Abstract
Purpose
Clinical trials have demonstrated the benefits of exercise for cancer survivors. This investigation determined the effectiveness and safety of a disseminated community-based exercise program for cancer survivors who had completed treatment.
Methods
Personal trainers from regional YMCAs received training in cancer rehabilitation and supervised twice-a-week, 12-week group exercise sessions for survivors. At baseline and post-program, validated measures assessed patient-reported outcomes (PRO) and physiologic measurements.
Results
Data were collected from 221 survivors from 13 YMCA sites and 36 separate classes. All participants had data available at one time point, while matched baseline and post-program PRO and physiologic data were available for 85% (N=187). Participants with matched data were largely female (82%), with mean age of 58 (range, 28–91 years). Time since diagnosis ranged from 1 to 48 (mean, 5.6 years), and mean time since last treatment was 3.0 (range, 1–33 years). Physiological improvements were significant in systolic (P<0.001) and diastolic (P=0.035) blood pressure, upper and lower body strength, the 6-min walk test (P= 0.004), and flexibility (P<0.001). Participants reported improvements in overall health-related quality of life (P< 0.001), social support (P=0.019), body pain (P=0.016), fatigue (P<0.001), insomnia (P<0.001), and overall musculoskeletal symptoms (P=<0.001). Few injuries or lymphedema events occurred during classes.
Conclusions
Community-based exercise groups for cancer survivors of mixed diagnoses and ages, who have completed active treatment, have physiologic and psychosocial benefits, and are safe.
Implications for cancer survivors
Survivors may expect significant benefit from participating in a community-based exercise program tailored to meet their individual needs as a survivor.
Keywords: Cancer survivors, Exercise, Physical activity, Community, YMCA
Introduction
Cancer is now a disease that most people diagnosed can expect to survive. Each year, more people benefit from early detection of cancer and effective medical treatments. Approximately 66% of adult cancer patients and 80% of childhood cancer patients are expected to live at least 5 years after diagnosis [1]. For breast cancer, the 5-year survival rate ranges from 93% for DCIS to 49% for stage IIIC; for colon cancer from 74% for stage I to 25% for stage IIIC; and for lymphoma from 82% for localized to 77% for regional disease [2]. As of 01 January 2007, there were approximately 11.7 million cancer survivors in the USA, representing about 3.9% of the population, with this number estimated to grow to near 20 million by 2020 [3]. As a result, cancer can be considered a chronic disease, and the physical functioning and psychological well-being of this population is of considerable public health importance. Although cured from their cancer, many survivors who have completed their medical treatment face distressing physical and psychosocial problems as a result of their illness and treatment [4, 5]. These late and long-term effects of cancer treatment can include fatigue [6–9], muscle aches and joint pain [4, 10], cardiovascular and pulmonary issues [11–14], body pain [15, 16], as well as decreased strength and flexibility [17]. Cancer survivors can also experience diminished quality of life after cancer stemming from increased anxiety, depression, stress, insecurity, decreased self-esteem, and social isolation [18–20]. These physical difficulties and psychosocial problems have been shown to lead to decreased physical and social functioning [21–23], illustrating the need for interventions within this population.
Recent research has illustrated significant post-treatment benefits of exercise suggesting that physical activity may be a particularly appropriate intervention for cancer survivors [24, 25]. Accumulating evidence indicates that exercise can ameliorate the clinical treatment sequelae of cancer including cancer-related fatigue [26, 27], lymphedema [28, 29], and osteoporosis [30, 31]. Studies have also shown that regular exercise plays a role in improving quality of life [28, 32, 33], preventing recurrence [34, 35], improving overall survival [25, 36–38], and reducing both cancer-specific and all-cause mortality [34, 39, 40].
Despite these and other well-documented benefits of physical activity, a cancer diagnosis is typically followed by a decrease in physical activity. A large proportion of cancer survivors do not participate in recommended duration or intensity of physical activity [41–43], suggesting that intervention is necessary in this population. Furthermore, cancer survivors themselves have shown a high level of interest in health promotion exercise programs [41, 44, 45], and these programs have been demonstrated safe and effective [24, 33, 46], providing strong evidence to support the development of such interventions.
In 2007, the LIVESTRONG™ Foundation and YMCA of the USA joined forces to create LIVESTRONG at the YMCA, a program to support people affected by cancer in reaching their health and well-being goals. In 2008, the LIVESTRONG at the YMCA program was piloted in ten cities across the country. As one of the ten pilot sites, the YMCA of Greater Seattle partnered with the Fred Hutchinson Cancer Research Center Survivorship Program to offer Exercise and Thrive (E&T), a 12-week exercise program available to cancer survivors in Western Washington who have completed cancer treatment. The program was designed to be easily disseminated across the country through the YMCAs of America. The first year of the program implementation focused on developing and refining the training curriculum and program, determining effective and safe criteria for program eligibility, and training the YMCA personal trainers in reliable and consistent physiologic and patient-reported outcomes testing administration. Using a pre- and post-testing study design, this paper reports on the E&T program effectiveness and safety for survivors participating in the second and third years of the program implementation.
Methods
Participants
Cancer survivors in this research component of the E&T program enrolled between February 2009 and September 2010. Survivors were self-referred to the E&T program after hearing about it through the media, from a friend, family member or health care provider, or brochures at community seminars for survivors. Cancer survivors were eligible to participate if they were over the age of 21 years, had been off cancer treatment (excluding hormone suppression therapies) for at least 90 days, and had no evidence of active disease. Prior to enrollment, eligible participants were required to have their oncologist or primary health care provider review and sign a medical clearance form allowing them to participate. The clearance form excluded patients with metastatic disease in their bones, comorbidities that would prohibit participation in moderate physical activity, or any other reason that might restrict their safe participation in exercise in a group format.
Exercise procedure
All YMCA personal trainers involved in the program were required to have at least 1 year of personal training experience. They received a 16-h group training led by a cancer rehabilitation physical therapist (AL). This training included an overview of cancer statistics, terminology, staging, and standard treatment options as well as education on common metastatic spread patterns and cancer treatment late effects such as lymphedema, peripheral neuropathy, and fatigue. A licensed clinical psychologist with expertise in cancer survivorship (KLS) provided additional training to address psychological issues facing cancer survivors as well as guidelines for responses if these or other personal issues that may arise during the exercise sessions. She also addressed emotional issues and needs of the personal training staff who were leading the program. The physical therapist and a YMCA chronic disease prevention program director (LG) provided instruction on how to apply the standardized exercise protocol.
Over a 12-week period, participants exercised in a group format at the YMCA during designated 90-min sessions, 2 days/week, supervised by the trained YMCA personal trainers. All survivor participants and their immediate family members received a full 12-week YMCA membership, which included access to facility branches within their participating YMCA association. They were allowed to access the YMCA facilities on days other than the designated sessions and were encouraged to exercise outside of the designated sessions.
During the 12-week program, the participants in the group each followed an individualized resistance training program developed by their personal trainer that was based on their health history and baseline testing. Specific exercise precautions or contraindicated movements were noted in each participant’s training program allowing the personal trainers to provide appropriate changes and additions to exercise protocols as the participant progressed through the program. Attendance was tracked at each session. Prior to the start of each session participants were asked to complete a daily symptom ‘check-in’, rating how they felt physically and any signs of lymphedema for those at risk.
The exercise protocol followed a group format with a ratio of one personal trainer to seven participants with a maximum group size of 14. Aerobic warm-up occupied the first 10 min of the program, followed by resistance training for the rest of the hour. The last 30 min incorporated ‘community building’ time with sharing personal experiences, thoughts, or didactic and experiential training in breathing, relaxation, stress management, nutrition, and complementary treatments.
Data collection procedure
All data collection procedures were reviewed and approved by the Fred Hutchinson Cancer Research Center Institutional Review Board. Personal trainers were trained in administration of the PRO and the physiologic measures during their E&T program training. Data collection for each participant included baseline completion of PRO forms and physiologic testing and repeated post-program completion of PRO and physiologic testing. Additional questions assessing incidence of injuries (including lymphedema) during program participation were added to the post-program PRO for data collected after January, 2010. Prior to January 2010, injury and lymphedema questions were asked in 6-month follow-up phone calls for which results could not be matched to individual program participants. Therefore, these phone call results are not reported here.
Measures
Physiologic testing
Resting heart rate, blood pressure, weight, and waist circumference were measured at baseline and post-program by YMCA personal trainers using standardized procedures for each test. At baseline and post-program, functional capacity was measured using the 6-min walk test following the American Thoracic Society guidelines [47, 48], and upper and lower strength was measured by the maximum amount of weight that could be lifted once for the horizontal chest press (upper) and leg press (lower) using the testing methodology of Kraemer and Fry [49]. The One Repetition Maximum assessments are considered acceptable in strength evaluations for properly supervised and medically screened patients [50, 51]. At both time points, flexibility of the lower back and hamstring was measured using the Sit and Reach test [52].
Patient-reported outcomes (PRO)
Validated PRO measures were used to assess health-related quality of life, fatigue, physical activity level, muscle and joint problems, and social support. Questions evaluating injuries and report of any lymphedema during the time frame of the classes were developed for this study. Quality of life was measured using the SF-36, version 2, a 36-item, well-validated and reliable instrument with population norms [53], widely used in cancer survivorship studies [54]. This measure includes eight scales: Physical Functioning, Role-Physical, Bodily Pain, General Health, Vitality, Social Functioning, Role-Emotional, and Mental Health. In addition, two summary measures: the Physical Component Summary and the Mental Component Summary are calculated based on the eight scales. The SF-36 is scored so that higher scores represent better functioning, and scores are standardized using normative values for the general US population, with a score of 50 representing the national average and ten points above or below the mean representing a difference of 1 standard deviation from the national average.
Fatigue was measured using the Fatigue Symptom Inventory (FSI), a scale designed for use in the cancer population, and evidence supports its reliability and validity [55, 56]. The FSI has a total score based on 13 items that assess the duration, intensity, and disruptiveness of fatigue and its impact on quality of life. Higher scores on the FSI indicate greater fatigue.
A brief insomnia rating with a mean of three items was used for this study. Each item was rated from 0=rarely or never to 4=nearly every day for: “Does it take you more than half an hour to fall asleep at night?” “Do you wake during sleep and have difficulty falling back to sleep?” and “Do you wake earlier than you want in the morning and are you unable to get back to sleep?” Principal components analysis with a Promax rotation revealed a one-factor solution (eigenvalue=1.90) that explained 63.35% of the variance. Internal consistency reliability in the study participants was adequate, with alpha=0.70.
Assessment of musculoskeletal symptoms was completed using the Muscle and Joint Measure with four subscales assessing muscle aches or stiffness (myalgias), joint pain, stiffness or swelling (arthralgias), muscle cramps, and muscle weakness [10]. Higher scores on the MJM indicated increased symptom severity.
The social support measure was first used in the ENRICHD study testing a psychosocial intervention on post acute myocardial infarction patients [57]. The measure is brief, with seven items, but has strong reliability and validity and correlates well with longer measures of support. With participants in this study, the scale had excellent internal consistency with alpha=0.87.
Injuries and lymphedema were assessed in the post-program PRO using several yes/no response items including: “During your participation in Exercise and Thrive did you have any injuries?” and “As far as you know did you have any swelling or lymphedema that developed during Exercise and Thrive?” For those participants responding positive to the lymphedema question, subsequent questions were asked to determine if the lymphedema symptoms developed before or during the E&T program and (if lymphedema developed during the program) if the symptoms were a flare of existing lymphedema or a new site of lymphedema.
Program evaluation items were developed for this study and included five items (content can be seen in Table 4). Response options ranged from 1=not at all to 5=very much, 1=very difficult to 5= very easy, or 1=definitely not to 5= definitely would.
Table 4.
Evaluation ratings
| Question: | Mean (SD) | % Mostly and very much or probably and definitely or quite easy and very easy |
|---|---|---|
| How satisfied are you with your participation in the program overall? | 4.65 (0.62) | 97.2% |
| Does the environment in the YMCA support your pursuit of health and well-being? | 4.74 (0.60) | 96.6% |
| Were the staff leading the program competent and knowledgeable? | 4.87 (0.35) | 99.4% |
| Would you recommend the program to other cancer survivors? | 4.90 (0.39) | 97.7% |
| How easy or difficult was it for you to participate in the program? | 3.97 (0.82) | 72.9% |
Statistical analysis
Descriptive and inferential analyses were performed using Statistical Package for the Social Sciences version 18.0 (SPSS; SPSS Inc, Chicago, IL). We calculated descriptive statistics for the demographic and treatment characteristics of the participants and the evaluation reports at the post-intervention time point. Chi-square or independent t tests compared those who were represented by matched pre- and post- intervention data with those represented by data at only one time point. Paired t tests compared pre to post-performance on physiologic outcomes and PRO. Effect sizes (d) were calculated by computing difference scores between the pre- and post-timepoints and dividing the mean of the difference scores by the standard deviation of the difference scores.
Results
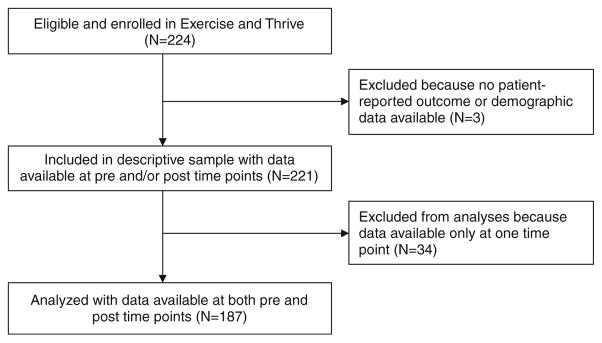
Data was collected on 221 survivors who participated from 13 YMCA sites and 36 separate classes over 2 years from February 2009 through December 2010. For a sample size of 221, and an effect size of 0.15, there was 60% power to detect a significant difference, and for an effect size of .25, there was 95% power. Both analyses are two-sided tests. All participants had data available at one time point, while matched baseline and post-program PRO and physiologic data were available for 85% (N=187). Figure 1 provides a flow diagram for the study.
Fig 1.
Study flow diagram
Participant characteristics
Table 1 presents demographic and medical characteristics of the study sample with data matched for both time points (N=187). The participants were largely female (82.4% female). Mean age was 57.7 (range, 28–91 years). Time since initial diagnosis ranged from 1 to 48 (mean, 5.6 years), and the mean time since last treatment was 3.0 (range, 1–33 years). Breast cancer survivors represented over half of the cohort (N=111, 55.5%). The majority self-identified as Caucasian (N=177, 97.3%) and non-Hispanic, non-Latino (N=184, 98.4%), with over two thirds (N=68, 36.4%) reporting a 2-year college or trade degree education or less. About half reported working full or part time for pay (N=93, 49.7%). Differences between groups with data matched for both time points (N=187), and those without matched data (N=34) were not detected in demographic or medical characteristics, with three exceptions: Those without assessments at two time points were younger (P=0.007), were likely to have been diagnosed more recently (P=0.011), and were more common in diagnostic groups of lung or colorectal cancer and those classified as ‘other’ diagnoses (P=0.031).
Table 1.
Participant characteristics
| Cases with matched pre and post data: N=187 | |
|---|---|
| Age, mean (SD) | 57.7 (10.3) |
| Range | 28–91 |
| Gender, N (%) | |
| Male | 33 (17.6) |
| Female | 154 (82.4) |
| Race, N (%) a | |
| African American | 4 (3.6) |
| Asian | 5 (4.6) |
| Native American or Alaska Native | 4 (3.6) |
| East Indian | 0 (0) |
| Native Hawaiian | 0 (0) |
| Pacific Islander | 1 (0.9) |
| Caucasian | 177 (97.3) |
| Other | 3 (2.8) |
| Ethnicity: Hispanic/Latino, N (%) | 3 (1.6) |
| Education, N (%) | |
| High school or less | 6 (3.2) |
| Some vocational or college credit | 40 (21.4) |
| 2-year college or trade degree | 22 (11.8) |
| 4-year college degree | 70 (37.4) |
| Graduate degree | 49 (26.2) |
| Diagnosis, N (%) b | |
| Breast | 111 (55.5) |
| Lymphoma | 15 (7.5) |
| Colorectal | 9 (4.5) |
| Prostate | 10 (5.0) |
| Ovarian | 9 (4.5) |
| Leukemia | 10 (5.0) |
| Lung | 5 (2.5) |
| Head and neck | 5 (2.5) |
| Thyroid | 3 (1.5) |
| Sarcoma | 2 (1.0) |
| Other | 24 (12.0) |
| Years since first diagnosis, mean (SD) | 5.6 (6.9) |
| Range | 0–48 |
| Years since last treatment, mean (SD) | 3.0 (4.5) |
| Range | 0–33 |
| Work status, N (%) | |
| In school, part time | |
| In school, full time | 4 (2.1) |
| Working part time for pay | 44 (23.5) |
| Working full time for pay | 49 (26.2) |
| Homemaker | 28 (15.0) |
| Not working for pay, not going to school, and not working as homemaker as job | 62 (33.2) |
Some participants indicated more than one race
Some participants had more than one cancer
Variables were recoded as the following for chi-square analysis comparison of cases with and without matched pre and post data:
Race: Caucasian vs. non-Caucasian
Work status: full time work or school or work/school vs. others
Diagnosis: breast vs. leukemia/lymphoma vs. colorectal vs. prostate vs. ovarian vs. lung vs. others
Education: high school or less, more than high school but not college degree, college degree or higher
Physiologic outcomes
As Table 2 indicates, physiological improvements were significant in systolic (P<0.001, d=0.27) and diastolic (P=0.035, d=0.16) blood pressure, the 6-min walk test (P=0.004, d=0.34), upper and lower body strength on the One Repetition Maximum test (P<0.001, d=0.94, d=0.83, respectively) and flexibility on the Sit and Reach test (P< 0.001, d=0.31). Significant improvement was not seen in resting heart rate, weight, or waist circumference.
Table 2.
Physiologic measures
| Baseline mean (SD) | 12 weeks mean (SD) | Paired t test | P value | |
|---|---|---|---|---|
| 6-min walk test, total distance in meters, N=77 a | 444.54 (147.79) | 481.56 (170.97) | −2.98 | 0.004 |
| Resting heart rate | 73.43 (11.73) | 74.13 (12.55) | −0.88 | 0.379 |
| Blood pressure | ||||
| Systolic | 127.63 (20.24) | 123.21 (19.54) | 3.69 | <0.001 |
| Diastolic | 79.39 (13.60) | 77.22 (13.22) | 2.12 | 0.035 |
| Waist circumference | 36.14 (6.10) | 36.13 (7.19) | 0.02 | 0.987 |
| Weight | 178.05 (41.76) | 176.58 (43.64) | 1.77 | 0.079 |
| Sit and reach (flexibility) | 8.34 (8.51) | 9.72 (8.32) | −4.01 | <0.001 |
| Strength | ||||
| Upper body | 45.94 (31.86) | 60.01 (38.43) | −12.20 | <0.001 |
| Lower body | 99.79 (67.79) | 133.82 (87.89) | −10.81 | <0.001 |
Sample size of the 6 min walk test are lower because the testing protocol was not administered consistently across the sites until after retraining of the testers. The data presented are for those participating after the retraining
Patient-reported outcomes
As seen in Table 3, participants reported improvements in nearly all PRO measured, with the exception of muscle cramps (P=0.171). Participants reported improvements in both the Physical Component Summary and Mental Component Summary Scores for the SF-36 (P<0.001 for both, d=0.41, d=0.39, respectively). Relevant subscales of the SF-36 that were compared improved for physical function, general health, mental health, and social function (all P< 0.001, d=0.41, d=0.44, d=0.30, respectively). Social support also improved (P =0.019. d =0.18). Participants reported significant reductions in body pain on the SF-36 (P=0.016, d=0.18) as well as fatigue (P<0.001, d=0.56), insomnia (P <0.001, d =0.40), overall musculoskeletal symptoms (P=<0.001, d=0.29), and three out of the four subscales of the Muscle and Joint Measure (arthralgias, myalgias, and weakness).
Table 3.
Patient reported outcomes
| Baseline mean (SD) | 12 weeks mean (SD) | Paired t test | P value | |
|---|---|---|---|---|
| SF-36 physical component summary score | 44.95 (9.77) | 47.74 (8.49) | −5.57 | <0.001 |
| SF-36 bodily pain | 47.02 (9.22) | 48.43 (9.14) | −2.43 | 0.016 |
| SF-36 physical function | 44.95 (9.10) | 47.95 (7.97) | −5.56 | <0.001 |
| SF-36 mental component summary score | 44.44 (10.74) | 48.06 (8.88) | −5.26 | <0.001 |
| SF-36 general health | 45.14 (9.65) | 48.51 (9.83) | −6.00 | <0.001 |
| SF-36 mental health | 47.08 (10.0) | 49.97 (8.16) | −4.08 | <0.001 |
| SF-36 social function | 39.22 (10.63) | 42.17 (9.18) | −3.79 | <0.001 |
| Muscle and joint measure overall | 1.04 (0.68) | 0.90 (0.65) | 3.99 | <0.001 |
| Muscle cramps | 0.79 (0.86) | 0.72 (0.86) | 1.38 | 0.171 |
| Muscle weakness | 0.94 (1.01) | 0.71 (0.87) | 4.14 | <0.001 |
| Myalgias | 1.30 (0.99) | 1.13 (0.93) | 2.24 | 0.026 |
| Arthralgias | 1.19 (0.85) | 1.09 (0.85) | 2.25 | 0.026 |
| Fatigue symptom inventory | 3.30 (1.76) | 2.47 (1.53) | 7.58 | <0.001 |
| Insomnia | 1.68 (0.93) | 1.43 (0.85) | 5.39 | <0.001 |
| Social support | 17.74 (5.13) | 18.32 (5.25) | −2.38 | 0.019 |
Injuries and lymphedema
Injuries were reported during the class time frame by 11 of 80 (13.8%) participants for whom post-program data was available and who were asked about injuries and lymphedema at the end of classes. Three of these injuries were not attributed to participation in the program but rather occurred outside of the class but during the time frame of the class (“slammed left knee into the car door”, “walked off pavement and twisted ankle not during Exercise and Thrive,” “cartilage damage, the current injury happened when I simply straightened my leg”). Another three were previous injuries that influenced exercise capacity or were aggravated by the classes (“chronic vertigo,” “bursitis,” “Baker’s cyst”). The five injuries occurring during the classes were related to weight lifting (“pulled back muscle,” “sore hips,” “shoulder efforts too much,” “legs and arms out of shape”, and “sore wrist”). For the injuries related to weight lifting during the classes, only the pulled back muscle was reported have continued residual effects by the end of the class. Two participants reported swelling or lymphedema during the program, however, both of these instances were flares of lymphedema that had been diagnosed before the program began and did not represent a new site of lymphedema that developed during the class.
Participant attendance and evaluation
A high percentage of participants (88%) attended more than half of the sessions. Few of those who attended less than half the sessions completed both pre- and post-treatment assessments, therefore, they did not have evaluable data. Outcome differences between those completing less or more than half the sessions, for whom pre- and post-data were available and were not significant, although the study was not powered to confidently test this question. Satisfaction with the classes was generally high (Table 4). All evaluation components were above 95%. However, over a quarter of the participants did not find it easy to participate in the classes, selecting responses of either “quite difficult” or “a little difficult.” Reasons for difficulty, when specified, were largely about scheduling challenges, though most participants did not explain what was difficult for themselves. Recommendations for improving the program largely included requests for more frequent classes, additional types of training, or longer duration of the program.
Discussion
This study indicates that a community-based exercise program has important beneficial effects on physiologic, symptom, and quality of life health outcomes for cancer survivors and is safe to implement. The YMCA-based program, with personal trainers who receive 2 days of group training from cancer specialists, effectively and safely provides a program that improves the lives of cancer survivors within their home communities. Based on these findings, we believe this model of support for physical activity holds promise for wide dissemination to the physically inactive population of cancer survivors.
Overall our findings provide preliminary evidence that the 12-week E&T program is helpful for improving fatigue, insomnia, physical function, overall musculoskeletal symptoms, mental health, social support, and physical activity in cancer survivors. Additionally, the exercise program indicates notable improvements in blood pressure, upper and lower body strength, walking endurance, and flexibility. These results are consistent with the building body of recent research illustrating significant post-treatment benefits of exercise for cancer survivors. Also consistent with other research, study results did not indicate significant average weight loss as a benefit of class participation, which is consistent with findings from studies of other resistance-based, strength training focus (rather than aerobic) exercise programs [29].
Importantly, this program was implemented with widely diverse groups of survivors, at community sites by trainers who received a limited amount of cancer-specific training. Nearly all of the trainers and most of the participants were highly enthusiastic about the program. The infrequent rates of injury or lymphedema flares, together with the extensive changes in physiologic and PRO improvements, support the value of this type of exercise program as having broad-spectrum reach in a well-identified area of need for many cancer survivors. Although rates of injuries in usual exercise programs with previously inactive participants have not been documented, we believe the rate of injury in this program was low, with all but one event healing by the end of the classes. This study adds further evidence that weight resistance exercise can be safe for women with lymphedema risk following breast cancer treatment [29].
The mean age of our study sample represents a working-age population, which is further supported by our finding that over half were working full- or part-time while participating in the exercise program. This characteristic of our cancer survivor population highlights the importance of offering the structured exercise class sessions on varying days of the week and time of day, including weekends and evenings. The age range of the participants from 28 to 91 years is noteworthy, especially for those completing the classes, as indicated by those having assessments at both baseline and post-program time points. Given the large proportion of elderly cancer survivors, exercise programs are needed for the older population of survivors that are susceptible to inactivity after cancer diagnosis.
Our predominantly female study cohort is consistent with research indicating that males are underrepresented in research on lifestyle interventions [58], suggesting that recruitment strategies targeting male cancer survivors may be warranted. Past research has also detailed the benefits of sex-specific exercise groups among male patients, as they allow the development of comradeship, “male trust,” and action-oriented togetherness [59]. The lack of racial diversity in our populations also supports recent research suggesting the use of population-based cancer registries to recruit racially and geographically diverse participants into a community-based exercise intervention [60]. While results of this program may not represent the experiences of all post-treatment cancer survivors due to its voluntary nature and the homogeneity of our cohort, the findings make an important contribution to the understanding of the benefits as well as safety of exercise within this population.
In our study population, most participants were over 5 years out from their cancer diagnosis and almost 3 years out from finishing cancer treatment. The benefits of physical activity with cancer patients have been demonstrated as early as at the time of diagnosis, suggesting that earlier invention may be warranted. Cancer survivors themselves advocate an earlier start to exercise, beginning either at diagnosis or soon after treatment, with emphasis placed on the physical and psychological benefits that could be gained from exercise [61]. However, studies examining physical activity promotion practices of oncologists, including oncologists’ perceptions of the benefits of physical activity for cancer survivors and barriers for promotion of physical activity, have suggested that only half of oncologists inquire about their patients’ physical activity on some or most visits with “insufficient time” rated as the highest barrier to promotion of physical activity [62].
Our group-based, supervised, individually tailored, and gradually progressing format for the exercise intervention was consistent with participant-reported factors that have been shown to facilitate exercise in cancer survivors [61]. Additionally, the self-management approach (daily check-ins, managing up and down increments of weight resistance changes) of the exercise program, may have improved participants’ self-efficacy in the training protocol. These factors may have contributed to high satisfaction with the program along with the group support experienced by the participants.
Offering this physical activity intervention at numerous YMCA facilities throughout the region encouraged varied participants by not limiting potential participants to those who lived close to a cancer or rehabilitation facility or those with personal resources available to overcome financial and proximity challenges. Furthermore, when the 12-week structured program was over, the facility and the personal trainers were still available to participants, encouraging continued exercise. This community-based approach thus allowed us to avoid numerous barriers to sustaining and disseminating a physical activity program that have proved challenging in other similar studies [60, 63].
Limitations of the reported design and analyses should be noted. The pre–post clinical trial design of the E&T program did not allow for a randomized control group, and the convenience sampling provides a population of participants who are motivated to self-initiate participation in an exercise program. In addition, generalizability of the findings is restricted by the female gender as well as non-Hispanic Caucasian ethnic and racial homogeneity of the sample. Finally, the reported analyses lack long-term follow-up.
Conclusions
This investigation supports the physical and psychosocial value of community-based exercise groups for cancer survivors of mixed diagnoses and ages who have completed active treatment. Given the large and expanding number of cancer survivors, more research is needed that tests interventions against a comparison group. These interventions need to provide health behavior change technologies that can be widely disseminated to this population. Programs are needed that focus on high-reach, sustainable exercise that is accessible to diverse survivors with varying incomes, accessible within communities and responsive to cultural diversity.
Acknowledgments
Support for this research was provided by the LIVESTRONG Foundation and the Amgen Foundation of Washington State. We thank the survivors participating in this study and each of the Exercise and Thrive personal trainers and sites participating in this project, including:
Northshore YMCA, Bothell, WA
Bellevue Family YMCA, Bellevue, WA
West Seattle Family YMCA, Seattle, WA
Downtown Seattle YMCA, Seattle, WA
Dale Turner Family YMCA, Shoreline, WA
Meredith Mathews-East Madison YMCA, Seattle, WA
Auburn Valley YMCA, Auburn, WA
Gig Harbor Family YMCA, Gig Harbor, WA
Marysville Family YMCA, Marysville, WA
University Family YMCA, Seattle, WA
Briggs Community YMCA, Olympia, WA
Olympia Downtown YMCA, Olympia, WA
Clallam County Family YMCA, Port Angeles, WA
Contributor Information
Emily Jo Rajotte, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D5-220, P.O. Box 19024, Seattle, WA 98109, USA.
Jean C. Yi, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D5-220, P.O. Box 19024, Seattle, WA 98109, USA
K. Scott Baker, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D5-220, P.O. Box 19024, Seattle, WA 98109, USA.
Lindsey Gregerson, YMCA of Greater Seattle, Seattle, WA, USA.
Andréa Leiserowitz, Oncology Physical Therapy, Eugene, OR, USA.
Karen L. Syrjala, Email: ksyrjala@fhcrc.org, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D5-220, P.O. Box 19024, Seattle, WA 98109, USA, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave N, D5-220, P.O. Box 19024, Seattle, WA 98109, USA
References
- 1.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975–2008. National Cancer Institute; Bethesda, MD: [Accessed 14 Nov 2011]. http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975–2008. National Cancer Institute; Bethesda, MD: [Accessed 14 Nov 2011]. http://seer.cancer.gov/csr/1975_2008/ [Google Scholar]
- 3.Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, et al. SEER Cancer Statistics Review, 1975–2006. National Cancer Institute; Bethesda, MD: [Accessed 14 Nov 2011]. http://seer.cancer.gov/csr/1975_2006/ [Google Scholar]
- 4.Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112:2577–92. doi: 10.1002/cncr.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brearley SG, Stamataki Z, Addington-Hall J, Foster C, Hodges L, Jarrett N, et al. The physical and practical problems experienced by cancer survivors: a rapid review and synthesis of the literature. Eur J Oncol Nurs. 2011;15:204–12. doi: 10.1016/j.ejon.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Servaes P, Verhagen S, Schreuder B, Veth R, Bleijenberg G. Fatigue after treatment for malignant and benign bone and soft tissue tumors. J Pain Symptom Manage. 2003;26:1113–22. doi: 10.1016/j.jpainsymman.2003.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Hjermstad SD, Fosså SD, Oldervoll L, Holte H, Jacobsen AB, Loge JH. Fatigue in long-term Hodgkin’s disease survivors: a follow-up study. J Clin Oncol. 2005;23:6587–95. doi: 10.1200/JCO.2005.09.936. [DOI] [PubMed] [Google Scholar]
- 8.Bower JE, Ganz PA, Desmond KA, Bernaards C, Rowland JH, Meyerowitz BE, et al. Fatigue in long-term breast carcinoma survivors. A longitudinal investigation. Cancer. 2006;106:751–8. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 9.Servaes P, Gielissen MFM, Verhagen C, Bleijenberg G. The course of severe fatigue in disease-free breast cancer patients: a longitudinal study. Pycho-Oncol. 2007;16:787–95. doi: 10.1002/pon.1120. [DOI] [PubMed] [Google Scholar]
- 10.Syrjala K, Yi JC, Artherholt SB, Stover AC, Abrams JR. Measuring musculoskeletal symptoms in cancer survivors who receive hematopoietic cell transplantation. J Cancer Surviv. 2010;4:225–35. doi: 10.1007/s11764-010-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yahalom J, Portlock CS. Long-term cardiac and pulmonary complications of cancer therapy. Heart Fail Clin. 2011;7:403–11. doi: 10.1016/j.hfc.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Abouassaly R, Fossa SD, Giwercman A, Kollmannsberger C, Motzer RJ, Schmoll HJ, et al. Sequelae of treatment in long-term survivors of testis cancer. Eur Urol. 2011;60:516–26. doi: 10.1016/j.eururo.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 13.Baker KS, Chow E, Steinberger J. Metabolic syndrome and cardiovascular risk in survivors after hematopoietic cell transplantation. Bone Marrow Transplant. 2011 doi: 10.1038/bmt.2011.118. [DOI] [PubMed] [Google Scholar]
- 14.Pliarchopoulou K, Pectasides D. Late complications of chemotherapy in testicular cancer. Cancer Treat Rev. 2010;36:262–7. doi: 10.1016/j.ctrv.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Sharma N, Hansen CH, O'Connor M, Walker J, Kleiboer A, Murray G, et al. Sleep problems in cancer patients: prevalence and association with distress and pain. Psycho-Oncol. 2011 doi: 10.1002/pon.2004. [DOI] [PubMed] [Google Scholar]
- 16.Farquhar-Smith P. Chemotherapy-induced neuropathic pain. Curr Opin Support Palliat Care. 2011;5:1–7. doi: 10.1097/SPC.0b013e328342f9cc. [DOI] [PubMed] [Google Scholar]
- 17.Harrington S, Padua D, Battaglini C, Michener LA, Giuliani C, Myers J, et al. Comparison of shoulder flexibility, strength, and function between breast cancer survivors and healthy participants. J Cancer Surviv. 2011;5:167–74. doi: 10.1007/s11764-010-0168-0. [DOI] [PubMed] [Google Scholar]
- 18.Jansen L, Hermann A, Stegmaier C, Singer S, Brenner H, Arndt V. Health-related quality of life during the 10 years after diagnosis of colorectal cancer: a population-based study. J Clin Oncol. 2011;29:3263–9. doi: 10.1200/JCO.2010.31.4013. [DOI] [PubMed] [Google Scholar]
- 19.Bencová V, Bella V, Svec J. The dynamics of psychosocial burden development in breast cancer survivors: clinical success with psychosocial consequences. Klin Onkol. 2011;24:203–8. [PubMed] [Google Scholar]
- 20.Oerlemans S, Mols F, Nijziel MR, Lybeert M, van de Poll-Franse LV. The impact of treatment, sociodemographic and clinical characteristics on health-related quality of life among Hodgkin's and non-Hodgkin's lymphoma survivors: a systematic review. Ann Hematol. 2011;90:993–1004. doi: 10.1007/s00277-011-1274-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ness K, Wall MM, Oakes JM, Robison LL, Gurney JG. Physical performance limitations and participation restrictions among cancer survivors: a population-based study. Ann Epidemiol. 2006;16:197–205. doi: 10.1016/j.annepidem.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 22.Drake D, Falzer P, Xistris D, Robinson G, Roberge M. Physical fitness training: outcomes for adult oncology patients. Clin Nurs Res. 2004;13:245–64. doi: 10.1177/1054773804265673. [DOI] [PubMed] [Google Scholar]
- 23.Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5:353–60. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 24.Pekmezi D, Demark-Wahnefried W. Updated evidence in support of diet and exercise interventions in cancer survivors. Acta Oncol. 2011;50:167–78. doi: 10.3109/0284186X.2010.529822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Demark-Wahnefried W. Cancer survival: time to get moving? Data accumulate suggesting a link between physical activity and cancer survival. J Clin Oncol. 2006;24:3517–8. doi: 10.1200/JCO.2006.06.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown J, Huedo-Medina TB, Pescatello LS, Pescatello SM, Ferrer RA, Johnson BT. Efficacy of exercise interventions in modulating cancer-related fatigue among adult cancer survivors: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2011;20:123–33. doi: 10.1158/1055-9965.EPI-10-0988. [DOI] [PubMed] [Google Scholar]
- 27.Hede K. Supportive care: large studies ease yoga, exercise into mainstream oncology. J Natl Cancer Inst. 2011;103:11–2. doi: 10.1093/jnci/djq536. [DOI] [PubMed] [Google Scholar]
- 28.Kim D, Sim YJ, Jeong HJ, Kim GC. Effect of active resistive exercise on breast cancer-related lymphedema: a randomized controlled trial. Arch Phys Med Rehabil. 2010;91:1844–8. doi: 10.1016/j.apmr.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Schmitz K, Ahmed R, Troxel A, Cheville A, Smith R, Lewis-Grant L, et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med. 2009;361:664–73. doi: 10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 30.Winters-Stone K, Dobek J, Nail L, Bennett JA, Leo MC, Naik A, et al. Strength training stops bone loss and builds muscle in postmenopausal breast cancer survivors: a randomized, controlled trial. Breast Cancer Res Treat. 2011;127:447–56. doi: 10.1007/s10549-011-1444-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loprinzi C, Wolf SL, Barton DL, Laack NN. Symptom management in premenopausal patients with breast cancer. Lancet Oncol. 2008;9:993–1000. doi: 10.1016/S1470-2045(08)70256-0. [DOI] [PubMed] [Google Scholar]
- 32.Ferrer R, Huedo-Medina TB, Johnson BT, Ryan S, Pescatello LS. Exercise interventions for cancer survivors: a meta-analysis of quality of life outcomes. Ann Behav Med. 2011;41:32–47. doi: 10.1007/s12160-010-9225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Speck R, Courneya KS, Mâsse LC, Duval S, Schmitz KH. An update of controlled physical activity trials in cancer survivors: a systematic review and meta-analysis. J Cancer Surviv. 2010;4:87–100. doi: 10.1007/s11764-009-0110-5. [DOI] [PubMed] [Google Scholar]
- 34.Ibrahim E, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28:753–65. doi: 10.1007/s12032-010-9536-x. [DOI] [PubMed] [Google Scholar]
- 35.Wiggins M, Simonavice EM. Cancer prevention, aerobic capacity, and physical functioning in survivors related to physical activity: a recent review. Cancer Manag Res. 2010;2:157–64. doi: 10.2147/cmar.s7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holmes MD, Chen WY, Feskanich D, Kroenke CH, Colditz GA. Physical activity and survival after breast cancer diagnosis. JAMA. 2005;293:2479–86. doi: 10.1001/jama.293.20.2479. [DOI] [PubMed] [Google Scholar]
- 37.Meyerhardt JA, Giovannucci EL, Holmes MD, Chan AT, Chan JA, Colditz GA, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24:3527–34. doi: 10.1200/JCO.2006.06.0855. [DOI] [PubMed] [Google Scholar]
- 38.Meyerhardt JA, Heseltine D, Niedzwiecki D, Hollis D, Saltz LB, Mayer RJ, et al. Impact of physical activity on cancer recurrence and survival in patients with stage III colon cancer: findings from CALGB 89803. J Clin Oncol. 2006;24:3535–41. doi: 10.1200/JCO.2006.06.0863. [DOI] [PubMed] [Google Scholar]
- 39.Irwin ML, McTiernan A, Manson JE, Thomson CA, Sternfeld B, Stefanick ML, et al. Physical activity and survival in postmenopausal women with breast cancer: results from the Women's Health Initiative. Cancer Prev Res. 2011;4:522–9. doi: 10.1158/1940-6207.CAPR-10-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirkegaard H, Johnsen NF, Christensen J, Frederiksen K, Overvad K, Tjønneland A. Association of adherence to lifestyle recommendations and risk of colorectal cancer: a prospective Danish cohort study. BMJ. 2010 doi: 10.1136/bmj.c5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Demark-Wahnefried W, Peterson B, McBride C, Lipkus I, Clipp E. Current health behaviors and readiness to pursue life-style changes among men and women diagnosed with early stage prostate and breast carcinomas. Cancer. 2000;88:674–84. [PubMed] [Google Scholar]
- 42.Irwin ML, McTiernan A, Bernstein L, Gilliland FD, Baumgartner R, Baumgartner K, et al. Physical activity levels among breast cancer survivors. Med Sci Sports Exerc. 2004;36:1484–91. [PMC free article] [PubMed] [Google Scholar]
- 43.Irwin M, Crumley D, McTiernan A, Bernstein L, Baumgartner R, Gilliland FD, et al. Physical activity levels before and after a diagnosis of breast carcinoma: the Health, Eating, Activity, and Lifestyle (HEAL) study. Cancer. 2003;97:1746–57. doi: 10.1002/cncr.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones LW, Courneya KS. Exercise counseling and programming preferences of cancer survivors. Cancer Pract. 2002;10:208–15. doi: 10.1046/j.1523-5394.2002.104003.x. [DOI] [PubMed] [Google Scholar]
- 45.Fink B, Weiner JG, Jordan TR, Thompson AJ, Salvage TC, Coman M, et al. Early stage breast cancer and its association with diet and exercise-related perceptions and behaviors to prevent recurrence. Breast Cancer. 2010;4:65–72. doi: 10.4137/BCBCR.S6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haines TP, Sinnamon P, Wetzig NG, Lehman M, Walpole E, Pratt T, et al. Multimodal exercise improves quality of life of women being treated for breast cancer, but at what cost? Randomized trial with economic evaluation. Breast Cancer Res Treat. 2010;124:163–75. doi: 10.1007/s10549-010-1126-2. [DOI] [PubMed] [Google Scholar]
- 47.American Thoracic Society. ATS Statement: Guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–7. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 48.Enright PL. The six-minute walk test. Respir Care. 2003;48:783–5. [PubMed] [Google Scholar]
- 49.Kraemer WJ, Fry AC. Strength testing: development and evaluation of methodology. In: Maud PJ, Foster C, editors. Physiological assessment of human fitness. Champaign, IL: Human Kinetics; 1995. [Google Scholar]
- 50.Shaw CE, McCully KK, Posner JD. Injuries during the one repetition maximum assessment in the elderly. J Cardiopulm Rehabil. 1995;15:283–7. doi: 10.1097/00008483-199507000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Barnard KL, Adams KJ, Swank AM, Mann E, Denny DM. Injuries and muscle soreness during the one repetition maximum assessment in a cardiac rehabilitation population. J Cardiopulm Rehabil. 1999;19:52–8. doi: 10.1097/00008483-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Wells KF, Dillon EK. The sit and reach. A test of back and leg flexibility. Res Q. 1952;23:115–8. [Google Scholar]
- 53.Ware JE, Kosinski M, Dewey JE. How to score version 2 of the SF-36 health survey. Lincoln, RI: QualityMetric Incorporated; 2000. [Google Scholar]
- 54.Hawthorne G, Osborne RH, Taylor A, Sansoni J. The SF36 version 2: critical analyses of population weights, scoring algorithms and population norms. Qual Life Res. 2007;16:661–73. doi: 10.1007/s11136-006-9154-4. [DOI] [PubMed] [Google Scholar]
- 55.Hann DM, Jacobsen PB, Azzarello LM, Martin SC, Curran SL, Fields KK, et al. Measurement of fatigue in cancer patients: development and validation of the fatigue symptom inventory. Qual Life Res. 1998;7:301–10. doi: 10.1023/a:1024929829627. [DOI] [PubMed] [Google Scholar]
- 56.Hann DM, Denniston MM, Baker F. Measurement of fatigue in cancer patients: further validation of the fatigue symptom inventory. Qual Life Res. 2000;9:847–54. doi: 10.1023/a:1008900413113. [DOI] [PubMed] [Google Scholar]
- 57.ENRICHD Investigators. Enhancing recovery in coronary heart disease (ENRICHD): baseline characteristics. Am J Cardiol. 2001;88:316–22. doi: 10.1016/s0002-9149(01)01652-6. [DOI] [PubMed] [Google Scholar]
- 58.Pagoto S, Schneider KL, Oleski JL, Luciani JM, Bodenlos JS, Whited MC. Male inclusion in randomized controlled trials of lifestyle weight loss interventions. Obesity. 2011 doi: 10.1038/oby.2011.140. [DOI] [PubMed] [Google Scholar]
- 59.Adamsen L, Rasmussen JM, Pedersen LS. “Brothers in arms”: how men with cancer experience a sense of comradeship through group intervention which combines physical activity with information relay. J Clin Nurs. 2001;10:528–37. doi: 10.1046/j.1365-2702.2001.00514.x. [DOI] [PubMed] [Google Scholar]
- 60.Rogerino A, Grant LL, Wilcox H, Schmitz KH. Geographic recruitment of breast cancer survivors into community-based exercise interventions. Med Sci Sports Exerc. 2009;41:1413–20. doi: 10.1249/MSS.0b013e31819af871. [DOI] [PubMed] [Google Scholar]
- 61.Blaney J, Lowe-Strong A, Rankin J, Campbell A, Allen J, Gracey J. The cancer rehabilitation journey: barriers to and facilitators of exercise among patients with cancer-related fatigue. Phys Ther. 2010;90:1135–47. doi: 10.2522/ptj.20090278. [DOI] [PubMed] [Google Scholar]
- 62.Karvinen K, DuBose KD, Carney B, Allison RR. Promotion of physical activity among oncologists in the United States. J Support Oncol. 2010;8:35–41. [PubMed] [Google Scholar]
- 63.Ahmed RL, Thomas W, Yee D, Schmitz K. Weight training does not increase incidence of lymphedema in breast cancer survivors. J Clin Oncol. 2006;24:2765–72. doi: 10.1200/JCO.2005.03.6749. [DOI] [PubMed] [Google Scholar]