nutraceuticals because the presence of bioactive molecules that play biological activities such as, antioxidant, anti-inflammatory, antimicrobial, antitumor, among others.[2,3] With the current upsurge of interest in the efficacy of functional foods and nutraceuticals, they have been received much attention especially fruits with antioxidant potential.
Within fruits broadly consumed because of its nutraceuticals properties include, Lycium barbarum (Goji berry), Vaccinium myrtillus (Blueberry or Bilberry) and Vaccinium macrocarpon (Cranberry), and they are used by folk medicine for several diseases. Goji berry (GOJI) is consumed as juice or dried, and it has been used as a traditional Chinese herbal medicine for the treatment of infertility, cancer, diabetes, abdominal pain, dry cough, fatigue, and headache, also to increase longevity. Previous studies have shown that polysaccharides and polyphenols are molecules responsible for its biological activities.[4] Blueberry (BLUE) is native from Northern Europe and used in as unspecific treatment of acute diarrhea, blood purifier, inflammation of mouth and throat.[5] BLUE is rich in anthocyanins, exhibiting intense blue color and phenolic compounds present in this fruit shows antioxidant, anti-inflammatory, antitumor, and antimicrobial activity.[6,7,8] One fruit belonging to Ericaceae family, the Cranberry (CRAN), is a native fruit of North America used as a folk remedy by native inhabitants to clean kidneys, bladder and to treat urinary tract and gastrointestinal infections.[9,10] CRAM is exported to other regions of the world dried or processed into juice, like other berry fruits, is rich in phenolic acids, flavonoids, and terpenes. The presence of these phytochemicals wide range of biological effects, including, antioxidant, anticarcinogenic, antibacterial.[11,12,13] Although the antioxidant effects of berry fruit is widely reported, the information about the effects of berries on DNA damage and endogenous antioxidant enzyme activities are poor understood, including their effects on the acute inflammation.[14] Inflammatory processes is an important hallmark of diseases or pathological conditions described above and many plants have demonstrated anti-inflammatory effects by modulating oxidative stress.[15,16]
The consumption of this fruits has been increasing in natural or industrialized forms, including its use in bakery products, different types of syrups and alcoholic beverages, preserves, and coloring additives. We conducted this study in response to recent interest in the nutritional and health benefits of Blueberry, Cranberry, and Goji berry. The objectives of this study were the investigation and comparison of chemical composition, antioxidant activity “in vitro” and “in vivo” and anti-inflammatory property of berry fruits bought dry form.
MATERIALS AND METHODS
Animal care
Animals were supplied by Biotery of Universidade do Oeste de Santa Catarina. Forty-six adult Swiss male mice, weighing 25–35 g, were maintained in room under controlled conditions at 22°C ± 2°C with a 12 h light/dark cycle was maintained, with lights on from the time of 07:00 to 19:00, with free access to water and food. All animals have been acclimatized into the Laboratory of Pharmacology, 24 h prior experiments. All procedures were approved by the Institutional Ethics Committee of Universidade do Oeste de Santa Catarina (document number: 008/2014). In all experiments, animals were managed following the principles and guidelines for the care of laboratory animals, according to Zimmermann.[17]
Drugs and reagents
Carrageenan type IV, Indomethacin, 2-[4-(2-hydroxyethyl) piperazin-1-yl] ethanesulfonic acid (HEPES), Folin-Ciocalteu, trichloroacetic acid (TCA), thiobarbituric acid (TBA), soybean trypsin inhibitor (SBTI), H2O2, tetramethylbenzidine (TMB), Tris-HCl, 2,2-diphenyl-1-picrylhydrazyl (DPPH), potassium ferricyanide, diethylenetriaminepentaacetic acid (DPTA), 5,5-ditiobis 2-nitrobenzoic (DTNB), MgCl2, galic acid, 2,2',2'',2'''-(Ethane-1,2-diyldinitrilo) tetraacetic acid (EDTA), rutin and quercetin were all purchased from Sigma-Aldrich® (St. Louis, MO, USA). Aprotinin, leupeptin, and phenylmethanesulfonyl fluoride (PMSF) from Santa Cruz Biotechnology® (Dallas, Texas, USA). Dimethylsulfoxide from Fluka® (St. Gallen, Switzerland). Ketamine and xilasine from CEVA® (Paulínia, SP, Brazil). The methanol high-performance liquid chromatography (HPLC) grade was purchased from Tedia® Brasil (Rio de Janeiro, RJ, Brazil) and ultrapure water was obtained from Milli® Q apparatus.
Plant materials and sample preparation
The dry fruit GOJI, BLUE, and CRAN were investigated. Berry fruits were purchased at the local market; all fruits were imported in dry form by the company Natividade Trade Importação and Exportação LTDA with certificates of analysis. Extraction was performed according to standard, commonly used procedures.[18] A 100 g sample of the dried berry fruit was crushed and mixed with around 250 mL methanol for 7 days at −4°C. The extracts were filtered using a Whatman no. 1 filter paper. After removal of the methanol in a rotary evaporator at a temperature below 40°C, the extracts were weighed and showed a yield of 64%, 54%, and 30% (Blueberry, Cranberry, and Goji berry, respectively). The extracts result was used for experiments “in vitro” and “in vivo,” being dissolved in methanol.
Chemical analysis
High-performance liquid chromatography instrumentation and chromatographic conditions
An aliquot of 1 mg of each extract and compounds were diluted in 1 mL with methanol. Then, the solutions were filtered through a microfilter of 0.45 μm before HPLC analysis. Chromatographic analysis was performed on the Shimadzu® LC AC - 20, equipped with quaternary pump, ultraviolet (UV) detector, and auto sampler injection SIL-20a. The analyses were separated on a Phenomenex® Luna-phase C18 analytical column (250 mm × 4.6 mm × 5 μm) protected by a C18 guard column. The column temperature was set at 30°C with a flow of 1 mL/min. The better condition of the mobile phase was obtained by the combination of acidified water pH = 2.5 (A) and methanol (B). The gradient elution was programmed as follows: 0–40 min, (60:40–0:100) and 40–50 min, (60:40) than return to the initial condition. In this chromatographic condition, rutin and quercetin had retention time (Rt) of 11.19 ± 0.1 min and 17.84 ± 0.1 min, respectively. The UV − visible spectra were recorded between 190 and 800 nm, and the chromatographic profiles were registered at 355 nm.
Total phenolic content
The total phenolic content was estimated using the Folin-Ciocalteu colorimetric method. Briefly, the appropriate dilutions of the extracted of berry fruits (0.4 ml) were oxidized with 2 ml of 0.5 mol/L Folin-Ciocalteu reagent for 4 min at room temperature. Then, the reaction was neutralized with 2 ml of 75 mg/mL saturated sodium carbonate. The absorbance was measured at 760 nm after incubation for 2 h at room temperature in the dark. Quantification was done on the basis of the standard curve of gallic acid. Results were expressed as gallic acid equivalent, i.e., mg gallic acid/100 g the extract.
Total flavonoid content
The total flavonoid content was measured using a modified colorimetric method. A 1 ml sample of appropriately diluted filtered plant extract was mixed with 0.1 ml 0.05 g/mL NaNO2. After 6 min, 0.1 mL 0.1 g/mL AlCl3·6H2O solution was added. Then 1 ml of 1 mol/L NaOH was added to the mixture after another 5 min. The reactive solution was well mixed and allowed to stand for 15 min before the absorbance at 510 nm was measured. Quantification was done on the basis of the standard curve of gallic acid. The total flavonoid content was calculated and expressed as quercetin equivalents, i.e., mg quercetin/100 g extract.
Antioxidant capacity: “In vitro” assays
2,2-diphenyl-1-picrylhydrazyl radical scavenging activity
The scavenging activity for DPPH radicals was determined using spectrophotometric analysis. A quantity consisting of 0.8 mL of 0.2 mM DPPH solution was mixed with a 0.2 mL different concentration of the extracts (25–3200 μg/mL). The mixture was shaken and left to stand for 30 min. The absorbance was measured at 517 nm using a spectrophotometer. The DPPH radical scavenging activity (%) was calculated with the following equation: 1− (AAss/AAcc) × 100, where AAss is the absorbance in the presence of sample and AAcc is the absorbance in the absence of sample. Quercetin was used as positive standards.
Reducing power assay
Each extract was mixed with 2.5 mL of 200 mM sodium phosphate buffer (pH 6.6) and 2.5 mL of 1% potassium ferricyanide, and the mixture was incubated at 50°C for of 20 min. After, 2.5 mL of 10% TCA was added, and the mixture was centrifuged at 650 rpm for 10 min. The upper layer (2.5 mL) was mixed with 2.5 mL of deionized water, and 0.5 mL of 0.1% ferric chloride and absorbance was measured at 700 nm: Higher absorbance indicates higher reducing power. The assays were carried out in triplicate, and the results are expressed as mean values ± standard deviations. The extract concentration providing 0.5 of absorbance (IC50) was calculated from the graph of absorbance at 700 nm plotted against the extract concentration. Quercetin has used a standard.
“In vivo” assays
Animals treatment
Mice were divided into 8 experimental groups (5–6 animal each group) and treated with GOJI, CRAN and BLUE, in doses of 50 and 200 mg/kg, 12/12 h, by oral route (p.o.) for 10 days. Control animals received only vehicle (distilled water; 0.1 mL/10 g), and indomethacin (10 mg/kg; i.p. 1 h after the experiments) was used such as positive control on inflammation. After treatment, the animals were killed by an overdose of ketamine/xilasine and right paws and liver were removed and storage at − 80°C until determination of thiobarbituric acid reactive substances (TBARS), catalase (CAT), and reduced glutathione (GSH).
Antioxidant activity “ex vivo”
Preparation of tissues homogenates
Liver or paws were rapidly removed and homogenized (1:10 w/v) in a buffer containing 0.1% triton X-100, 0.12 M NaCl, 30 mM sodium phosphate, pH 7.4. After were homogenized with turrax, for 30 s at 4°C followed by centrifugation at 10,000 ×g for 10 min. Lipid peroxidation, reactive species formation, GSH contents, and CAT were measured with freshly liver prepared samples. In paws was determined only by lipid peroxidation.
Lipid peroxidation
TBA-reactive species levels were determined according to the method in which malondialdehyde (MDA), a product of fatty acid peroxidation, reacts with TBA to form a colored complex. Freshly prepared tissue homogenates were sequentially mixed in test tubes with equal volumes of 60 mM Tris-HCl, pH 7.4, 0.1 mM DPTA buffer, 12% TCA and 0,73% TBA, stirring after each addition. The tubes were kept at 100°C for 1 h and subsequently cooled and centrifuged (10,000 g for 5 min). The absorbance of the supernatant was measured at 535 nm. Lipid peroxidation was calculated using the molar extinction coefficient of MDA (153/M/cm) at 535 nm. All samples were analyzed in duplicate, and results were expressed in μmol/g tissue.
Reduced glutathione
Determination of reduced GSH concentration in the samples is based on the reaction of GSH with DTNB, generating a thiolate anion (TNB), which yellow color is measured spectrophotometrically at 412 nm. The test was made in standard 96-well plates. To determine the GSH content, the sample was added to a reaction medium containing 2.525 mM DTNB in 200 mM sodium phosphate buffer, pH 8.0. The formation of TNB was monitored after 10 min at 412 nm. The results were expressed mmol/g tissue.
Catalase activity
CAT activity was measured by quantifying, at 240 nm, the rate of hydrogen peroxide decomposition by the enzyme present in the sample. For this purpose, an aliquot of the sample was added to the reaction medium containing 10 μM hydrogen peroxide in 50 mM phosphate buffer (pH 7.0), freshly prepared. The decrease in absorbance was monitored at 240 nm for 60 s with 10 s intervals. Enzyme activity was calculated using the molar extinction coefficient of hydrogen peroxide (43.6/M/cm) at 240 nm. All samples were analyzed in duplicate. The results were expressed μmol/g/min.
Anti-inflammatory activity
Paw edema induced by carrageenan test
To paw edema procedure, the mice were slightly anesthetized with halothane, and carrageenan (450 μg/paw) was injected into the right paw. The contralateral paw received the same volume of sterile phosphate-buffered saline (PBS) and served as control. The paw volume was measured with a plethysmometer (Panlab, Barcelona, Spain) immediately after carrageenan or PBS administration, at specified time points (30, 90, 180, and 300 min after injection of phlogistic agent). At the end of experiment, the right paw was removed and maintained at − 80°C. The results were expressed as the mean ± standard error of mean (SEM) values, of the difference in volume (in milliliters) between the carrageenan- and saline-treated paws.
Determination of myeloperoxidase activity in paw
A fragment of right paw (100 mg) was homogenized in ice-cold buffer 50 mM HEPES, 1 mM MgCl2, 10 mM EDTA, and 1% Triton X-100, pH 6.4, containing 1 μg/mL each protease inhibitors, aprotinin, leupeptin, and SBTI and 1 mM PMSF. Homogenate was centrifuged for 30 min (10.000 G). The supernatant (10 μL) was mixed for enzyme activity with 15 μL of H2O2 (0.017%), and 10 μL of TMB in 185 μL of phosphate buffer saline (80 mM, pH 5.4). After 3 min of incubation, 100 μL de H2SO4 (1M) was added and optical density (OD) at 450 nm was measured. The results were expressed as the mean ± SEM of OD at 450 nm.
Statistical analysis
Data were presented as mean ± SEM. Statistical analysis was performed by one-way ANOVA followed by Tukey's post hoc test when P < 0.05 was considered statistically significant.
RESULTS
Phytochemical analysis
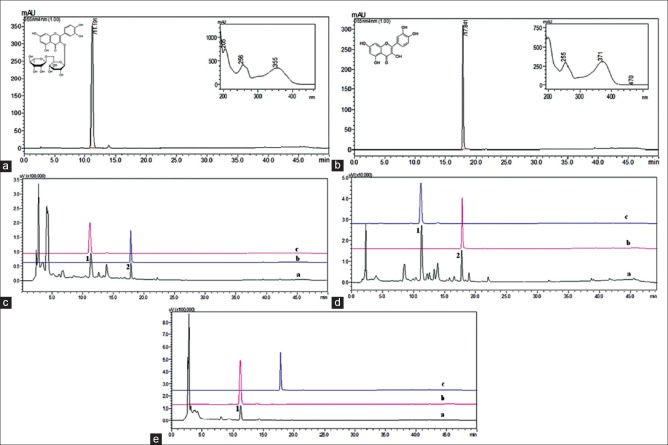
The phytochemical analysis conducted on the methanolic extract of berry fruits revealed the presence of phenolic compounds. GOJI showed the maximum phenolic content (142.2 ± 1.7 mg/100 g) and flavonoids (74.5 ± 2.2 mg/100 g) as compared to BLUE or CRAN [Table 1]. Qualitative analysis of these extracts was done by HPLC for identification of phenolic constituents. The chromatogram peaks with Rt of 11.19 and 17.84 min in extracts of different berry fruits [Figure 1c and d] indicates the presence of rutin and quercetin, respectively, similar to their standards profiles [Figure 1a and b].
Table 1.
Determination of the total phenolic and flavonoid in methanolic extracts
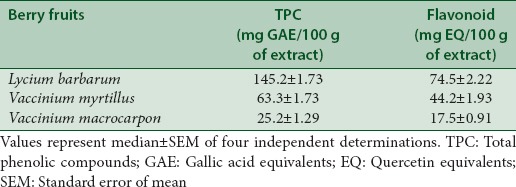
Figure 1.
(a) Chromatographic profile of the standard rutin; (b) chromatographic profile of the standard quercetin; (c) overlay chromatogram Goji berry extract, (d) overlay chromatogram Blueberry extract, (e) overlay chromatogram cranberry extract
Antioxidant activity
Determination of DPPH scavenging activity represents a rapid method to characterize the antioxidant capacity of extracts against oxidation caused by free radicals. According to this method, the color of the reaction mixture changed from mauve to light yellow, depending on the antioxidant capacity of the extracts. Berry fruits produced moderate DPPH radical scavenging activity, GOJI has the highest scavenging activity (56.1% ±1.5%) and was 3.9 and 1.9 higher as compared to CRAN and BLUE, respectively [Table 2].
Table 2.
Effect of berry fruits in 2,2-diphenyl-1-picrylhydrazyl radical inhibition “in vitro”
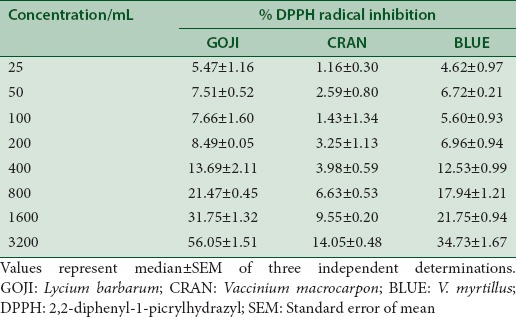
Figure 2 shows the reducing power of the three methanolic extracts of berry fruits as a function of their concentration and quercetin as standard. Following the tests performed, the color of the solution changed from yellow to various shades of green and blue, depending on the reducing power of each compound. The reducing power increased in direct proportion to extract concentration, and the reducing power of GOJI extract was 164.8 and 220.3 higher as compared to BLUE and CRAN, respectively, in the concentration of 1.200 μg/ml.
Figure 2.
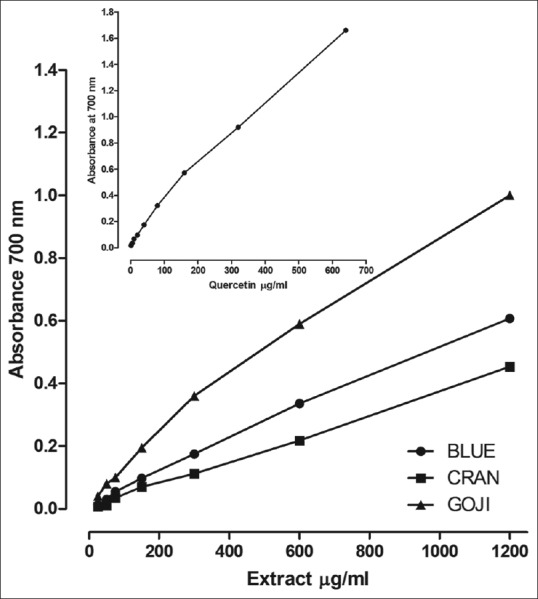
Reducing power activities of methanolic extracts of Lycium barbarum (GOJI), Vaccinium macrocarpon (CRAN) and Vaccinium myrtillus (BLUE). Methanol used for extraction of berries was used for measurement of reducing power activity, as described in the text
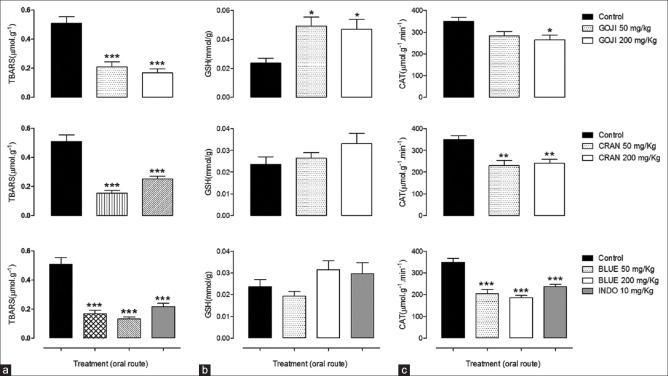
Front of the high content in phenolic compounds in fruit in question tested the raw methanol extracts in rats, for both was administered a daily dose of 50 and 200 mg/kg for 10 days. After the treatment, animals were sacrificed, and liver was collected for determination of lipid peroxidation, CAT activity, and reduced GSH. Berry fruits reduced TBARS levels in the liver of all tested groups. Animals treated with BLUE showed the largest reduction (75.3%) in TBARS when compared to CRAN (70.7%) and GOJI (65.3%), respectively [Figure 3a]. In the same way, CAT activity was lower in BLUE group than others. However, hepatic concentrations of GSH were significantly higher in animals treated with GOJI (125.4% and 103.4% of 50 and 200 mg/kg doses, respectively) than with CRAN and BLUE [Figure 3b and c]. Curiously, indomethacin reduced TBARS levels and CAT activity in liver but did not change the level of reduced GSH significantly [Figure 3].
Figure 3.
Antioxidant effect of Lycium barbarum (GOJI), Vaccinium macrocarpon (CRAN) and Vaccinium myrtillus (BLUE). Mice were treated during 10 days, twice a day (50 and 200 mg/kg, p.o.). Control animals received only vehicle and positive control indomethacin (10 mg/kg, i.p., INDO). After treatment, antioxidant activity was determined in liver. (a) Represents effect on thiobarbituric acid reactive substances, in (b) reduced glutathione concentration and in (c) catalase activity. Bars represent mean ± standard error of mean of 5–6 animals for group. ANOVA followed by Dunnett's post hoc test, *P < 0.05
Anti-inflammatory activity
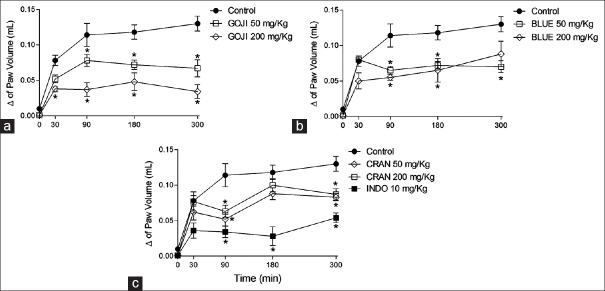
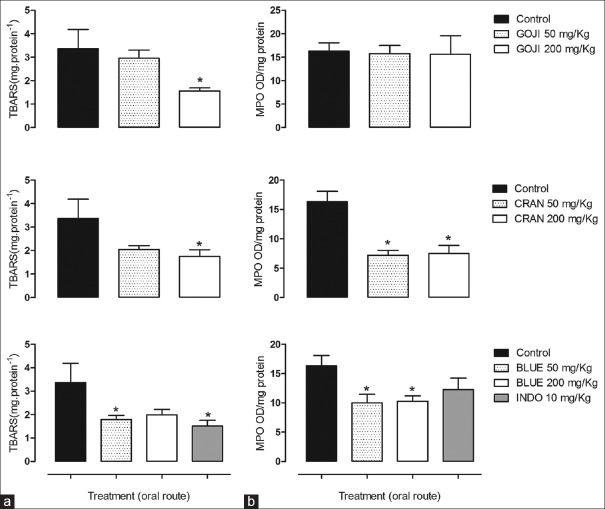
Doses of 50 and 200 mg/kg of GOJI, CRAN, and BLUE extracts lead to a reduction in paw edema of 38.9% and 63.8% (GOJI), 35.4% and 28.8% (BLUE), and 42.9% and 36.9% (CRAN), respectively, of the doses. Indomethacin also significantly inhibited the carrageenan-induced paw edema [Figure 4]. Carrageenan increased the lipid peroxidation and influx of neutrophils, measured by myeloperoxidase (MPO) activity, in inflamed paws [Figure 5a and b]. Treatment of animals with berries extracts reduced TBARS levels only in high dose (200 mg/kg) [Figure 5a]. However, only BLUE and CRAN were able to reduce MPO activity [Figure 5b] and indomethacin reduced only TBARS [Figure 5a].
Figure 4.
Antioxidant effect of Lycium barbarum (GOJI), Vaccinium macrocarpon (CRAN) and Vaccinium myrtillus (BLUE). Mice were treated during 10 days, twice a day (50 and 200 mg/kg, p.o.). Control animals received only vehicle and positive control indomethacin (10 mg/kg, i.p., INDO). After treatment, antioxidant activity was determined in liver. (a) Represents effect on thiobarbituric acid reactive substances, in (b), reduced glutathione concentration and in (c), catalase activity. Bars represent mean ± standard error of mean of 5–6 animals for group. ANOVA followed by Dunnett's post hoc test, *P < 0.05
Figure 5.
Effect of Lycium barbarum (GOJI), Vaccinium macrocarpon (CRAN) and Vaccinium myrtillus (BLUE) on levels of thiobarbituric acid reactive substances and myeloperoxidase. Mice were treated with extracts in the doses of 50 and 200 mg/kg; p.o., twice a day, for 10 days. Indomethacin (10 mg/kg; i.p.) was used as positive control. 300 min after carrageenan (450 μg) injection. Levels of thiobarbituric acid reactive substances (a) and myeloperoxidase (b) was determined. The graphs shows mean ± standard error of mean of thiobarbituric acid reactive substances in μmol/g or myeloperoxidase in optical density. mg protein-1. n = 5–6 animals per group. ANOVA followed by Dunnett's post hoc test, *P < 0.05
DISCUSSION
In summary, two main findings can be addressed with this study: (1) Berry fruits presented antioxidant and anti-inflammatory activities “in vitro” and “in vivo;” (2) the extracts of GOJI, CRAN, and BLUE modulate the inflammatory process by different mechanisms.
Oxidative stress occurs due to an imbalance between pro-oxidants and antioxidants as consequent the excessive production of reactive oxygen species (ROS). ROS are implicated in disease processes whereby they mediate damage to cell structures, including lipids, membranes, proteins, and DNA, however, also playing a role in the normal physiological process. There are many chemical classes of antioxidants found in nutraceuticals, such flavonoid polyphenols, nonflavonoid polyphenols, phenolic acids, and several of these molecules have biological activities.[19] Consumption of berry fruits contributes to reduces ROS and consequently diseases related to oxidative stress. Berries are an excellent source of bioactive compounds, includes polyphenols and anthocyanins, that are responsible of beneficial effects of berries in the modulation of numerous cell functions related to oxidative stress and/or antioxidant protection.[14]
In the present study, GOJI extracts show the higher quantity of polyphenols, mainly flavonoids, rather than CRAN and BLUE extracts. Although some authors have shown this compounds in species of berry fruits as well as attributed to antioxidant activity, it was not able to observe the quercetin in the Goji berry extracts [Figure 1].[20,21,22] However, secondary metabolites are frequently affected by environmental conditions which may subject to variations in the total content or relative proportions.[23] Based on the above and considering the presence of these compounds in our extracts, we can suggest that they are acting in part by the biological effect observed “in vitro” and “in vivo.”
Based on chromatogram [Figure 1], the antioxidant effect of CRAN is directly related to the content of quercetin and rutin present in the extract. Although BLUE also present significant amounts of quercetin and rutin, its highest antioxidant potential may be associated with the presence of other active compounds, which could be present in GOJI and is responsible for the antioxidant and anti-inflammatory potential. Some authors attribute the biological effect, especially antioxidant effect of BLUE and GOJI, mainly due to the presence of carotenoids and anthocyanins.[21,24,25]
This large amount of antioxidants was responsible for the highest DPPH radical scavenging activity and reduction power of GOJI when compared to CRAN and BLUE. Polyphenols exhibit powerful antioxidant activity by acting as free radical scavengers, namely, hydrogen donating compounds, singlet oxygen quenchers, and metal ion chelators.[26] The three berry fruits had a similar scavenging activity against DPPH radicals, but higher total antioxidant capacity than GOJI, which may be attributable containing more hydrophilic antioxidants.
CAT, superoxide dismutase (SOD), and glutathione peroxidase (GPX) play important roles in antioxidant defense mechanism by scavenging ROS as well as removing free radicals. In the current study, all extracts were able to promote a significant decrease CAT activity compared with control group [Figure 4c]. In a study reported by Kim et al.,[27] SOD and xanthine oxidase activities were insignificantly lowered when rats were fed a diet containing mandarin peel. They suggested that SOD activity was decreased because a number of flavonoids in mandarin peel could lower the activity of xanthine oxidase and consequently decrease levels of superoxide radicals and hydrogen peroxide. Therefore, we might assume that higher content of phenolic compound's present in all extract utilized in the current study acted as antioxidants, which suppress the production of oxygen radicals, resulting in decrease in activities of antioxidant enzymes such as CAT. These authors showed that mice treated with an atherogenic diet and supplemented with 10% cranberry had a reduction in the antioxidant status and a decrease in lipid peroxidation, the authors suggest that these results are related to the content of phenolic compounds present in cranberry fruit.
Berry fruits diets help improve neuronal functioning, restore the protective ability of the brain against oxidative or inflammatory stressors. However, the effects of each berry fruits are modulated differently, and this is presumably a result of the variety of phytochemicals in each berry fruit. Anthocyanins and other polyphenolic phytochemicals contained in these berry fruits extracts have, in addition to antioxidant, antidiabetic effects, hypolipidemic action can control their weight, improving the redox balance as well as can better other factors.[27,28,29,30] Although further investigations may be necessary to verify the pharmacological properties of the extracts, the evaluation of antioxidant effects “in vitro” and “in vivo” allows us to infer that the berry fruit consumption may be beneficial to health, especially GOJI that presented a great antioxidant potential when compared to the other studied extracts.
Paw edema induced by carrageenan is an acute model of inflammation widely used to evaluated anti-inflammatory effect of natural products.[31,32,33] After injection, carrageenan immediately produces edema, hyperalgesia, and erythema, resulting from the action of proinflammatory agents such as bradykinin, histamine, tachykinins, and complement.[34] Our results showed that all extracts present anti-inflammatory effects, being GOJI extract more effective in reducing paw edema than BLUE and CRAN extracts.
In fact, several studies demonstrate that berry fruit shows anti-inflammatory properties. Blueberry blocked the activation of nuclear factor kβ and expression of nitric oxide synthase-2, cyclooxygenase-2, interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) in H2O2-stimulated macrophages.[6,35] Cranberry (CRAN) increased SOD, CAT and GPX activity in liver and reduce TNF-α, after exposition to gamma radiation.[36] Moreover, polyphenols from Cranberry improved mitochondrial dysfunctions in intestinal Caco-2/15 cells stimulated with com lipopolysaccharide (LPS), which alleviate intestinal oxidative stress and inflammation.[37] Likewise, Goji berry protected DNA damage induced by H2O2 in mouse testicular cells and decreased the production of inflammatory mediators, such, prostaglandin E2, nitric oxide, IL-1β, and IL-6 in macrophages LPS-stimulated.[38,39] However, most of this studies are performed in cultured cells, even when using an animal model, the authors use only a single dose of berry fruits before the inflammatory stimulus. Our work shows that GOJI extracts, CRAN, and BLUE have anti-inflammatory activity even when administered by the sub-chronic way. Furthermore, continued consumption of these plants can exert a protective role also in acute inflammation.
After carrageenan injection, neutrophils migrate to inflamed paw and release enzymes such as MPO. This enzyme is a member of hem peroxidase found in azurophilic granules of neutrophils. MPO activity is significantly increased in inflamed tissues indicating that neutrophils accumulation can exacerbate the inflammatory response.[40,41] Activated neutrophils increase ROS production, such as hydrogen peroxide, superoxide, and hydroxyl radicals. It is known that alterations in ROS level contributed to characteristic microvascular inflammation responses, such as leukocyte recruitment and increased vascular permeability. In addition, ROS attacks polyunsaturated fatty acids in the plasma membrane and create products such as MDA. The products of lipid peroxidation may be measured by TBARS reaction and is commonly used as a marker of free radical-mediated damage.[36,41]
Our results showed that berry fruits modulate the inflammation by distinct mechanisms while BLUE and CRAN reduced TBARS and MPO, GOJI reduce the infiltration of neutrophils. In the same way, indomethacin reduces only TBARS, without interfering with the migration of neutrophils. Together, this results indicating that BLUE and CRAN exhibit an acute anti-inflammatory activity by reducing damage mediated by ROS while GOJI has compounds with activity more similar to the classic nonsteroidal anti-inflammatory drugs, such indomethacin. However, the antioxidant capacity of GOJI cannot be disregarded in acute inflammation, because this extract showed the highest antioxidant activity both “in vitro” and “in vivo” in our experimental conditions.
CONCLUSION
The results above suggest that quercetin, rutin, and other polyphenolic phytochemicals contained in these berry fruits extracts have, in addition to antioxidant effects, important anti-inflammatory effect in analyzed doses specially GOJI that shows similar indomethacin effect. This study also suggests that the consumer of these berry fruits is value against inflammatory diseases and could be one pharmaceutical ingredient for prevention of diseases associated with oxidative stress and inflammation such, diabetes, cancer, and neurodegenerative disorders. However, our study is limited in the context that it is valid for animals and transfer of these results for humans must be done carefully.
Financial support and sponsorship
This work was supported by grants from FAPESC/CNPq (Edital Universal 2012, PRONEM 2012 and Jovem Pesquisador, 2011) and UNOESC – Videira.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Claudriana Locatelli
Claudriana Locatelli, Graduated in Pharmaceutical Sciences from the Federal University of Santa Catarina (1999), Master (2002) and PhD (2009). Professor at School of Pharmaceutical Sciences, University of the West of Santa Catarina (Unoesc). Contribute as professor at Unoesc Postgraduate Program of Bioscience and Health, and Program of Science and Biotecnology. It has experience in cancer and oxidative strees.
REFERENCES
- 1.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90:7915–22. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tapsell LC, Hemphill I, Cobiac L, Patch CS, Sullivan DR, Fenech M, et al. Health benefits of herbs and spices: The past, the present, the future. Med J Aust. 2006;185(4 Suppl):S4–24. doi: 10.5694/j.1326-5377.2006.tb00548.x. [DOI] [PubMed] [Google Scholar]
- 3.Benzie IF. Evolution of dietary antioxidants. Comp Biochem Physiol A Mol Integr Physiol. 2003;136:113–26. doi: 10.1016/s1095-6433(02)00368-9. [DOI] [PubMed] [Google Scholar]
- 4.Potterat O. Goji (Lycium barbarum and L. Chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010;76:7–19. doi: 10.1055/s-0029-1186218. [DOI] [PubMed] [Google Scholar]
- 5.Menkovic N, Savikin K, Tasic S, Zdunic G, Stesevic D, Milosavljevic S, et al. Ethnobotanical study on traditional uses of wild medicinal plants in Prokletije mountains (Montenegro) J Ethnopharmacol. 2011;133:97–107. doi: 10.1016/j.jep.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Pomari E, Stefanon B, Colitti M. Effect of plant extracts on H2O2-induced inflammatory gene expression in macrophages. J Inflamm Res. 2014;7:103–12. doi: 10.2147/JIR.S61471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsunaga N, Tsuruma K, Shimazawa M, Yokota S, Hara H. Inhibitory actions of bilberry anthocyanidins on angiogenesis. Phytother Res. 2010;24(Suppl 1):S42–7. doi: 10.1002/ptr.2895. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto M, Yamaura K, Ishiwatari M, Ueno K. Difficulty for consumers in choosing commercial bilberry supplements by relying only on product label information. Pharmacognosy Res. 2013;5:212–5. doi: 10.4103/0974-8490.112432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavender A. Folk medical uses of plant foods in Southern Appalachia, United States. J Ethnopharmacol. 2006;108:74–84. doi: 10.1016/j.jep.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 10.Vattem DA, Ghaedian R, Shetty K. Enhancing health benefits of berries through phenolic antioxidant enrichment: Focus on cranberry. Asia Pac J Clin Nutr. 2005;14:120–30. [PubMed] [Google Scholar]
- 11.Wu H, Guo H, Zhao R. Effect of Lycium barbarum polysaccharide on the improvement of antioxidant ability and DNA damage in NIDDM rats. Yakugaku Zasshi. 2006;126:365–71. doi: 10.1248/yakushi.126.365. [DOI] [PubMed] [Google Scholar]
- 12.Vidlar A, Vostalova J, Ulrichova J, Student V, Stejskal D, Reichenbach R, et al. The effectiveness of dried cranberries (Vaccinium macrocarpon) in men with lower urinary tract symptoms. Br J Nutr. 2010;104:1181–9. doi: 10.1017/S0007114510002059. [DOI] [PubMed] [Google Scholar]
- 13.Seeram NP, Adams LS, Hardy ML, Heber D. Total cranberry extract versus its phytochemical constituents: Antiproliferative and synergistic effects against human tumor cell lines. J Agric Food Chem. 2004;52:2512–7. doi: 10.1021/jf0352778. [DOI] [PubMed] [Google Scholar]
- 14.Del Bo' C, Martini D, Porrini M, Klimis-Zacas D, Riso P. Berries and oxidative stress markers: An overview of human intervention studies. Food Funct. 2015;6:2890–917. doi: 10.1039/c5fo00657k. [DOI] [PubMed] [Google Scholar]
- 15.da Rosa RL, Nardi GM, Januário AG, Boçois R, Bagatini KP, Bonatto SJ, et al. Anti-inflammatory, analgesic, and immunostimulatory effects of Luehea divaricata Mart. & Zucc. (Malvaceae) bark. Braz J Pharm Sci. 2014;50:599–610. [Google Scholar]
- 16.Nardi GM, Siqueira Junior JM, Delle Monache F, Pizzolatti MG, Ckless K, Ribeiro-do-Valle RM. Antioxidant and anti-inflammatory effects of products from Croton celtidifolius Bailon on carrageenan-induced pleurisy in rats. Phytomedicine. 2007;14:115–22. doi: 10.1016/j.phymed.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 18.Puttaraju NG, Venkateshaiah SU, Dharmesh SM, Urs SM, Somasundaram R. Antioxidant activity of indigenous edible mushrooms. J Agric Food Chem. 2006;54:9764–72. doi: 10.1021/jf0615707. [DOI] [PubMed] [Google Scholar]
- 19.Kelsey NA, Wilkins HM, Linseman DA. Nutraceutical antioxidants as novel neuroprotective agents. Molecules. 2010;15:7792–814. doi: 10.3390/molecules15117792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duan H, Chen Y, Chen G. Far infrared-assisted extraction followed by capillary electrophoresis for the determination of bioactive constituents in the leaves of Lycium barbarum Linn. J Chromatogr A. 2010;1217:4511–6. doi: 10.1016/j.chroma.2010.04.069. [DOI] [PubMed] [Google Scholar]
- 21.Huang WY, Zhang HC, Liu WX, Li CY. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J Zhejiang Univ Sci B. 2012;13:94–102. doi: 10.1631/jzus.B1100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.White BL, Howard LR, Prior RL. Proximate and polyphenolic characterization of cranberry pomace. J Agric Food Chem. 2010;58:4030–6. doi: 10.1021/jf902829g. [DOI] [PubMed] [Google Scholar]
- 23.Gobbo Neto L, Lopes NP. Medicinal plants: factors of influence on the content of secondary metabolites. Quim Nova. 2007;30:374–81. [Google Scholar]
- 24.Liu Y, Zeng S, Sun W, Wu M, Hu W, Shen X, et al. Comparative analysis of carotenoid accumulation in two goji (Lycium barbarum L. and L. ruthenicum Murr.) fruits. BMC Plant Biol. 2014;4:269. doi: 10.1186/s12870-014-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zoratti L, Sarala M, Carvalho E, Karppinen K, Martens S, Giongo L, et al. Monochromatic light increases anthocyanin content during fruit development in bilberry. BMC Plant Biol. 2014;14:377. doi: 10.1186/s12870-014-0377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vladimir-Kneževic S, Blažekovic B, Štefan MB, Alegro A, Koszegi T, Petrik J. Antioxidant activities and polyphenolic contents of three selected Micromeria species from Croatia. Molecules. 2011;16:1454–70. doi: 10.3390/molecules16021454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim MJ, Chung JY, Kim JH, Kwak HK. Effects of cranberry powder on biomarkers of oxidative stress and glucose control in db/db mice. Nutr Res Pract. 2013;7:430–8. doi: 10.4162/nrp.2013.7.6.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khoo HE, Azlan A, Ismail A, Abas F, Hamid M. Inhibition of oxidative stress and lipid peroxidation by anthocyanins from defatted Canarium odontophyllum pericarp and peel using in vitro bioassays. PLoS One. 2014;9:e81447. doi: 10.1371/journal.pone.0081447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nair AR, Elks CM, Vila J, Del Piero F, Paulsen DB, Francis J. A blueberry-enriched diet improves renal function and reduces oxidative stress in metabolic syndrome animals: Potential mechanism of TLR4-MAPK signaling pathway. PLoS One. 2014;9:e111976. doi: 10.1371/journal.pone.0111976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shukitt-Hale B, Galli RL, Meterko V, Carey A, Bielinski DF, McGhie T, et al. Dietary supplementation with fruit polyphenolics ameliorates age-related deficits in behavior and neuronal markers of inflammation and oxidative stress. Age (Dordr) 2005;27:49–57. doi: 10.1007/s11357-005-4004-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Azza Z, Oudghiri M. In vivo anti-inflammatory and antiarthritic activities of aqueous extracts from Thymelaea hirsuta. Pharmacognosy Res. 2015;7:213–6. doi: 10.4103/0974-8490.150510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chattopadhyay P, Hazarika S, Dhiman S, Upadhyay A, Pandey A, Karmakar S, et al. Vitex negundo inhibits cyclooxygenase-2 inflammatory cytokine-mediated inflammation on carrageenan-induced rat hind paw edema. Pharmacognosy Res. 2012;4:134–7. doi: 10.4103/0974-8490.99072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta R, Kaur J. Evaluation of analgesic, antipyretic and anti-inflammatory activity on Cordia dichotoma G. Forst. Leaf. Pharmacognosy Res. 2015;7:126–30. doi: 10.4103/0974-8490.147227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morris CJ. Carrageenan-induced paw edema in the rat and mouse. Methods Mol Biol. 2003;225:115–21. doi: 10.1385/1-59259-374-7:115. [DOI] [PubMed] [Google Scholar]
- 35.Lietti A, Cristoni A, Picci M. Studies on Vaccinium myrtillus anthocyanosides. I. Vasoprotective and antiinflammatory activity. Arzneimittelforschung. 1976;26:829–32. [PubMed] [Google Scholar]
- 36.Azab KS, Salama AF, Magdy A, Rizk M. Cranberry extract modulate inflammation in irradiated rats. Sci Res Essays. 2014;2:392–8. [Google Scholar]
- 37.Denis MC, Desjardins Y, Furtos A, Marcil V, Dudonné S, Montoudis A, et al. Prevention of oxidative stress, inflammation and mitochondrial dysfunction in the intestine by different cranberry phenolic fractions. Clin Sci (Lond) 2015;128:197–212. doi: 10.1042/CS20140210. [DOI] [PubMed] [Google Scholar]
- 38.Song MY, Jung HW, Kang SY, Kim KH, Park YK. Anti-inflammatory effect of lycii radicis in LPS-stimulated RAW 264.7 macrophages. Am J Chin Med. 2014;42:891–904. doi: 10.1142/S0192415X14500566. [DOI] [PubMed] [Google Scholar]
- 39.Luo Q, Li Z, Huang X, Yan J, Zhang S, Cai YZ. Lycium barbarum polysaccharides: Protective effects against heat-induced damage of rat testes and H2O2-induced DNA damage in mouse testicular cells and beneficial effect on sexual behavior and reproductive function of hemicastrated rats. Life Sci. 2006;79:613–21. doi: 10.1016/j.lfs.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Malle E, Furtmüller PG, Sattler W, Obinger C. Myeloperoxidase: A target for new drug development? Br J Pharmacol. 2007;152:838–54. doi: 10.1038/sj.bjp.0707358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prokopowicz Z, Marcinkiewicz J, Katz DR, Chain BM. Neutrophil myeloperoxidase: Soldier and statesman. Arch Immunol Ther Exp (Warsz) 2012;60:43–54. doi: 10.1007/s00005-011-0156-8. [DOI] [PubMed] [Google Scholar]