Abstract
Brown tumors are focal bone lesions, encountered in patients with uncontrolled hyperparathyroidism. They can be located in any part of the skeleton. Clinically significant lesions in the craniofacial bones are rare. Craniofacial involvement may cause facial disfiguration and compromise social ease of the patient and normal functions, such as chewing, talking, and breathing. In this case report, we present a patient with a brown tumor of the craniofacial bones provoked by secondary hyperparathyroidism and review the last 10 years of craniofacial brown tumors associated with secondary hyperparathyroidism in the English literature.
Key Words: Brown tumors, Secondary hyperparathyroidism, Craniofacial involvement, Treatment
Introduction
Brown tumors are focal bone lesions, caused by increased osteoclastic activity and fibroblastic proliferation, encountered in patients with uncontrolled hyperparathyroidism (HPT). They can be located in any part of the skeleton, but are most frequently encountered in the ribs, clavicles, extremities, and pelvic girdle. Clinically significant lesions in the craniofacial bones are rare [1].
Here, we present a patient with a brown tumor of the craniofacial bones provoked by secondary HPT. When we searched PubMed for English literature of the last 10 years, there were 26 cases of craniofacial brown tumors associated with secondary HPT. Of the 26 cases, full texts of 24 cases were available and a summary of these cases is shown in table 1.
Table 1.
Cases of craniofacial brown tumors associated with secondary HPT
| Study (first author) | Age, years | F/M | HD duration, years | Tumor age, months | Location | iPTH | Ca2+ | ALP | PO4 | Treatment | Response | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Leal 2006 [2] | 31 | F | 9 | 8 | Maxilla | 3,086 | 8.4 | 1,333 | 7.1 | Total parathyroidectomy and 2 years later local excision of the tumor | Tumor regressed, able to breathe and feed, improvement of patient's appearance |
| 2 | Zwick 2006 [3] | 29 | M | – | 1 | Frontal calvarium and orbital wall | 450 | 9.6 | – | 7.3 | Complete excision of the tumor | – |
| 3 | Triantafillidou 2006 [4] | 70 | F | – | – | Mandible | 412.5 | – | – | – | Local excision of the lesion | No recurrence |
| 4 | Triantafillidou 2006 [4] | 68 | F | – | – | Mandible | 389.2 | – | – | – | Local excision of the lesion | No recurrence |
| 5 | Triantafillidou 2006 [4] | 21 | F | – | – | Mandible | 481.61 | – | – | – | Local excision of the lesion | Recurrence after 1 year, 7 years after kidney transplantation there was no tumor |
| 6 | Tarrass 2008 [1] | 18 | M | 6 | 2 | Mandible | 1,335 | 8.2 | 568 | 5.7 | Subtotal parathyroidectomy | Progressive decrease in the size of tumor |
| 7 | Karabekmez 2008 [5] | 11 | M | 4 | – | Maxilla and mandible | 2,528 | 8.4 | 1,869 | 9.7 | – | Died before operation |
| 8 | Monteiro 2009 [6] | 40 | F | 7 | 24 | Orbit | Removal of parathyroid glands | Symptoms and signs disappeared | ||||
| 9 | Di Daniele 2009 [7] | 40 | F | – | – | Maxilla | 1,700 | 10.5 | 319 | 5.3 | Total parathyroidectomy and implantation of parathyroid fragment | Regression of the tumor, but with residual hyperostosis |
| 10 | Fatma 2010 [8] | 19 | F | 144 | – | Mandible | 870 | 2.27 | 2,706 | 2.08 | Subtotal parathyroidectomy | Regression of the tumor |
| 11 | Fatma 2010 [8] | 37 | F | 120 | – | Mandible | 3,687 | 2.29 | – | 1.8 | Subtotal parathyroidectomy, local excision of the lesion | Insufficient regression, so excision of the tumor |
| 12 | Fatma 2010 [8] | 57 | F | 88 | – | Maxilla | 1,500 | 2.25 | 945 | 1.98 | Subtotal parathyroidectomy | Regression of the tumor |
| 13 | Fatma 2010 [8] | 30 | F | CKD | – | Mandible | 1,115 | 2.13 | 2,493 | 2.38 | Total parathyroidectomy, implantation of parathyroid fragment | Regression of the tumor |
| 14 | Fatma 2010 [8] | 29 | M | 84 | – | Maxilla | 1,450 | 2.63 | 628 | 2.63 | Subtotal parathyroidectomy | Regression of the tumor |
| 15 | Fatma 2010 [8] | 32 | F | 84 | – | Maxilla | 1,142 | 2.56 | 318 | 2.56 | Subtotal parathyroidectomy | Regression of the tumor |
| 16 | Fatma 2010 [8] | 52 | F | 216 | – | Maxilla | 1,700 | 2 | 568 | 2 | Subtotal parathyroidectomy, local excision of the lesion | Insufficient regression, so excision of the tumor |
| 17 | Pinto 2010 [9] | 37 | F | 8 | 4 | Maxilla and mandible | 1,927 | – | 1,831 | – | Total parathyroidectomy | Regressed significantly but at 18 months remains stable |
| 18 | Nabi 2010 [10] | 24 | F | 10 | 2 | Maxilla and ipsilateral paranasal sinus | 1,591 | 3.26 | 352 | 0.88 | Total Parathyroidectomy | Significant regression |
| 19 | Jakubowski 2011 [11] | 49 | F | 10 | 12 | Mandible | – | – | – | – | – | – |
| 20 | Pechalova 2013 [12] | 19 | M | 6 | – | Maxilla and mandible | 1,409.3 | – | – | 2,204 | Local excision | – |
| 21 | Pechalova 2013 [12] | 19 | F | 6 | – | Maxilla | 2,595.8 | – | – | 3,227 | Local excision | – |
| 22 | Artul 2013 [13] | 46 | F | 11 | No | Maxillary frontal bone | 1,282 | 8.5 | 406 | 4.1 | Medical | – |
| 23 | Verma 2014 [14] | 31 | F | CKD | 13 | Mandible | 234.1 | 14.3 | 1,963 | – | Referred for treatment in higher medical center | – |
| 24 | Jafari-Pozve 2014 [15] | 29 | M | 8 | 3 | Mandible, zygoma, maxilla, palate | 3,552 | 8.7 | 2,800 | 6.3 | Parathyroidectomy | Symptoms relieved |
F = Female; M = male; HD = hemodialysis; CKD = chronic kidney disease; iPTH = intact PTH; Ca2+ = calcium; PO4 = phosphorus.
Case Report
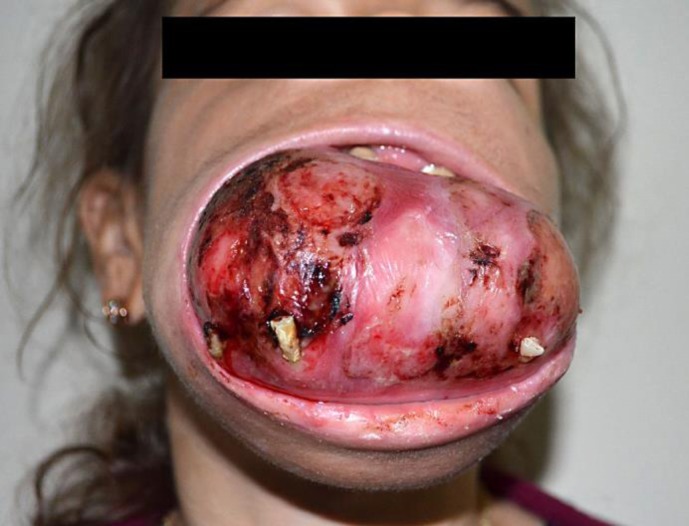
A 30-year-old Albanian female patient was referred to our hospital for living donor kidney transplantation. She was unable to walk without help and was restricted to a wheelchair. Her medical history included chronic renal insufficiency, hypertension, anemia, and hepatitis C. She had been undergoing dialysis three times per week for 19 years. A tumor was protruding through her oral cavity; it had appeared insidiously 10 years ago and had increased gradually in size. The lesion was fixed to the underlying mandible, was tender to the touch, and was covered with oral mucosa. The teeth were severely affected, and bleeding sites were observed on the fragile mucosa (fig. 1). The patient had functional problems with chewing and speech. Serum chemistry revealed an elevated parathyroid hormone (PTH) level of 2,930.6 pg/dl (normal range, 15–65 pg/dl), serum calcium 9.08 mg/dl (normal range, 8.8–11 mg/dl), phosphorus 4.2 mg/dl (normal range, 2.5–5.0 mg/dl), and alkaline phosphatase (ALP) 1,753 IU/l (normal range, 65–300 IU/l).
Fig. 1.
Apperance of the brown tumor before surgery.
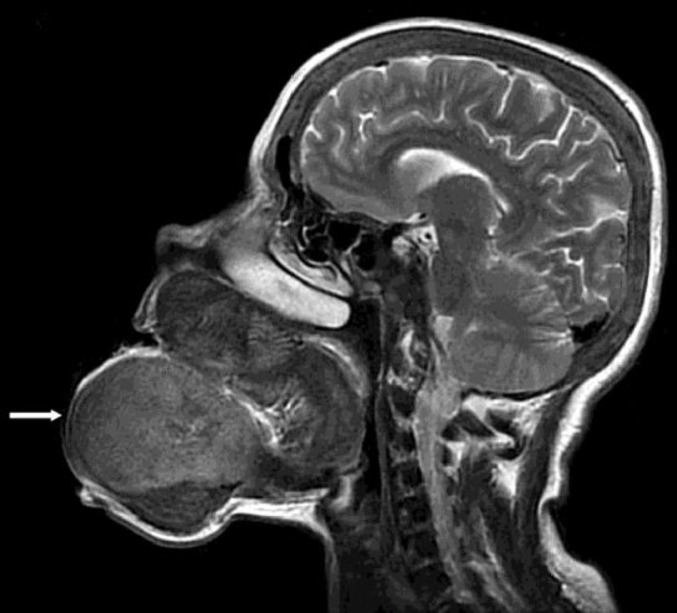
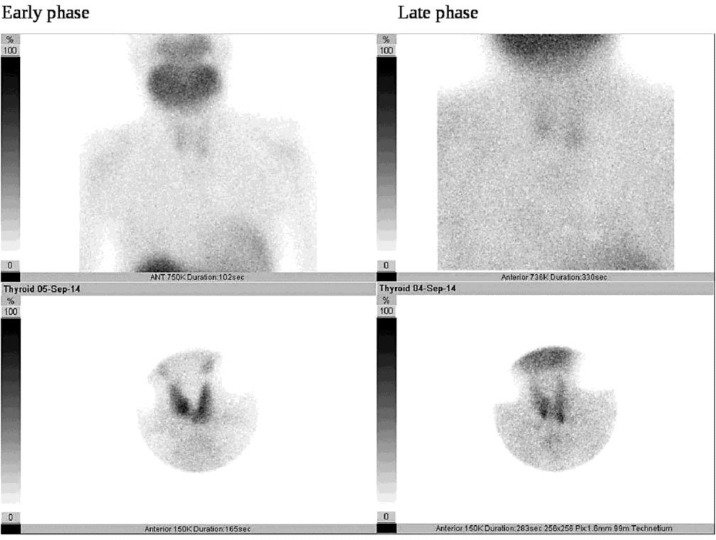
A sagittal magnetic resonance imaging scan of the head showed a brown tumor originating from the mandibular and maxillary bones (fig. 2). Ultrasonography of the neck revealed no parathyroid mass; however, a scan with 20 mCi Tc-99-sestamibi showed increased uptake by the parathyroid glands (fig. 3). Based on the medical history, clinical manifestations, and laboratory tests, the final diagnosis was brown tumor with HPT. A whole-body scan with 20 mCi Tc-99m-HDP was performed to look for multifocal disease, which may have been missed. However, only maxillary and mandibular accumulation was observed.
Fig. 2.
Sagittal magnetic resonance imaging scan showed that the brown tumor originated from the mandibular and maxillar bones (white arrow).
Fig. 3.
Parathyroid scan showed increased uptake in the parathyroid glands.
A total parathyroidectomy was performed without implantation of a parathyroid fragment into the forearm muscle. PTH was monitored intraoperatively, and the PTH level decreased (355 pg/dl). A histopathological examination of the mass confirmed the diagnosis of parathyroid adenoma. Serum PTH level continued to decrease after surgery, and the laboratory findings 1 month postoperatively were PTH 127.2 pg/dl, ALP 247 IU/l, calcium 8.4 mg/dl, and phosphorus 2 mg/dl.
The lesion failed to regress 6 months postoperatively, despite optimal treatment for HPT. Kidney transplantation was performed with a living donor kidney from her cousin and creatinine levels decreased to normal postoperatively. No complications were observed during the first postoperative week, and the patient was discharged from the hospital.
Discussion
Here, we report a case of a huge brown tumor originating from the mandible and protruding through the oral cavity, which was provoked by secondary HPT. This tumor is of the longest duration reported among brown tumors provoked by secondary HPT that affected craniofacial bones (table 1). In fact, these tumors are not malignant, and there are options for management. Treating the HPT is the first step, and normalizing PTH level with drugs, dialysis, parathyroidectomy, or kidney transplantation will often cause the tumor to regress or resolve [1]. Surgical resection of a brown tumor is generally not recommended and should only be considered if the patient wants quick resolution, if the bony lesion is com promising body functions or promoting facial deformation, or if the lesion fails to regress after 1–2 years of follow-up [2, 3]. The 10-year duration of our case was too long, reflecting the inadequacy of preventing and managing brown tumors in underdeveloped countries.
Brown tumors may be located in any part of the skeleton. Involvement of the mandible, maxilla, palate, nasal cavity, paranasal sinuses, and orbital and temporal bones has been reported [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15]. The mandible is more commonly affected than the maxilla, and these tumors are usually asymptomatic except when large. Brown tumors may cause facial disfiguration and compromise social ease of the patient and normal functions, such as chewing, talking, and breathing. Other complications include headaches, visual impairment, proptosis of the eyes, displacement and mobility of the teeth, and nasal or intraoral bleeding [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15]. The huge tumor in our case originated from the mandible and caused teeth to fall out and severely affected chewing. Talking and the appearance of our patient were also affected. This tumor caused surgical difficulties due to its location. Nasotracheal intubation and a tracheotomy were necessary for the surgery, but our team completed the procedure successfully.
Conclusion
This case was unique considering the severity and the absolute lack of response to parathyroidectomy. Despite the parathyroidectomy and optimal control of calcium/phosphate metabolism, the brown tumor did not decrease in size during follow-up, which was confirmed by computed tomography of the lesion. The mass remained also unchanged 1 year after transplantation and the surgical option will be assessed during the follow-up of the patient.
Statement of Ethics
The authors have no ethical conflicts to disclose. Written informed consent was obtained from the patient for publication of this case report and the accompanying images.
Disclosure Statement
There are neither conflicts of interest nor financial support to declare.
References
- 1.Tarrass F, Benjelloun M, Bensaha T. Severe jaw enlargement associated with uremic hyperparathyroidism. Hemodial Int. 2008;12:316–318. doi: 10.1111/j.1542-4758.2008.00273.x. [DOI] [PubMed] [Google Scholar]
- 2.Leal CT, Lacativa PG, Gomes EM, et al. Surgical approach and clinical outcome of a deforming brown tumor at the maxilla in a patient with secondary hyperparathyroidism due to chronic renal failure. Arq Bras Endocrinol Metabol. 2006;50:963–967. doi: 10.1590/s0004-27302006000500021. [DOI] [PubMed] [Google Scholar]
- 3.Zwick OM, Vaqefi MR, Cockerham KP, McDermott MW. Brown tumor of secondary hyperparathyroidism involving the superior orbit and frontal calvarium. Ophthal Plast Reconstr Surg. 2006;22:304–306. doi: 10.1097/01.iop.0000225744.19613.3d. [DOI] [PubMed] [Google Scholar]
- 4.Triantafillidou K, Zouloumis L, Karakinaris G, Kalimeras E, Iordanidis F. Brown tumors of the jaws associated with primary or secondary hyperparathyroidism. A clinical study and review of the literature. Am J Otolaryngol. 2006;27:281–286. doi: 10.1016/j.amjoto.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Karabekmez FE, Duymaz A, Keskin M, Tosun Z. Huge deforming brown tumour of the maxilla and mandible in a patient with secondary hyperparathyroidism. J Plast Reconstr Aesthet Surg. 2008;61:1404–1405. doi: 10.1016/j.bjps.2008.02.035. [DOI] [PubMed] [Google Scholar]
- 6.Monteiro ML. Multiple brown tumors of the orbital walls: case report. Arq Bras Oftalmol. 2009;72:116–118. doi: 10.1590/s0004-27492009000100025. [DOI] [PubMed] [Google Scholar]
- 7.Di Daniele N, Condò S, Ferrannini M, et al. Brown tumour in a patient with secondary hyperparathyroidism resistant to medical therapy: case report on successful treatment after subtotal parathyroidectomy. Int J Endocrinol. 2009;2009:827652. doi: 10.1155/2009/827652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fatma LB, Barbouch S, Fethi BH, et al. Brown tumors in patients with chronic renal failure and secondary hyperparathyroidism: report of 12 cases. Saudi J Kidney Dis Transpl. 2010;21:772–777. [PubMed] [Google Scholar]
- 9.Pinto MC, Sass SM, Sampaio CP, Campos DS. Brown tumor in a patient with hyperparathyroidism secondary to chronic renal failure. Braz J Otorhinolaryngol. 2010;76:404. doi: 10.1590/S1808-86942010000300022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nabi Z, Algailani M, Abdelsalam M, Asaad L, Albaqumi M. Regression of brown tumor of the maxilla in a patient with secondary hyperparathyroidism after a parathyroidectomy. Hemodial Int. 2010;14:247–249. doi: 10.1111/j.1542-4758.2010.00436.x. [DOI] [PubMed] [Google Scholar]
- 11.Jakubowski JM, Velez I, McClure SA. Brown tumor as a result of hyperparathyroidism in an end-stage renal disease patient. Case Report Radiol. 2011;2011:415476. doi: 10.1155/2011/415476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pechalova PF, Poriazova EG. Brown tumor at the jaw in patients with secondary hyperparathyroidism due to chronic renal failure. Acta Medica (Hradec Kralove) 2013;56:83–86. doi: 10.14712/18059694.2014.29. [DOI] [PubMed] [Google Scholar]
- 13.Artul S, Bowirrat A, Yassin M, Armaly Z. Maxillary and frontal bone simultaneously involved in brown tumor due to secondary hyperparathroidism in a hemodialysis patient. Case Rep Oncol Med. 2013;2013:909150. doi: 10.1155/2013/909150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verma P, Verma KG, Verma D, Patwardhan N. Craniofacial brown tumor as a result of secondary hyperparathyroidism in chronic renal disease patient: a rare entity. J Oral Maxillofac Pathol. 2014;18:267–270. doi: 10.4103/0973-029X.140779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jafari-Pozve N, Ataie-Khorasgani M, Jafari-Pozve S, Ataie-Khorasgani M. Maxillofacial brown tumors in secondary hyperparathyroidism: a case report and literature review. J Res Med Sci. 2014;19:1099–1102. [PMC free article] [PubMed] [Google Scholar]