Abstract
Background:
Woodfordia fruticosa, a plant of Indian origin, is extensively used in folk medicine for the treatment of various ailments.
Objective:
The aim of the present study was to standardize the flowers of W. fruticosa, Kurz (Lythraceae), an important plant of Indian origin and explore the chemical constituents contributing to its anti-ulcer activity.
Materials and Methods:
High-performance thin layer chromatography (HPTLC) and high-performance liquid chromatography (HPLC) profiling of the three samples of W. fruticosa flowers purchased from three different markets was done using ellagic acid as the biomarker. Two doses of the aqueous extract of the W. fruticosa (AEWF) flowers were evaluated for anti-ulcer activity by ethanol-induced ulcer model in Wistar albino rats. Omeprazole was used as the positive control. The parameters used for the assessment of the anti-ulcer potential were total titratable acidity (TTA), ulcer index, and percentage protection.
Results:
The HPTLC and HPLC studies confirmed the presence of ellagic acid in all the three drug samples. The AEWF showed significant reduction in terms of TTA at both doses of 100 mg/kg and 200 mg/kg. The gastroprotection indicated by a lower ulcer index and higher percentage protection was significant for 200 mg/kg dose of AEWF, better than the protection afforded by omeprazole (10 mg/kg).
Conclusion:
The chromatographic profiling and the anti-ulcer studies served as an efficient tool in the characterization of ellagic acid as an important biomarker for the flowers of W. fruticosa and a probable contributor to the gastroprotective capacity of the drug. The bioactivity studies further supported the traditional use of W. fruticosa in the treatment of ulcers.
SUMMARY
HPTLC & HPLC fingerprinting of W. fruticosa using ellagic acid as a biomarker.
Evaluation of W. fruticosa for gastroprotection potential in ethanol induced gastric ulcer in rats model.
Aqueous extract of the drug showed better gastroprotection than the standard drug omeprazole at a dose of 200 mg/kg.
Keywords: Anti-ulcer activity, ellagic acid, ethanol-induced ulcer, high-performance thin layer chromatography, Woodfordia fruticosa
INTRODUCTION
There is a balance in the stomach between the aggressive digestive capabilities of the acid plus pepsin and the mucosal barrier. The disturbance of the normal equilibrium caused by either enhanced aggression or diminished mucosal resistance leads to ulceration. Several factors such as increased acid pepsin secretion or impaired bicarbonate neutralization or mucus secretion and precipitated lesions on the mucosal layer are implicated in the pathogenesis of gastric ulcer.[1]
Woodfordia fruticosa is one of two species of the genus Woodfordia, Salisbury, in the family Lythraceae. It is well-known in many parts of Asia as a plant possessing medicinal values.[2] The generic name honors E. James Alexander Woodford (e), a botanist, physician, and contemporary of Salisbury, Edinburgh.[3] This plant is commonly known by various vernacular names such as fire-flame bush, dhai ke phul, dhataki, and bahupushpi.[4] The species are long-lived shrubs, typically 2–3 m in height, with a sprawling, untidy appearance which is the result of an irregular sympodial branching pattern. The Asian species W. fruticosa is distributed principally along the lower Southern slopes of the Himalayas from Kashmir into Yun-nan Province of China and extends southward along the Western Ghats in India.[3] The flowers and leaves of the plant W. fruticosa Kurz (Lythraceae) have been a source of great value in traditional systems of medicines.[5] The leaves and flowers of W. fruticosa are popularly employed in folk medicine for fever, hemothermia, persistent dysentery, menorrhagia, and seminal weakness.[6] The dried flowers are powdered and dusted over ulcers and wounds to eliminate discharge and promote granulation.[7] While chemical investigations on various extracts of the flowers of W. fruticosa have shown the presence of several bioactive compounds such as flavonoids, saponins,[8] polyphenols, tannins,[9] and alkaloids.[5] In separate studies, bioactivity studies have demonstrated the gastroprotective, gastric healing, and anti-diarrheal activities of these phytoconstituents, viz., flavonoids,[10] saponins,[11] polyphenols,[12] alkaloids,[13] in other plants.
An herbal product contains multiple constituents that might be responsible for its therapeutic effects. It thus becomes necessary to define as many of the constituents as possible to understand and explain the bioactivity. The concept of “phytoequivalence” has been introduced in Germany to ensure consistency of phytotherapeuticals. According to this concept, a chemical profile for an herbal product is constructed and compared with the profile of a clinically proven reference product.[14]
Thus, the present study is aimed at evaluating the gastroprotective potential of W. fruticosa flower extracts. The alcoholic and hydro-alcoholic extracts of W. fruticosa flowers along with the ellagic acid standard have been subjected to chromatographic analyses followed by bioactivity studies in the conquest to explore the chemical compounds probably responsible for the gastroprotective activity.
MATERIALS AND METHODS
Drugs, chemicals, and materials
The three drug samples of the dried flowers of W. fruticosa were collected from the local market in Hyderabad, Telangana; Bangalore, Karnataka; and Nahan, Himachal Pradesh, respectively. Ellagic acid was purchased from Sigma-Aldrich. Potassium dihydrogen orthophosphate and acetonitrile were obtained from Loba Chemie, India, and Merck, India, respectively. Solvents and mobile phases used for high-performance liquid chromatography (HPLC) were of HPLC grade obtained from Rankem, India. All solvents used were of analytical grade.
Thin layer chromatography
Thin layer chromatography (TLC) profiling of the three samples of W. fruticosa purchased from Hyderabad, Telangana; Bangalore, Karnataka; and Nahan, Himachal Pradesh, along with the ellagic acid (standard biomarker) were subjected to TLC profiling to optimize the solvent systems and conditions.
Method of extraction
Powdered sample (1 g) was refluxed with 50 ml 70% ethanol for 30 min. The solution was filtered and again the marc was extracted with the same menstruum until colorless. The extracts were pooled and evaporated on a water bath and the residue was redissolved in the same solvent and made up to 50 ml. Freshly prepared extracts were used for TLC studies.[15]
Preparation of standards
Ellagic acid solution was prepared by dissolving 3 mg of the standard in 5 ml of HPLC water (3 mg/5 ml).
Solvent systems
The solvent system toluene: chloroform: ethyl acetate: formic acid (2:6:6:2) designated as solvent system A and toluene: ethyl acetate: methanol: formic acid (6:6:1.6:0.4) designated as solvent system B were optimized for the drug samples with respect to the standard used.[16,17]
High-performance thin layer chromatography fingerprinting
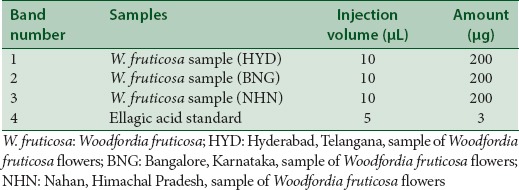
TLC procedure was standardized with the solvent systems A and B which were used for the high-performance TLC (HPTLC) fingerprinting of the three samples of the W. fruticosa flowers along with the standard ellagic acid. The sequence of the tracks is given in Table 1. HPTLC fingerprinting analysis was done using the CAMAG linomat 5 sample applicator using micro syringe (100 μl, Hamilton), CAMAG reprostar 3, Twin trough chamber, Dip tank, Win cat software - Version 1.3.3., with a development distance of 70 mm. Precoated silica gel plates 60F254 from Merck (20 cm × 10 cm) were used. Samples and standards were applied as bands on the plates in volumes 10 μl and detection was done under ultraviolet (UV) 254, UV 366, and after derivatization with 5% methanolic ferric chloride reagent.
Table 1.
Application pattern of samples and standard ellagic acid on high-performance thin layer chromatography plate
High-performance liquid chromatography
Standard solution
The stock solution of ellagic acid (standard biomarker) was prepared in HPLC grade water. The stock solution was diluted in the mobile phase to make the final concentration of 3 μg/5 ml.
Method of extraction
The sample of W. fruticosa flowers weighing 0.125 g pulverized (16 mesh size) was extracted using HPLC grade water (25 ml) as solvent and sonicated for 30 min. The extract was filtered and made up to 25 ml with the same solvent and filtered through 0.45 μ membrane filter and used for HPLC analysis.[18]
High-performance liquid chromatography conditions
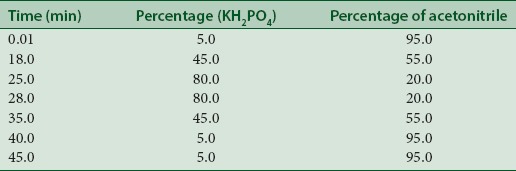
The liquid chromatographic system used was a gradient type HPLC Shimadzu system comprising of a pump (LC-10ATVP, Shimadzu, Japan), a UV-visible detector (SPD-10A Shimadzu) equipped with CLASS-VP6 software, and a rheodyne sample injector fitted with a 20 μl sample loop. The chromatographic separation was carried out on Merck C18 (250 mm × 4.6 mm) column with 5 μ particle size. The solvent system was acetonitrile: 5 mM potassium dihydrogen orthophosphate (KH2PO4) (95:5, v/v) buffer adjusted to pH 2.5 with dilute orthophosphoric acid and filtered with Whatman filter paper. The total run time for sample analysis was 18 min with a flow rate of 1.5 ml/min at room temperature. The UV detector was operated at 254 nm. The time program is given in Table 2.
Table 2.
Time program for gradient elution of Woodfordia fruticosa samples and ellagic acid standard in high-performance liquid chromatography
Acute toxicity studies
Acute oral toxicity of the aqueous extract of the W. fruticosa (AEWF) in mice was carried out as per OECD guidelines (425). Healthy adult albino mice (25–30 g) were used for the study. They were divided into two groups each containing six animals. Group I animals were treated with distilled water (2 ml/kg), and group II administered with single dose of 2000 mg/kg extract by gastric intubation using a soft catheter. The animals were observed continuously after 2 h and then intermittently at gaps of 1 h and observed for mortality during 48 h study period toxicity (short-term). The short-term profile of the drug was used to determine the dose of the next animals as per OECD guideline 425 with minor modifications. Calculation of the LD50 of the test extract was done using AOT 425 software provided by environmental protection agency, USA.[19]
Experimental animals
Thirty male Wistar rats weighing 180–200 g were used for the study. The animals were housed in five groups of six animals in standard laboratory cages and fed on balanced rat chow and water ad libitum with temperature (22°C ± 3°C) and lighting (12 h light/dark cycle; lights on 0700 h) controlled.[20] All experimental animals were carried out according to the guidelines of the Institutional Animals Ethics Committee, MESCO College of Pharmacy, Hyderabad. The rats in group I served as normal control group which received distilled water (1 ml) orally and were not subjected to ulcer induction. Rats in group II were left untreated after ulcer induction by absolute ethanol (1 ml/200 g of rat) and designated as negative control. Rats in group III were administered with omeprazole (10 mg/kg) as positive control. Rats in group IV and V received the AEWF at doses of 100 mg/kg and 200 mg/kg, respectively.[21]
Selection of dose
The dose was selected as prescribed by The Ayurvedic Pharmacopoeia of India, 2007, i.e. for W. fruticosa flowers - 3 − 6 g.[4]
Approximately 4 g of the human dose of raw drug was calculated for rats by using dose conversion factor reported by Gosh.[22]
It is expressed as follows:
Animal dose (for 200 g animal) = Human dose × 0.018, where 0.018 is conversion factor
= 4000 mg × 0.018
= 72 mg/200 g of rat i.e. 360 mg/kg
Ethanol-induced ulcer model
The gastric ulcers were induced in rats by administering absolute ethanol (1 ml/200 g of rat) orally. All the animals fasted for 18 h before administration of ethanol. The rats were treated with the extract/omeprazole (10 mg/kg) orally using gastric lavage, 30 min before administration of 1 ml absolute ethanol. Untreated animals were included as negative controls. The animals were anesthetized 1 h later with anesthetic ether and sacrificed by cervical dislocation. The ulcers were examined by an incision on the stomach along the greater curvature end. Mean ulcer score for each animal was expressed as ulcer index. Percentage protection was also calculated with the ulcer index. Gastric juice was collected and gastric secretion studies were performed in terms of total titratable acidity (TTA).[23,24,25]
Ulcer score
The ulcers were scored as follows:
0 = Normal colored stomach,
0.5 = Red coloration,
1 = Spot ulcers
1.5 = Hemorrhagic streaks,
2 = Ulcers ≥3 but ≤5,
3 = Ulcers >5
The mean ulcer score was expressed as ulcer index for each animal.
Percentage protection

Total titratable acidity
The TTA is expressed as follows:
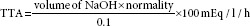
Statistical analysis
The data were expressed as the mean ± standard error of mean (SEM), and statistical analysis was performed using the Dunnet's t-test and ANOVA.
RESULTS
Preliminary studies
In our previous study, the W. fruticosa flowers purchased from three different geographical locations had been subjected to proximate analysis, successive solvent extraction, phytochemical screening, and quantification of total alkaloids, total saponins, and total polyphenols for the assessment of stability, repeatability, accuracy, and chief active phytoconstituents. The results have been discussed in detail in our previous publication.[5]
High-performance thin layer chromatography fingerprinting
Ellagic acid was used as the biomarker for the standardization of the three W. fruticosa samples using HPTLC fingerprint profiles. The HPTLC conditions described in the material and methods section allowed good separation for all the three W. fruticosa samples. Various solvent systems were tried for the separation. Good separation and resolution was achieved with two solvent systems; toluene: chloroform: ethyl acetate: formic acid (2:6:6:2) and toluene: ethyl acetate: methanol: formic acid (6:6:1.6:0.4) which were designated as solvent systems A and B. Development was done under UV 254, UV 366 nm, and after spraying with 5% methanolic ferric chloride.
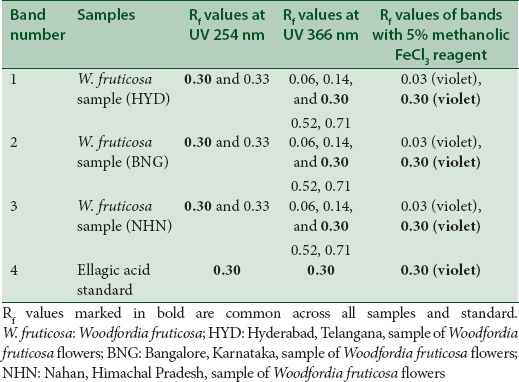
The chromatograms developed using solvent system A and solvent system B for the three samples along with the standard ellagic acid are shown in Figures 1 and 2 under UV 254 nm, UV 366 nm, and after spraying with 5% methanolic FeCl3, respectively. The results summarized in Tables 3 and 4 for solvent systems A and B, respectively, reveal that the standard ellagic acid showed a single band at Rf 0.21 and 0.30, respectively. Apart from other bands, all the three W. fruticosa samples also showed a common corresponding band with the same Rf value as that of ellagic acid. After spraying with 5% methanolic FeCl3, brown bands were observed for ellagic acid and the three W. fruticosa samples with solvent system A at the same Rf value. The plate developed with solvent system B showed violet color bands for ellagic acid and the corresponding bands in the three W. fruticosa samples after spraying with 5% methanolic FeCl3. The results confirmed the presence of ellagic acid [Tables 3 and 4].
Figure 1.
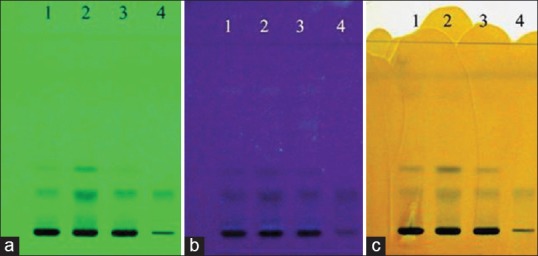
High-performance thin layer chromatography fingerprint of Woodfordia fruticosa samples and ellagic acid standard with solvent system A. (a) At ultraviolet 254 nm; (b) at ultraviolet 366 nm; (c) after spraying with 5% methanolic FeCl3 reagent. 1: Hyderabad, Telangana, sample of Woodfordia fruticosa flowers; 2: Bangalore, Karnataka, sample of Woodfordia fruticosa flowers; 3: Nahan, Himachal Pradesh, sample of Woodfordia fruticosa flowers; 4: Standard ellagic acid
Figure 2.
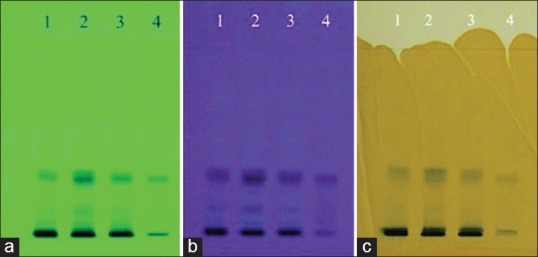
High-performance thin layer chromatography fingerprint of Woodfordia fruticosa samples and standard ellagic acid with solvent system B. (a) At ultraviolet 254 nm; (b) at ultraviolet 366 nm; (c) after spraying with 5% methanolic FeCl3 reagent. 1: Hyderabad, Telangana, sample of Woodfordia fruticosa flowers; 2: Bangalore, Karnataka, sample of Woodfordia fruticosa flowers; 3: Nahan, Himachal Pradesh, sample of Woodfordia fruticosa flowers; 4: Standard ellagic acid
Table 3.
High-performance thin layer chromatography fingerprinting of Woodfordia fruticosa samples and standard ellagic acid with solvent system A
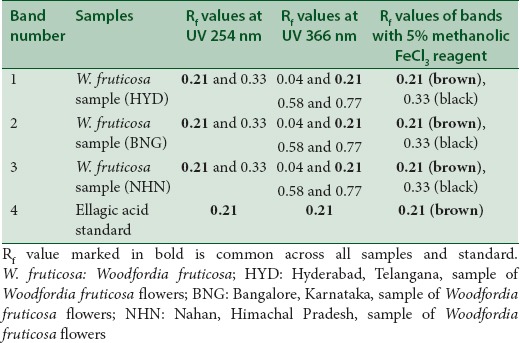
Table 4.
High-performance thin layer chromatography fingerprinting of Woodfordia fruticosa samples and standard ellagic acid with solvent system B
High-performance liquid chromatography fingerprinting
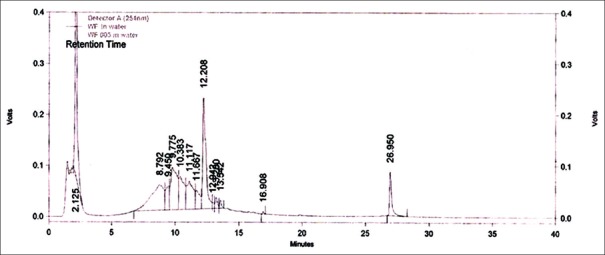
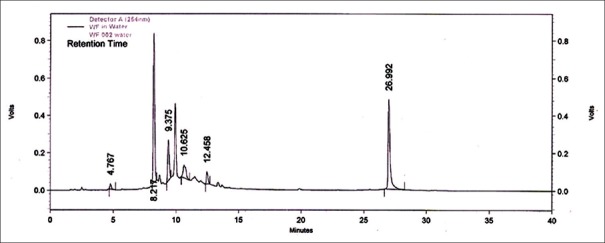
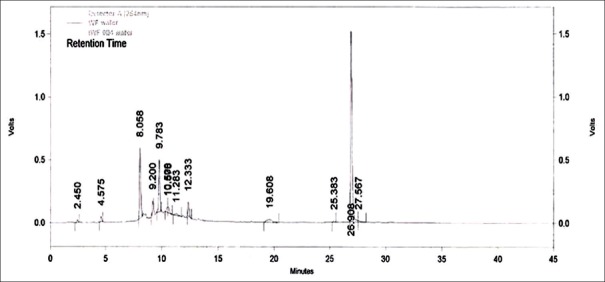
An attempt has been made to standardize the flowers of W. fruticosa along with ellagic acid by HPLC. The solvent system composition was optimized to acetonitrile and 5 mM potassium dihydrogen orthophosphate buffer pH 2.5 (95:5, v/v) by considering resolution, peak symmetry, and shape. Typical chromatograms of samples and ellagic acid [Figures 3–6] showed that all compounds were completely separated in 27 min. The chromatographic peaks of the ellagic acid in the three drug samples were identified by comparing their retention time with that of the ellagic acid standard. It can be observed in Table 5 that the peak at Rt = 12.3 min is common among all the drug samples and ellagic acid [Figure 7].
Figure 3.
High-performance liquid chromatography chromatogram of aqueous extract of Woodfordia fruticosa flower sample (Hyderabad). HYD: Hyderabad, Telangana, sample of Woodfordia fruticosa flowers
Figure 6.
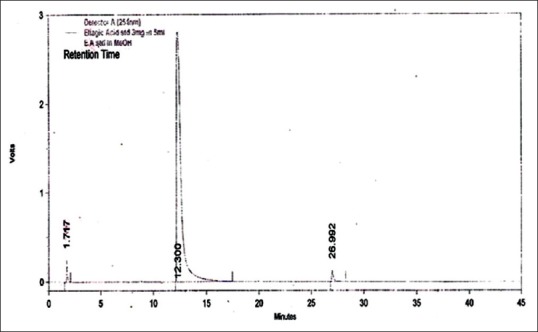
High-performance liquid chromatography chromatogram of standard ellagic acid
Table 5.
Comparison of high-performance liquid chromatography peak profiles of the three Woodfordia fruticosa flower samples
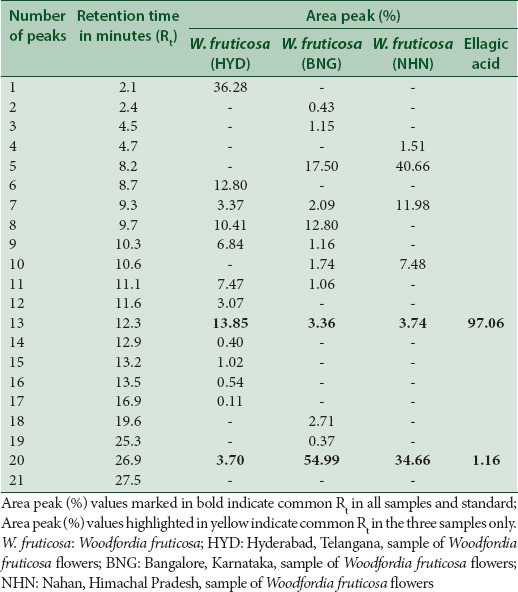
Figure 7.
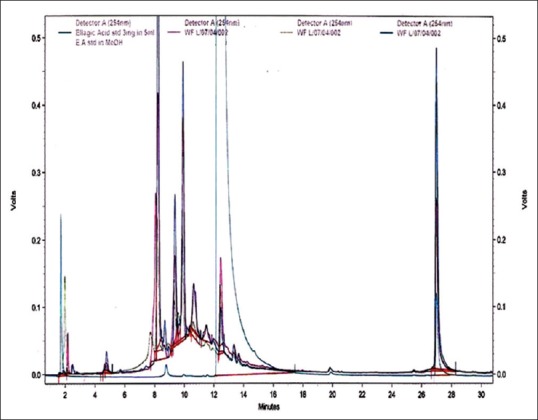
Overlay high-performance liquid chromatography chromatogram of aqueous extracts of Woodfordia fruticosa flower samples with standard ellagic acid
Figure 4.
High-performance liquid chromatography chromatogram of aqueous extract of Woodfordia fruticosa flower sample (Bangalore). BNG: Bangalore, Karnataka, sample of Woodfordia fruticosa flowers
Figure 5.
High performance liquid chromatography chromatogram of aqueous extract of Woodfordia fruticosa flower sample (Nahan, Himachal Pradesh). NHN: Nahan, Himachal Pradesh, sample of Woodfordia fruticosa flowers
The HPLC method of standardization of W. fruticosa described herein is simple, sensitive, and precise and may be of value in standardizing the raw material for preparation of formulations containing this plant.
Acute toxicity studies
The AEWF flowers were found to be safe up to a dose of 2000 mg/kg body weight in adult albino mice.
Anti-ulcer activity
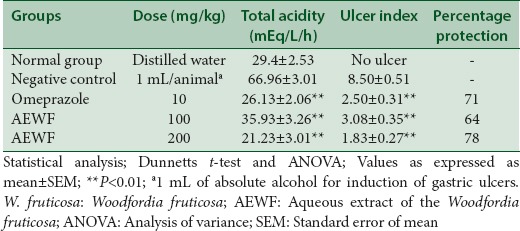
To evaluate the biological effect of W. fruticosa flowers for gastroprotective action and the probable contribution of ellagic acid to this effect, the aqueous extract of the flowers was subjected to ethanol-induced ulcer model in albino rats. The results of this study of antiulcer properties of W. fruticosa in ethanol-induced ulcer model show a significant reduction in TTA in the aqueous extract treated animals [Table 6]. The reduction in TTA is highly significant at both doses of 100 mg/kg as well as 200 mg/kg body weight. With respect to ulcer index, the extract showed gastroprotective effects at both doses of 100 mg/kg and 200 mg/kg body weight [Table 6]. The gastroprotection indicated by a low ulcer index was significant for 200 mg/kg dose of AEWF and has offered better protection than omeprazole 10 mg/kg dose. The AEWF showed significant protection index of 78% and 64% with the doses of 100 and 200 mg/kg, respectively. Omeprazole as reference standard drug showed a protection index of 71% [Table 6].
Table 6.
Effects of omeprazole and Woodfordia fruticosa flowers on total titratable acidity, ulcer index, and percentage protection in ethanol-induced ulcer in rats
DISCUSSION
This study deals with the chromatographic fingerprinting of the hydro-alcoholic and aqueous extracts of the flowers of W. fruticosa using ellagic acid as a biomarker and investigation of the gastroprotective activity of the water extract of the flowers of W. fruticosa on gastric lesions induced by alcohol as well as acid secretion parameters. This is the first report standardizing the different extracts of the flowers of W. fruticosa using ellagic acid as a biomarker in HPTLC and HPLC studies.
Standardization of herbal drugs aims at developing authentic analytical methods which can efficiently and reliably profile the phytochemical composition of medicinal plants, along with the quantitative analyses of marker/bioactive compounds and other major constituents. Standardization also serves as a tool for the establishment of a consistent biological activity and chemical profile.[26]
Results of the present chromatographic investigation confirm the presence of an important polyphenol ellagic acid in W. fruticosa flowers pointed out in the previous literature.[27] In HPTLC analysis very similar to that of the present study (solvent system A), Heeshma et al.[17] reported the presence of a spot at Rf value of 0.20 at UV 254 nm and a bluish-black spot at Rf value of 0.21. In the present study, we compared the spots at UV 254 nm, UV 366 nm, and after derivatization with brown spot of ellagic acid at Rf value of 0.21 in all the three hydro-alcoholic extract samples. To further substantiate the presence of ellagic acid in the extracts of W. fruticosa flowers solvent, system B reported by Bagul et al.[16] was tried which gave very clear bands at Rf value of 0.30 at UV 254 and 366 nm and a violet spot with 5% methanolic FeCl3 reagent. This solvent system B reported for the quantification of ellagic acid content in a different study yielded precise, specific, and accurate results in the chromatographic profiling of W. fruticosa flowers.
The HPLC method developed by Bala et al. was tried with respect to resolution of peaks which showed good results.[28] For improving separations in HPLC, gradient elution systems instead of isocratic elution are universally recommended.[29] The optimizing of 5 mM potassium dihydrogen orthophosphate (pH 2.5) – acetonitrile (5:95, v/v) overcame the problem of peak tailing (asymmetry at 10% height of the peak reduced to 1.1) in C18 column with a retention time of 12.3 min which was not accomplished in previous studies. To the best of our knowledge, no paper has been published on HPLC studies of W. fruticosa flowers using ellagic acid as a standard.
The evaluation of the biological activity of the whole plant is one of the most significant parameters in standardization of herbal raw drugs along with the passport data of raw plant parts, botanical authentication, microscopic examination, and chemical profiling by various chromatographic techniques.[30] In the present study, the ethanol-induced ulcer model in male Wistar albino rats was employed for the evaluation of gastroprotective capacity of the flowers of W. fruticosa. The genesis of ethanol-induced gastric lesions is multifactorial with the depletion of gastric wall mucous content as one of the involved factors. Another factor to this effect is the significant production of free radicals which results in increased oxidative stress and damage to the cell and cell membrane.[31] Although several factors have been suggested as being responsible for ulceration, recent data[32] indicate the involvement of histamine release in this process, which explains the high efficacy of H2-antagonists in this model. Our results suggest parallelism in the mechanism of action for both standard omeprazole[33] and the test drug (i.e., interaction with H2-receptors of parietal cells). In the experiments presented here, both standard and the test drugs reduced ulcer incidence, in a dose-dependent manner, in the stomach, with a potency of AEWF 200 mg/kg higher than that of omeprazole. Furthermore, alcohol stimulates acid secretion and reduces blood flow leading to microvascular injuries, by the disruption of the vascular endothelium and facilitation of vascular permeability; it also increases activity of xanthine oxidase.[34] The acid secretion studies also exhibited significant reduction in the TTA. Ethanol also triggers imbalances in cellular antioxidant processes. Thus, the ethanol-induced ulcer model is useful for studying the efficacy of potential drugs or testing agents that have cytoprotective and antioxidant activities.[35] The correlation between ellagic acid in particular and the phenolic compounds in general from different sources and their anti-ulcer capacity has been evidenced by several researchers.[36,37,38] Whether or not ellagic acid present in AEWF 150 and AEWF 300 contributes to the anti-ulcer activity is not possible to determine from this experiment. As AEWF contains flavonoids, saponins, polyphenols, tannins, alkaloids for which bioactivity studies have demonstrated the gastroprotective, gastric healing, and anti-diarrheal activities of these phytoconstituents in separate studies,[5,8,9] we conclude that in addition to ellagic acid these phytoconstituents may be contributing to the anti-ulcer activity in this animal model.
CONCLUSION
The developed HPTLC and HPLC methods proved to be quite simple, precise, and robust. The proposed methods are suitable for the chromatographic profiling of the flowers of W. fruticosa with respect to ellagic acid. These methods can be used for the quality control of marketed preparations containing W. fruticosa as one of the ingredients. The bioactivity studies further supported the role of ellagic acid as a contributor in the gastroprotective capacity of W. fruticosa flowers. Nevertheless, further study is required to isolate, characterize, quantify, and evaluate the other active constituents present in the flowers of W. fruticosa for their contribution to the overall gastroprotective potential. Clinical studies should be performed to evaluate the effect as a remedy against gastric ulcer in human.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
ABOUT AUTHOR

Yousuf Hussain Syed
Mr. Yousuf Hussain Syed, completed his M.Pharm from Al-Ameen College of Pharmacy, Bangalore and is presently pursuing his Ph.D from JNTU, Hyderabad. His research area of interest is standardization of natural products, isolation & building development paths of phytopharmaceuticals and new drug discovery. He has published 4 research papers and 2 review articles in reputed peer reviewed international and national journals.
REFERENCES
- 1.Borrelli F, Izzo AA. The plant kingdom as a source of anti-ulcer remedies. Phytother Res. 2000;14:581–91. doi: 10.1002/1099-1573(200012)14:8<581::aid-ptr776>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 2.Shirley AG. Systematics of Woodfordia (Lythraceae) Syst Bot. 1995;20:482–502. [Google Scholar]
- 3.Stafleu FA, Cowan RS. 2nd ed. Vol. 7. Utrecht: Bohn-Scheltema & Holkema; 1988. Taxonomic Literature. [Google Scholar]
- 4.1st ed. Vol. 1. New Delhi: Government of India, Ministry of health and Family Welfare, Department of Indian Systems of Medicine & Homeopathy; 2007. Government of India, Ministry of health and Family Welfare, Department of Indian Systems of Medicine & Homeopathy. The Ayurvedic Pharmacopeia of India. Part 1; pp. 32–3. [Google Scholar]
- 5.Syed YH, Khan M, Bhuvaneshwari J, Ansari JA. Phytochemical investigation and standardization of extracts of flowers of Woodfordia fruticosa; a preliminary study. J Pharm Biosci. 2013;1:134–40. [Google Scholar]
- 6.Khare CP. New York, Berling, Heidelberg: Springer-Verlag; 2007. Encyclopedia of Indian Medicinal Plants; pp. 483–4. [Google Scholar]
- 7.Kirtikar KR, Basu BD. 2nd ed. Allahabad: Lalith Mohan Basu; 1992. Indian Medicinal Plants; pp. 1074–6. [Google Scholar]
- 8.Gyawali R, Jnawali D, Chang-Hun S, Kyong-Su K. Evaluation of the phytochemical profile and uterine contractile effect of Woodfordia fruticosa (L.) Kurz and Dipsacus mitis D. Don. J Nepal Pharm Assoc. 2012;26:1–11. [Google Scholar]
- 9.Khushalani H, Tatke P, Singh KK. Antifertility activity of dried flowers of Woodfordia fruticosa kurz. Indian J Pharm Sci. 2006;68:528–9. [Google Scholar]
- 10.Mota KS, Dias GE, Pinto ME, Luiz-Ferreira A, Souza-Brito AR, Hiruma-Lima CA, et al. Flavonoids with gastroprotective activity. Molecules. 2009;14:979–1012. doi: 10.3390/molecules14030979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yesilada E, Takaishi Y. A saponin with anti-ulcerogenic effect from the flowers of Spartium junceum. Phytochemistry. 1999;51:903–8. doi: 10.1016/s0031-9422(99)00198-3. [DOI] [PubMed] [Google Scholar]
- 12.Sumbul S, Ahmad MA, Mohd A, Mohd A. Role of phenolic compounds in peptic ulcer: An overview. J Pharm Bioallied Sci. 2011;3:361–7. doi: 10.4103/0975-7406.84437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Sousa Falcão H, Leite JA, Barbosa-Filho JM, de Athayde-Filho PF, de Oliveira Chaves MC, Moura MD, et al. Gastric and duodenal antiulcer activity of alkaloids: A review. Molecules. 2008;13:3198–223. doi: 10.3390/molecules13123198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen ØM, Markham KR. USA: Taylor and Francis Group; 2006. Flavonoids: Chemistry, Biochemistry, and Applications. [Google Scholar]
- 15.Rajpal V. New Delhi: Eastern Publishers; 2002. Standardization of Botanicals. [Google Scholar]
- 16.Bagul M, Srinivasa H, Padh H, Rajani M. A rapid densitometric method for simultaneous quantification of gallic acid and ellagic acid in herbal raw materials using HPTLC. J Sep Sci. 2005;28:581–4. doi: 10.1002/jssc.200301695. [DOI] [PubMed] [Google Scholar]
- 17.Heeshma K, Pratima T, Singh KK. Antifertility activity of dried flowers of Woodfordia fruticosa kurz. Indian J Pharm Sci. 2006;68:528–9. [Google Scholar]
- 18.Rauchensteiner F, Matsumura Y, Yamamoto Y, Yamaji S, Tani T. Analysis and comparison of Radix Glycyrrhizae (licorice) from Europe and China by capillary-zone electrophoresis (CZE) J Pharm Biomed Anal. 2005;38:594–600. doi: 10.1016/j.jpba.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 19.Agrawal R, Garg HK, Garg U, Singh SK. Anti-ulcer activity of Smithia conferta in various animal. J Saudi Chem Soc. 2010;14:307–10. [Google Scholar]
- 20.Glavin GB, Rockman GE. Acute ethanol administration: Effects on stress-induced gastric and duodenal ulcer in rats. Alcohol. 1985;2:651–3. doi: 10.1016/0741-8329(85)90141-7. [DOI] [PubMed] [Google Scholar]
- 21.Shirode D, Patel T, Roy SP, Jyothi TM, Rajendra SV, Prabhu K, et al. Anti-ulcer properties of 70% ethanolic extract of leaves of Albizzia lebbeck. Pharmacogn Mag. 2008;4:228–31. [Google Scholar]
- 22.Gosh MN. 3rd ed. Kolkata: Hilton & Company; 2005. Fundamentals of Experimental Pharmacology; pp. 192–3. [Google Scholar]
- 23.Singh S, Majumdar DK. Evaluation of the gastric antiulcer activity of fixed oil of Ocimum sanctum (Holy Basil) J Ethnopharmacol. 1999;65:13–9. doi: 10.1016/s0378-8741(98)00142-1. [DOI] [PubMed] [Google Scholar]
- 24.Gupta J, Kumar D, Gupta A. Evaluation of gastric anti-ulcer activity of methanolic extract of Cayratia trifolia in experimental animals. Asian Pac J Trop Dis. 2012;2:99–102. [Google Scholar]
- 25.Kulkarni SK. 3rd ed. Delhi: Vallabh Prakashan; 2005. Handbook of Experimental Pharmacology; pp. 148–50. [Google Scholar]
- 26.Choudhary N, Sekhon BS. An overview of advances in the standardization of herbal drugs. J Pharm Educ Res. 2011;2:55–70. [Google Scholar]
- 27.Nair RA, Kotiyal JP, Ramesh P, Subramanian SS. Polyphenols of the flowers and leaves of Woodfordia fruticosa. Indian J Pharm. 1976;38:110–1. [Google Scholar]
- 28.Bala I, Bhardwaj V, Hariharan S, Kumar MN. Analytical methods for assay of ellagic acid and its solubility studies. J Pharm Biomed Anal. 2006;40:206–10. doi: 10.1016/j.jpba.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Islam MN, Chung HJ, Kim DH, Yoo HH. A simple isocratic HPLC method for the simultaneous determination of bioactive components of Scutellariae radix extract. Nat Prod Res. 2012;26:1957–62. doi: 10.1080/14786419.2011.631134. [DOI] [PubMed] [Google Scholar]
- 30.Patel PM, Patel NM, Goyal RK. Quality control of herbal products. Indian Pharm. 2006;5:26–30. [Google Scholar]
- 31.Narayan S, Devi RS, Jainu M, Sabitha KE, Devi CS. Protective effect of a polyherbal drug, ambrex in ethanol-induced gastric mucosal lesions in experimental rats. Indian J Pharmacol. 2004;36:34–7. [Google Scholar]
- 32.Del Soldato P. A rat model based on the histamine H2-receptor-activation-induced gastric pathology. Pharmacol Res Commun. 1982;14:175–85. doi: 10.1016/s0031-6989(82)80098-2. [DOI] [PubMed] [Google Scholar]
- 33.Hsu TC, Su CF, Leu SC, Huang PC, Wang TE, Chu CH. Omeprazole is more effective than a histamine H2-receptor blocker for maintaining a persistent elevation of gastric pH after colon resection for cancer. Am J Surg. 2004;187:20–3. doi: 10.1016/j.amjsurg.2002.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Glavin GB, Szabo S. Experimental gastric mucosal injury: Laboratory models reveal mechanisms of pathogenesis and new therapeutic strategies. FASEB J. 1992;6:825–31. doi: 10.1096/fasebj.6.3.1740232. [DOI] [PubMed] [Google Scholar]
- 35.Adinortey MB, Ansah C, Galyuon I, Nyark A. In vivo models used for evaluation of potential antigastroduodenal ulcer agents. Ulcers. 2013;2013:1–12. [Google Scholar]
- 36.Beserra AM, Calegari PI, Souza Mdo C, Dos Santos RA, Lima JC, Silva RM, et al. Gastroprotective and ulcer-healing mechanisms of ellagic acid in experimental rats. J Agric Food Chem. 2011;59:6957–65. doi: 10.1021/jf2003267. [DOI] [PubMed] [Google Scholar]
- 37.Iino T, Nakahara K, Miki W, Kiso Y, Ogawa Y, Kato S, et al. Less damaging effect of whisky in rat stomachs in comparison with pure ethanol. Role of ellagic acid, the nonalcoholic component. Digestion. 2001;64:214–21. doi: 10.1159/000048864. [DOI] [PubMed] [Google Scholar]
- 38.Sen S, Chakraborty R, De B, Mazumder J. Plants and phytochemicals for peptic ulcer: An overview. Pharmacogn Rev. 2009;3:270–9. [Google Scholar]