Abstract
Pd(II)-catalyzed arylation of γ-C(sp3)–H bond of aliphatic acid-derived amides is developed by using quinoline-based ligands. Various γ-aryl-α-amino acids are prepared from natural amino acids using this method. The influence of ligand structure on reactivity is also systematically investigated.
Keywords: γ-C–H activation, arylation, quinoline ligand, palladium, carboxylic acids
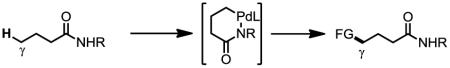
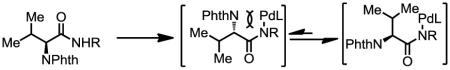
Transitional metal catalyzed C–H functionalization of readily accessible and structurally simple chemicals provides a compelling method for expanding molecular diversity.[1] The diverse reactivity of the carbon-palladium bond renders Pd(II)-catalyzed C–H funcationalization reactions highly suitable for this endeavor. In this context, late-stage functionalization of sp2 C–H bonds has been demonstrated.[2] Great efforts have also been made towards directed C(sp3)–H activation over the past decade, specifically, functionalizations of carboxylic acids and derivatives,[3] amines,[3c,4] imines[5] via five-membered metallacycles have been extensively demonstrated. Development of C–H activation reactions via six-membered metallacycles to functionalize more distal carbon centers will significantly broaden the synthetic utility of C(sp3)–H activation reactions. For example, γ-C–H functionalization and β-C–H functionalizations of carboxylic acid derivatives provides two distinct synthetic disconnections respectively (Eq. 1–2). While substantial progress has been made towards β-C–H activation using Pd catalysts, γ-C–H activation has met with difficulties due to the less favored six-membered cyclopalladation of alkyl C–H bonds. Corey et al. established that the presence of a phthalimido group at α-position was beneficial for the arylation of γ-C–H bonds in valine using a bidentate directing group (Eq. 3).[6] The sterically bulky phthalimido group could favor the assembly of the desired conformation as shown in Eq. 3. An improved protocol for γ-C–H arylation of phthalimide protected valine was also reported more recently.[7] However, γ-C–H arylation of simple carboxylic acids derivatives not containing a sterically bulky phthalimido group has not been reported to date. Herein we report a ligand-enabled γ-arylation of primary C–H bonds attached to quaternary carbon centers of simple aliphatic acids derivatives. The protocol is also successfully applied to the diversification of valine, isoleucine and tert-leucine.
Our initial effort was focused on the γ-arylation of simple aliphatic acids. 3,3-dimethyl-butanamide 1a was chosen as the model substrate as it contains nine γ-C–H bonds which would result in better reactivity. However, only trace amount of product was formed using 20 mol% of Pd(OAc)2 under various conditions. Encouraged by our recent results of γ-olefination of amide
 |
(1) |
 |
(2) |
 |
(3) |
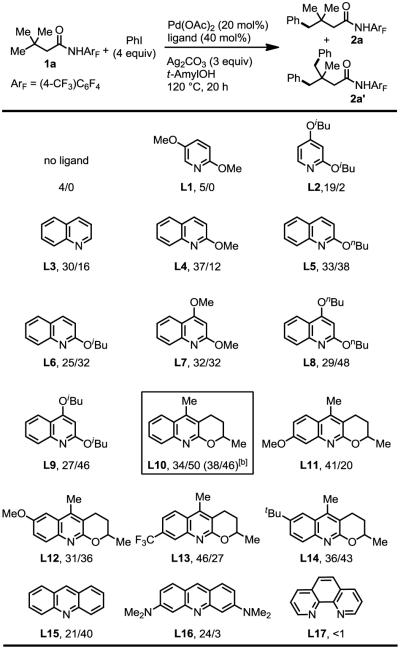
derivatives that were enabled by ligands in combination with a weakly coordinating amide directing group (CONHArF, ArF = 2,3,5,6-tetrafluoro-4-(trifluoromethyl)phenyl),[8] we wondered whether these types of ligands would promote arylation of γ-C–H bond. Systematic ligand screening was carried out using pyridine, quinoline, acridine and phenanthroline derivatives (Table 1). Various substituted pyridines (L1–L2) were tested and gave no noticeable improvement. To our delight, quinoline significantly promoted the reaction to give mono-arylated and di-arylated products in 46% total yield (L3). The yield was improved when an alkoxyl group was introduced to the 2-position of quinoline (L4–L6).[9] 2,4-dialkoxyl substituted quinolines (L7–L9) further increases the yield, indicating that electron-donating substituent will enhance the efficiency of the ligand. Therefore, we started to test tricyclic quinoline (L10–L14) with 2-alkoxy moiety constrained in the ring where one of the oxygen lone pairs is parallel to the π-system of the quinoline ring. Such conformational orientation will result in a more electron-rich ligand.[10] After a systematic examination of the tricyclic quinolines, ligand L10 was found to be the most effective. Simple acridine (L15) also gave moderate yield, while dimethylamine substituted acridine (L16) decreased the efficiency. Bidentate 1,10-phenanthroline (L17) inhibited the reaction. Using the most effective ligand L10, we performed extensive optimization and found that this γ-C–H arylation can proceed with 10 mol% Pd catalyst under an oxygen atmosphere at 90 °C to give comparable yield (see supporting information). Ligand can also be recycled. The replacement of air by molecular oxygen is crucial. A range of other amide directing groups are also tested with these new conditions and gave significantly inferior results (see supporting information). While the rationalization of the ligand effect would need further investigations, the observed influence of the ligand structure on this reaction indicates the superiority of electron-rich quinoline ligands. These type of ligands have also been shown to accelerate the C–H activation step in the C(sp3)–H borylation reaction.[11]
Table 1.
Ligand effect.[a]
Reaction conditions: 0.1 mmol of substrate, 0.4 mmol of PhI (45 μL), 0.02 mmol of Pd(OAc)2 (20 mol%), 0.04 mmol of Ligand (40 mol%), 0.3 mmol of Ag2CO3, 1 mL of t-AmylOH, air, 50 mL sealed tube, 120 °C (oil), 20 h. Yield was determined by the 1H NMR spectroscopy using CH2Br2 as the internal standard. The ratios are the yields of mono-arylated product to di-arylated product (2a%/2a'%).
0.2 mmol of substrate, 0.8 mmol of PhI (90 μL), 0.02 mmol of Pd(OAc)2 (10 mol%), 0.04 mmol of Ligand (20 mol%), 0.6 mmol of Ag2CO3, 1 mL of t-AmylOH, O2 (1 atm), 50 mL sealed tube, 90 °C (oil), 20 h.
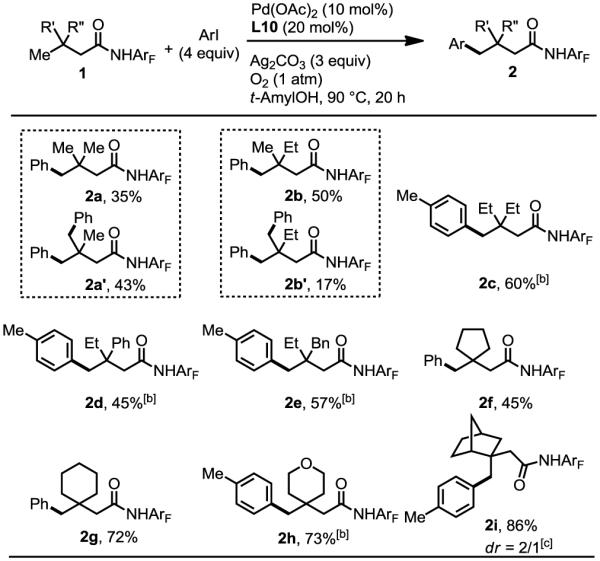
With these optimized conditions in hand, we proceeded to survey the substrates scope of the reaction (Table 2). This protocol is compatible with substrates containing one or two β-methyl groups affording comparable yields (2b and 2b', 2c). Phenyl and benzyl groups at the β-positions are also tolerated affording the desired products in moderate yields (2d, 2e). The single methyl group in the cyclic substrates can also be arylated to give desired products in 45–73% yields (2f–h). Notably, tetrahydropyran and substituted norbornane structural motif are also compatible with this arylation protocol (2h, 2i), affording 73% and 86% yields respectively. Unfortunately, the amide derived from 3-methylbutanoic acid is not reactive, which is consistent with the well-known Thorpe-Ingold effect. Further development of ligand will be required to overcome this limitation.
Table 2.
Substrates scope.[a]
|
0.2 mmol of substrate, 0.8 mmol of ArI, 0.02 mmol of Pd(OAc)2 (10 mol%), 0.04 mmol of L10 (20 mol%), 0.6 mmol of Ag2CO3, 1 mL of t-AmylOH, 50 mL sealed tube, 90 °C (oil), 20 h. The yields are isolated yields.
1 equiv of AgO2CnBu was added and the reaction temperature was 100 °C.
The dr value of 1i is 2/1; The configuration in the table is the major isomer.
In light of the broad interests in modifying natural amino acids[3p,4,6,7,12] and oligopeptides,[13] we performed the γ-functionalization of valine under these conditions. Unfortunately, only 36% of arylated product was obtained under the newly established conditions (Eq. 4).
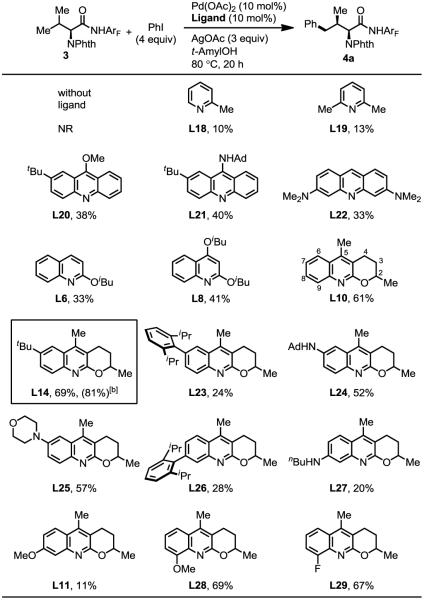
However, the dramatic ligand effect indicated by the control experiment (no product was formed in the absence of ligand) (Table 3) encouraged us to further optimize the ligand effect. We found that reducing the ligand/palladium ratio from 2:1 to 1:1 increased the yield to 61% (see supporting information). Using these new conditions, we re-investigated a series of ligands. 2-picoline (L18) and 2,6-lutidine (L19) are poor ligands. Acridine (L20–L22) ligands gave the arylated products in 30~40% yields. While 2-alkoxyl or
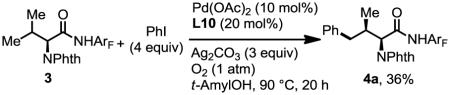 |
(4) |
2,4-dialkoxyl quinoline (L6, L8) did not improve further, the tricyclic quinoline ligand 10 afforded 61% yield. Thus, we prepared more substituted tricyclic quinoline ligands to establish the structure-reactivity relationship. Electronic donating and bulky tert-butyl substituted at 7-position (L14) could enhance the reactivity. On the other hand, extremely bulky 2,6-diisopropyl phenyl at the same position (L23) decreases the effectiveness. Quinolines containing amino groups at the 7-positions (L24–25) gave moderate yields. Either electron withdrawing (L26) or electron donating groups (L27, L11) at 8-position decreases the yields. However, Ligands containing either electron-donating or –withdrawing groups at the 9-positions (L28, L29) are comparable to the best ligand L14. Through further screening, we found that the use of 1 equivalent of AgO2CnBu and raising reaction temperature to 90 °C increased the yield to 81% (see supporting information).
Table 3.
Ligand development.[a]
Reaction conditions: 0.1 mmol of substrate, 0.4 mmol of PhI (45 μL), 0.01 mmol of Pd(OAc)2 (10 mol%), 0.01 mmol of Ligand (10 mol%), 0.3 mmol of AgOAc, 0.5 mL of t-AmylOH, 10 mL sealed tube, 80 °C (oil), 20 h. Yield was determined by the 1H NMR spectroscopy using CH2Br2 as the internal standard.
0.1 mmol of substrate, 0.4 mmol of ArI, 0.01 mmol of Pd(OAc)2 (10 mol%), 0.01 mmol of Ligand (10 mol%), 0.2 mmol of AgOAc, 0.1 mmol of AgO2CnBu, 0.5 mL of t-AmylOH, 10 mL sealed tube, 90 °C (oil), 20 h.
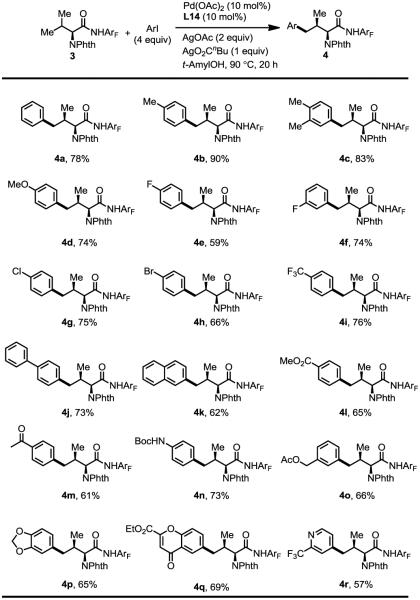
Variously substituted aryl iodides are compatible with this ligand-enabled γ-C–H arylation reaction (Table 4). Aryl iodides containing methyl and methoxy groups reacted well under the standard conditions giving the products in good to excellent yields (4a–4d). Fluoro, chloro, bromo and trifluoromethyl substituents produced moderate to good yields of modified valine derivatives. These halide substituents could be used for further transformation (4e–4i). 4-iodo-1,1'-biphenyl and 2-iodonaphthalene worked well in the reaction (4j–4k). A range of common functional groups including ester, ketone and acetate are also compatible affording 61~73% of the desired products (4l–4o). A number of heterocyclic moieties are also successfully attached to valine via this arylation reaction (4p–4r).
Table 4.
Arylation of valine.[a]
Reaction conditions: 0.1 mmol of substrate, 0.4 mmol of ArI, 0.01 mmol of Pd(OAc)2 (10 mol%), 0.01 mmol of L14 (10 mol%), 0.2 mmol of AgOAc, 0.1 mmol of AgO2CnBu, 0.5 mL of t-AmylOH, 10 mL sealed tube, 90 °C (oil), 20 h. The yields are isolated yields. dr > 20:1 in all examples.
4-Aryl isoleucine 6 could also be prepared by arylation of γ-C–H bond of isoleucine in good yield (Scheme 1). Arylation of tert-leucine produced 30% of mono-arylated product (8a) and 44% of diarylated product (8b). In these arylation reactions, the stereochemistry of the α-chiral center is maintained (ee > 98%, see supporting information). In addition, high diastereoselectivity (dr > 20:1) is obtained in the arylation reaction, due to the favored trans-conformation and disfavored cis-conformation regarding the methyl and phthalimide groups (Figure 1). The amide auxiliary could be converted to thioester in high yield using LiSEt (Eq. 5). Alternatively, formation of the carbamate followed by treatment with LiOH/H2O2 affords the carboxlic acid 10 (Eq. 6). The phthalimide group is readily removed by treating with ethylenediamine to generate the free amine and subsequently protected with Boc2O to give 11.
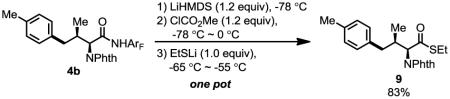 |
(5) |
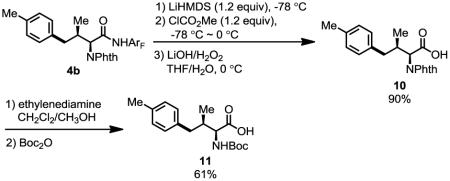 |
(6) |
Scheme 1.
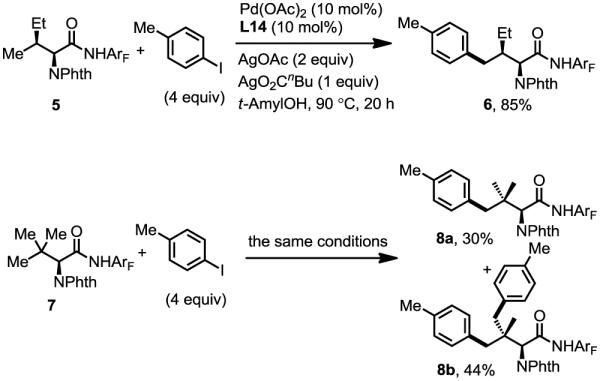
Arylation of amino acids.
Figure 1.
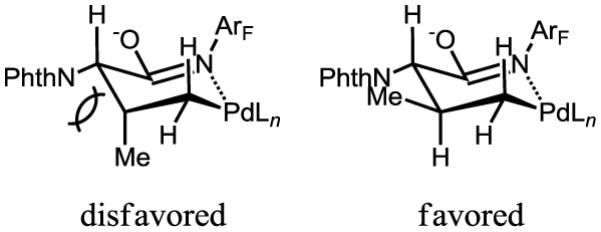
The origin of the high diastereoselectivity.
In summary, we have developed a protocol for γ-arylation of simple aliphatic acids containing β-quaternary carbon centers, as well as amino acids including valine, isoleucine and tert-leucine. The use of tricyclic quinoline ligands is crucial for the reactivity, providing useful information for developing more efficient ligand that will enable γ-C-H activation.
Supplementary Material
Footnotes
We gratefully acknowledge The Scripps Research Institute and the NIH (NIGMS, 2R01GM084019) for financial support.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201xxxxxx.
References
- [1].Engle KM, Yu J-Q. J. Org. Chem. 2013;78:8927. doi: 10.1021/jo400159y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].For selected examples, see: Wang D-H, Yu J-Q. J. Am. Chem. Soc. 2011;33:5767. doi: 10.1021/ja2010225.; Dai H-X, Stepan AF, Plummer MS, Zhang Y-H, Yu J-Q. J. Am. Chem. Soc. 2011;133:7222. doi: 10.1021/ja201708f.; Rosen BR, Simke LR, Thuy-Boun PS, Dixon DD, Yu J-Q, Baran PS. Angew. Chem. Int. Ed. 2013;52:7317. doi: 10.1002/anie.201303838.; Yang G, Lindovska P, Zhu D, Kim J, Wang P, Tang R-Y, Movassaghi M, Yu J-Q. J. Am. Chem. Soc. 2014;136:10807. doi: 10.1021/ja505737x.; Wencel-Delord J, Glorius F. Nature Chem. 2013;5:369. doi: 10.1038/nchem.1607.
- [3].For selected examples, see: Giri R, Chen X, Yu J-Q. Angew. Chem. Int. Ed. 2005;44:2112. doi: 10.1002/anie.200462884.; Giri R, Liang J, Lei J-G, Li J-J, Wang D-H, Chen X, Naggar IC, Guo C, Foxman BM, Yu J-Q. Angew. Chem. Int. Ed. 2005;44:7420. doi: 10.1002/anie.200502767.; Zaitsev VG, Shabashov D, Daugulis O. J. Am. Chem. Soc. 2005;127:13154. doi: 10.1021/ja054549f.; Giri R, Maugel NL, Li J-J, Wang D-H, Breazzano SP, Saunders LB, Yu J-Q. J. Am. Chem. Soc. 2007;129:3510. doi: 10.1021/ja0701614.; Wang D-H, Wasa M, Giri R, Yu J-Q. J. Am. Chem. Soc. 2008;130:7190. doi: 10.1021/ja801355s.; Yoo EJ, Wasa M, Yu J-Q. J. Am. Chem. Soc. 2010;132:17378. doi: 10.1021/ja108754f.; Shabashov D, Daugulis O. J. Am. Chem. Soc. 2010;132:3965. doi: 10.1021/ja910900p.; Hasegawa N, Charra V, Inoue S, Fukumoto Y, Chatani N. J. Am. Chem. Soc. 2011;133:8070. doi: 10.1021/ja2001709.; Nadres ET, Santos GIF, Shabashov D, Daugulis O. J. Org. Chem. 2013;78:9689. doi: 10.1021/jo4013628.; Zhang S-Y, Li Q, He G, Nack WA, Chen G. J. Am. Chem. Soc. 2013;135:12135. doi: 10.1021/ja406484v.; Chen F-J, Zhao S, Hu F, Chen K, Zhang Q, Zhang S-Q, Shi B-F. Chem. Sci. 2013;4:4187.; Shang R, Ilies L, Matsumoto A, Nakamura E. J. Am. Chem. Soc. 2013;135:6030. doi: 10.1021/ja402806f.; Zhou L, Lu W. Org. Lett. 2014;16:508. doi: 10.1021/ol403393w.; Aihara Y, Chatani N. J. Am. Chem. Soc. 2014;136:898. doi: 10.1021/ja411715v.; Wu X, Zhao Y, Ge H. J. Am. Chem. Soc. 2014;136:1789. doi: 10.1021/ja413131m.; Chen G, Shigenari T, Jain P, Zhang Z, Jin Z, He J, Li S, Mapelli C, Miller MM, Poss MA, Scola PM, Yeung K-S, Yu J-Q. J. Am. Chem. Soc. 2015;137:3338. doi: 10.1021/ja512690x.
- [4].For selected examples, see: He G, Chen G. Angew. Chem. Int. Ed. 2011;50:5192. doi: 10.1002/anie.201100984.; He G, Zhao Y, Zhang S, Lu C, Chen G. J. Am. Chem. Soc. 2012;134:3. doi: 10.1021/ja210660g.; Zhang S-Y, He G, Nack WA, Zhao Y, Li Q, Chen G. J. Am. Chem. Soc. 2013;135:2124. doi: 10.1021/ja312277g.; Zhang S-Y, He G, Zhao Y, Wright K, Nack WA, Chen G. J. Am. Chem. Soc. 2012;134:7313. doi: 10.1021/ja3023972.; Rodríguez N, Romero-Revilla JA, Fernández-Ibáñez MÁ, Carretero JC. Chem. Sci. 2013;4:175.; Fan M, Ma D. Angew. Chem. Int. Ed. 2013;52:12152. doi: 10.1002/anie.201306583.; Chan KSL, Wasa M, Chu L, Laforteza BN, Miura M, Yu J-Q. Nature Chem. 2014;6:146. doi: 10.1038/nchem.1836.
- [5].a) Desai LV, Hull KL, Sanford MS. J. Am. Chem. Soc. 2004;126:9542. doi: 10.1021/ja046831c. [DOI] [PubMed] [Google Scholar]; b) Kang T, Kim Y, Lee D, Wang Z, Chang S. J. Am. Chem. Soc. 2014;136:4141. doi: 10.1021/ja501014b. [DOI] [PubMed] [Google Scholar]; c) Ren Z, Mo F, Dong G. J. Am. Chem. Soc. 2012;134:16991. doi: 10.1021/ja3082186. [DOI] [PubMed] [Google Scholar]; d) Thompson SJ, Thach DQ, Dong G. J. Am. Chem. Soc. 2015;137:11586. doi: 10.1021/jacs.5b07384. [DOI] [PubMed] [Google Scholar]
- [6].Reddy BVS, Reddy LR, Corey EJ. Org. Lett. 2006;8:3391. doi: 10.1021/ol061389j. [DOI] [PubMed] [Google Scholar]
- [7].a) He G, Zhang S-Y, Nack WA, Li Q, Chen G. Angew. Chem. Int. Ed. 2013;52:11124. doi: 10.1002/anie.201305615. [DOI] [PubMed] [Google Scholar]; b) He G, Zhang S-Y, William AN, Ryan P, Javon R-L, Chen G. Org. Lett. 2014;16:6488. [Google Scholar]
- [8].Li S, Chen G, Feng C-G, Gong W, Yu J-Q. J. Am. Chem. Soc. 2014;136:5267. doi: 10.1021/ja501689j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wasa M, Chan KSL, Zhang X-G, He J, Miura M, Yu J-Q. J. Am. Chem. Soc. 2012;134:18570. doi: 10.1021/ja309325e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].For insightful discussions on the conformation of 2- methoxypyridine: Chein R-J, Corey EJ. Org. Lett. 2010;12:132. doi: 10.1021/ol9025364.
- [11].He J, Jiang H, Takise R, Zhu R-Y, Chen G, Dai H-X, Dhar TGM, Shi J, Zhang H, Cheng PTW, Yu J-Q. Angew. Chem. Int. Ed. 2016;55:785. doi: 10.1002/anie.201509996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].For selected examples, see: Dangel BD, Johnson JA, Sames D. J. Am. Chem. Soc. 2001;123:8149. doi: 10.1021/ja016280f.; Li Q, Zhang S-Y, He G, Nack WA, Chen G. Adv. Synth. Catal. 2014;356:1544.; Tran LD, Daugulis O. Angew. Chem. Int. Ed. 2012;51:5188. doi: 10.1002/anie.201200731.; Aspin S, Goutierre A-S, Larini P, Jazzar R, Baudoin O. Angew. Chem. Int. Ed. 2012;51:10808. doi: 10.1002/anie.201206237.; Zhang L-S, Chen G, Wang X, Guo Q-Y, Zhang X-S, Pan F, Chen K, Shi Z-J. Angew. Chem. Int. Ed. 2014;53:3899. doi: 10.1002/anie.201310000.; Zhang Q, Chen K, Rao W, Zhang Y, Chen F-J, Shi B-F. Angew. Chem. Int. Ed. 2013;52:13588. doi: 10.1002/anie.201306625.; Chen K, Shi B-F. Angew. Chem. Int. Ed. 2014;53:11950. doi: 10.1002/anie.201407848.. Zhang Q, Yin X-S, Chen K, Zhang S-Q, Shi B-F. J. Am. Chem. Soc. 2015;137:8219. doi: 10.1021/jacs.5b03989.; Chen K, Zhang S-Q, Jiang H-Z, Xu J-W, Shi B-F. Chem. Eur. J. 2015;21:3264. doi: 10.1002/chem.201405942.; He J, Li S, Deng Y, Fu H, Laforteza BN, Spangler JE, Homs A, Yu J-Q. Science. 2014;343:1216. doi: 10.1126/science.1249198.; Zhu R-Y, He J, Wang X-C, Yu J-Q. J. Am. Chem. Soc. 2014;136:13194. doi: 10.1021/ja508165a.; Zhu R-Y, Tanaka K, Li G-C, He J, Fu H-Y, Li S, Yu J-Q. J. Am. Chem. Soc. 2015;137:7067. doi: 10.1021/jacs.5b04088.
- [13].Gong W, Zhang G, Liu T, Giri R, Yu J-Q. J. Am. Chem. Soc. 2014;136:16940. doi: 10.1021/ja510233h. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.