Abstract
Diabetic retinopathy (DR), one of the leading causes of preventable blindness, is associated with many systemic factors that contribute to the development and progression of this microvascular complication of diabetes. While the duration of diabetes is the major risk factor for the development of DR, the main modifiable systemic risk factors for development and progression of DR are hyperglycemia, hypertension, and dyslipidemia. This review article looks at the evidence that control of these systemic factors has significant benefits in delaying the onset and progression of DR.
Keywords: Control of systemic factors, diabetic retinopathy, glycemic control
With the prevalence of Type 2 diabetes now reaching pandemic proportions, there is a concomitant increase in the prevalence of diabetic retinopathy (DR). There are approximately 93 million people with DR, 17 million with proliferative DR (PDR), 21 million with diabetic macular edema (DME), and 28 million with vision-threatening DR worldwide.[1] More than 75% of people who have diabetes for more than 20 years will have some form of DR despite all the advances in diabetes care.[1] The World Health Organisation has declared DR, as the sixth leading cause of blindness, and as an important cause of avoidable blindness.[2] DR is associated with many systemic factors that contribute to its development, severity, and progression. Although the development of microvascular complications of diabetes including DR is dependent on the duration of diabetes to a great extent, there are other modifiable risk factors too, that influence the development of retinopathy.[1,3,4,5]
The Chennai Urban Rural Epidemiology Study (CURES) Eye Study showed that the major systemic risk factors associated with DR are the duration of diabetes, hyperglycemia, male gender, and macroalbuminuria.[3] Therapeutic approaches in people with retinopathy or at risk for DR include drug therapy to reduce modifiable risk factors, laser photocoagulation, and surgery. Recently, there have also been significant developments in pharmacotherapy in the management of DR. In this article, we look at the evidence for various systemic factors in the development of DR.
Glycemic Control in Diabetic Retinopathy
It is well known that hyperglycemia is one of the most important determinants of diabetic microvascular complications.[5,6] Hence, good glycemic control should indeed have a beneficial effect on the microvascular complications including retinopathy.
The CURES Eye Study demonstrated a significant increase in the prevalence of DR with increasing glycated hemoglobin (HbA1c) levels.[3] The multiple logistic regression analysis carried out using DR as a dependent variable showed a significant trend of increasing retinopathy at different quartiles of HbA1c (trend χ2= 51.6, P < 0.0001) as shown in Fig. 1.
Figure 1.
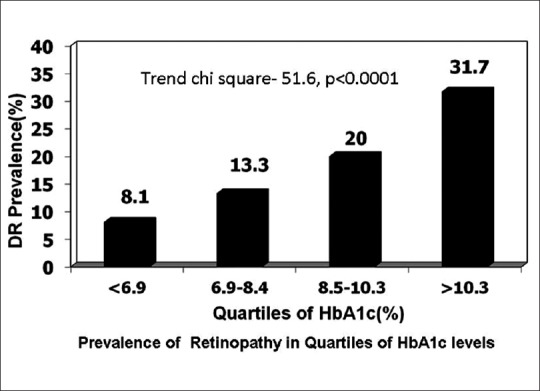
The Chennai Urban Rural Epidemiological Study: Prevalence of retinopathy based on quartiles of glycated hemoglobin levels
The long-term benefit of glycemic control has been evaluated by two large studies: The Diabetes Control and Complications Trial (DCCT) in Type 1 diabetes,[7] and the United Kingdom Prospective Diabetes Study (UKPDS) in Type 2 diabetes.[8] The DCCT and the UKPDS have demonstrated that intensive glycemic control (HbA1c ≤7%) reduced both the development and progression of DR, with the beneficial effects of intensive glycemic control persisting up to 10–20 years.
The DCCT was performed in 1441 patients with Type 1 diabetes with no DR or with mild to moderate non-PDR (NPDR).[7] The DCCT reported that during the average treatment period of 6.5 years, the risk of developing DR was substantially lower in the intensive treatment group treated compared to the conventionally treated group.[7] The benefits of intensive therapy were greater in patients with shorter duration of diabetes. Patients with no retinopathy at baseline (primary prevention cohort), the intensive treatment reduced the risk of the development of DR by 76% compared with conventional therapy (P < 0.001). In the secondary prevention cohort, intensive treatment slowed the DR progression by 54% relative to conventional treatment (P < 0.001). A 10% reduction in HbA1c level from baseline (for example from 8% to 7.2%) was associated with a significant reduction in progression of DR both in the intensive treatment group (43%) as well as in conventional treatment group (45%).[9] The study also showed that the level of HbA1c at the start of the trial as well as the level achieved during the trial influenced the rate of progression of DR. Total glycemic exposure was a dominant factor associated with risk of retinopathy progression.
Many of the DCCT patients participated in follow-up trial namely, the Epidemiology of Diabetes Interventions and Complications (EDIC) study.[10] The main aim of the EDIC was to determine if the benefits achieved in the DCCT with intensive insulin therapy persisted. These risk reductions achieved at a median HbA1c level difference of 9.1% for conventional treatment versus 7.3% for intensive treatment, were maintained through the 7 years follow-up of EDIC, though the difference in mean HbA1c levels of the two randomized treatment groups continued to narrow, and became statistically nonsignificant by 5 years (8.1% vs. 8.2%, P = 0.09). The EDIC showed the benefit of early tight control on the protection against progression of retinopathy being maintained, despite subsequent equalization of the HbA1c values between the groups, a concept of “metabolic memory.”
The UKPDS studied the impact of tight control versus conventional control on the microvascular and macrovascular complications in Type 2 diabetes.[8] After 6 years follow-up, the intensive treatment group had significantly lower rate of the two-step progression of DR and a 25% risk reduction in microvascular endpoints, including the need for retinal laser photocoagulation. UKPDS showed that intensive blood glucose control, irrespective of the antidiabetic agents used, substantially decreased the risk of microvascular complications.
Studies like the Kumamoto study also evaluated the relationship between glycemic control and DR.[11] In this study, the glycemic threshold to prevent the onset and progression of diabetic microvascular complications was mentioned as: HbA1c <6.5%, fasting blood glucose concentration <110 mg/dl and 2-h postprandial blood glucose concentration < 180 mg/dl.[11]
Two more clinical trials, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Eye Study, and the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) Retinal Measurements study, examined the effect of aggressive blood glucose lowering (HbA1c ≤6.5%) in people with Type 2 diabetes. In the ACCORD study, intensive glycemic control was associated with a lower rate of DR progression.[12] While the ACCORD Eye Study[13] showed a 33% reduction in the progression of DR in the intensive control group, after a period of 4 year follow-up, the ADVANCE study[14] and the Veterans Affairs Diabetes Trial[15] did not show any significant difference in the progression of microvascular changes after tight glycemic control.
Reversal of early retinopathy changes was seen with good glycemic control.[16] Tight glycemic control is most effective when initiated early in the course of diabetes. Intensive glycemic control can at times have adverse effects, including worsening of DR, possibly attributable to a rapid reduction of plasma glucose levels.[17] Early worsening of DR has been attributed to up-regulation of insulin-like growth factor-1.[18]
Agents for glycemic control and diabetic retinopathy
Insulin therapy has always been believed to be beneficial for delaying onset and progression of DR as it not only helps in achieving good glycemic control but also improves retinal blood flow and the vascular tone of retinal microvasculature.[19] The UKPDS[8] showed that it was not the particular anti-diabetic drug used that was important, i.e., sulfonylurea or metformin or insulin but the degree of glycemic control which mattered for prevention of retinopathy.
There are some experimental studies which show that newer anti-diabetic drugs like sitagliptin (dipeptidyl peptidase-4 inhibitor) may decrease the retinal inflammatory state and neuronal apoptosis, thus suggesting a possible protective effect on diabetic retinal cells. However, no clinical studies have evaluated the effect of gliptins on retinopathy endpoints.[20]
Glitazones are a class of oral hypoglycemic agents that result in the activation of peroxisome proliferator-activated receptor-γ, a transcription factor located in the adipose tissue and retina. Glitazones should be used with caution in patients with DME. Fluid retention occurs in 5–15% of patients taking glitazones. Its use appears to be a cause for macular edema, and drug cessation appears to result in rapid resolution of DME.[21] It is important for ophthalmologists to check if the patients are on glitazones when treating DME.
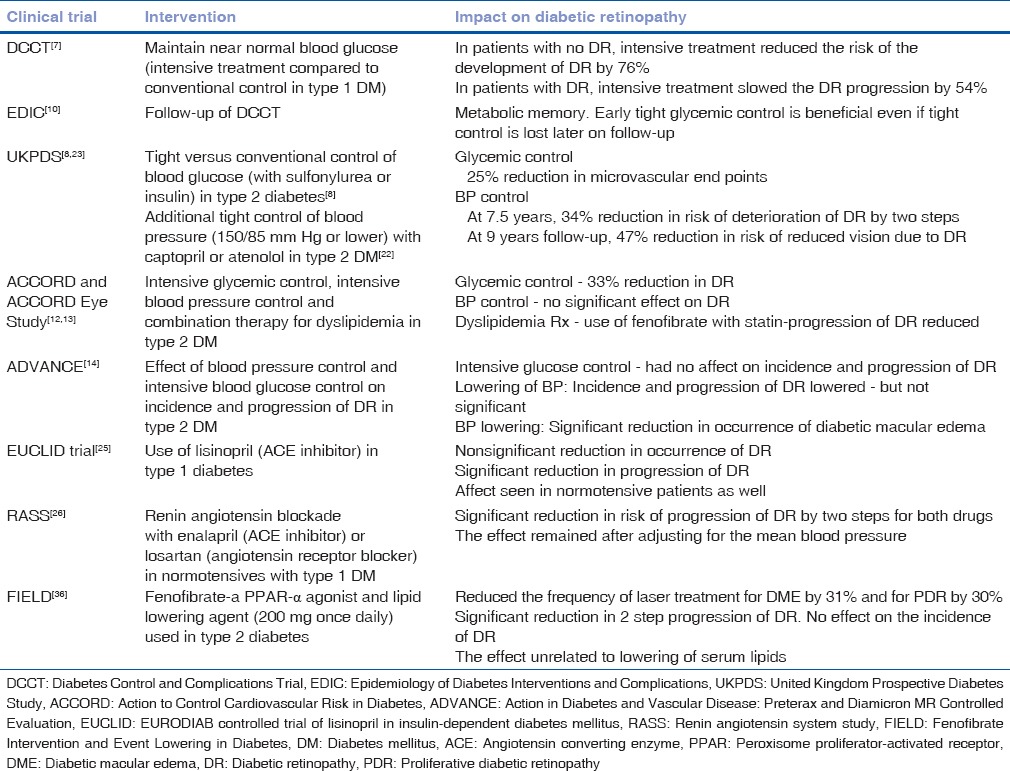
There is a very little evidence that any particular class of anti-diabetic drug is either beneficial or detrimental with respect to DR, independent of the glycemic control, i.e., the level of HbA1c achieved. The overall inference from various trials [Table 1] is that good glycemic control right from the time of diagnosis of diabetes is beneficial in preventing the onset of DR and in delaying its progression. The long-term benefits of glycemic control outweigh the small risk of “early worsening” of DR. Targeting HbA1c level of <7% is recommended for slowing down the progression of DR.[12]
Table 1.
Randomized controlled trials evaluating systemic control in diabetic retinopathy
Blood Pressure Control in Diabetic Retinopathy
Blood pressure (BP) control is an important component of risk factor modification in reducing the risk of retinopathy progression. Hypertension is very often coexistent with diabetes. The incidence of hypertension is 3 times greater in people with Type 2 diabetes when compared those without diabetes. A review article showed that diabetes and hypertension coexist in people with Type 2 diabetes ranging from 20.6% in India to 78.4% in Thailand in South-East Asia.[22] The vascular damage due to hypertension has an additive affect on the severity of DR.
The UKPDS showed that, among patients with Type 2 diabetes, tight BP control (mean BP 144/82 mm Hg) resulted in a significant reduction in progression of DR (35%) as well as a significant decrease in vision loss and need for laser photocoagulation compared to less control (mean BP 154/87 mm Hg).[23] At 9 years follow-up, the group with tight control of BP had a 47% reduction in risk of loss of three or more lines in the Early Treatment DR Study (ETDRS) visual acuity chart [Table 1]. Gallego et al.[24] identified systolic and diastolic BP as predictors for the onset of DR in adolescents with Type 1 diabetes. A linear association was noted, an increase in systolic BP by 10 mm Hg was associated with 3–20% increase in risk for DR and an increase in diastolic BP by 10 mm Hg increased the risk by 2–30%.
The ACCORD and ADVANCE studies, where the mean BP was <140/80 mm Hg in both the active intervention and control groups, active treatment did not show any additional benefit on preventing progression of DR.[12,14] The ADVANCE study, however, showed the reduction in the occurrence of macular edema to be more significant.[14]
The Role of the Renin-Angiotensin System in Diabetic Retinopathy
A local renin-angiotensin system (RAS) in the eye is been found to be up-regulated in patients with DR resulting in increased vascular endothelial growth factor (VEGF). Hence, an RAS blockade appears to be a logical approach to control the progression of retinopathy. A number of trials have examined the effect of RAS blockade on DR development and progression. The findings of the EURODIAB Controlled Trial of Lisinopril in Insulin-Dependent Diabetes suggested that blockade of the RAS with the angiotensin converting enzyme (ACE) inhibitor lisinopril could reduce both incidence and progression of retinopathy in Type 1 diabetes.[25] This benefit on the progression of retinopathy was noted even in normotensive patients [Table 1]. This drug possibly has a direct effect on retinopathy, outside of its BP-lowering mechanism.
In the RAS Study,[26] taking enalapril, an ACE inhibitor or losartan, an angiotensin receptor blocker (ARB), reduced DR progression independent of BP change in normotensive, normoalbuminuric participants with Type 1 DM. This study showed that the night ambulatory diastolic BP was associated with increasing severity of DR and the protective effect of ACE inhibitors and ARBs was probably due to the effect on the BP at night.[26,27]
The DR Candesartan Trials (DIRECT) Program, which evaluated the effect of the angiotensin II ARB candesartan 32 mg daily on the incidence of new DR in Type 1 diabetes (DIRECT-Prevent 1),[28] and on the progression of DR in patients with Type 1 and Type 2 diabetes showed a change toward less severe retinopathy with the use of candesartan.[29]
A conclusion from these studies is that control of BP reduces the risk of progression of DR. Optimizing BP helps to reduce the risk of vision loss and the necessity of laser photocoagulation.[30] Inhibition of the RAS by an ACE inhibitor or ARB, have effects on decreasing DR. It is important to remember that reduction of BP is more important than the type of BP-lowering medication. BP levels of about 135/80 mm Hg should be aimed for to reduce the risk for DR.[31]
Lipids Management in Diabetic Retinopathy
The Wisconsin Epidemiologic Study of DR XIII study,[32] and ETDRS,[33] have shown that total cholesterol (TC) and serum low-density lipoprotein cholesterol (LDL-C) are associated with the presence of hard exudates in patients with DR. Estimation of serum TC and lipids is essential in DR, especially in patients with DME. Association between dyslipidemia and DR has also been studied in the CURES Eye Study,[34] which showed that TC, nonhigh-density lipoprotein cholesterol (HDL-C) and serum triglycerides (TGs) were associated with DR. DME was associated with non-HDL-C and LDL-C.
A recent meta-analysis of case–control studies revealed that mean levels of serum TC, LDL-C, and TG were significantly higher in patients with DME compared with those without DME (TC: 30.08; 95% confidence interval [CI], 21.14–39.02; P < 0.001; LDL: 18.62; 95% CI, 5.80–31.43; P < 0.05; TG: 24.82; 95% CI, 9.21–40.42; P < 0.05).[35]
Fenofibrate, a lipid-lowering drug has been studied for its role in DME. The Fenofibrate Intervention and Event Lowering in Diabetes Study studied whether lipid-lowering therapy with fenofibrate could reduce the progression of retinopathy and the need for laser treatment.[35] Fenofibrate (200 mg once daily) reduced the frequency of laser photocoagulation for DME by 31% and for PDR by 30%.
The requirement for first laser treatment for retinopathy was significantly lower in the fenofibrate group than in the placebo group as shown in Fig. 2 (164 [3.4%] patients on fenofibrate vs. 238 [4.9%]) on placebo; hazard ratio (0·69, 95% CI, 0·56–0·84; P = 0.0002; absolute risk reduction 1·5% [0·7–2·3]). In patients with preexisting DR, the incidence of two-step ETDRS progression of retinopathy was significantly reduced in the fenofibrate group relative to placebo (from 14.6% to 3.1%, P = 0.004).[36]
Figure 2.
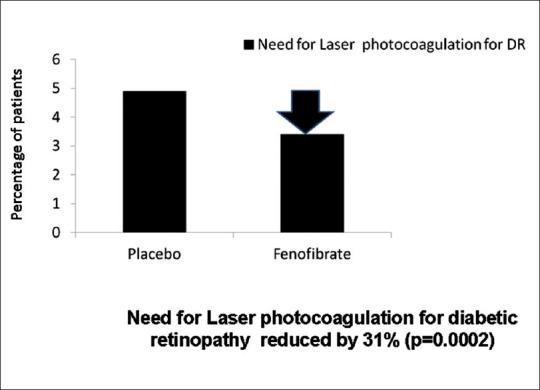
Fenofibrate Intervention and Event Lowering in Diabetes study-effect of fenofibrate on the need for laser photocoagulation for diabetic retinopathy
In the ACCORD Study, patients were randomly assigned to receive simvastatin with fenofibrate (160 mg daily) or a matching placebo [Table 1].[13] Treatment with fenofibrate (n = 806) was associated with a 40% decrease in DR progression, defined as three or more steps on the ETDRS scale or PDR that needed either laser or vitrectomy treatment (from 10.2% to 6.5%, P = 0.006). In both the studies, patients with preexisting DR derived greater benefit. The effect of fenofibrate was found to be independent of its lipid-lowering action.
Nontraditional lipid markers in diabetic retinopathy
Studies have also been done to look into the serum lipoprotein(a) (Lp[a]) and serum apolipoproteins (Apo) profiles in DR. In patients with PDR, serum Lp(a) was significantly higher compared to patients without DR.[37] In the retina, Apo A1 is proposed as a key factor for preventing lipid accumulation and a potent scavenger of oxygen-reactive species for protecting the retina from the oxidative stress caused by diabetes. Apo B is the main component of LDL-C and is a reflection of atherogenicity. Low Apo A1/Apo B ratio in serum was found to be associated with PDR in Type 2 diabetic patients of long duration.[38]
Renal Status and Diabetic Retinopathy
Microalbuminuria is found to be a reliable marker of DR.[39] In the Sankara Nethralaya DR Epidemiology and Molecular Genetic Study (SN-DREAMS), subjects with microalbuminuria had a 2-fold higher risk of DR compared to those without microalbuminuria, and this risk increased to almost 6 times, in the presence of macroalbuminuria.[40] In the CURES study, the risk of nephropathy was found to be significantly higher in sight-threatening DR group compared to the no retinopathy group (odds ratio [OR] 5.3, P < 0.0001).[41] Hence assessment of the renal parameters-blood urea, serum creatinine and microalbumuria, is important, especially if DR is present. In a study done to assess the course of DR after renal transplant, it was found that renal transplant stabilized retinopathy in the majority (60%) of the diabetic patients and significant improvement in visual acuity was seen during the first 20 months postrenal transplant.[42]
The presence of DR is also a major risk factor for progression to overt diabetic nephropathy.[43] The EURODIAB study in Type 1 diabetes showed that DR in association with increased BP is an important independent risk factor for diabetic nephropathy progression.[44] Albuminuria and DR are considered important for renal prognosis in Type 2 diabetic patients.[45]
Anemia and Diabetic Retinopathy
Severity of DR was found to increase with severity of anemia.[46] In a recent study from China,[47] the prevalence of anemia was significantly higher in people with DR (27.3%) compared to those without retinopathy (19.7%, P < 0.001). The SN-DREAMS study identified the duration of diabetes >5 years (OR 1.56 [95% CI, 1.09–2.69]) and the presence of retinopathy (OR 1.82 [95% CI, 1.22–2.69]) as independent predictors for anemia in people with diabetes.[48]
Anemia leads to progression of DR by aggravating hypoxia in the retina, resulting in the production of growth factors such as VEGF.[49] In the ETDRS, low hematocrit was found to an independent risk factor for high-risk PDR and visual impairment.[50] Anemia is an important risk factor for clinically significant macular edema (CSME).[51] Hence, assessment of Hb levels is of utmost importance, especially in diabetic patients with sight-threatening retinopathy, PDR and CSME.
Intravenous administration of erythropoietin to treat anemia in diabetic patients with renal impairment showed a beneficial effect in DME and improvement in visual acuity.[52]
Cardiovascular Diseases and Diabetic Retinopathy
Association of cardiovascular disease (CVD) events and DR has also been established in many epidemiological studies worldwide.[53] DR in Type 2 diabetes is found to be associated with a 1.7-fold increased risk of cardiovascular events, such as stroke, coronary artery disease (CAD), and heart failure.[54] Presence of any DR doubled the risk of mortality and CVD events (OR 2.34) in people with Type 2 diabetes and quadruplicated the risk in those with Type 1 diabetes (OR 4.1).[53]
The CURES study showed that the prevalence of CAD to be higher in DR versus no DR (P = 0.007).[55] Significant association was observed in subjects with HbA1c levels >7% (P = 0.002). It is likely that endothelial dysfunction, low-grade inflammation, and rheological abnormalities are common mechanistic denominators.[54]
DR has also been independently associated with the presence of carotid plaques (P = 0.045), an early sign of atherosclerotic burden.[56] Mean values of carotid intima-media thickness (0.93 ± 0.36 vs. 0.85 ± 0.21 mm, P = 0.001) and augmentation index (27.9 ± 8.9 vs. 25.8 ± 9.6%, P = 0.031) were also found to be significantly higher among patients with retinopathy compared with those without DR.[57]
Antiplatelet Therapy- Aspirin in Diabetic Retinopathy
A systematic review suggests that acetylsalicylic acid-aspirin therapy neither decreases nor increases the incidence or progression of DR.[58] Aspirin use does not appear to be associated with an increase in the risk of vitreous hemorrhage.
Pregnancy and Diabetic Retinopathy
Diabetic women in child-bearing age should be counseled regarding the risk of development and progression of DR.[59] Pregnancy may promote the onset of DR in about 10% of cases, as well as contribute to its worsening when already present.[60] Increasing systolic BP at first visit (OR 1.03, CI, 1.01–1.06, P = 0.02) and a greater drop in HbA1c between first and third trimesters of pregnancy (OR 2.05, CI, 1.09–3.87, P = 0.003) significantly increased the odds of retinopathy progression.[61] DR progression during pregnancy was higher in women with Type 1 diabetes than those with Type 2 diabetes (31.3% vs. 11.7%, P = 0.001).
Established sight-threatening DR should be treated at an earlier stage in pregnant women.[62] In a study of patients with no DR at onset, who then developed mild NPDR during pregnancy, 50% had complete regression, and 30% had partial regression of DR after delivery.[63] In another study,[61] two-third of women who experienced DR progression developed only mild NPDR. None of the women with normal retinal examination during the first trimester developed laser requiring sight-threatening DR during pregnancy.
Obesity and Diabetic Retinopathy
Obesity has been identified as an independent risk factor for DR. Persons with higher body mass index and larger neck circumference were found more likely to have DR and more severe DR.[64] In a study carried out in urban South Indian population, abdominal obesity and higher waist-to-hip ratio were associated with DR in women.[65] Obesity increases the prevalence of several risk factors involved in DR onset and development including inflammatory markers.[66] Elevated angiogenic factors including VEGF have also been observed in the serum of obese individuals.[67] Prevalence of retinopathy increased significantly with higher body weight (P < 0.05) with correlation to quality of metabolic control and systolic BP.[68] The Gutenberg Health Study has shown an association between vision-threatening DR and obesity.[69]
Conclusion
Good glycemic control and BP control are essential for the successful ophthalmic care of patients with diabetes. Early screening and tight glycemic control from the time of diagnosis of diabetes play an important role in the prevention of vision impairment due to sight-threatening retinopathy. Reduction of serum lipid levels by use of statins and fenofibrate and blockade of the RAS also add the beneficial effect in preventing development and progression of retinopathy. Association between microvascular and macrovascular complications of diabetes is well established. Optimal control of various systemic parameters that affect the onset and progression of DR through a multidisciplinary healthcare team approach involving the physician, the ophthalmologist and the dietician/counselor could help in reducing the morbidity due to DR.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Initiative for the Elimination of Avoidable Blindness. WHO/PBL/97.61 Rev 2. 2006. [Last accessed on 2016 Jan 05]. Available from: http://www.who.int/blindness/Vision2020_report.pdf .
- 3.Pradeepa R, Anitha B, Mohan V, Ganesan A, Rema M. Risk factors for diabetic retinopathy in a South Indian type 2 diabetic population – The Chennai urban rural epidemiology study (CURES) eye study 4. Diabet Med. 2008;25:536–42. doi: 10.1111/j.1464-5491.2008.02423.x. [DOI] [PubMed] [Google Scholar]
- 4.Raman R, Rani PK, Reddi Rachepalle S, Gnanamoorthy P, Uthra S, Kumaramanickavel G, et al. Prevalence of diabetic retinopathy in India: Sankara Nethralaya diabetic retinopathy epidemiology and molecular genetics study report 2. Ophthalmology. 2009;116:311–8. doi: 10.1016/j.ophtha.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Aiello LP, Cahill MT, Wong JS. Systemic considerations in the management of diabetic retinopathy. Am J Ophthalmol. 2001;132:760–76. doi: 10.1016/s0002-9394(01)01124-2. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez-Fontal M, Kerrison JB, Alfaro DV, Jablon EP. Metabolic control and diabetic retinopathy. Curr Diabetes Rev. 2009;5:3–7. doi: 10.2174/157339909787314176. [DOI] [PubMed] [Google Scholar]
- 7.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 8.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 9.The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995;44:968–83. [PubMed] [Google Scholar]
- 10.Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med. 2000;342:381–9. doi: 10.1056/NEJM200002103420603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000;23(Suppl 2):B21–9. [PubMed] [Google Scholar]
- 12.ACCORD Study Group; ACCORD Eye Study Group. Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–44. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chew EY, Davis MD, Danis RP, Lovato JF, Perdue LH, Greven C, et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: The action to control cardiovascular risk in diabetes (ACCORD) eye study. Ophthalmology. 2014;121:2443–51. doi: 10.1016/j.ophtha.2014.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beulens JW, Patel A, Vingerling JR, Cruickshank JK, Hughes AD, Stanton A, et al. Effects of blood pressure lowering and intensive glucose control on the incidence and progression of retinopathy in patients with type 2 diabetes mellitus: A randomised controlled trial. Diabetologia. 2009;52:2027–36. doi: 10.1007/s00125-009-1457-x. [DOI] [PubMed] [Google Scholar]
- 15.Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 16.White NH, Waltman SR, Krupin T, Santiago JV. Reversal of abnormalities in ocular fluorophotometry in insulin-dependent diabetes after five to nine months of improved metabolic control. Diabetes. 1982;31:80–5. doi: 10.2337/diab.31.1.80. [DOI] [PubMed] [Google Scholar]
- 17.Early worsening of diabetic retinopathy in the diabetes control and complications trial. Arch Ophthalmol. 1998;116:874–86. doi: 10.1001/archopht.116.7.874. [DOI] [PubMed] [Google Scholar]
- 18.Chantelau E. Evidence that upregulation of serum IGF-1 concentration can trigger acceleration of diabetic retinopathy. Br J Ophthalmol. 1998;82:725–30. doi: 10.1136/bjo.82.7.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silva PS, Cavallerano JD, Sun JK, Aiello LM, Aiello LP. Effect of systemic medications on onset and progression of diabetic retinopathy. Nat Rev Endocrinol. 2010;6:494–508. doi: 10.1038/nrendo.2010.122. [DOI] [PubMed] [Google Scholar]
- 20.Avogaro A, Fadini GP. The effects of dipeptidyl peptidase-4 inhibition on microvascular diabetes complications. Diabetes Care. 2014;37:2884–94. doi: 10.2337/dc14-0865. [DOI] [PubMed] [Google Scholar]
- 21.Ryan EH, Jr, Han DP, Ramsay RC, Cantrill HL, Bennett SR, Dev S, et al. Diabetic macular edema associated with glitazone use. Retina. 2006;26:562–70. doi: 10.1097/00006982-200605000-00011. [DOI] [PubMed] [Google Scholar]
- 22.Mohan V, Seedat YK, Pradeepa R. The rising burden of diabetes and hypertension in Southeast Asian and African regions: Need for effective strategies for prevention and control in primary health care settings. Int J Hypertens 2013. 2013:409083. doi: 10.1155/2013/409083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703–13. [PMC free article] [PubMed] [Google Scholar]
- 24.Gallego PH, Craig ME, Hing S, Donaghue KC. Role of blood pressure in development of early retinopathy in adolescents with type 1 diabetes: Prospective cohort study. BMJ. 2008;337:a918. doi: 10.1136/bmj.a918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaturvedi N, Sjolie AK, Stephenson JM, Abrahamian H, Keipes M, Castellarin A, et al. Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The EUCLID Study Group. EURODIAB controlled trial of lisinopril in insulin-dependent diabetes mellitus. Lancet. 1998;351:28–31. doi: 10.1016/s0140-6736(97)06209-0. [DOI] [PubMed] [Google Scholar]
- 26.Klein R, Moss SE, Sinaiko AR, Zinman B, Gardiner R, Suissa S, et al. The relation of ambulatory blood pressure and pulse rate to retinopathy in type 1 diabetes mellitus: The renin-angiotensin system study. Ophthalmology. 2006;113:2231–6. doi: 10.1016/j.ophtha.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 27.Mauer M, Zinman B, Gardiner R, Suissa S, Sinaiko A, Strand T, et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N Engl J Med. 2009;361:40–51. doi: 10.1056/NEJMoa0808400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaturvedi N, Porta M, Klein R, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: Randomised, placebo-controlled trials. Lancet. 2008;372:1394–402. doi: 10.1016/S0140-6736(08)61412-9. [DOI] [PubMed] [Google Scholar]
- 29.Sjølie AK, Klein R, Porta M, Orchard T, Fuller J, Parving HH, et al. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): A randomised placebo-controlled trial. Lancet. 2008;372:1385–93. doi: 10.1016/S0140-6736(08)61411-7. [DOI] [PubMed] [Google Scholar]
- 30.Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. UK Prospective Diabetes Study Group. BMJ. 1998;317:713–20. [PMC free article] [PubMed] [Google Scholar]
- 31.American Diabetes Association. Microvascular complications and foot care. Diabetes Care. 2015;38(Suppl 1):S58–66. doi: 10.2337/dc15-S012. [DOI] [PubMed] [Google Scholar]
- 32.Klein BE, Moss SE, Klein R, Surawicz TS. The Wisconsin epidemiologic study of diabetic retinopathy. XIII. Relationship of serum cholesterol to retinopathy and hard exudate. Ophthalmology. 1991;98:1261–5. doi: 10.1016/s0161-6420(91)32145-6. [DOI] [PubMed] [Google Scholar]
- 33.Chew EY, Klein ML, Ferris FL, 3rd, Remaley NA, Murphy RP, Chantry K, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy. Early treatment diabetic retinopathy study (ETDRS) report 22. Arch Ophthalmol. 1996;114:1079–84. doi: 10.1001/archopht.1996.01100140281004. [DOI] [PubMed] [Google Scholar]
- 34.Rema M, Srivastava BK, Anitha B, Deepa R, Mohan V. Association of serum lipids with diabetic retinopathy in urban South Indians – The Chennai urban rural epidemiology study (CURES) eye study-2. Diabet Med. 2006;23:1029–36. doi: 10.1111/j.1464-5491.2006.01890.x. [DOI] [PubMed] [Google Scholar]
- 35.Das R, Kerr R, Chakravarthy U, Hogg RE. Dyslipidemia and diabetic macular edema: A systematic review and meta-analysis. Ophthalmology. 2015;122:1820–7. doi: 10.1016/j.ophtha.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 36.Keech AC, Mitchell P, Summanen PA, O’Day J, Davis TM, Moffitt MS, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): A randomised controlled trial. Lancet. 2007;370:1687–97. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- 37.Suehiro M, Ohkubo K, Kato H, Kido Y, Anzai K, Oshima K, et al. Analyses of serum lipoprotein(a) and the relation to phenotypes and genotypes of apolipoprotein(a) in type 2 diabetic patients with retinopathy. Exp Clin Endocrinol Diabetes. 2002;110:319–24. doi: 10.1055/s-2002-34997. [DOI] [PubMed] [Google Scholar]
- 38.Hu A, Luo Y, Li T, Guo X, Ding X, Zhu X, et al. Low serum apolipoprotein A1/B ratio is associated with proliferative diabetic retinopathy in type 2 diabetes. Graefes Arch Clin Exp Ophthalmol. 2012;250:957–62. doi: 10.1007/s00417-011-1855-x. [DOI] [PubMed] [Google Scholar]
- 39.Manaviat MR, Afkhami M, Shoja MR. Retinopathy and microalbuminuria in type II diabetic patients. BMC Ophthalmol. 2004;4:9. doi: 10.1186/1471-2415-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rani PK, Raman R, Gupta A, Pal SS, Kulothungan V, Sharma T. Albuminuria and diabetic retinopathy in type 2 diabetes mellitus Sankara Nethralaya diabetic retinopathy epidemiology and molecular genetic study (SN-DREAMS, report 12) Diabetol Metab Syndr. 2011;3:9. doi: 10.1186/1758-5996-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pradeepa R, Anjana RM, Unnikrishnan R, Ganesan A, Mohan V, Rema M. Risk factors for microvascular complications of diabetes among South Indian subjects with type 2 diabetes – The Chennai urban rural epidemiology study (CURES) eye study-5. Diabetes Technol Ther. 2010;12:755–61. doi: 10.1089/dia.2010.0069. [DOI] [PubMed] [Google Scholar]
- 42.Roy R, Das MK, Pal BP, Ganesan S, Raman R, Sharma T. The effects of renal transplantation on diabetic retinopathy: Clinical course and visual outcomes. Indian J Ophthalmol. 2013;61:552–6. doi: 10.4103/0301-4738.121067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh SK, Behre A, Singh MK. Diabetic retinopathy and microalbuminuria in lean type 2 diabetes mellitus. J Assoc Physicians India. 2001;49:439–41. [PubMed] [Google Scholar]
- 44.Stephenson JM, Fuller JH, Viberti GC, Sjolie AK, Navalesi R. Blood pressure, retinopathy and urinary albumin excretion in IDDM: The EURODIAB IDDM complications study. Diabetologia. 1995;38:599–603. doi: 10.1007/BF00400730. [DOI] [PubMed] [Google Scholar]
- 45.Moriya T, Tanaka S, Kawasaki R, Ohashi Y, Akanuma Y, Yamada N, et al. Diabetic retinopathy and microalbuminuria can predict macroalbuminuria and renal function decline in Japanese type 2 diabetic patients: Japan diabetes complications study. Diabetes Care. 2013;36:2803–9. doi: 10.2337/dc12-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qiao Q, Keinänen-Kiukaanniemi S, Läärä E. The relationship between hemoglobin levels and diabetic retinopathy. J Clin Epidemiol. 1997;50:153–8. doi: 10.1016/s0895-4356(96)00335-6. [DOI] [PubMed] [Google Scholar]
- 47.He BB, Xu M, Wei L, Gu YJ, Han JF, Liu YX, et al. Relationship between anemia and chronic complications in Chinese patients with type 2 diabetes mellitus. Arch Iran Med. 2015;18:277–83. [PubMed] [Google Scholar]
- 48.Ranil PK, Raman R, Rachepalli SR, Pal SS, Kulothungan V, Lakshmipathy P, et al. Anemia and diabetic retinopathy in type 2 diabetes mellitus. J Assoc Physicians India. 2010;58:91–4. [PubMed] [Google Scholar]
- 49.McGill JB, Bell DS. Anemia and the role of erythropoietin in diabetes. J Diabetes Complications. 2006;20:262–72. doi: 10.1016/j.jdiacomp.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Davis MD, Fisher MR, Gangnon RE, Barton F, Aiello LM, Chew EY, et al. Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: Early treatment diabetic retinopathy study report #18. Invest Ophthalmol Vis Sci. 1998;39:233–52. [PubMed] [Google Scholar]
- 51.Jew OM, Peyman M, Chen TC, Visvaraja S. Risk factors for clinically significant macular edema in a multi-ethnics population with type 2 diabetes. Int J Ophthalmol. 2012;5:499–504. doi: 10.3980/j.issn.2222-3959.2012.04.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li W, Sinclair SH, Xu GT. Effects of intravitreal erythropoietin therapy for patients with chronic and progressive diabetic macular edema. Ophthalmic Surg Lasers Imaging. 2010;41:18–25. doi: 10.3928/15428877-20091230-03. [DOI] [PubMed] [Google Scholar]
- 53.Kramer CK, Rodrigues TC, Canani LH, Gross JL, Azevedo MJ. Diabetic retinopathy predicts all-cause mortality and cardiovascular events in both type 1 and 2 diabetes: Meta-analysis of observational studies. Diabetes Care. 2011;34:1238–44. doi: 10.2337/dc11-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosenson RS, Fioretto P, Dodson PM. Does microvascular disease predict macrovascular events in type 2 diabetes? Atherosclerosis. 2011;218:13–8. doi: 10.1016/j.atherosclerosis.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 55.Pradeepa R, Surendar J, Indulekha K, Chella S, Anjana RM, Mohan V. Relationship of diabetic retinopathy with coronary artery disease in Asian Indians with type 2 diabetes: The Chennai urban rural epidemiology study (CURES) eye study-3. Diabetes Technol Ther. 2015;17:112–8. doi: 10.1089/dia.2014.0141. [DOI] [PubMed] [Google Scholar]
- 56.Alonso N, Traveset A, Rubinat E, Ortega E, Alcubierre N, Sanahuja J, et al. Type 2 diabetes-associated carotid plaque burden is increased in patients with retinopathy compared to those without retinopathy. Cardiovasc Diabetol. 2015;14:33. doi: 10.1186/s12933-015-0196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rema M, Mohan V, Deepa R, Ravikumar R. Chennai urban rural epidemiology study. Association of carotid intima-media thickness and arterial stiffness with diabetic retinopathy: The Chennai urban rural epidemiology study (CURES-2) Diabetes Care. 2004;27:1962–7. doi: 10.2337/diacare.27.8.1962. [DOI] [PubMed] [Google Scholar]
- 58.Bergerhoff K, Clar C, Richter B. Aspirin in diabetic retinopathy. A systematic review. Endocrinol Metab Clin North Am. 2002;31:779–93. doi: 10.1016/s0889-8529(02)00017-8. [DOI] [PubMed] [Google Scholar]
- 59.Samra KA. The eye and visual system in pregnancy, what to expect?. An in-depth review. Oman J Ophthalmol. 2013;6:87–91. doi: 10.4103/0974-620X.116626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pescosolido N, Campagna O, Barbato A. Diabetic retinopathy and pregnancy. Int Ophthalmol. 2014;34:989–97. doi: 10.1007/s10792-014-9906-z. [DOI] [PubMed] [Google Scholar]
- 61.Egan AM, McVicker L, Heerey A, Carmody L, Harney F, Dunne FP. Diabetic retinopathy in pregnancy: A population-based study of women with pregestational diabetes. J Diabetes Res 2015. 2015:310239. doi: 10.1155/2015/310239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bhatnagar A, Ghauri AJ, Hope-Ross M, Lip PL. Diabetic retinopathy in pregnancy. Curr Diabetes Rev. 2009;5:151–6. doi: 10.2174/157339909788920929. [DOI] [PubMed] [Google Scholar]
- 63.Schocket LS, Grunwald JE, Tsang AF, DuPont J. The effect of pregnancy on retinal hemodynamics in diabetic versus nondiabetic mothers. Am J Ophthalmol. 1999;128:477–84. doi: 10.1016/s0002-9394(99)00234-2. [DOI] [PubMed] [Google Scholar]
- 64.Dirani M, Xie J, Fenwick E, Benarous R, Rees G, Wong TY, et al. Are obesity and anthropometry risk factors for diabetic retinopathy?. The diabetes management project. Invest Ophthalmol Vis Sci. 2011;52:4416–21. doi: 10.1167/iovs.11-7208. [DOI] [PubMed] [Google Scholar]
- 65.Raman R, Rani PK, Gnanamoorthy P, Sudhir RR, Kumaramanikavel G, Sharma T. Association of obesity with diabetic retinopathy: Sankara Nethralaya diabetic retinopathy epidemiology and molecular genetics study (SN-DREAMS Report no 8) Acta Diabetol. 2010;47:209–15. doi: 10.1007/s00592-009-0113-8. [DOI] [PubMed] [Google Scholar]
- 66.Kaštelan S, Tomic M, Gverovic Antunica A, Ljubic S, Salopek Rabatic J, Karabatic M. Body mass index: A risk factor for retinopathy in type 2 diabetic patients. Mediators Inflamm 2013. 2013:436329. doi: 10.1155/2013/436329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Miyazawa-Hoshimoto S, Takahashi K, Bujo H, Hashimoto N, Saito Y. Elevated serum vascular endothelial growth factor is associated with visceral fat accumulation in human obese subjects. Diabetologia. 2003;46:1483–8. doi: 10.1007/s00125-003-1221-6. [DOI] [PubMed] [Google Scholar]
- 68.Katusic D, Tomic M, Jukic T, Kordic R, Sikic J, Vukojevic N, et al. Obesity – A risk factor for diabetic retinopathy in type 2 diabetes? Coll Antropol. 2005;29(Suppl 1):47–50. [PubMed] [Google Scholar]
- 69.Raum P, Lamparter J, Ponto KA, Peto T, Hoehn R, Schulz A, et al. Prevalence and cardiovascular associations of diabetic retinopathy and maculopathy: Results from the Gutenberg health study. PLoS One. 2015;10:e0127188. doi: 10.1371/journal.pone.0127188. [DOI] [PMC free article] [PubMed] [Google Scholar]


