Abstract
Popeye domain containing (Popdc) proteins are a unique family, which combine several different properties and functions in a surprisingly complex fashion. They are expressed in multiple tissues and cell types, present in several subcellular compartments, interact with different classes of proteins, and are associated with a variety of physiological and pathophysiological processes. Moreover, Popdc proteins bind the second messenger cAMP with high affinity and it is thought that they act as a novel class of cAMP effector proteins. Here, we will review the most important findings about the Popdc family, which accumulated since its discovery about 15 years ago. We will be focussing on Popdc protein interaction and function in striated muscle tissue. However, as a full picture only emerges if all aspects are taken into account, we will also describe what is currently known about the role of Popdc proteins in epithelial cells and in various types of cancer, and discuss these findings with regard to their relevance for cardiac and skeletal muscle.
Keywords: Popeye domain containing genes, cAMP, Protein–protein interaction, Ion channel, Cardiac arrhythmia, Animal models
1. The Popeye domain containing gene family
The first member of the Popdc gene family was independently discovered by two groups through subtractive hybridisation aiming at the identification of novel transcripts with a cardiac muscle-restricted expression pattern (Andrée et al., 2000, Reese et al., 1999). Reese and colleagues named the novel gene Blood vessel epicardial substance (Bves), which was based on the expression pattern they observed by immunolabelling (Reese et al., 1999). Due to the high expression level in striated muscle tissue, Andrée et al. gave it the name Popeye1 (Pop1), which is a reference to the comic-strip hero “Popeye the sailor”, who is most famous for his supernatural muscle strength (Andrée et al., 2000). They also discovered two other related genes expressed in higher vertebrates, and referred to them as Pop2 and Pop3, in order to indicate their membership to the same gene family. Today, these genes are known as the Popeye domain containing (Popdc) genes Popdc1, Popdc2, and Popdc3 (Brand et al., 2014). In addition, the name Bves is still used as a synonym for Popdc1.
2. Evolutionary background
Popdc genes are found throughout the animal kingdom and are already present in Hydra and other Cnidaria indicating that their roots lie at the base of metazoan evolution (reviewed in Brand et al., 2014). Significantly, at the sequence level Popdc proteins are highly conserved suggesting that they have an important and essential role. In vertebrates three Popdc genes are present, while in lower chordates two genes are found (Brand, 2005). In man, Popdc1 and Popdc3 are found on human chromosome 6q21 as tandem arrayed genes, while Popdc2 is localised on human chromosome 3q13.33 (Andrée et al., 2000). The tandem array organisation of Popdc1 and Popdc3 genes is already present in lower chordates, suggesting that this genomic organisation may have some role at the gene regulatory level. However, it is noteworthy in this context that in addition to gene-specific transcripts a number of vertebrate species are predicted to generate also transcripts, which do not obey the gene boundaries. These transcripts would encode a Popdc3/Popdc1 fusion protein (Andrée et al., 2000), however, presently the functional significance thereof is unknown. Drosophila is unique in a having a single Popdc gene, while most invertebrates have two genes. Some species show a higher level of gene duplication (Brand et al., 2014). Interestingly, Popdc proteins show some sequence homology to the bacterial transcription factors Catabolite Activator Protein (CAP) and cAMP Receptor Protein (CRP), which are involved in metabolic regulation. It is therefore possible that these prokaryotic transcription factors and the transmembrane Popdc proteins, which are found in metazoans have a common evolutionary origin (Schindler et al., 2012, Simrick et al., 2013).
3. Expression pattern of Popdc proteins
All three members of the Popdc gene family are expressed in cardiac and skeletal muscle (Andrée et al., 2000, Breher et al., 2004). In the heart, Popdc1 expression in the embryonic heart is equally strong in both atrial and ventricular chambers, whereas postnatally, Popdc1 expression is weaker in ventricular compared to atrial myocardium. Popdc2 on the other hand is expressed at equal levels in both chamber types. The highest expression level for Popdc1 and Popdc2 is observed in the cardiac conduction system including the sinoatrial (SAN) and atrioventricular nodes (AVN) (Froese et al., 2012).
There still exists some controversy with regard to the cell types in the heart that express Popdc1. The first report of the cardiac Popdc1 expression pattern at the protein level by David Bader and colleagues described an expression in the epicardium and the coronary vasculature (Reese et al., 1999). However, β-galactosidase (LacZ) staining of tissues from a Popdc1-LacZ knockin mouse, revealed expression in cardiac myocytes and absence of staining in the epicardium, coronary arteries and other nonmuscle cell types (Andrée et al., 2002a). Moreover, immunohistochemistry, in situ hybridisation, and RT-PCR analysis of the chick and mouse heart did not reveal any expression in the proepicardium, epicardium or coronary vasculature (Andrée et al., 2002a, Andrée et al., 2002b, Torlopp et al., 2006).
It is noteworthy that in addition to the abundant expression in striated muscle tissue, Popdc1 is also present in smooth muscle cells of bladder, uterus, and the gastrointestinal tract, as well as in the brain, various epithelia, spinal ganglia, thymus, testes, stomach, lungs, kidneys, and spleen (Andrée et al., 2002a, Andrée et al., 2002b; Hager and Bader, 2009, Osler and Bader, 2004, Osler et al., 2006, Reese et al., 1999, Ripley et al., 2004, Smith and Bader, 2006, Torlopp et al., 2006, Vasavada et al., 2004). The expression pattern of Popdc2 has also been analysed and display strong overlap with Popdc1, however some differences have also been observed (Froese and Brand, 2008, Froese et al., 2012). Due to a lack of immunoreagents and appropriate mouse models, Popdc3 expression has not yet been extensively studied. However, preliminary data revealed an expression pattern similar to Popdc1 (Andrée et al., 2002a, Andrée et al., 2002b). Immunofluorescent analysis of the subcellular localisation of Popdc1 and Popdc2 proteins in cardiac myocytes established a strong labelling of the plasma membrane, with all three membrane compartments being labelled, i.e. the intercalated disk, the lateral membrane and the t-tubules (Froese et al., 2012).
4. Structure and biochemical properties of Popdc proteins
Popdc proteins are three-pass transmembrane proteins with a short extracellular amino-terminus, which contains up to two N-glycosylation sites (Andrée et al., 2000, Knight et al., 2003). Glycosylation is quite extensive in these proteins and significantly affecting the electrophoretic mobility in SDS-PAGE. Popdc1 for example runs at 58 kDa, while the protein sequence predicts a molecular weight of about 42 kDa. Interestingly, the extent of glycosylation and therefore electrophoretic mobility is tissue-dependent (Vasavada et al., 2004). Thus, POPDC1 protein isolated from chicken heart and skeletal muscle runs at 58 and 70 kDa, respectively. Possibly the size differences are based on tissue-specific regulation of glycosylation. The impact of glycosylation on Popdc function is presently unknown, however, it has been hypothesised that it may play a role in membrane localisation of Popdc proteins or protect them from proteolytic decay (Hager and Bader, 2009). Importantly, Popdc proteins form homodimers, which are stabilised by disulfide bonds and may be necessary for the maintenance of epithelial integrity and junctional stability (Hager and Bader, 2009). How homodimerisation is mediated is presently unclear. Although it has been previously reported that conserved lysines at the carboxy-terminal end of the Popeye domain of Popdc1 mediate homodimerisation (Kawaguchi et al., 2008), it was subsequently shown that Popdc1 protein lacking this sequence motif is still able to homodimerise, suggesting that there are probably also other protein domains involved, which have not yet been identified (Russ et al., 2011). The C-terminus of Popdc proteins is located in the cytoplasm and contains the Popeye domain (PFAM: PF04831), which consists of about 150 amino acids and shows high sequence conservation (Andrée et al., 2002a, Andrée et al., 2002b). The Popeye domain harbours a functional cyclic nucleotide binding domain (CNBD), which enables Popdc proteins to specifically bind to and be modulated by adenosine 3′,5′-cyclic monophosphate (cAMP). Popdc proteins probably do not bind guanosine 3′,5′-cyclic monophosphate (cGMP), since the affinities for both cyclic nucleotides differ by a factor of about 40 (Froese et al., 2012). Thus, Popdc proteins are one of only five classes of eukaryotic cAMP effector proteins, which, apart from protein kinase A (PKA), include exchange protein directly activated by cAMP (Epac), and hyperpolarisation-activated cyclic nucleotide-gated cation (HCN) channels (Rehmann et al., 2007). Recently, a sperm-specific novel cyclic nucleotide receptor (CRIS) has been reported (Krahling et al., 2013). Although the Popeye domain is predicted to be structurally similar to other cAMP binding domains, at the sequence level only very limited similarity is present. The actual phosphate binding cassette (PBC), which makes contact to the cyclic nucleotide is very different and does not resemble the PBC found in the other cAMP effector proteins (Brand et al., 2014). Using a radioligand binding assay and by FRET analysis, Froese and colleagues have demonstrated that the cAMP affinity of Popdc proteins is about 10-fold higher than that of Epac1 and similar to that of PKA (Froese et al., 2012). Charge-to-alanine mutations of an invariant aspartate residue (D200 in Popdc1 and D184 in Popdc2), which is part of the ultra-conserved DSPE sequence motif present in most POPDC proteins and thought to be part of the CNBD, eradicated cAMP binding, suggesting that this residue is crucial for cyclic nucleotide binding (Froese et al., 2012). Carboxy-terminal to the Popeye domain is a sequence, which is variable in length amongst Popdc family members. In Popdc1 this carboxy-terminal sequence is rich in acidic amino acids and contains an array of serine/threonine residues, which are subject to phosphorylation after β-adrenergic stimulation (Lundby et al., 2013).
5. Functional impact of the Popeye domain containing protein family
5.1. Popdc1 is involved in cell–cell contact formation and regulates epithelial function
Popdc1 has been shown to be an essential component of tight junctions and to be important for proper epithelial function. In mature epithelia including murine small intestine epithelium, Popdc1 was found to co-localise with constituents of the tight junction complex such as occludin, and a direct interaction of Popdc1 and ZO-1 has been established by GST pull-down (Osler et al., 2005). It has been hypothesised that via this protein–protein interaction Popdc1 plays an important role in the formation and maintenance of epithelial monolayers. In human corneal epithelial cells, Popdc1, presumably through interaction with ZO-1, sequesters GEF-H1, an activator of RhoA, and ZONAB/DbpA at tight junctions thereby regulating the junctional localisation of these proteins and their downstream signalling (Russ et al., 2011). Also in trabecular meshwork cells, Popdc1 inhibits RhoA signalling, leading to a decreased phosphorylation of myosin light chain, a downstream target of the RhoA-ROCK signalling pathway (Russ et al., 2010). It is thought that Popdc1 regulates trabecular meshwork cell contraction and thereby aqueous outflow and intraocular pressure in the eye. Furthermore, overexpression of Popdc1 causes an increase in tight junction formation and transepithelial resistance (Russ et al., 2010, Russ et al., 2011). While RhoA-ROCK signalling in the heart has been associated with several functions, including cardiac conduction and repolarization (Sugiyama et al., 2003), it is presently unknown whether Popdc proteins are modulating this pathway in the heart as well. In zebrafish an interaction of Popdc1 with atypical protein kinase C (aPKC) has recently been reported (Wu et al., 2012) and aPKC controls tight junctional integrity by recruiting and tethering components of the tight junction signalling complex. In addition to tight junctions, Popdc1 also regulates adherens junction formation in epithelial cells. In human corneal epithelial cells as well as colorectal cancer cells, E-cadherin expression and β-catenin localisation correlate with the level of Popdc1 expression (Williams et al., 2011). By modulating both, tight junctions and adherens junctions, Popdc1 has been implicated in the control of epithelial–mesenchymal transition (EMT) (Williams et al., 2011, Han et al., 2014). The important role of Popdc1 in cell–cell contact formation has also been established in the context of eye development (Ripley et al., 2004, Wu et al., 2014). Interestingly, Popdc1 is rapidly recruited to cell–cell contact sites, and forced expression of Popdc1 in non-adherent L-cells increased adhesiveness (Wada et al., 2001). It was proposed that Popdc1 might act as a cell adhesion molecule. However, the extracellular domain of the Popdc proteins is only 20–40 residues long, it is therefore unlikely that Popdc proteins are directly involved in establishing cell–cell adhesion. However, it is possible, that adhesion is mediated indirectly through interacting proteins, or that the extensive glycosylation of Popdc proteins is of importance in this context.
During early Drosophila embryogenesis, the epithelial function of Popdc1 seems to be important. Knockdown of Popdc1 (DmBves) in Drosophila causes impaired pole cell migration and abnormal gastrulation (Lin et al., 2007). Likewise, in Xenopus, Popdc1 morpholino injection caused a developmental arrest at gastrulation (Ripley et al., 2006). However in contrast to these reports, zebrafish popdc1 or popdc2 morphants revealed no gastrulation phenotype (Kirchmaier et al., 2012, Schindler et al., 2016). Moreover, the lack of an embryonic phenotype in the Popdc1 or Popdc2 null mutant in mice do not support an essential function of Popdc genes during early development (Andrée et al., 2002b, Froese et al., 2012). However, it cannot be ruled out that Popdc genes show some species-specific functional differences.
Although Popdc function in contact structures has not yet been studied in cardiac tissue, it is reasonable to assume that this protein family is also involved in the regulation of intercellular junctions in the heart. The intercalated disks (ICD) are the contact structures that mediate mechanical and electrical coupling between myocytes. They consist of adherens junctions, desmosomes, and gap junctions (Palatinus et al., 2011). An important constituent of the ICD is the Popdc1-associated protein ZO-1 (Osler et al., 2005). ZO-1 interacts with the main components of both adherens junctions such as N-cadherin and gap junctions such as Connexin43 as well as with the actin cytoskeleton, and is important for the structural organisation of intercalated disks (Palatinus et al., 2011). Given the fundamental role of intercalated discs for the function of cardiomyocytes, as well as pathologies associated with dysfunctions of intercalated disk constituents, the role of Popdc proteins in this context deserves further studies.
In addition to modulating cell–cell adhesion Popdc1 also regulates epithelial morphogenesis and cell movements in epithelia. With the help of a yeast two-hybrid screen and GST pull-down, GEFT has been established as an interaction partner of Popdc1 (Smith et al., 2008). A domain, which seems to be necessary but not sufficient to mediate interaction has been mapped to an intracellular portion between aa 250 and aa 300 that partially overlaps with the Popeye domain of Popdc1 (Smith et al., 2008). GEFT, an N-terminally truncated isoform of p63RhoGEF, is a guanine nucleotide exchange factor (GEF) for Rho-family GTPases Rac1 and Cdc42, activating these small G proteins by catalysing the exchange of GDP to GTP. GEFT is highly expressed in brain, heart and skeletal muscle. Overexpression of GEFT leads to a re-organisation of the actin cytoskeleton and alteration in cell morphology and cell migration. GEFT overexpression also promotes cell proliferation and may therefore have a role in tumourigenesis (Guo et al., 2003, Lutz et al., 2004). Transfection of a truncated Popdc1 construct lacking the transmembrane domains into NIH3T3 cells resulted in a reduced activation of Rac1 and Cdc42, but not of RhoA. Furthermore, cells transfected with Popdc1 showed a more prominent roundness and exhibited a reduced speed of cell locomotion compared to controls without alteration of directionality of cell movement. Interestingly, GEFT overexpression in C2C12 cells induced differentiation (Bryan et al., 2005), whereas the transfection of a carboxy-terminal Popdc1 construct resulted in reduced differentiation of C2C12 cells (Smith et al., 2008). However, it has not been shown that this observation was a direct result of altered GEFT and Rac1/Cdc42 activity induced by the carboxy-terminus of Popdc1. Therefore, the impact of Popdc1-GEFT function in muscle cells remains unknown. Furthermore, it is presently unclear by which mechanism Popdc1 modulates GEFT. Direct control of GEFT and its nucleotide binding ability, sequestering of GEFT and therefore preventing it from activating its downstream effectors, as well as regulating proper subcellular localisation of GEFT have been proposed as potential mechanisms (Hager and Bader, 2009, Smith et al., 2008). Although there is no experimental proof as yet, it is plausible to assume that Popdc1 interacts with the p63RhoGEF protein. In the heart, p63RhoGEF protein is predominantly expressed in non-myocytes, however, it is also present at the I-band of the sarcomere in cardiomyocytes (Souchet et al., 2002, Wuertz et al., 2010). In this context it is noteworthy that Popdc proteins have been associated with structural abnormalities in the sinus node of Popdc1 and Popdc2 null mutant mice (Froese et al., 2012).
5.2. Popdc1 controls vesicular transport and fusion
Using CoIP and GST pull-down experiments Popdc1 has been established as a interacting partner of the vesicle-associated membrane proteins Vamp2 and Vamp3 (Hager et al., 2010). Vamp3, also known as cellubrevin, is a ubiquitously expressed vesicular SNARE protein that plays a role in vesicle fusion and in trafficking of several membrane proteins such as transferrin or β-integrin, and thereby regulates cell motility (Galli et al., 1994, Luftman et al., 2009, McMahon et al., 1993, Proux-Gillardeaux et al., 2005, Tayeb et al., 2005). Interestingly, overexpression of a dominant-negative form of Popdc1 in MDCK cells, containing only the first 118 amino acids of the protein (Bves118), resulted in decreased transferrin uptake and β1-integrin internalisation as well as in impaired exocytosis. Similar effects were also seen in Xenopus embryos by antisense morpholino-mediated depletion of either Popdc1 or Vamp3. As a consequence, cell spreading, a process, which depends on integrin recycling, was impaired in these models. Furthermore, Popdc1 knockdown led to morphological defects comparable to integrin depletion (Hager et al., 2010). It has therefore been concluded that Popdc1, via interaction with Vamp3, controls important cellular and physiological processes where vesicular transport is required. Intriguingly, in the heart, Vamp2 is involved in secretion of atrial natriuretic peptide (ANP) from atrial myocytes (Ferlito et al., 2010). Interestingly, also the Popdc protein interaction partner TREK-1 (see below) has been associated with ANP release (McGrath and de Bold, 2009).
A role for Popdc1 in vesicular trafficking is also supported by the fact that Popdc1 interacts with the N-myc Downstream Regulated Gene 4 (NDRG4) and thereby controls the fusion of Vamp3-positive recycling endosomes with the cell surface membrane. If this interaction was disrupted, directional movement of epicardial cells was randomised and accelerated due to interfering with the autocrine ECM deposition pathway, by which internalised fibronectin is recycled and eventually re-secreted (Benesh et al., 2013). It is noteworthy that the binding site for NDRG4 has been mapped to a region between amino acids 307 and 316 of the Popdc1 protein, which is outside of the Popeye domain and not shared by either Popdc2 or Popdc3 suggesting that probably only Popdc1 is able to interact with NDRG4 (Benesh et al., 2013).
5.3. Popdc genes and cancer
Several studies have demonstrated that POPDC1 and -3 expression is down-regulated in various types of cancer. This finding is consistent with the impact of POPDC1 on cell motility and cell adhesion, and its role in junctional structures and EMT, a hallmark of cancer and key process for invasiveness of tumours (Micalizzi et al., 2010). Even though at the first glance these findings may appear to be irrelevant for the heart, it is likely that there are overlapping functions of Popdc proteins in different tissue types. Moreover, some of the principal disease-causing mechanisms may be similar.
The POPDC1 promoter in tumour tissue from non-small cell lung cancer patients is significantly more frequently and at higher levels methylated in comparison to matched normal tissue resulting in reduced expression of POPDC1 in the cancerous tissue (Feng et al., 2008). Hypermethylation of the POPDC1 promoter was not detected in cancer-free lung tissue, including pre-cancerous lungs of tobacco smokers, suggesting a role of POPDC1 at advanced cancer stages (Feng et al., 2008, Salskov et al., 2011). Similarly, down-regulation of POPDC1 and -3, but not of POPDC2, has been revealed in many gastric cancer cell lines and in gastric tumour samples. Again, gene silencing by promoter hypermethylation as well as histone deacetylation was observed. In addition to promoter hypermethylation, also EGF treatment, which is known to promote cell migration and invasion by positive regulation of Rac1 and Cdc42, resulted in an immediate decrease of POPDC1 and -3 expression probably through repression by SnaiI, which is up-regulated after EGF stimulation (Kim et al., 2010a, Lee et al., 2008). Likewise, in hepatocellular cancer cell lines and tumour samples POPDC1 was found to be down-regulated (Han et al., 2014). Moreover, reduced POPDC1 expression was shown to increase the expression of mesenchymal markers such as vimentin, and to reduce epithelial proteins like E-cadherin, which was accompanied by a significant increase of the EMT-promoting transcription factors Snail1 and Twist1. Interestingly, in Drosophila the EGF homologue gurken suppresses Popdc1/DmBves expression in the dorsal follicular epithelium of the egg chamber (Lin et al., 2007). Similarly, down-regulation of Popdc1 and -3 but not of Popdc2 expression by EGF has been observed in rat neonatal cardiac myocytes (Parnes et al., 2007). It has been shown that loss of POPDC3 in gastric cancer leads to enhanced cell migration and invasion. This is accompanied by a reduction of ZO-1 and occludin expression. It has therefore been proposed that down-regulation of POPDC1 and -3 contributes to a loss of cell–cell contacts as well as to enhanced cell motility by up-regulation of Rac1 and Cdc42, resulting in increased cell migration and invasion (Kim et al., 2010a). Furthermore, it was demonstrated that POPDC1 expression is down-regulated via AKT activation by netrin-1 in hepatocellular carcinoma (Han et al., 2015). It can therefore be concluded that POPDC1 expression is regulated by several different signalling pathways. In addition, the regulation of GEFT, Vamp3, and NDRG4 by Popdc1 and its modulatory effects on cell shape and motility as well as on vesicle trafficking including integrin-dependent cell movements may mechanistically be involved in cancer progression (Benesh et al., 2013, Hager et al., 2010, Kim et al., 2010b). It is noteworthy that both, POPDC1 and POPDC3 showed a predominant cytoplasmic localisation in tumours. Thus, in addition to differences in expression levels, altered subcellular localisation is also supposed to play a role in gastric cancer (Luo et al., 2012). In addition to gastric and lung cancer, abnormal POPDC1 and -3 expression and its impact on tumourigenesis has been described for colorectal cancer (Williams et al., 2011) and uveal melanoma (Jayagopal et al., 2011).
5.4. Popdc proteins interact with ion channels
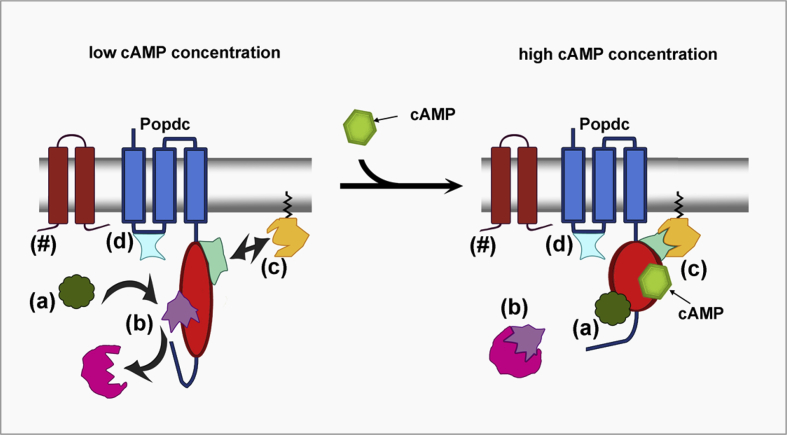
Due to the prominent expression of Popdc proteins at the plasma membrane it was hypothesised that Popdc proteins may modulate electrogenic proteins in cardiomyocytes. To test this hypothesis a screen was performed using Xenopus oocytes to analyse conductivity changes of ion channels in the presence of Popdc proteins. This led to the identification of the potassium channel TWIK-related K+ channel 1 (TREK-1) as novel Popdc interacting protein (PIP) and as the first protein interaction partner of Popdc2 and -3 (Froese et al., 2012). TREK-1 is a mechano-sensitive member of the two-pore domain potassium channel (K2P) family, which consists of 6 subfamilies: TWIK, TREK, TASK, TALK, THIK, and TRESK channels (Enyedi and Czirjak, 2010). K2P channels form dimers consisting of two subunits with each showing the arrangement of two pore-forming units that are flanked by two transmembrane helices. TREK-1 is a highly regulated protein that is sensitive to various stimuli including membrane stretch, intracellular pH, temperature, phosphorylation, and protein–protein interactions (reviewed in Honore, 2007). The main region by which these regulatory mechanisms are mediated is the so-called post-M4 regulatory region located in the C-terminal part of the protein just next to the fourth transmembrane domain. The post-M4 regulatory region also harbours a binding site for A kinase anchoring protein 150 (AKAP150). Binding of AKAP150 changes the conductivity of TREK-1 and modulates inhibition of the channel via Gs and Gq-coupled receptor signalling, with an acceleration of the Gs transduced inhibition and an increase of inhibition via Gq-coupled receptors (Sandoz et al., 2006). Moreover, TREK-1 is also regulated by the interaction with microtubule-associated protein 2 (Mtap2), which is involved in microtubule-dependent transport of the channel to the plasma membrane. Mtap2 and AKAP150 can bind to TREK-1 simultaneously and modulate the channel synergistically (Sandoz et al., 2008). Another interaction partner, which promotes surface localisation and whole-cell currents of TREK-1, is β-COP. This protein is part of the COPI complex and involved in vesicular transport. Interestingly, β-COP protein interaction is not mediated by the C-terminus of TREK-1 but by its N-terminus (Kim et al., 2010b). Intriguingly, co-expression of both Popdc1 (or Popdc2 or -3) and TREK-1 in the Xenopus oocyte system results in a current, which is about 2-fold higher than the current when TREK-1 is expressed alone. This involves direct physical interaction of TREK-1 and the C-terminus of Popdc proteins as demonstrated for Popdc1 by a GST pull-down (Froese et al., 2012). Similar to other CNBDs (Rehmann et al., 2007), the Popeye domain is supposed to undergo a conformational change upon cAMP binding. Likewise a bi-molecular sensor consisting of Popdc1-CFP and YFP-TREK-1 demonstrated direct physical interaction. Moreover, the FRET ratio obtained under baseline condition decreased after raising the intracellular cAMP levels with isoproterenol or forskolin, indicating a structural rearrangement of the sensor proteins to one another caused by a conformational change in the Popeye domain (Froese et al., 2012). This conformational change could possibly have several effects on Popdc proteins and their interaction partners (Fig. 1). It can be imagined that the conformational change induced by cAMP binding results in the exposure of a binding site on Popdc proteins, which is not accessible under baseline conditions. Conversely, other binding sites may only be accessible when no cAMP is bound and cAMP binding would result in a release of the PIP, which may then become accessible for other proteins including modulating enzymes, proteases, etc. Furthermore, it is possible that the conformational change in Popdc proteins may not alter the interaction with the PIP itself but may induce conformational rearrangements in directly interacting proteins, and thus enabling or disabling the binding of the PIP to a third protein. Therefore, cAMP binding may have effects not only on proteins directly interacting with Popdc protein but also those, which are only indirectly associated with them. In addition to the modulation of protein–protein interactions, cAMP binding to Popdc proteins could also have an impact on the activity of other cAMP effector proteins by regulating local cAMP concentrations. The functional consequences of these mechanisms have previously been described in the “cargo model” and the “switch model” (Brand et al., 2014). The cargo model describes that cAMP binding to Popdc proteins could enhance surface expression of associated proteins either by docking to an anchor protein in the cell surface membrane or by modulating intracellular trafficking. The switch model postulates that Popdc proteins might directly affect the function of interacting proteins, e.g. the conductivity in the case of an ion channel such as TREK-1 (Froese et al., 2012). Moreover, additional functions could be proposed such as preventing proteins from proteolytic decay or from phosphorylation or from other types of posttranslational modification, etc. as recently proposed in the shielding model (Schindler et al., 2016).
Fig. 1.
Potential mechanisms by which cAMP binding may modulate Popdc proteins and PIPs. It is supposed that binding of cAMP leads to a conformational change in the Popeye domain (red), which may result in the exposure of a binding site for PIPs that do not interact with Popdc proteins when cAMP is not bound (a). In contrast, other PIPs may only be able to bind to Popdc proteins under baseline conditions and may be released upon cAMP binding to the Popeye domain. In turn, these proteins may then be accessible to interact with other proteins (b). It could also be assumed that conformational changes in the Popeye domain do not directly affect binding of a PIP but may induce a conformational change in it, thereby enabling or disabling it to bind to and regulate other proteins, which are only indirectly associated with Popdc proteins (c). In addition, it is likely that protein–protein interactions exist, which are not affected by cAMP binding (d). Importantly, protein–protein interactions are not necessarily confined to the cytoplasmic portion of Popdc proteins but could also involve the transmembrane domains and the extracellular parts (#). Also these interactions may be affected by cAMP binding.
Importantly, the increase in TREK-1 conductivity is accompanied by an about 2-fold higher membrane localisation of TREK-1, suggesting that Popdc proteins modulate membrane trafficking of TREK-1 (Froese et al., 2012). However, it is presently unclear how this is achieved and whether Mtap2 or β-COP are involved. Interestingly, this modulation seems to be specific for TREK-1 as the closely related TASK-1 channel was not affected by co-expression with Popdc1 (Froese et al., 2012). Even though the physiological impact of Popdc-TREK-1 interaction is still unclear, it is believed that Popdc proteins modulate the resting membrane potential and thereby the excitability of cardiac myocytes (Boukens and Christoffels, 2012, Froese et al., 2012). In epithelia and cancer cells, Popdc1 has been shown to modulate fundamental cellular processes like vesicular trafficking, which may have a profound impact also for membrane localisation of ion channels, transporters, and pumps. Also the complexity of phenotypes in animal models lacking Popdc proteins points to the involvement of other mechanism and interactions, by which Popdc proteins modulate cardiac excitability under baseline condition and under stress. In this context it is also noteworthy that the mechano-sensitive TREK-1 protein may provide a substrate for Popdc-mediated regulation of mechanical properties in cardiac myocytes and neurons and thereby regulate stretch-related processes like the secretion of atrial natriuretic peptide (ANP) (McGrath and de Bold, 2009). Thus, Popdc proteins may be involved in mechanical and electrical coupling. In favour for this hypothesis is also the multiple direct and indirect involvement of the cytoskeleton. Popdc1 has been shown to regulate the organisation of actin filaments via interaction with GEFT (Smith et al., 2008). Importantly, also TREK-1 is involved in cytoskeletal arrangement (Lauritzen et al., 2005). Moreover, the actin-associated protein ßIV-spectrin regulates TREK-1 membrane targeting (Hund et al., 2014) and so do Popdc proteins (Froese et al., 2012). Therefore, a complex regulatory network involving cytoskeletal components, mechanosensors, and Popdc proteins exist, which deserves further scientific attention.
5.5. Popdc1 interacts with Caveolin-3 and controls number and size of caveolae
A novel PIP in the heart is the caveolae protein Caveolin-3 (Alcalay et al., 2013). Caveolae are flask-shaped membrane invaginations and play important roles in different physiological processes including vesicular trafficking and signal transduction (reviewed in Gazzerro et al., 2010). It is supposed that Popdc1 regulates number and size of caveolae as in Popdc1 null mutant mice fewer caveolae were present, which were larger in size compared to wildtype controls. Moreover, alteration in Ca2+ transients and increased vulnerability of the null mutant heart to ischemia-reperfusion injury have been observed (Alcalay et al., 2013). Importantly, the binding site of Popdc1 for Caveolin-3 has been mapped to a motif located in the Popeye domain, which is predicted to form the lid structure of the CNBD (Alcalay et al., 2013), which is supposed to undergo profound conformational changes after cAMP binding (Rehmann et al., 2007). It may therefore be possible that conformational changes in the Popeye domain have a strong effect on the Caveolin-3/Popdc1 complex and other associated proteins. Interestingly, the protein composition of caveolae is dynamic and regulated by beta-adrenergic stimulation (Wypijewski et al., 2015). It may be possible that Popdc proteins are involved in this by mechanisms described in Fig. 1. Moreover, depletion of Popdc proteins may result in uncoordinated caveolar organisation, which may contribute to the abnormal stress-induced arrhythmia and the vulnerability to ischemia-reperfusion injury. However, thorough investigation under baseline conditions and after β-adrenergic stimulation will be necessary to better understand the role of Popdc proteins in regulation of caveolar number, size, and function. Interestingly, caveolae have been implicated in mechano-sensing and mechano-transduction (reviewed in Calaghan, 2011), and via interaction with Caveolin-3, Popdc1 may contribute to that functions. However, one must not forget that interaction is unlikely to be a unidirectional process with Popdc1 regulating Caveolin-3. In fact, Caveolin-3 may also modulate Popdc1, e.g. with regard to its subcellular localisation and potential clustering with other proteins involved in β-adrenergic signalling processes (Xiang, 2011).
5.6. Popdc proteins are important players in cardiac and skeletal muscle physiology
Popdc proteins are most abundantly present in striated muscle tissue and indeed, genetic inactivation of Popdc1 and -2 in mouse models demonstrated their important physiological roles in these tissues (Andrée et al., 2002a, Froese et al., 2012). In the heart, loss of either Popdc1 or Popdc2 resulted in severe arrhythmia phenotypes with the presence of sinus pauses (temporary loss of sinus node activity) leading to a strong reduction in mean heart rate and therefore to a bradycardia phenotype with a high heart rate variability (Table 1) (Froese et al., 2012). Importantly, this bradycardia did not occur at rest but was provoked by either physical (swimming) or mental (hot air jet) stress. Moreover, also isoproterenol treatment led to the stress-induced bradycardia phenotype in the null mutants, similar to what was seen after physical or mental stress. In contrast, carbachol treatment resulted in a comparable drop in heart rate in both genotypes, suggesting that parasympathetic stimulation is not altered in the null mutants (Froese et al., 2012). Importantly, in both Popdc1 and Popdc2 null mutants, the bradycardia develops in an age-dependent manner, which means that young animals (3 months old) do not have the phenotype but the older the animals grow the more severe the phenotype becomes (Froese et al., 2012). Due to the similarities of the phenotypes observed in animal models Popdc genes have tentatively been associated with the sick sinus syndrome (SSS) in human patients. The bradycardia is independent of the autonomic nervous system as it also present in isolated null mutant hearts. Interestingly, in these mouse models no functional alterations of the atrioventricular (AV) node were seen (Froese et al., 2012). However, in Popdc1/Popdc2 double null mutants, AV block has been observed (Simrick et al., 2012). Electrical activity in the working myocardium seems to be unaffected in younger Popdc single null mutant mice (Froese et al., 2012), however, in Popdc1/Popdc2 double null mutants functional impairments of the working myocardium with atrial fibrillation, polymorphic ventricular tachycardia, and extrasystoles has been observed in addition to sinus pauses. In isolated ventricular myocytes, β-adrenergic stimulation caused delayed-after-depolarisations (DADs), spontaneously generated action potentials, and shortening of action potential durations (Simrick et al., 2012, Simrick et al., 2013). It has been described that in older (8 months) Popdc1−/− and Popdc2−/− mice the dysfunction on the electrophysiological level is accompanied by degeneration of the sinoatrial node, particularly in the inferior part, which is important for pacemaking after adrenergic stimulation, and by structural abnormalities such as a reduction of cellular extensions (Froese et al., 2012, Opthof, 1988). The molecular basis for these changes has yet to be determined. It may be possible that mechanisms similar to what was described for epithelial cells may also play a role in this context such as the effect of interaction with GEFT or Vamp3 etc (see above), however, experimental evidence has not yet been obtained. Also the relative contribution of the electrophysiological and the structural abnormalities is not fully understood and requires further studies. Furthermore, abnormal Ca2+ transients are present in Popdc1 null mutants, and an increase vulnerability to ischemia-reperfusion injury has been documented (Alcalay et al., 2013).
Table 1.
Cardiac and skeletal muscle functions of Popdc proteins.
| Function | Reference |
|---|---|
| Popdc1, Popdc2, and Popdc3 are predominantly expressed in heart and skeletal muscle | (Andrée et al., 2000) |
| POPDC1 and POPDC3 are down-regulated in human heart failure | (Gingold-Belfer et al., 2011) |
| POPDC1 expression is upregulated in patients with ventricular septal defects | (Zhang et al., 2006) |
| Popdc1 is associated with atrial fibrillation | (Tan et al., 2013) |
| Popdc1 has been implicated in the development of Tetralogy of Fallot | (Wu et al., 2013) |
| Popdc1 interacts with Caveolin-3 and Popdc1−/− mice display a reduction in number and an increase in the size of caveolae | (Alcalay et al., 2013) |
| Loss of Popdc1 results in increased vulnerability to ischemia-reperfusion injury | (Alcalay et al., 2013) |
| Popdc1, Popdc2, and Popdc3 interact with the K2P channel TREK-1 | (Froese et al., 2012) |
| Popdc1 controls membrane trafficking of TREK-1 | (Froese et al., 2012) |
| Depletion of either Popdc1 or Popdc2 results in an age-dependent stress-induced bradycardia phenotype in mice | (Froese et al., 2012) |
| Depletion of popdc2 results in AV-block and muscular dystrophy in zebrafish morphants | (Kirchmaier et al., 2012) |
| Depletion of either Popdc1 or Popdc2 in mice results in structural remodelling of SAN tissue | (Froese et al., 2012) |
| The point mutation POPDC1S191F causes cardiac arrhythmia and muscular dystrophy in patients | (Schindler et al., 2016) |
| Popdc1 and Popdc2 interacts with Dystrophin, Dysferlin, | (Schindler et al., 2016) |
| Popdc1 controls the formation of the myotendinous junction | (Schindler et al., 2016) |
In zebrafish morphants depletion of popdc2 and popdc1 leads to electrophysiological phenotypes such as cardiac conduction defects causing 2:1 and 3:1 atrioventricular (AV)-block (Table 1) (Kirchmaier et al., 2012, Schindler et al., 2016). The morphants showed high variability in atrial and ventricular heart rate and irregular action potential durations. If higher morpholino concentrations were used, morphological changes were seen including looping defects, and abnormal heart chambers characterised by reduced myofibrillar content and a lack of trabeculation. Moreover, large pericardial oedemas were often seen, which is indicative for embryonic heart failure (Kirchmaier et al., 2012).
5.7. A mutation in POPDC1 causes cardiac arrhythmia and muscular dystrophy by affecting protein trafficking
Recently, a family suffering from AV-block and limb-girdle muscular dystrophy was identified, which carries a recessive serine 201 to phenylalanine (S201F) point mutation (Schindler et al., 2016). This S201 residue is ultra-conserved and invariable present in all Popeye domains. It is part of the DSPE motif in the PBC which directly interacts with cAMP (Froese et al., 2012). Measuring cAMP binding revealed a significant loss in binding affinity of the mutant POPDC1S201F protein. Co-expression of the of the mutant POPDC1S201F protein together with TREK-1 in Xenopus oocytes revealed an augmented TREK-1 current, which was not altered in response to raising cAMP levels (Schindler et al., 2016). Despite the increased membrane current, TREK-1 protein showed a reduction in membrane localisation, suggesting impaired membrane trafficking. Skeletal muscle biopsies of family members carrying the S201F mutation displayed an aberrant plasma membrane localisation of POPDC1, which was significantly reduced, while the mutant protein accumulated in a perinuclear expression domain. Aberrant membrane trafficking was not confined to the mutant POPDC1 protein but was also observed for POPDC2 (Schindler et al., 2016). A S191F mutation was introduced into the popdc1 gene in zebrafish and the resulting homozygous mutant also displayed muscular dystrophy and cardiac arrhythmia, and therefore resembled the mutant phenotype in patients carrying the homologous S201F mutation in homozygosity. Moreover, also the zebrafish popdc1S191F mutant and wildtype popdc2 proteins displayed aberrant membrane trafficking (Schindler et al., 2016). The rather mild limb-girdle muscular dystrophy phenotype present in the family carrying the S201F mutation, which display a late onset, was associated with fibre size variability, central nuclei, and plasma membrane discontinuities, which are often found in patients with dysferlinopathies and related muscle disorders (Bansal et al., 2003, Magri et al., 2012). However, membrane localisation of Dystrophin, Dysferlin, and Caveolin-3, which all are PIPs (Table 2), was not altered in the muscle of homozygous patients (Schindler et al., 2016). The S201F mutation is rare and screening of an additional 104 patients with a similar phenotype displaying cardiac arrhythmia and muscular dystrophy revealed no additional case of a patient carrying a POPDC1 mutation (Schindler et al., 2016) therefore further work is required to search for mutations of POPDC genes in different patient cohorts with cardiac and skeletal muscle disease. Recently POPDC1 has been associated with atrial fibrillation (AF) using a bioinformatics approach but mutations have not yet been identified in AF patients (Tan et al., 2013). POPDC1 mutations have been implicated in the development of Tetralogy of Fallot (Wu et al., 2013). It has been hypothesised that extracellular matrix disorders may be involved, which, however, remains speculative as neither animal models harbouring these mutations were produced nor functional experiments were carried out (Wu et al., 2013). A study has recently been published assessing the expression levels of POPDC proteins in heart failure samples and a down-regulation of POPDC1 and POPDC3 and to a lesser degree also of POPDC2 in failing compared to normal control hearts has been reported (Gingold-Belfer et al., 2011). In contrast, POPDC1 expression was upregulated in patients with ventricular septal defects (Zhang et al., 2006) suggesting complex regulation of POPDC protein expression in different human cardiac diseases.
Table 2.
Popdc interaction partners.
| Protein | Evidence | Reference |
|---|---|---|
| TREK-1 | GST-PD, Co-IP, Co-IF, FRET, TEVC | (Froese et al., 2012) |
| Caveolin-3 | Co-IP, Co-IF | (Alcalay et al., 2013) |
| Dystrophin | Co-IP, Co-IF | (Schindler et al., 2016) |
| Dysferlin | Co-IP, Co-IF | (Schindler et al., 2016) |
| VAMP2, VAMP3 | Y2H, GST-PD, Co-IF | (Hager et al., 2010) |
| GEFT | Y2H, GST-PD, Co-IF | (Smith et al., 2008) |
| NDRG4 | Y2H, GST-PD, Co-IP, Co-IF | (Benesh et al., 2013) |
| ZO1 | GST-PD, Co-IF, IG-EM | (Osler et al., 2005) |
Abbreviations: Co-IF – co-localisation by immunofluorescence, Co-IPT – co-immunoprecipitation, FRET– fluorescence resonance energy transfer, GST-PD – Glutathione S-transferase pull down, IG-EM- Immunogold electron microscopy, TEVC – two electrode voltage clamp, Y2H – Yeast two hybrid.
In addition to the cardiac phenotypes seen in Popdc null mutant mice, zebrafish morphants, and patients (see above) also skeletal muscle physiology is affected. It has been reported that hindlimb muscles (gastrocnemius and soleus) of Popdc1 null mutants have a reduced regenerative capacity with a delay of muscle fibre regeneration and maturation after injection of the snake venom cardiotoxin, which causes muscle damage and subsequent regeneration (Andree et al., 2002a). In zebrafish, morpholino oligonucleotide-mediated knockdown of popdc2 results in aberrant tail morphology with abnormal formation of myotomal segment boundaries, myofibrillar misalignment and ruptured myofibrils (Kirchmaier et al., 2012). The level of disorganisation increased with the age of the embryos. Interestingly, both fast and slow muscle fibres were affected by the knockdown but specification of fibre types was not altered in popdc2 morphants. In addition, myotendinous junctions were severely affected, myotomal borders abnormally thin and disorganised, and proteins important for cell-matrix adhesion like the Focal Adhesion Kinase (FAK) and vinculin were expressed discontinuously in the junction region. In addition to these impairments of the tail musculature, craniofacial muscles were smaller and abnormally shaped (Kirchmaier et al., 2012). In contrast to the developmental defects seen in zebrafish morphants, mouse null mutants do not show any embryonic phenotype, suggesting that zebrafish are much more sensitive to depletion of Popdc protein expression. It will be important to study these skeletal muscle phenotypes in both animal models in further detail to unravel the underlying pathomechanisms. Although it has been proposed that the muscular phenotype in the animal models results from the role of Popdc1 on cell motility and involves the interaction with GEFT and Vamp2 and -3, which have been implicated in muscle regeneration before (Hager and Bader, 2009, Hager et al., 2010, Smith et al., 2008), other mechanisms are likely to be involved as well, such as functions in the nuclear envelope where Popdc proteins are also present (Korfali et al., 2012, Wilkie et al., 2011).
6. Summary and outlook
In the past 15 years, impressive progress has been made in studying Popdc protein function, which nicely demonstrate the complex but highly important nature of this protein family for a plethora of physiological processes in several organs and tissue types. However, many aspects remain still unclear and deserve further studies. These include for example the role of Popdc3, which is the least studied member of the Popdc family. Moreover, it has yet to be determined how the different Popdc family members are functionally related, as the phenotypes that occur after depletion of either Popdc1 or Popdc2 show significant functional overlap indicating some form of “crosstalk”. Given the high expression levels of Popdc proteins in striated muscle and the complexity of the phenotypes in skeletal muscle and heart arising from inactivation and mutation makes it necessary to study the underlying molecular mechanisms in more detail and identify the protein networks that are modulated by Popdc proteins in different muscle cell types. For a better understanding of how Popdc proteins work and to define their precise role, it will be mandatory to understand the role of cAMP-binding for Popdc protein function and in particular its effect on protein–protein interaction and how this might have an impact at the structural and functional level. It will also be important to understand better how Popdc proteins are regulated, e.g. the role of alternative splicing or posttranslational modification such as glycosylation, phosphorylation or proteolytic processing. This will result in a better understanding of the various physiological and pathophysiological processes Popdc proteins are associated with in striated muscle tissue and beyond.
Editors' note
Please see also related communications in this issue by Schultz et al. (2016) and Moghtadaei et al. (2016).
Acknowledgements
Research in the authors' laboratory was financially supported by the Medical Research Council (MR/J010383/1), the British Heart Foundation (PG/14/46/30911, PG/14/83/31128), and the Magdi Yacoub Institute (HSC324/14, HSC326/14).
References
- Alcalay Y., Hochhauser E., Kliminski V., Dick J., Zahalka M.A., Parnes D., Schlesinger H., Abassi Z., Shainberg A., Schindler R.F., Brand T., Kessler-Icekson G. Popeye domain containing 1 (popdc1/bves) is a caveolae-associated protein involved in ischemia tolerance. PLoS One. 2013;8:e71100. doi: 10.1371/journal.pone.0071100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrée B., Hillemann T., Kessler-Icekson G., Schmitt-John T., Jockusch H., Arnold H.H., Brand T. Isolation and characterization of the novel popeye gene family expressed in skeletal muscle and heart. Dev. Biol. 2000;223:371–382. doi: 10.1006/dbio.2000.9751. [DOI] [PubMed] [Google Scholar]
- Andrée B., Fleige A., Arnold H.H., Brand T. Mouse Pop1 is required for muscle regeneration in adult skeletal muscle. Mol. Cell. Biol. 2002;22:1504–1512. doi: 10.1128/mcb.22.5.1504-1512.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrée B., Fleige A., Hillemann T., Arnold H.H., Kessler-Icekson G., Brand T. Molecular and functional analysis of Popeye genes: a novel family of transmembrane proteins preferentially expressed in heart and skeletal muscle. Exp. Clin. Cardiol. 2002;7:99–103. [PMC free article] [PubMed] [Google Scholar]
- Bansal D., Miyake K., Vogel S.S., Groh S., Chen C.C., Williamson R., McNeil P.L., Campbell K.P. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- Benesh E.C., Miller P.M., Pfaltzgraff E.R., Grega-Larson N.E., Hager H.A., Sung B.H., Qu X., Baldwin H.S., Weaver A.M., Bader D.M. Bves and NDRG4 regulate directional epicardial cell migration through autocrine extracellular matrix deposition. Mol. Biol. Cell. 2013;24:3496–3510. doi: 10.1091/mbc.E12-07-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukens B.J., Christoffels V.M. Popeye proteins: muscle for the aging sinus node. J. Clin. Investig. 2012;122:810–813. doi: 10.1172/JCI62588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand T. The Popeye domain-containing gene family. Cell Biochem. Biophys. 2005;43:95–103. doi: 10.1385/CBB:43:1:095. [DOI] [PubMed] [Google Scholar]
- Brand T., Simrick S.L., Poon K.L., Schindler R.F. The cAMP-binding Popdc proteins have a redundant function in the heart. Biochem. Soc. Trans. 2014;42:295–301. doi: 10.1042/BST20130264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breher S.S., Mavridou E., Brenneis C., Froese A., Arnold H.H., Brand T. Popeye domain containing gene 2 (Popdc2) is a myocyte-specific differentiation marker during chick heart development. Dev. Dyn. 2004;229:695–702. doi: 10.1002/dvdy.20015. [DOI] [PubMed] [Google Scholar]
- Bryan B.A., Mitchell D.C., Zhao L., Ma W., Stafford L.J., Teng B.B., Liu M. Modulation of muscle regeneration, myogenesis, and adipogenesis by the Rho family guanine nucleotide exchange factor GEFT. Mol. Cell. Biol. 2005;25:11089–11101. doi: 10.1128/MCB.25.24.11089-11101.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calaghan S. Role of caveolae in stretch-sensing: implications for mechano-electric coupling. In: Kohl P., Sachs F., Franz M.R., editors. Cardiac Mechano-electric Coupling and Arrhythmias. Oxford University Press; Oxford: 2011. pp. 50–56. [Google Scholar]
- Enyedi P., Czirjak G. Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol. Rev. 2010;90:559–605. doi: 10.1152/physrev.00029.2009. [DOI] [PubMed] [Google Scholar]
- Feng Q., Hawes S.E., Stern J.E., Wiens L., Lu H., Dong Z.M., Jordan C.D., Kiviat N.B., Vesselle H. DNA methylation in tumor and matched normal tissues from non-small cell lung cancer patients. Cancer Epidemiol. Biomarkers Prev. 2008;17:645–654. doi: 10.1158/1055-9965.EPI-07-2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlito M., Fulton W.B., Zauher M.A., Marban E., Steenbergen C., Lowenstein C.J. VAMP-1, VAMP-2, and syntaxin-4 regulate ANP release from cardiac myocytes. J. Mol. Cell. Cardiol. 2010;49:791–800. doi: 10.1016/j.yjmcc.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froese A., Brand T. Expression pattern of Popdc2 during mouse embryogenesis and in the adult. Dev. Dyn. 2008;237:780–787. doi: 10.1002/dvdy.21431. [DOI] [PubMed] [Google Scholar]
- Froese A., Breher S.S., Waldeyer C., Schindler R.F., Nikolaev V.O., Rinne S., Wischmeyer E., Schlueter J., Becher J., Simrick S., Vauti F., Kuhtz J., Meister P., Kreissl S., Torlopp A., Liebig S.K., Laakmann S., Muller T.D., Neumann J., Stieber J., Ludwig A., Maier S.K., Decher N., Arnold H.H., Kirchhof P., Fabritz L., Brand T. Popeye domain containing proteins are essential for stress-mediated modulation of cardiac pacemaking in mice. J. Clin. Investig. 2012;122:1119–1130. doi: 10.1172/JCI59410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli T., Chilcote T., Mundigl O., Binz T., Niemann H., De Camilli P. Tetanus toxin-mediated cleavage of cellubrevin impairs exocytosis of transferrin receptor-containing vesicles in CHO cells. J. Cell Biol. 1994;125:1015–1024. doi: 10.1083/jcb.125.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzerro E., Sotgia F., Bruno C., Lisanti M.P., Minetti C. Caveolinopathies: from the biology of caveolin-3 to human diseases. Eur. J. Hum. Genet. 2010;18:137–145. doi: 10.1038/ejhg.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingold-Belfer R., Bergman M., Alcalay Y., Schlesinger H., Aravot D., Berman M., Salman H., Brand T., Kessler-Icekson G. Popeye domain-containing 1 is down-regulated in failing human hearts. Int. J. Mol. Med. 2011;27:25–31. doi: 10.3892/ijmm.2010.558. [DOI] [PubMed] [Google Scholar]
- Guo X., Stafford L.J., Bryan B., Xia C., Ma W., Wu X., Liu D., Songyang Z., Liu M. A Rac/Cdc42-specific exchange factor, GEFT, induces cell proliferation, transformation, and migration. J. Biol. Chem. 2003;278:13207–13215. doi: 10.1074/jbc.M208896200. [DOI] [PubMed] [Google Scholar]
- Hager H.A., Bader D.M. Bves: ten years after. Histol. Histopathol. 2009;24:777–787. doi: 10.14670/hh-24.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager H.A., Roberts R.J., Cross E.E., Proux-Gillardeaux V., Bader D.M. Identification of a novel Bves function: regulation of vesicular transport. EMBO J. 2010;29:532–545. doi: 10.1038/emboj.2009.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P., Fu Y., Liu J., Wang Y., He J., Gong J., Li M., Tan Q., Li D., Luo Y., Han J., Tu W., Tian D., Yan W. Netrin-1 promotes cell migration and invasion by down-regulation of BVES expression in human hepatocellular carcinoma. Am. J. Cancer Res. 2015;5:1396–1409. [PMC free article] [PubMed] [Google Scholar]
- Han P., Fu Y., Luo M., He J., Liu J., Liao J., Tian D., Yan W. BVES inhibition triggers epithelial-mesenchymal transition in human hepatocellular carcinoma. Dig. Dis. Sci. 2014;59:992–1000. doi: 10.1007/s10620-013-2992-3. [DOI] [PubMed] [Google Scholar]
- Honore E. The neuronal background K2P channels: focus on TREK1. Nat. Rev. Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- Hund T.J., Snyder J.S., Wu X., Glynn P., Koval O.M., Onal B., Leymaster N.D., Unudurthi S.D., Curran J., Camardo C., Wright P.J., Binkley P.F., Anderson M.E., Mohler P.J. beta(IV)-Spectrin regulates TREK-1 membrane targeting in the heart. Cardiovasc. Res. 2014;102:166–175. doi: 10.1093/cvr/cvu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayagopal A., Yang J.L., Haselton F.R., Chang M.S. Tight junction-associated signaling pathways modulate cell proliferation in uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2011;52:588–593. doi: 10.1167/iovs.10-5746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi M., Hager H.A., Wada A., Koyama T., Chang M.S., Bader D.M. Identification of a novel intracellular interaction domain essential for Bves function. PLoS One. 2008;3:e2261. doi: 10.1371/journal.pone.0002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M., Jang H.R., Haam K., Kang T.W., Kim J.H., Kim S.Y., Noh S.M., Song K.S., Cho J.S., Jeong H.Y., Kim J.C., Yoo H.S., Kim Y.S. Frequent silencing of popeye domain-containing genes, BVES and POPDC3, is associated with promoter hypermethylation in gastric cancer. Carcinogenesis. 2010;31:1685–1693. doi: 10.1093/carcin/bgq144. [DOI] [PubMed] [Google Scholar]
- Kim E., Hwang E.M., Yarishkin O., Yoo J.C., Kim D., Park N., Cho M., Lee Y.S., Sun C.H., Yi G.S., Yoo J., Kang D., Han J., Hong S.G., Park J.Y. Enhancement of TREK1 channel surface expression by protein-protein interaction with beta-COP. Biochem. Biophys. Res. Commun. 2010;395:244–250. doi: 10.1016/j.bbrc.2010.03.171. [DOI] [PubMed] [Google Scholar]
- Kirchmaier B.C., Poon K.L., Schwerte T., Huisken J., Winkler C., Jungblut B., Stainier D.Y., Brand T. The Popeye domain containing 2 (popdc2) gene in zebrafish is required for heart and skeletal muscle development. Dev. Biol. 2012;363:438–450. doi: 10.1016/j.ydbio.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R.F., Bader D.M., Backstrom J.R. Membrane topology of Bves/Pop1A, a cell adhesion molecule that displays dynamic changes in cellular distribution during development. J. Biol. Chem. 2003;278:32872–32879. doi: 10.1074/jbc.M301961200. [DOI] [PubMed] [Google Scholar]
- Korfali N., Wilkie G.S., Swanson S.K., Srsen V., de Las Heras J., Batrakou D.G., Malik P., Zuleger N., Kerr A.R., Florens L., Schirmer E.C. The nuclear envelope proteome differs notably between tissues. Nucleus. 2012;3:552–564. doi: 10.4161/nucl.22257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahling A.M., Alvarez L., Debowski K., Van Q., Gunkel M., Irsen S., Al-Amoudi A., Strunker T., Kremmer E., Krause E., Voigt I., Wortge S., Waisman A., Weyand I., Seifert R., Kaupp U.B., Wachten D. CRIS-a novel cAMP-binding protein controlling spermiogenesis and the development of flagellar bending. PLoS Genet. 2013;9:e1003960. doi: 10.1371/journal.pgen.1003960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritzen I., Chemin J., Honore E., Jodar M., Guy N., Lazdunski M., Jane Patel A. Cross-talk between the mechano-gated K2P channel TREK-1 and the actin cytoskeleton. EMBO Rep. 2005;6:642–648. doi: 10.1038/sj.embor.7400449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.Y., Chou C.Y., Tang M.J., Shen M.R. Epithelial-mesenchymal transition in cervical cancer: correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clin. Cancer Res. 2008;14:4743–4750. doi: 10.1158/1078-0432.CCR-08-0234. [DOI] [PubMed] [Google Scholar]
- Lin S., Zhao D., Bownes M. Blood vessel/epicardial substance (bves) expression, essential for embryonic development, is down regulated by Grk/EFGR signalling. Int. J. Dev. Biol. 2007;51:37–44. doi: 10.1387/ijdb.052108sl. [DOI] [PubMed] [Google Scholar]
- Luftman K., Hasan N., Day P., Hardee D., Hu C. Silencing of VAMP3 inhibits cell migration and integrin-mediated adhesion. Biochem. Biophys. Res. Commun. 2009;380:65–70. doi: 10.1016/j.bbrc.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundby A., Andersen M.N., Steffensen A.B., Horn H., Kelstrup C.D., Francavilla C., Jensen L.J., Schmitt N., Thomsen M.B., Olsen J.V. In vivo phosphoproteomics analysis reveals the cardiac targets of β-adrenergic receptor signaling. Sci. Signal. 2013;6:rs11. doi: 10.1126/scisignal.2003506. [DOI] [PubMed] [Google Scholar]
- Luo D., Lu M.L., Zhao G.F., Huang H., Zheng M.Y., Chang J., Lv L., Luo J.B. Reduced Popdc3 expression correlates with high risk and poor survival in patients with gastric cancer. World J. Gastroenterol. 2012;18:2423–2429. doi: 10.3748/wjg.v18.i19.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz S., Freichel-Blomquist A., Rumenapp U., Schmidt M., Jakobs K.H., Wieland T. p63RhoGEF and GEFT are Rho-specific guanine nucleotide exchange factors encoded by the same gene. Naunyn Schmiedeb. Arch. Pharmacol. 2004;369:540–546. doi: 10.1007/s00210-004-0926-5. [DOI] [PubMed] [Google Scholar]
- Magri F., Del Bo R., D'Angelo M.G., Sciacco M., Gandossini S., Govoni A., Napoli L., Ciscato P., Fortunato F., Brighina E., Bonato S., Bordoni A., Lucchini V., Corti S., Moggio M., Bresolin N., Comi G.P. Frequency and characterisation of anoctamin 5 mutations in a cohort of Italian limb-girdle muscular dystrophy patients. Neuromuscul. Disord. 2012;22:934–943. doi: 10.1016/j.nmd.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath M.F., de Bold A.J. Transcriptional analysis of the mammalian heart with special reference to its endocrine function. BMC Genom. 2009;10:254. doi: 10.1186/1471-2164-10-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon H.T., Ushkaryov Y.A., Edelmann L., Link E., Binz T., Niemann H., Jahn R., Sudhof T.C. Cellubrevin is a ubiquitous tetanus-toxin substrate homologous to a putative synaptic vesicle fusion protein. Nature. 1993;364:346–349. doi: 10.1038/364346a0. [DOI] [PubMed] [Google Scholar]
- Moghtadaei M., Polina I., Rose R. Electrophysiological effects of natriuretic peptides in the heart are mediated by multiple receptor subtypes. Prog. Biophys. Mol. Biol. 2016;120(1-3):39–47. doi: 10.1016/j.pbiomolbio.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Micalizzi D.S., Farabaugh S.M., Ford H.L. Epithelial-mesenchymal transition in cancer: parallels between normal development and tumor progression. J. Mammary Gland. Biol. Neoplasia. 2010;15:117–134. doi: 10.1007/s10911-010-9178-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opthof T. The mammalian sinoatrial node. Cardiovasc. Drugs Ther. 1988;1:573–597. doi: 10.1007/BF02125744. [DOI] [PubMed] [Google Scholar]
- Osler M.E., Bader D.M. Bves expression during avian embryogenesis. Dev. Dyn. 2004;229:658–667. doi: 10.1002/dvdy.10490. [DOI] [PubMed] [Google Scholar]
- Osler M.E., Chang M.S., Bader D.M. Bves modulates epithelial integrity through an interaction at the tight junction. J. Cell Sci. 2005;118:4667–4678. doi: 10.1242/jcs.02588. [DOI] [PubMed] [Google Scholar]
- Osler M.E., Smith T.K., Bader D.M. Bves, a member of the Popeye domain-containing gene family. Dev. Dyn. 2006;235:586–593. doi: 10.1002/dvdy.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatinus J.A., O'Quinn M.P., Barker R.J., Harris B.S., Jourdan J., Gourdie R.G. ZO-1 determines adherens and gap junction localization at intercalated disks. Am. J. Physiol. Heart Circ. Physiol. 2011;300:H583–H594. doi: 10.1152/ajpheart.00999.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnes D., Jacoby V., Sharabi A., Schlesinger H., Brand T., Kessler-Icekson G. The Popdc gene family in the rat: molecular cloning, characterization and expression analysis in the heart and cultured cardiomyocytes. Biochim. Biophys. Acta. 2007;1769:586–592. doi: 10.1016/j.bbaexp.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Proux-Gillardeaux V., Gavard J., Irinopoulou T., Mege R.M., Galli T. Tetanus neurotoxin-mediated cleavage of cellubrevin impairs epithelial cell migration and integrin-dependent cell adhesion. Proc. Natl. Acad. Sci. U. S. A. 2005;102:6362–6367. doi: 10.1073/pnas.0409613102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reese D.E., Zavaljevski M., Streiff N.L., Bader D. bves: a novel gene expressed during coronary blood vessel development. Dev. Biol. 1999;209:159–171. doi: 10.1006/dbio.1999.9246. [DOI] [PubMed] [Google Scholar]
- Rehmann H., Wittinghofer A., Bos J.L. Capturing cyclic nucleotides in action: snapshots from crystallographic studies. Nat. Rev. Mol. Cell Biol. 2007;8:63–73. doi: 10.1038/nrm2082. [DOI] [PubMed] [Google Scholar]
- Ripley A.N., Chang M.S., Bader D.M. Bves is expressed in the epithelial components of the retina, lens, and cornea. Investig. Ophthalmol. Vis. Sci. 2004;45:2475–2483. doi: 10.1167/iovs.04-0013. [DOI] [PubMed] [Google Scholar]
- Ripley A.N., Osler M.E., Wright C.V., Bader D. Xbves is a regulator of epithelial movement during early Xenopus laevis development. Proc. Natl. Acad. Sci. U. S. A. 2006;103:614–619. doi: 10.1073/pnas.0506095103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ P.K., Kupperman A.I., Presley S.H., Haselton F.R., Chang M.S. Inhibition of RhoA signaling with increased Bves in trabecular meshwork cells. Investig. Ophthalmol. Vis. Sci. 2010;51:223–230. doi: 10.1167/iovs.09-3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russ P.K., Pino C.J., Williams C.S., Bader D.M., Haselton F.R., Chang M.S. Bves modulates tight junction associated signaling. PLoS One. 2011;6:e14563. doi: 10.1371/journal.pone.0014563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salskov A., Hawes S.E., Stern J.E., Feng Q., Jordan C.D., Wiens L., Rasey J., Lu H., Kiviat N.B., Vesselle H. Hypermethylation of CCND2 may reflect a smoking-induced precancerous change in the lung. J. Oncol. 2011;2011:950140. doi: 10.1155/2011/950140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz G., Tardy M.P., Thummler S., Feliciangeli S., Lazdunski M., Lesage F. Mtap2 is a constituent of the protein network that regulates twik-related K+ channel expression and trafficking. J. Neurosci. 2008;28:8545–8552. doi: 10.1523/JNEUROSCI.1962-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoz G., Thummler S., Duprat F., Feliciangeli S., Vinh J., Escoubas P., Guy N., Lazdunski M., Lesage F. AKAP150, a switch to convert mechano-, pH- and arachidonic acid-sensitive TREK K(+) channels into open leak channels. EMBO J. 2006;25:5864–5872. doi: 10.1038/sj.emboj.7601437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler R.F., Poon K.L., Simrick S., Brand T. The Popeye domain containing genes: essential elements in heart rate control. Cardiovasc. Diagn. Ther. 2012;2:308–319. doi: 10.3978/j.issn.2223-3652.2012.12.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler R.F., Scotton C., Zhang J., Passarelli C., Ortiz-Bonnin B., Simrick S.L., Schwerte T., Poon K.L., Fang M., Rinné S., Froese A., Nikolaev V.O., Grunert C., Müller T., Tasca G., Sarathchandra P., Drago F., Dallapiccola B., Rapezzi C., Arbustini E., Romana Di Raimo F., Neri M., Selvatici R., Gualandi F., Fattori F., Pietrangolo A., Li W., Jiang H., Xun X., Bertini E., Decher N., Wang J., Brand T., Ferlini A. POPDC1S201F causes muscular dystrophy and arrhythmia by affecting protein trafficking. J. Clin. Investig. 2016;126:239–253. doi: 10.1172/JCI79562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz F., Hasan A., Alvarez-Laviada A., Miragoli M., Bhogal N., Wells S., Poulet C., Chambers J., Williamson C., Gorelik J. The protective effect of ursodeoxycholic acid in the human fetal heart occurs via targeting cardiac fibroblasts. Prog. Biophys. Mol. Biol. 2016;120(1-3):149–163. doi: 10.1016/j.pbiomolbio.2016.01.003. [DOI] [PubMed] [Google Scholar]
- Simrick S., Kreutzer R., Rao C., Terracciano C.M., Kirchhof P., Fabritz L., Brand T. Pronounced stress-induced lethality in popdc1/2 null mutants. Cardiovasc. Res. 2012;93:S53. [Google Scholar]
- Simrick S., Schindler R.F., Poon K.L., Brand T. Popeye domain-containing proteins and stress-mediated modulation of cardiac pacemaking. Trends Cardiovasc. Med. 2013;23:257–263. doi: 10.1016/j.tcm.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T.K., Bader D.M. Characterization of Bves expression during mouse development using newly generated immunoreagents. Dev. Dyn. 2006;235:1701–1708. doi: 10.1002/dvdy.20739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith T.K., Hager H.A., Francis R., Kilkenny D.M., Lo C.W., Bader D.M. Bves directly interacts with GEFT, and controls cell shape and movement through regulation of Rac1/Cdc42 activity. Proc. Natl. Acad. Sci. U. S. A. 2008;105:8298–8303. doi: 10.1073/pnas.0802345105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souchet M., Portales-Casamar E., Mazurais D., Schmidt S., Leger I., Javre J.L., Robert P., Berrebi-Bertrand I., Bril A., Gout B., Debant A., Calmels T.P. Human p63RhoGEF, a novel RhoA-specific guanine nucleotide exchange factor, is localized in cardiac sarcomere. J. Cell Sci. 2002;115:629–640. doi: 10.1242/jcs.115.3.629. [DOI] [PubMed] [Google Scholar]
- Sugiyama A., Takahara A., Yatomi Y., Satoh Y., Nakamura Y., Hashimoto K. Constitutional rho-kinase regulates atrioventricular nodal conduction and ventricular repolarization of the canine heart. J. Cardiovasc. Pharmacol. 2003;41:930–933. doi: 10.1097/00005344-200306000-00015. [DOI] [PubMed] [Google Scholar]
- Tan N., Chung M.K., Smith J.D., Hsu J., Serre D., Newton D.W., Castel L., Soltesz E., Pettersson G., Gillinov A.M., Van Wagoner D.R., Barnard J. Weighted gene coexpression network analysis of human left atrial tissue identifies gene modules associated with atrial fibrillation. Circ. Cardiovasc. Genet. 2013;6:362–371. doi: 10.1161/CIRCGENETICS.113.000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayeb M.A., Skalski M., Cha M.C., Kean M.J., Scaife M., Coppolino M.G. Inhibition of SNARE-mediated membrane traffic impairs cell migration. Exp. Cell Res. 2005;305:63–73. doi: 10.1016/j.yexcr.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Torlopp A., Breher S.S., Schluter J., Brand T. Comparative analysis of mRNA and protein expression of Popdc1 (Bves) during early development in the chick embryo. Dev. Dyn. 2006;235:691–700. doi: 10.1002/dvdy.20687. [DOI] [PubMed] [Google Scholar]
- Vasavada T.K., DiAngelo J.R., Duncan M.K. Developmental expression of Pop1/Bves. J. Histochem. Cytochem. 2004;52:371–377. doi: 10.1177/002215540405200308. [DOI] [PubMed] [Google Scholar]
- Wada A.M., Reese D.E., Bader D.M. Bves: prototype of a new class of cell adhesion molecules expressed during coronary artery development. Development. 2001;128:2085–2093. doi: 10.1242/dev.128.11.2085. [DOI] [PubMed] [Google Scholar]
- Wilkie G.S., Korfali N., Swanson S.K., Malik P., Srsen V., Batrakou D.G., de las Heras J., Zuleger N., Kerr A.R., Florens L., Schirmer E.C. Several novel nuclear envelope transmembrane proteins identified in skeletal muscle have cytoskeletal associations. Mol. Cell. Proteom. 2011;10 doi: 10.1074/mcp.M110.003129. M110 003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C.S., Zhang B., Smith J.J., Jayagopal A., Barrett C.W., Pino C., Russ P., Presley S.H., Peng D., Rosenblatt D.O., Haselton F.R., Yang J.L., Washington M.K., Chen X., Eschrich S., Yeatman T.J., El-Rifai W., Beauchamp R.D., Chang M.S. BVES regulates EMT in human corneal and colon cancer cells and is silenced via promoter methylation in human colorectal carcinoma. J. Clin. Investig. 2011;121:4056–4069. doi: 10.1172/JCI44228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Li Y., He X., Shao X., Yang F., Zhao M., Wu C., Zhang C., Zhou L. Mutational and functional analysis of the BVES gene coding region in Chinese patients with non-syndromic tetralogy of Fallot. Int. J. Mol. Med. 2013;31:899–903. doi: 10.3892/ijmm.2013.1275. [DOI] [PubMed] [Google Scholar]
- Wu Y.C., Chen R.F., Liu C.Y., Hu F.R., Huang C.J., Wang I.J. Knockdown of zebrafish blood vessel epicardial substance results in incomplete retinal lamination. ScientificWorldJournal. 2014;2014:803718. doi: 10.1155/2014/803718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.C., Liu C.Y., Chen Y.H., Chen R.F., Huang C.J., Wang I.J. Blood vessel epicardial substance (Bves) regulates epidermal tight junction integrity through atypical protein kinase C. J. Biol. Chem. 2012;287:39887–39897. doi: 10.1074/jbc.M112.372078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuertz C.M., Lorincz A., Vettel C., Thomas M.A., Wieland T., Lutz S. p63RhoGEF–a key mediator of angiotensin II-dependent signaling and processes in vascular smooth muscle cells. FASEB J. 2010;24:4865–4876. doi: 10.1096/fj.10-155499. [DOI] [PubMed] [Google Scholar]
- Wypijewski K.J., Tinti M., Chen W., Lamont D., Ashford M.L., Calaghan S.C., Fuller W. Identification of caveolar resident proteins in ventricular myocytes using a quantitative proteomic approach: dynamic changes in caveolar composition following adrenoceptor activation. Mol. Cell. Proteom. 2015;14:596–608. doi: 10.1074/mcp.M114.038570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y.K. Compartmentalization of beta-adrenergic signals in cardiomyocytes. Circ. Res. 2011;109:231–244. doi: 10.1161/CIRCRESAHA.110.231340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Zhou L., Yang R., Sheng Y., Sun W., Kong X., Cao K. Identification of differentially expressed genes in human heart with ventricular septal defect using suppression subtractive hybridization. Biochem. Biophys. Res. Commun. 2006;342:135–144. doi: 10.1016/j.bbrc.2006.01.113. [DOI] [PubMed] [Google Scholar]