Abstract
BACKGROUND & AIMS
Patients with cholestatic disease have increased systemic concentrations of bile acids (BAs) and profound pruritus. The G-protein–coupled BA receptor 1 TGR5 (encoded by GPBAR1) is expressed by primary sensory neurons; its activation induces neuronal hyperexcitability and scratching by unknown mechanisms. We investigated whether the transient receptor potential ankyrin 1 (TRPA1) is involved in BA-evoked, TGR5-dependent pruritus in mice.
METHODS
Co-expression of TGR5 and TRPA1 in cutaneous afferent neurons isolated from mice was analyzed by immunofluorescence, in situ hybridization, and single-cell polymerase chain reaction. TGR5-induced activation of TRPA1 was studied in in HEK293 cells, Xenopus laevis oocytes, and primary sensory neurons by measuring Ca2+ signals. The contribution of TRPA1 to TGR5-induced release of pruritogenic neuropeptides, activation of spinal neurons, and scratching behavior were studied using TRPA1 antagonists or Trpa1−/− mice.
RESULTS
TGR5 and TRPA1 protein and messenger RNA were expressed by cutaneous afferent neurons. In HEK cells, oocytes, and neurons co-expressing TGR5 and TRPA1, BAs caused TGR5-dependent activation and sensitization of TRPA1 by mechanisms that required Gβγ, protein kinase C, and Ca2+. Antagonists or deletion of TRPA1 prevented BA-stimulated release of the pruritogenic neuropeptides gastrin-releasing peptide and atrial natriuretic peptide B in the spinal cord. Disruption of Trpa1 in mice blocked BA-induced expression of Fos in spinal neurons and prevented BA-stimulated scratching. Spontaneous scratching was exacerbated in transgenic mice that overexpressed TRG5. Administration of a TRPA1 antagonist or the BA sequestrant colestipol, which lowered circulating levels of BAs, prevented exacerbated spontaneous scratching in TGR5 overexpressing mice.
CONCLUSIONS
BAs induce pruritus in mice by co-activation of TGR5 and TRPA1. Antagonists of TGR5 and TRPA1, or inhibitors of the signaling mechanism by which TGR5 activates TRPA1, might be developed for treatment of cholestatic pruritus.
Keywords: Liver, Mouse Model, Itching, Signal Transduction
Itch induces the protective reflex of scratching that removes irritants and parasites from the skin. However, chronic itch that is associated with disease (eg, hepatic, renal, neurological disorders, eczema, certain cancers) is debilitating, poorly understood, and difficult to treat.1 A deeper understanding of the mechanisms of disease-associated itch is required to develop more effective therapies.
Pruritogens induce itch by activating G-protein–coupled receptors (GPCRs) and transient receptor potential (TRP) ion channels on cutaneous afferent nerve endings. Histamine from mast cells activates the histamine H1 receptor that, via phospholipase-C signaling, sensitizes TRP vanilloid 1 (TRPV1), which is necessary for neuronal excitation and scratching.2 Although antagonists of the H1 receptor are useful therapies for this acute allergic itch, antihistamines are ineffective for chronic itch. Pruritogens that induce histamine-independent itch include the anti-malarial drug chloroquine, which activates the Mas-related GPCR MrgprA3, the endogenous peptide pruritogen BAM8-22, which activates MrgprC11,3 and proteases that activate protease-activated receptor-2.4 MrgprA3 and MrgprC11 respectively activate Gβγ and phospholipase-C signaling pathways that sensitize TRP ankyrin 1 (TRPA1), which is required for the acute excitatory and pruritogenic actions of chloroquine and BAM8-22.5 TRPA1 is also necessary for the exacerbated scratching of mice with dry skin.6 However, the contribution of TRPA1 to acute and chronic itch that is induced by disease-relevant endogenous pruritogens has not been studied, and the contribution of TRPA1 to the release of neuropeptides that transmit itch, including gastrin-releasing peptide (GRP)7 and natriuretic polypeptide B (NPPB),8 is unknown.
We recently identified the bile acid (BA) GPCR TGR5 as a major mediator of BA-evoked scratching in mice.9 Patients with cholestatic liver disease, which is characterized by deficient bile secretion into the intestine, can develop chronic pruritus that is so severe and intractable that it is an indication for liver transplantation.10 BAs may cause cholestatic pruritus because BA levels are increased in the circulation11 and skin12 of patients with cholestasis, BA chelators relieve cholestatic pruritus,13 and application of BAs to the skin causes itch.14 TGR5 is expressed by primary afferent neurons, and BAs induce neuronal excitability, stimulate release of GRP in the spinal cord, and evoke scratching by TGR5-dependent mechanisms.9 The role of TRPA1 in BA-evoked itch is unexplored.
Here we report on a major role for TGR5-dependent activation of TRPA1 in the pruritogenic actions of exogenous BAs, and define the importance of this pathway for the actions of a disease-relevant endogenous pruritogen.
Materials and Methods
See Supplementary Material for sources of animals and materials, and for detailed methods.
Animals
Institutional Animal Ethics Committees approved all studies.
Retrograde Tracing
1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI, 2%, 10 μL, intradermal) was injected to the nape of the neck and mice were recovered for 7 days.
Immunofluorescence, In Situ Hybridization
Dorsal root ganglia (DRG, C1-C7) were fixed, sectioned, and processed to detect TGR5, NeuN, and HuC/D by indirect immunofluorescence9 and TRPA1 by in situ hybridization.15
Single-Cell Reverse Transcription Polymerase Chain Reaction
DRG were dissociated and individual DiI-positive small diameter (<25 μm) neurons were aspirated.9 Polymerase chain reactions used intron-spanning mouse primers for TGR5, TRPA1, TRPV1, or β-actin.
Cell Lines
HEK293 cells stably expressing human TGR5 have been described.16 HEK293 cells expressing human TRPA1 alone or together with TGR5 were generated using a tetracycline-inducible system.
[Ca2+]i Assays in HEK Cells
HEK cells were loaded with Fura-2/AM, and fluorescence of populations of cells was measured (340/380 nm excitation, 530 nm emission). Cells were challenged with deoxycholic acid (DCA) (100 μM, 5 minutes) and then allyl isothiocyanate (AITC, 100 μM, 5 minutes). Cells were incubated with H89 (10 μM, protein kinase A [PKA], PKA inhibitor), GF109203X (10 μM, protein kinase C [PKC], PKC inhibitor), gallein (100 μM, Gβγ inhibitor), HC-030031 (100 μM, TRPA1 antagonist), or vehicle (control) (60-minute preincubation and inclusion throughout).
Isolation and Culture of Dorsal Root Ganglia Neurons
DRG from all spinal levels were dissociated and maintained for 24 hours before assays.9
[Ca2+]i Assays in Dorsal Root Ganglia Neurons
Neurons were loaded with Fura-2/AM and fluorescence of individual small diameter neurons (<25 μm) was measured (340/380 nm excitation, 530 nm emission). Neurons were challenged sequentially with AITC (100 μM), DCA (100 μM), capsaicin (1 μM), and KCl (50 mM). Neurons were incubated with GF109203X (10 μM), gallein (100 μM), HC-030031 (100 μM), or vehicle (control) (30- or 60-minute preincubation and inclusion throughout), or were assayed in Ca2+-free buffer containing 2 mM EDTA.
Extracellular Signal–Regulated Kinase 1/2 Activation in Dorsal Root Ganglia Neurons
Neurons were serum-starved for 6 hours, challenged with DCA (100 μM) or phorbol 12,13-dibutyrate (200 nM, positive control) for 30 minutes at 37°C, and lysed. Extracellular signal–regulated kinase 1/2 activation was assessed using AlphaScreen phosphor–extracellular signal–regulated kinase assay.
Adenosine 3′,5′-Cyclic Monophosphate Accumulation in Dorsal Root Ganglia Neurons
Neurons were serum-starved for 6 hours, incubated in activation buffer (Dulbecco's modified Eagle medium, 5 mM HEPES, 0.1% bovine serum albumin, 1 mM 3-isobutyl-1-methylxanthine) for 45 minutes, challenged with DCA (100 μM) or forskolin (10 μM, positive control) for 30 minutes at 37°C, and lysed. Adenosine 3′,5′-cyclic monophosphate accumulation was assessed using AlphaScreen cAMP Assay Kit (PerkinElmer, Waltham, MA).
Transient Receptor Potential Ankyrin 1 Currents in Xenopus laevis Oocytes
Oocytes were injected with complementary RNA encoding human TRPA1 alone (0.5 ng) or both TRPA1 (0.5 ng) plus humanTGR5 (2 ng). Oocytes were studied after 2 days by 2-electrode voltage-clamp.17 AITC (50 μM) and HC-030031 (15 μM) were used to activate and inhibit TRPA1 currents, respectively. DCA (500 μM) was used to activate TGR5.
Neuropeptide Release
Slices of rat spinal cord with attached dorsal roots (combined cervical, thoracic, lumbar-sacral segments) were prepared and superfused with Krebs solution.9 Slices were stimulated with taurolithocholic acid (TLCA) (500 μM), AITC (100 μM), or vehicle for 60 minutes. Some tissues were superfused with HC-030031 (50 μM) or vehicle wiping behavior for 20 minutes before and during the stimulus. GRP and NPPB release were determined by enzyme immunoassays, and peptide concentrations were calculated as fmol/g tissue wet weight.
c-fos in Spinal Neurons
Mice were sedated (isoflurane) and DCA (25 μg, 10 μL, subcutaneous [SC]) or vehicle (0.9% NaCl) was injected into the nape of the neck. They were either allowed to recover from sedation of were kept sedated to exclude scratching-induced activation of spinal neurons. Some groups were pretreated with HC-030031 (100 mg/kg per os [PO]) or vehicle (100 μL) 30 minutes before DCA. At 60 minutes after DCA, mice were anesthetized and transcardially fixed. Frozen sections of cervical spinal cord were made and processed to detect β-galactosidase.18 β-galactosidase (c-fos)–positive cells in dorsal horn laminae I-III were counted by an observer unaware of the treatment.
Scratching and Nociception
Behavior was assessed in mice by an investigator unaware of treatment groups.9,19 Mice were sedated (isoflurane) and DCA (25 μg, 10 μL, SC) was injected into the nape of the neck. Some groups were pretreated with HC-030031 (100 mg/kg, PO, in 2% dimethyl sulfoxide, 20% cyclodextrin), AMG-9810 (40 mg/kg, intraperitoneal, in 0.9% saline) or vehicle (100 μL) 30 minutes before DCA injection. Scratching behavior was recorded approximately 2 hours after food withdrawal for 60 minutes after DCA injection.9 The effectiveness of AMG-9810 as a TRPV1 antagonist was determined by its capacity to suppress pain-related wiping behavior in response to capsaicin (3 μg, 10 μL, SC) into the cheek.19
Bile Acid Sequestrant
Tgr5-tg mice were treated with the BA sequestrant colestipol hydrochloride (2.5 mg/kg, PO) or vehicle (0.9% NaCl, PO) at 8:00 AM and 2:00 PM for 5 days. After the final dose, spontaneous scratching behavior was recorded for 60 minutes. Some mice received HC-030031 (100 mg/kg) or vehicle 30 minutes before scratching behavior was recorded.
Plasma Bile Acids
Tgr5-tg mice treated with colestipol or vehicle were killed at the end of the scratching assays. Blood was collected at approximately 4 hours after food withdrawal by cardiac puncture for assay of total plasma BAs.
Statistics
Results are expressed as mean ± SEM. Data were compared statistically using a Student t test (2 groups) or analysis of variance and Bonferroni or Tukey-Kramer post-hoc test (multiple groups). P < .05 was considered significant.
Results
TGR5 is Co-expressed With Transient Receptor Potential Ankyrin 1 in a Population of Primary Afferent Neurons That Innervate the Skin
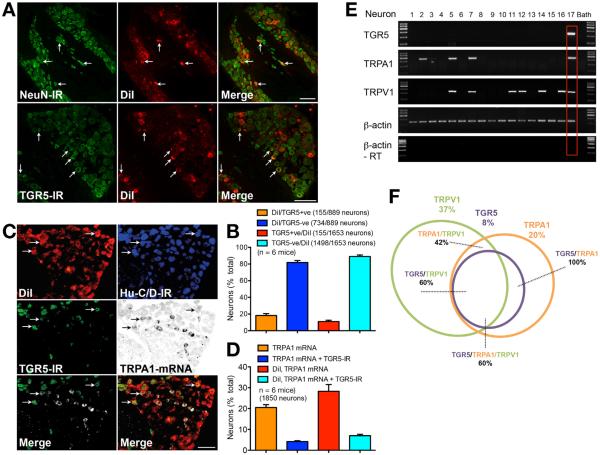
To identify DRG neurons that innervate the skin, we injected the retrograde tracer DiI intradermally (nape of neck) in mice. TGR5 was detected using an antibody that specifically detects TGR5 in small-diameter neurons of wild-type but not tgr5−/− mice.9 TGR5 and the pan-neuronal markers NeuN or Hu-C/D were localized by immunofluorescence, and TRPA1 was detected by in situ hybridization. All DiI-containing cells expressed NeuN immunoreactivity (IR) or Hu-C/D-IR, and are thus neurons (Figure 1). TGR5-IR was detected in a subpopulation of small-diameter (<25 μm) neurons that contained DiI, although some DiI-containing neurons did not express TGR5-IR (Figure 1A and B). Of the DiI-containing neurons, 18.2% ± 2.4% expressed TGR5-IR and 81.8% ± 2.4% did not express detectable TGR5 (889 neurons, n = 6 mice) (Figure 1B). TGR5-IR and TRPA1 messenger RNA (mRNA) were colocalized in a proportion of DiI-containing neurons (Figure 1C). TRPA1 mRNA was detected in 20.5% ± 1.4% and TGR5-IR and TRPA1 mRNA were co-expressed in 4.2% ± 0.4% of all DRG neurons (1850 neurons, n = 6 mice) (Figure 1D). TRPA1 mRNA was detected in 28.3% ± 3.2% and TGR5-IR and TRPA1 mRNA were co-expressed in 7.0% ± 0.7% of cutaneous afferent neurons (Figure 1D). By using single-cell reverse transcription polymerase chain reaction, we detected transcripts corresponding to TGR5 (236 bp), TRPA1 (393 bp), and TRPV1 (229 bp) in DiI-containing neurons (Figure 1E). Analysis of 60 small-diameter DiI-containing neurons from 6 mice revealed that 37% (22 of 60) expressed TRPV1, 20% (12 of 60) expressed TRPA1, and 8% (5 of 60) expressed TGR5 (Figure 1F). All TGR5-positive neurons (5 of 5) co-expressed TRPA1.
Figure 1.
Localization and expression of TGR5-IR, NeuN-IR, and TRPA1 mRNA in cutaneous afferent neurons. (A) Co-localization of NeuN-IR and TGR5-IR in DiI-positive cutaneous afferent DRG neurons (arrows). Scale bar = 100 μm. (B) Proportion of DiI-positive neurons with (TGR5-positive) or without (TGR5-negative) co-expression of TGR5-IR (889 or 1653 neurons, 6 mice). (C) Co-localization of Hu-C/D-IR, TGR5-IR, and TRPA1 mRNA in DiI-positive cutaneous afferent DRG neurons (arrows). Merged images show TGR5-IR + TRPA1 mRNA (left) and DiI + TGR5-IR + TRPA1 mRNA (right). Scale bar = 100 μm. (D) Proportion all neurons or of DiI-positive neurons expressing TGR5-IR alone or TGR5-IR + TRPA1 mRNA (1850 neurons, 6 mice). (E) Single-cell reverse transcription polymerase chain reaction of small-diameter, DiI-positive, cutaneous afferent neurons. Transcripts of TGR5, TRPA1, TRPV1, and β-actin were amplified. Results from 17 neurons are shown (60 neurons, 6 mice). Red box denotes co-expression of TGR5, TRPA1, and TRPV1. RT, reverse transcriptase. (F) Venn diagram indicating the proportion of small-diameter, Di-positive cutaneous afferent neurons expressing TRG5, TRPA1, and TRPV1.
TGR5 Activates and Sensitizes Transient Receptor Potential Ankyrin 1 in HEK293 Cells by a Gβγ- and Protein Kinase C-Mediated Mechanism
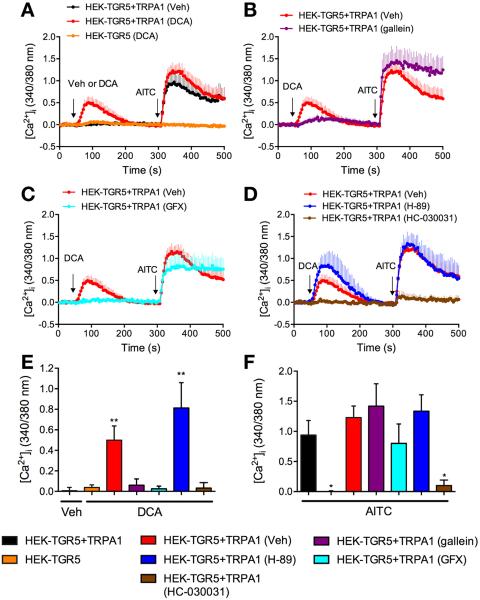
To examine the functional interactions between TGR5 and TRPA1, we expressed in HEK293 cells TGR5 and TRPA1 separately or together. We assessed TRPA1 activation by measuring [Ca2+]i. Cells were challenged with vehicle or DCA (100 μM), a TGR5 agonist, and 5 minutes later were stimulated with the TRPA1 agonist AITC (100 μM). In HEK-TGR5 cells, vehicle, DCA, or AITC did not affect [Ca2+]i (Figure 2A, E, and F). In contrast, in HEK-TGR5+TRPA1 cells, DCA but not vehicle induced a prompt increase in [Ca2+]i (Figure 2A and E). The response of HEK-TGR5+TRPA1 cells to AITC was larger in cells pretreated with DCA when compared with vehicle (Figure 2A and F). The observation that DCA increases [Ca2+]i in HEK-TGR5+TRPA1, but not in HEK-TGR5 cells indicates that TGR5 can activate TRPA1 to induce an influx of extracellular Ca2+ ions. In support of this proposal, DCA-evoked increases in [Ca2+]i in HEK-TGR5+TRPA1 cells were abolished by the TRPA1 antagonist HC-030031 (100 μM) (Figure 2D and F). The finding that DCA-pretreatment amplified the response of HEK-TGR5+TRPA1 cells to AITC suggests that activation of TGR5 by DCA can also sensitize TRPA1. DCA did not increase [Ca2+]i in HEK-TRPA1 cells, indicating that DCA cannot directly activate TRPA1 (not shown).
Figure 2.
BA and TGR5-induced activation and sensitization of TRPA1 in HEK293 cells. (A–D). [Ca2+]i measured in HEK-TGR5 and HEK-TGR5+TRPA1 cells treated with vehicle (Veh) or DCA (100 μM) and then AITC (100 μM). Some cells were treated with gallein (B), GF-109203X (GFX, C), H-89 or HC-030031 (D). (E, F) Pooled results showing maximal increase in [Ca2+]i over basal. Triplicate measurements, 3–8 independent experiments; *P < .05 compared with vehicle (E) or AITC (F) in HEK-TGR5 + TRPA1 cells. **P < .001.
GPCRs activate and sensitize TRP channels via Gα and Gβγ signaling. Gβγ subunits can directly open certain channels,20 and Gβγ mediates MrgprA3-evoked activation of TRPA1.5 Gα can activate PKA and PKC, which phosphorylate and activate TRP channels.21 The Gβγ inhibitor gallein (100 μM) and the PKC inhibitor GF109203X (10 μM) inhibited DCA-evoked increases in [Ca2+]i in HEK-TGR5+TRPA1 cells (Figure 2B, C, and E). In contrast, gallein and GF109203X did not prevent DCA-induced sensitization of the response to AITC (Figure 2B, C, and F). Thus, Gβγ and are required for TGR5-induced activation but not sensitization of TRPA1. The PKA inhibitor H89 (10 μM) amplified DCA-evoked increases in [Ca2+]i in HEK-TGR5+TRPA1 cells without affecting DCA-induced sensitization of the response to AITC (Figure 2D, E, and F). PKA may desensitize TGR5, which couples to adenosine 3′,5′-cyclic monophosphate and PKA.22
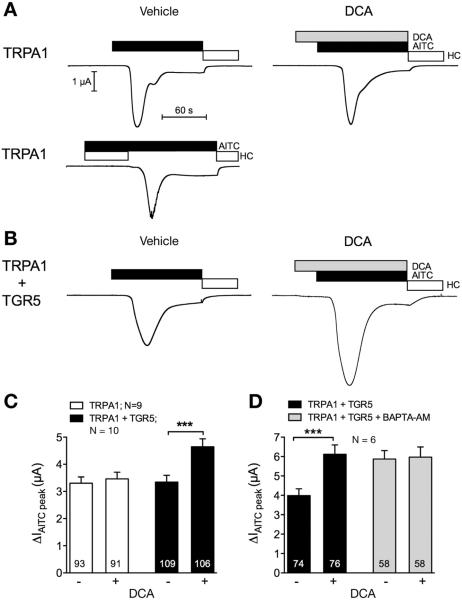
TGR5 Stimulates Transient Receptor Potential Ankyrin 1 Currents in Xenopus laevis Oocytes
In oocytes expressing TRPA1 alone or TRPA1 and TGR5, AITC (50 μM) elicited a transient inward current that was prevented or reversed by HC-030031 (15 μM) consistent with activation of TRPA1 (Figure 3A and B). To determine whether activation of TGR5 sensitizes TRPA1 currents, oocytes co-expressing TRPA1 and TGR5 were exposed to DCA (500 μM) or vehicle (control) for 30 seconds before AITC. Preincubation with DCA resulted in an approximately 40% increase of the AITC-evoked TRPA1 current compared with vehicle-treated oocytes (Figure 3C). In contrast, DCA did not increase the AITC current response in oocytes expressing TRPA1 alone. Thus, the stimulatory effect of DCA on TRPA1 currents requires co-expression of TGR5, and DCA does not directly activate TRPA1. Chelation of intracellular Ca2+ ions using BAPTA-AM (100 μM) prevented DCA-evoked sensitization of TRPA1, which is Ca2+-dependent (Figure 3D). BAPTA-AM increased the baseline AITC response by unknown mechanisms.
Figure 3.
BA and TGR5-induced activation of TRPA1 currents in Xenopus laevis oocytes. Oocytes expressing TRPA1 alone or TRPA1 and TGR5 were stimulated with the TRPA1 agonist AITC (50 μM, black bar) and the TRPA1 antagonist HC-030031 (open bar). TGR5 was activated by DCA (500 μM, gray bar). (A, B) Representative whole-cell current traces of oocytes expressing TRPA1 (A) or TRPA1 and TGR5 (B). Oocytes were treated with vehicle or DCA 30 seconds before AITC. (C, D) Change in AITC currents (ΔIAITC peak) in oocytes treated with DCA (+) or vehicle (−) (C). ΔIAITC peak in oocytes preincubated for 3 hours in BAPTA-AM or vehicle and subsequently treated with DCA (+) or vehicle (−) (D). Numbers inside the columns indicate the number of individual oocytes measured. N indicates the number of batches of oocytes. ***P < .001.
TGR5 Activates Transient Receptor Potential Ankyrin 1 in Primary Afferent Neurons by a Gβγ-and Protein Kinase C-Mediated Mechanism
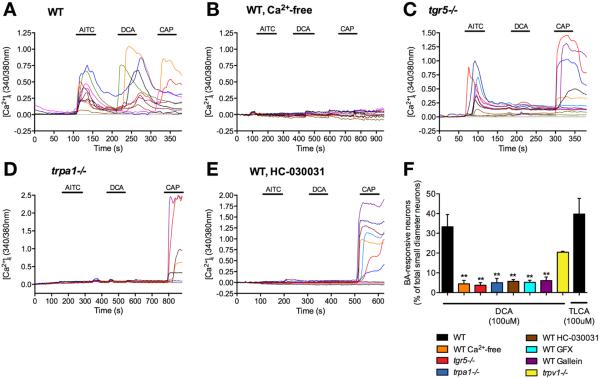
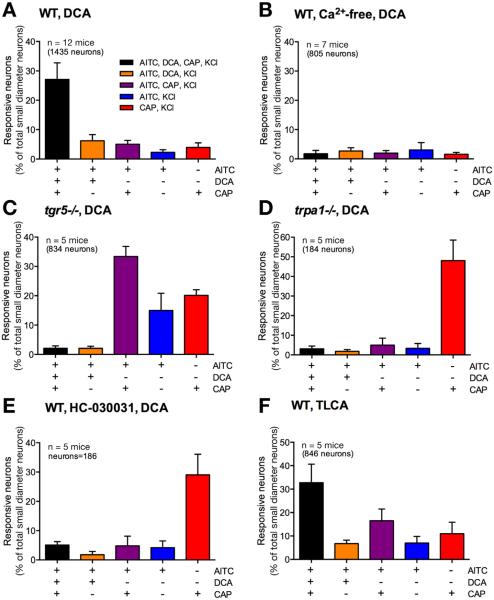
DCA (100 μM) stimulated generation of adenosine 3′,5′-cyclic monophosphate and activation of extracellular signal-regulated kinase 1/2 in DRG neurons isolated from wild-type but not tgr5−/− mice (Supplementary Figure 1A and B), which indicates that DCA signals to DRG neurons by a TGR5-dependent process. To assess the interactions between TGR5 and TRP channels in DRG neurons, we measured [Ca2+]i in individual neurons, and determined the magnitude of responses and the proportions of responsive neurons. Neurons were sequentially challenged with the TRPA1 agonist AITC (100 μM), the TGR5 agonists DCA or TLCA (100 μM), the TRPV1 agonist capsaicin (1 μM), and finally with KCl (50 mM). In wild-type mice, AITC, DCA, and capsaicin all caused a robust increase in [Ca2+]i in a subpopulation of neurons (Figure 4A). Of all small-diameter KCl-responsive neurons, 27.1% ± 5.6% responded to DCA and 32.8% ± 7.8% responded to TLCA (1435 neurons from 12 mice, 846 neurons from 5 mice, respectively) (Figure 4F). Removal of extracellular Ca2+ ions prevented AITC-, DCA-, and capsaicin-induced increases in [Ca2+]i (Figure 4B and F). The responses to AITC and capsaicin were retained in neurons from tgr5−/− mice, and TGR5 deletion abolished the response to DCA (Figure 4C and F; Supplementary Figure 2A). In neurons from trpa1−/− mice, the responses to both AITC and DCA were absent, and the response to capsaicin was retained (Figure 4D and F; Supplementary Figure 2A). In wild-type mice, the TRPA1 antagonist HC-030031 similarly prevented DCA- and AITC- but not capsaicin-evoked increases in [Ca2+]i (Figure 4E and F). The Gβγ-inhibitor gallein and the PKC inhibitor GF109203X both prevented DCA-evoked increases in [Ca2+]i in neurons from wild-type mice (Figure 4F, Supplementary Figure 2B and C). GF109203X, but not gallein, also inhibited the AITC response in neurons, but neither affected to capsaicin response. In neurons from trpv1−/− mice, the responses to AITC and DCA were preserved, and the response to capsaicin was absent (Figure 4F; Supplementary Figure 2D). Trpv1 deletion reduced the proportion of DCA-responsive neurons, but the change was not significant.
Figure 4.
TGR5- and TRPA1-dependent BA signaling in DRG neurons. [Ca2+]i was measured in small diameter neurons from wild-type (WT) mice (A), WT mice in Ca2+-free medium (B), tgr5−/− mice (C), trpa1−/− mice (D), and WT mice treated with the TRPA1 antagonist HC-030031 (E). They were challenged sequentially with AITC (100 μM), DCA (100 μM) and capsaicin (CAP, 1 μM). Responses of KCl (50 mM)-responsive neurons are shown. (F) Pooled data showing the proportion of DCA- or TLCA-responsive neurons in the different experimental groups. In (A–E), each line represents a single neuron. (F) Pooled data from 2281 neurons, n = 17 mice. **P < .01 to DCA in WT.
In wild-type mice, 33.3% ± 6.2% of AITC-responsive neurons also responded to DCA. In addition, 27.1% ± 5.6% of wild-type neurons responded to AITC, DCA, and capsaicin, whereas 6.2% ± 2.1% of neurons responded to AITC and DCA but not capsaicin (Figure 5A). Removal of extracellular Ca2+ ions suppressed responses to AITC, DCA, and capsaicin (Figure 5B). Deletion of TGR5 and deletion or antagonism of TRPA1 abolished responses to DCA (Figure 5C, D, and E). TRPA1 deletion and antagonism also blocked responses to AITC (Figure 5D and E). In wild-type mice, 32.8% ± 7.8% of neurons responded to AITC, TLCA, and capsaicin, and 6.8% ± 1.4% of neurons responded to AITC and TLCA but not capsaicin (Figure 5F).
Figure 5.
The proportion of DRG neurons responding to BAs and agonists of TRP channels. [Ca2+]i was measured in small diameter neurons from wild-type (WT) mice (A, F), WT mice in Ca2+-free medium (B), tgr5−/− mice (C), trpa1−/− mice (D), WT mice treated with the TRPA1 antagonist HC-030031 (E). They were challenged sequentially with AITC (100 μM), DCA (A–E) or TLCA (F) (both 100 μM), capsaicin (CAP, 1 μM), and finally KCl (50 mM). The proportion of small-diameter neurons that responded to the combinations of AITC, DCA or TLCA, and capsaicin are shown. Pooled data from 184–1435 neurons, n = 5–12 mice.
DCA and TLCA stimulate TGR5, which activates TRPA1 via Gβγ and PKC to promote the influx of extracellular Ca2+ ions in small-diameter DRG neurons of mice.
Bile Acids Induce Transient Receptor Potential Ankyrin 1–Dependent Release of the Itch Transmitters Gastrin-Releasing Peptide and Natriuretic Polypeptide B
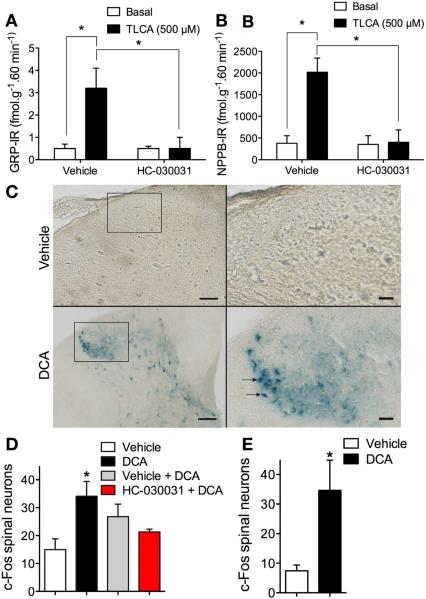
Pruritogens stimulate release of the itch-selective transmitters GRP and NPBB in the dorsal horn of the spinal cord by unknown mechanisms.7,8 To study neuropeptide release, we superfused slices of rat dorsal spinal cord with vehicle or the TGR5 agonist TLCA (500 μM, 60 minutes) and measured release of GRP and NPPB by enzyme-linked immunosorbent assay. Rats provided an adequate amount of tissue to reproducibly measure peptide release, and tissues were stimulated with TLCA, which is a more potent TGR5 agonist and stimulant of neuropeptide release than DCA.9,23,24 In vehicle-treated tissues, TLCA stimulated a 6.4-fold release of GRP-IR and a 5.3-fold release of NPPB-IR over baseline (Figure 6A and B). The TRPA1 agonist AITC (100 μM, 60 minutes) stimulated release of calcitonin gene-related peptide–IR, GRP-IR and NPPB-IR (Supplementary Figure 3A–C). HC-030031 (50 μM) abolished TLCA- and AITC-stimulated neuropeptide release. Thus, TRPA1 activity is necessary for BA-evoked release of pruritogenic peptides in the spinal cord.
Figure 6.
Contributions of TRPA1 to BA-evoked release of pruritogenic neuropeptides and activation of spinal neurons. (A, B) Release of GRP-IR (A) and NPPB-IR (B) from superfused slices of rat spinal cord under basal conditions and after stimulation with TLCA (500 μM, 60 minutes). Tissues were preincubated with vehicle or HC-030031 (50 μM). n = 4; *P < .05. (C–E) Expression of c-fos in neurons in the dorsal horn of c-fos reporter mice. (C) Photomicrographs of the dorsal horn of the spinal cord (C1–C7) 60 minutes after injection of DCA (25 μg, SC) or vehicle to the nape of the neck of conscious mice. Scale bar, left panel = 100 μm; right panel = 25 μm. (D) Quantification of c-fos–expressing neurons in superficial laminae of the spinal cord (C1–C7) 60 minutes after injection of DCA or vehicle. (E) Quantification of c-fos–expressing neurons 60 minutes after injection of DCA or vehicle in anesthetized mice. Subgroups of the DCA-treated mice were pretreated with HC-030031 or vehicle 30 minutes before DCA. n = 3 mice; *P < .05 to vehicle.
Bile Acids Activate Spinal Neurons by a Transient Receptor Potential Ankyrin 1–Dependent Mechanism
To determine whether cutaneous BAs pruritogens activate spinal neurons, we studied transgenic mice in which the c-fos promoter controls expression of a tau-lacZ reporter construct (FTL mice). DCA (25 μg, SC) or vehicle (control) was injected into the nape of the neck of sedated mice. Mice were either allowed to regain consciousness or were kept sedated, and 60 minutes later the cervical spinal cord was removed for analysis of β-galactosidase in the dorsal horn. In conscious mice receiving vehicle, β-galactosidase was detected in few neurons (Figure 6C and D). In DCA-treated mice, β-galactosidase was detected in the soma and processes of numerous neurons in laminae I, II, and III of the dorsal horn, as well as in some deeper laminae (Figure 6C and D). The number of β-galactosidase-positive neurons in superficial laminae increased from 15 ± 3.8 in vehicle-treated mice to 34 ± 5.3 in DCA-treated mice (P < .05, n = 3 mice). HC-030031 (100 mg/kg, PO), administered 30 minutes before DCA, prevented DCA-evoked activation of spinal neurons (Figure 6D). DCA similarly induced β-galactosidase in superficial spinal neurons of anesthetized mice, which indicates that scratching was not necessary for c-fos induction (Figure 6E). Thus, cutaneous DCA activates spinal neurons, and TRPA1 is required for the central transmission of the pruritogenic signal.
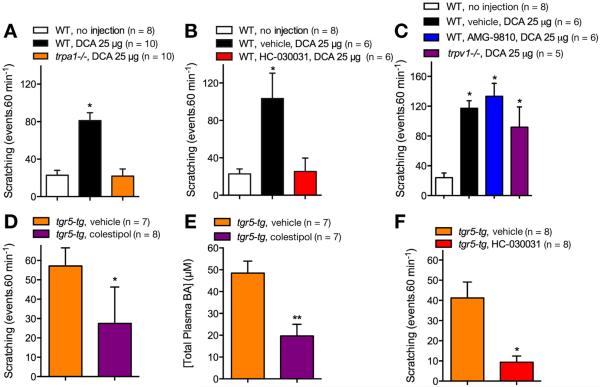
Bile Acids Cause Scratching by Transient Receptor Potential Ankyrin 1–Dependent Mechanisms
To evaluate the contribution of TRPA1 to BA-stimulated scratching, we injected DCA (25 μg, SC) into the nape of the neck in wild-type or trpa1−/− mice, and quantified scratching for 60 minutes. In wild-type mice, DCA stimulated a 4-fold increase in scratching over baseline (basal, 22.7 ± 5.2 scratches/60 min; DCA, 81.2 ± 8.3 scratches/60 min; P < .0001, n = 8–10 mice) (Figure 7A). DCA did not cause scratching in trpa1−/− mice (21.9 ± 7.6 scratches/60 min, n = 10 mice). To examine the therapeutic potential of a TRPA1 antagonist to treat BA-evoked pruritus, we administered the TRPA1 antagonist HC-030031 (100 mg/kg, PO) or vehicle (control) to wild-type mice 30 minutes before DCA. HC-030031 prevented DCA-evoked scratching (vehicle, 103.3 ± 27.2 scratches/60 min; HC-030031, 25.3 ± 14.4 scratches/60 min; P < .0001, n = 6 mice) (Figure 7B). In contrast, the TRPV1 antagonist AMG-9810 (40 mg/kg, intraperitoneal) did not affect DCA-evoked scratching (vehicle, 117.2 ± 10.03 scratches/60 min; AMG-9810, 133.3 ± 17.3 scratches/60 min; P = .4383, n = 6 mice) (Figure 7C). AMG-9810 prevented cheek-wiping responses to capsaicin (3 μg, SC), suggesting effective antagonism of TRPV1-mediated nociceptive behavior (vehicle, 46.4 ± 7.2 wipes/20 min; AMG-9810, 10 ± 3.8 wipes/20 min; P < .005, n = 5 mice) (Supplementary Figure 4). Thus, DCA causes scratching in mice by a mechanism that requires expression and activation of TRPA1 but not TRPV1.
Figure 7.
Contributions of TRPA1 to scratching evoked by exogenous and endogenous BAs. (A–C) DCA-evoked scratching in wild-type (WT) and trpa1−/− mice. DCA was injected to the nape of the neck (25 μg SC) and scratching was measured for 60 minutes in WT mice and trpa1−/− mice (A), in wild-type mice pretreated with HC-030031 or vehicle (B), and in wild-type mice pretreated with AMG-9810 or vehicle and in trpv1−/− mice (C). (D–F) Scratching and serum BAs levels in tgr5-tg mice. tgr5-tg mice were treated with colestipol or vehicle for 5 days before measurement of scratching (D) and plasma concentrations of total BAs (E). Scratching was also measured in tgr5-tg mice pretreated with HC-030031 or vehicle (F). n = 6–10 mice; *P < .05, **P < .01 to untreated wild-type mice (A–C) or vehicle (D–F).
Endogenous Bile Acids and Transient Receptor Potential Ankyrin 1 Can Induce Spontaneous Pruritus
Transgenic mice overexpressing mouse TGR5 (tgr5-tg) exhibit elevated TGR5 expression in tissues know to express TGR525 and demonstrate exacerbated spontaneous scratching by unknown mechanisms.9 Because the circulating levels of BAs fluctuate during feeding and fasting,26 we evaluated whether endogenous circulating BAs could be responsible for the exacerbated scratching in tgr5-tg mice. We treated tgr5-tg mice with the BA sequestrant colestipol (100 mg/kg, twice a day) or vehicle (control) by gavage for 5 days, and then assessed scratching behavior. Vehicle-treated tgr5-tg mice scratched 2.6-fold more frequently than wild-type mice (wild-type, 22.7 ± 5.7 scratches/60 min; tgr5-tg, 57.1 ± 9.4 scratches/60 min; P < .005, n = 7–8 mice) (Figure 7D). Colestipol reduced scratching of tgr5-tg mice by 2.1-fold (tgr5-tg, 57.1 ± 9.4 scratches/60 min; colestipol, 27.5 ± 6.6 scratches/60 min; P < .05 compared with vehicle-treated tgr5-tg mice, n = 7–8 mice) (Figure 7D). Colestipol also caused a 2.5-fold reduction in the plasma concentrations of total BAs (P < .01 compared with vehicle, n = 7 mice) (Figure 7E). HC-030031 strongly inhibited scratching of tgr5-tg mice when compared with vehicle (vehicle, 41.3 ± 7.8 scratches/60 min; HC-030031, 9.4 ± 3.0 scratches/60 min; P < .01 compared to vehicle-treated tgr5-tg mice, n = 8 mice) (Figure 7F). Thus, endogenous circulating BAs and TRPA1 are responsible for exacerbated spontaneous scratching in tgr5-tg. These results highlight the pathophysiological importance of TGR5- and TRPA1-induced itch caused by endogenous BAs.
Discussion
Our results reveal an essential role for TGR5-induced activation of TRPA1 for the pruritogenic actions of exogenous and endogenous BAs. We report that TGR5 and TRPA1 are co-expressed by a subpopulation of cutaneous afferent neurons, and that TGR5 activates TRPA1 via Gβγ and PKC (Supplementary Figure 5). TRPA1 is necessary for BA-evoked release of the pruritogenic neuropeptides in the spinal cord, activation of spinal neurons, and scratching behavior. A major finding is that the exacerbated scratching in mice overexpressing TGR5 is driven by endogenous BAs and requires TRPA1 activation.
TGR5 Activates and Sensitizes Transient Receptor Potential Ankyrin 1
We found that approximately 7% of cutaneous afferent neurons co-expressed TGR5 and TRPA1, determined by retrograde tracing, immunofluorescence, in situ hybridization, and single-cell reverse transcription polymerase chain reaction. BAs induced Ca2+ signals only in those DRG neurons that responded to the TRPA1 agonist AITC, confirming co-expression of functional TGR5 and TRPA1.
Our results show that BAs, via TGR5, activate and sensitize TRPA1. Although TGR5 does stimulate Gαq-dependent mobilization of intracellular Ca2+ stores, BAs increased [Ca2+]i in HEK cells and DRG neurons co-expressing TGR5 and TRPA1. This effect was abolished by TRPA1 antagonism or deletion. Thus, BAs stimulate TGR5, which in turn activates TRPA1 to cause an influx of extracellular Ca2+ ions. Gallein and GF109203X attenuated BA-evoked activation of TRPA1 in HEK-TGR5+TRPA1 cells and in DRG neurons, which implicates Gβγ and PKC in TGR5-evoked activation of TRPA1. Gβγ also mediates MrgprA3-induced activation of TRPA1 in NG108 cells,5 either by directly binding to TRPA120 or through other pathways. PKC can also mediate bradykinin-evoked activation of TRPV1, which in turn activates TRPA1 by a Ca2+-dependent process.27 However, we did not observe a major role for TRPV1 in BA-evoked Ca2+ signaling in DRG neurons. Alternatively, activated TGR5 could generate TRPA1 agonists, which remains to be investigated.
TGR5 can also sensitize TRPA1, because pretreatment of oocytes expressing TGR5 and TRPA1 with DCA amplified AITC-elicited TRPA1 currents. Chelation of intracellular Ca2+ ions in oocytes prevented this sensitization, which is consistent with the known ability of Ca2+ ions to activate TRPA1.28 DCA did not alter TRPA1 currents in oocytes expressing TRPA1 alone or increase [Ca2+]i in HEK-TRPA1 cells. BAs cannot directly regulate TRPA1.
Transient Receptor Potential Ankyrin 1 Is Required for TGR5-Dependent Itch
We report that TRPA1 is necessary for the central transmission of the pruritogenic signals from cutaneous BAs. TLCA stimulated GRP and NPPB release in the spinal cord, consistent with involvement of GRP in TGR5-dependent scratching.9 HC-030031 prevented TLCA-stimulated GRP and NPPB release, which reveals a key role for TRPA1 in BA-induced release of neuropeptides that mediate itch transmission in the dorsal horn. TRPA1 deletion and antagonism also prevented cutaneous DCA-evoked activation of spinal neurons, revealed by c-fos expression, and scratching. Although TGR5 was co-expressed in primary sensory neurons with both TRPA1 and TRPV1, TRPV1 antagonism or deletion had no effect on DCA-evoked scratching. This result is consistent with the retention of DCA-evoked Ca2+ signaling in neurons from trpv1−/− mice. With regard to its dependency for activation of TRPA1, but not TRPV1, for neuronal activation and scratching, TGR5 resembles MrgprA3 and MrgprC11, which also activate TRPA1 to induce itch.5
A key finding is that the BA sequestrant colestipol inhibits exacerbated spontaneous scratching in gain-of-function tgr5-tg mice. BAs that are secreted into the intestinal lumen during feeding are absorbed in the ileum and colon, and the circulating levels of BAs wax and wane during feeding and fasting.26 Our observation that treatment of tgr5-tg mice with a BA sequestrant reduced both spontaneous scratching and circulating BA levels by approximately 50% suggests that endogenous BAs are sufficient to drive exacerbated scratching in mice that overexpress TGR5. HC-030031 reduced spontaneous scratching of tgr5-tg mice to that observed under basal conditions in wild-type mice, which reveals a requirement for TRPA1 for the pruritogenic actions of endogenous BAs.
Contributions of Bile Acid, TGR5, and Transient Receptor Potential Ankyrin 1 to Cholestatic Pruritus
The contribution of BAs to cholestatic pruritus is debatable. There are increased circulating and tissue levels of BAs in cholestatic patients,11,12 injection of BAs into the skin causes scratching in humans,14 and BA sequestrants are a treatment for cholestatic pruritus.13 However, scratching severity does not correlate with circulating BAs levels in patients with cholestatic disease, and other factors have been implicated in cholestatic itch, including lysophosphatidic acid and opioids.29,30 Our finding that tgr5-tg mice exhibit spontaneous pruritus that depends on endogenous BAs suggests that the pathological up-regulation of TGR5 alone, in the absence of elevated levels of BAs, may be sufficient to induce pruritus. Whether TGR5 is up-regulated in patients with pruritus remains to be examined. We found that TRPA1 antagonism prevents exacerbated scratching of tgr5-tg mice. Thus, TRPA1 antagonists, in addition to TGR5 antagonists, may be considered to be new treatments for pruritus that accompanies liver diseases and other conditions, such as pregnancy, where certain up-regulated progesterone metabolites can also activate TGR5.31 These possibilities deserve further attention. Given the intractable nature of the severe pruritus that accompanies hepatic diseases, potentially leading to liver transplantation, the TGR5/TRPA1 pathway may be a therapeutic target for the histamine-independent chronic itch in which BAs are involved.
Supplementary Material
Acknowledgments
The authors thank Tao Yu and Cameron Nowell for technical assistance.
Funding Supported by NHMRC 63303, 1049682, 1031886 and Monash University (NWB).
Abbreviations used in this paper
- AITC
allyl isothiocyanate
- BA
bile acid
- DiI
dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate
- DCA
deoxycholic acid
- DRG
dorsal root ganglia
- GPCR
G-protein–coupled receptor
- GRP
gastrin-releasing peptide
- IR
immunoreactivity
- mRNA
messenger RNA
- NPPB
natriuretic polypeptide B
- PKA
protein kinase A
- PKC
protein kinase C
- PO
per os
- SC
subcutaneous
- TLCA
taurolithocholic acid
- TRP
transient receptor potential
- TRPA1
transient receptor potential ankyrin 1
- TRPV1
transient receptor potential vanilloid 1
Footnotes
Supplementary Material To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2014.08.042.
Conflicts of interest The authors disclose no conflicts.
References
- 1.Ikoma A, Steinhoff M, Stander S, et al. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- 2.Imamachi N, Park GH, Lee H, et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Q, Tang Z, Surdenikova L, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinhoff M, Neisius U, Ikoma A, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23:6176–6180. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson SR, Gerhold KA, Bifolck-Fisher A, et al. TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat Neurosci. 2011;14:595–602. doi: 10.1038/nn.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson SR, Nelson AM, Batia L, et al. The ion channel TRPA1 is required for chronic itch. J Neurosci. 2013;33:9283–9294. doi: 10.1523/JNEUROSCI.5318-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 8.Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–971. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alemi F, Kwon E, Poole DP, et al. The TGR5 receptor mediates bile acid-induced itch and analgesia. J Clin Invest. 2013;123:1513–1530. doi: 10.1172/JCI64551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51:237–267. doi: 10.1016/j.jhep.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Magyar I, Loi HG, Feher T. Plasma bile acid levels and liver disease. Acta Med Acad Sci Hung. 1981;38:109–115. [PubMed] [Google Scholar]
- 12.Schoenfield LJ, Sjorvall J, Perman E. Bile acids on the skin of patients with pruritic hepatobiliary disease. Nature. 1967;213:93–94. [Google Scholar]
- 13.Mela M, Mancuso A, Burroughs AK. Review article: pruritus in cholestatic and other liver diseases. Aliment Pharmacol Ther. 2003;17:857–870. doi: 10.1046/j.1365-2036.2003.01458.x. [DOI] [PubMed] [Google Scholar]
- 14.Kirby J, Heaton KW, Burton JL. Pruritic effect of bile salts. Br Med J. 1974;4:693–695. doi: 10.1136/bmj.4.5946.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho HJ, Callaghan B, Bron R, et al. Identification of enteroendocrine cells that express TRPA1 channels in the mouse intestine. Cell Tiss Res. 2014;356:77–82. doi: 10.1007/s00441-013-1780-x. [DOI] [PubMed] [Google Scholar]
- 16.Poole DP, Godfrey C, Cattaruzza F, et al. Expression and function of the bile acid receptor GpBAR1 (TGR5) in the murine enteric nervous system. Neurogastroenterol Motil. 2010;22:814–825. e227–e818. doi: 10.1111/j.1365-2982.2010.01487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haerteis S, Krappitz M, Diakov A, et al. Plasmin and chymotrypsin have distinct preferences for channel activating cleavage sites in the gamma subunit of the human epithelial sodium channel. J Gen Phys. 2012;140:375–389. doi: 10.1085/jgp.201110763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson Y, Nag N, Davern P, et al. Visualization of functionally activated circuitry in the brain. Proc Natl Acad Sci U S A. 2002;99:3252–3257. doi: 10.1073/pnas.042701199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dascal N. Ion-channel regulation by G proteins. TEM. 2001;12:391–398. doi: 10.1016/s1043-2760(01)00475-1. [DOI] [PubMed] [Google Scholar]
- 21.Amadesi S, Cottrell GS, Divino L, et al. Protease-activated receptor 2 sensitizes TRPV1 by protein kinase Cepsilon- and A-dependent mechanisms in rats and mice. J Physiol. 2006;575:555–571. doi: 10.1113/jphysiol.2006.111534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen DD, Godfrey CB, Niklas C, et al. The bile acid receptor TGR5 does not interact with beta-arrestins or traffic to endosomes but transmits sustained signals from plasma membrane rafts. J Biol Chem. 2013;288:22942–22960. doi: 10.1074/jbc.M113.455774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawamata Y, Fujii R, Hosoya M, et al. A G protein-coupled receptor responsive to bile acids. J Biol Chem. 2003;278:9435–9440. doi: 10.1074/jbc.M209706200. [DOI] [PubMed] [Google Scholar]
- 24.Maruyama T, Miyamoto Y, Nakamura T, et al. Identification of membrane-type receptor for bile acids (M-BAR) Biochem Biophys Res Commun. 2002;298:714–719. doi: 10.1016/s0006-291x(02)02550-0. [DOI] [PubMed] [Google Scholar]
- 25.Thomas C, Gioiello A, Noriega L, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab. 2009;10:167–177. doi: 10.1016/j.cmet.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angelin B, Bjorkhem I. Postprandial serum bile acids in healthy man. Evidence for differences in absorptive pattern between individual bile acids. Gut. 1977;18:606–609. doi: 10.1136/gut.18.8.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bautista DM, Jordt SE, Nikai T, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 28.Jordt SE, Bautista DM, Chuang HH, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 29.Bergasa NV. The itch of liver disease. Semin Cutan Med Surg. 2011;30:93–98. doi: 10.1016/j.sder.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 30.Kremer AE, Martens JJ, Kulik W, et al. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology. 2010;139:1008–1018. 1018, e1001. doi: 10.1053/j.gastro.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 31.Papacleovoulou G, Abu-Hayyeh S, Nikolopoulou E, et al. Maternal cholestasis during pregnancy programs metabolic disease in offspring. J Clin Invest. 2013;123:3172–3181. doi: 10.1172/JCI68927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.