Abstract
Background
Prenatal exposure to stress and selective serotonin reuptake inhibitors (SSRIs) alter hypothalamic–pituitary–adrenal (HPA) stress reactivity in offspring, however, the effects of combined exposure to HPA activity in human infants is unknown.
Objective
To examine HPA basal levels and stress responsiveness in 3-month olds with prenatal exposure to SSRIs.
Methods
Salivary cortisol levels in infants of SSRI treated mothers (n=31, mean exposure 230.2± 72.2 days) were compared with non-SSRI exposed (n=45) infants in response to a challenge (infant-controlled habituation task) and under basal conditions in the late afternoon/early evening. Mode of feeding, to account for possible postnatal drug exposure via breast milk, as well as measures of pre and postnatal maternal mood, were included as covariates.
Results
Lower post-stress cortisol levels were observed in non-SSRI exposed/non-breastfed infants compared with non-SSRI exposed infants who were breastfed at 3 months of age. Stress reactivity patterns among SSRI exposed infants did not differ with mode of feeding. The cortisol reactivity slope (CRS) was significantly lower among non-SSRI exposed non-breastfed infants compared with non-SSRI exposed breastfed infants. Early evening basal cortisol levels were lower in SSRI exposed infants than in non-SSRI exposed infants, controlling for maternal mood and mode of feeding. Postnatal SSRI exposure (infant SSRI drug levels) via breast milk was not associated with stress or basal cortisol levels. Total cortisol, reflected by the AUC measure, did not differ significantly between exposure groups.
Conclusions
Prenatal SSRI exposure altered HPA stress response patterns and reduced early evening basal cortisol levels. Stress challenge HPA response differences only became apparent when the moderating effect of method of feeding was accounted for. These findings suggest an early “programming” effect of antenatal maternal mood, prenatal SSRI exposure and postnatal maternal care giving on the HPA system.
Keywords: Prenatal SSRI exposure, Infant HPA stress reactivity, Prenatal depressed maternal mood
1. Introduction
The development and function of the hypothalamic–pituitary–adrenal (HPA) and the serotonergic (5HT) regulatory systems are highly interrelated [1] and are exquisitely sensitive to the effects of early adverse experience [2]. Among the earliest adverse experiences is exposure to maternal depression during pregnancy, which alters HPA and 5-HT function and potentially sets a life course of vulnerability to illness [3–5]. While, antenatal exposure to stress in animal models and to maternal mood disorders in humans might not constitute similar etiological or phenomenological adverse experiences, they both appear to share a final common pathway that affects HPA function in offspring, possibly via altered 5HT levels. In animals, prenatal and early postnatal stress is associated with increased corticosterone responses to mild stressors in adulthood [6], mediated by altered 5HTand 5HTreceptors [7]. In humans, offspring of depressed and anxious mothers may have an increased risk for neonatal neurobehavioral [4] and physiological disturbances, such as increased cortisol and norepinephrine levels and lower dopamine and 5-HT levels [8]. Central 5HT plays a key role in regulating the HPA system, and in turn, HPA hormones modulate 5-HT function [9,10]. Importantly, prenatal stress in animal models lowers plasma and hippocampal serotonergic activity [11,12] leading to reduced HPA adaptation to stressors [13].
Increasingly, the use of selective (and non-selective) serotonin reuptake inhibitor (SSRI) antidepressants which act by blocking the reuptake of 5HT, thereby increasing levels of central 5HT, are used to manage maternal mood disorders during pregnancy [14], raising concerns that such exposure may alter HPA development and function in offspring. Depression has been characterized by increased HPA activity and resistance to suppression of cortisol by dexamethasone [15] and SSRI antidepressants, which potentiate central 5HT activity, are thought to “normalized” HPA function [16]. In an animal model, Ishiwata et al. [17] observed that early postnatal SSRI (fluoxetine) treatment of prenatally stressed mice “normalized” corticosterone responses to a subsequent stressor, increased 5HT turnover in the hippocampus and restored the ability to learn spatial information compared with the effects of exposure to prenatal stress alone. This might be analogous to a “normalized” HPA activity that may following SSRI antidepressant treatment secondary to increased glucocorticoid receptor (GR) and GR mRNA levels [16]. To date, such effects have not been examined in humans following gestational antidepressant exposure. However, given serotonin's role as a trophic developmental signal [18], mediating hippocampal GR expression and subsequent HPA stress reactivity, changes in basal or stress HPA function following prenatal exposure to maternal depression alone or together with SSRI medication may occur.
Given the highly interrelated nature of the serotonergic and HPA systems, it is conceivable that prenatal SSRI exposure will lead to altered HPA regulation in human infants. The following study was undertaken to examine the effects of prenatal SSRI exposure on HPA activity, indexed by salivary cortisol levels, at 3 months of age in two settings: first, during a stress challenge (infant-controlled habituation and novelty response procedure) and second, under basal conditions in the late afternoon/early evening. Further, as some infants at 3 months also received postnatal SSRI exposure via breastfeeding, we examined the potential influence of mode of feeding and related infant drug levels as possible confounding variables. We hypothesized that prenatal SSRI exposure would have long term effects on HPA basal function and stress reactivity, even when accounting for SSRI drug levels at 3 months (i.e. via postnatal exposure in breastfeeding infants) and depressed maternal mood.
2. Methods
2.1. Subjects
With approval from the University of British Columbia Research Ethics Board, Children's and Women's Health Centre of British Columbia Research Review Committee, and informed parent consent, a cohort (n=98) of mothers was recruited during the second trimester of pregnancy as part of a study of effects of SSRI antidepressant medication exposure on infants during and following pregnancy. Mothers were included in the study if no other psychotropic, serotonergic or antidepressant medications were used during their pregnancies. Of the mothers recruited during their second trimester, 81 mothers and infants continued to the 3-month study (17 mothers moved or withdrew for personal reasons), and five infants could not be included in the final data analysis due to insufficient saliva samples at 3 months, leaving 76 infants, 45 in the control (non-SSRI exposed) group and 31 in the SSRI exposed group. All mothers in the SSRI treated group, with the exception of two who started medication during their third trimester, were already on medication at the time of recruitment, and all continued on medication up to the time of delivery (Table 1). The 3-month age was specifically selected for this study of HPA system function because by 3 months, infants have established regularized state cycles and have an increasing capacity for sustained attention, which are crucial for the infant's emerging capacity to regulate stress reactivity and interaction with the environment [19]. Because maternal mood varied over time among all mothers regardless of their original medication group assignment, maternal mood was considered a continuous rather than a categorical exposure variable that may have influenced infant behavior across all infants, regardless of SSRI exposure status and was controlled for maternal mood in all analyses. Further, mode of feeding was included as a covariate, as infant outcomes could have been potentially influenced by postnatal SSRI exposure via breastfeeding. Non-breastfeeders were defined as infants who had never been breastfed or for whom, at the time of the study, breast milk was reported by their mothers to constitute <75% of their daily diet (termed non-breastfed group).
Table 1.
Maternal demographic, medication and mood characteristics
| SSRI Treated (n = 31) (mean (SD)) | Controls (n = 45) (mean (SD)) | |
|---|---|---|
| Maternal demographics characteristics | ||
| Maternal age at birth (years) (range) | 32.1 (4.8) | 33.6 (4.7) |
| Delivery (n: vaginal/c-section) | 20/11 | 31/14 |
| First pregnancy (%) | 37* | 63 |
| Smoking (n) | 1 | 0 |
| Maternal education (years) | 15.1(2.5)* | 17.8 (3.2) |
| Alcohol use (drinks/pregnancy) (%) | ||
| • 0 | 74 | 53 |
| • 1–5 | 16 | 48 |
| • 6–10 | 3 | 11 |
| • 11–25 | 6 | 8 |
| Prenatal SSRI Medication Use (2nd Trimester) (median mg) (n = 30) | ||
| • Paroxetine (13) | 20 | n/a |
| • Fluoxetine (5) | 20 | |
| • Sertraline (5) | 50 | |
| • Venlafaxine (1) | 225 | |
| • Citalopram (6) | 25 | |
| Prenatal SSRI medication use (3rd Trimester) (median mg) (n = 31) | ||
| • Paroxetine (13) | 20 | n/a |
| • Fluoxetine (5) | 30 | |
| • Sertraline (5) | 50 | |
| • Venlafaxine (2) | 56 | |
| • Citalopram (6) | 25 | |
| Postnatal SSRI medication use (3 months (median mg) (n = 31) | ||
| • Paroxetine (13) | 20 | n/a |
| • Fluoxetine (5) | 30 | |
| • Sertraline (5) | 62.5 | |
| • Venlafaxine (2) | 37.5 | |
| • Citalopram (6) | 25 | |
| Maternal mood measures | ||
| Gestation age at time of 2nd trimester visit (weeks) | 23.3 (5.6) | 24.7 (3.6) |
| Maternal mood 2nd trimester | ||
| • Ham-A | 14.7 (8.0) * | 7.2 (7.7) |
| • Ham-D | 12.7 (6.9) * | 6.0 (7.5) |
| • EPDS | 10.1 (6.0) * | 5.2 (6.2) |
| Gestation age at time of 3rd trimester visit (weeks) | 33.7 (1.1) | 33.8 (1.3) |
| Maternal mood 3rd trimester | ||
| • Ham-A | 11.9 (7.4) * | 5.4 (4.7) |
| • Ham-D | 9.8 (6.3) * | 3.6 (4.6) |
| • EPDS | 8.2 (4.9) * | 4.3 (4.3) |
| Maternal Mood at 3 months | ||
| • Ham-A | 10.4 (7.4) * | 3.84 (3.8) |
| • Ham-D | 7.2 (5.6) * | 3.13 (4.0) |
| • EPDS | 7.5 (5.3) * | 3.77 (3.8) |
| • PSI | 70.5 (17.1) | 63.4 (18.5) |
| • |
p < 0.05 for differences between groups.
HAM-A: Hamilton Rating Scale for Anxiety.
HAM-D: Hamilton Rating Scale for Depression.
EPDS: Edinburgh Postnatal Depression Scale.
PSI: Parent Stress Index.
n/a: Not applicable.
SD: Standard Deviation.
2.2. Procedure
Cortisol was collected at 3 months of age under two different conditions: 1) Cortisol stress reactivity was evaluated using saliva obtained before, during and following an infant-controlled information processing task lasting approximately 20 min, followed by a mother–infant interaction task [20,21]. Testing took place with the infant in an awake alert state [22] in the morning (11:49±.07 h), mean of 103±51 min from last feed (p>0.5 for group differences). Saliva was collected at three time points: during a quiet awake alert period 10 min before the start of the information processing task (Baseline); approximately 40 min (39.2±14.0 min) after the onset of the task (Stress1, S1), and approximately 35 min (34.0±15.2 min) after the S1 sample (Recovery). 2) On a separate day, cortisol was obtained in the late afternoon/early evening (17:59±0.07 h) under basal conditions at home. Mothers were instructed to collect saliva within a 3-week period following the day of the 3-month habituation study visit. This sample was collected on a day separate from the stress challenge day to ensure that this basal measure would not be influenced by the trip to our laboratory or the study itself.
2.3. Salivary cortisol
Saliva was collected by placing a dental roll (Sullivan Dental Products, St. Laurent, Quebec) in the infant's mouth, for 3 min. Saliva was then extracted from the dental role by centrifugation at 3000 rpm for 6 min, and stored at −20 °C until assayed using the Salimetrics High Sensitivity Salivary Cortisol Enzyme Immunoassay Kit for quantitative determination of salivary cortisol (Salimetrics LLC, Philadelphia, PA) at the University of British Columbia (Weinberg laboratory). Intra and inter assay coefficients of variation were 2.92% and 3.41%, respectively.
2.4. Maternal mood
During pregnancy, maternal mood was assessed at the time of study enrollment (late 2nd and mid 3rd trimester), and at 3 months postpartum using three instruments, the Hamilton Rating Scale for Depression (HAM-D) and Anxiety (HAM-A) and the Edinburgh Postnatal Depression Scale. The Hamilton Rating Scale for Depression (HAM-D) [23] is a 21-item clinician-administered scale that measures the severity of depression in adults. Scores on this scale have a possible range of 0–63, with higher scores being associated with higher levels of depression. Scores ranging from 0–7 suggest no or minimal levels of depression, 8–17 indicate mild depression, 18–25 suggest moderate depression, and scores of 26 and above are associated with severe depression. The Hamilton Rating Scale for Anxiety (HAM-A) [24] is a 14-item clinician-administered scale that measures the severity of anxiety. Total scores on this scale have a possible range of 0–56, with higher scores being associated with higher levels of anxiety. Scores ranging from 0–7 suggest no or minimal levels of anxiety, 8–17 indicate mild anxiety, 18–25 suggest moderate anxiety, and scores of 26 and above are associated with severe anxiety. The Edinburgh Postnatal Depression Scale (EPDS) [25] is a 10-item, patient-rated instrument used to assess symptoms of depressed mood in both prenatal and postnatal settings [34].
2.5. Pharmacological data
At the time of the 3-month study, additional maternal (~5 mL) and infant (~1 mL) blood and breast milk (~10 mL) samples were collected for determination of antidepressant levels. Blood samples were collected in Vacutainer tubes without additives and allowed to clot for 30 min. The serum was then separated following centrifugation at 3000 g for 10 min and transferred into a glass tube. Milk samples were collected by manual expression or breast pump at the same time as blood sampling and were transferred to glass tubes. All serum and milk samples were stored at −80 °C until analysis for antidepressant drug levels.
Fluoxetine, norfluoxetine, paroxetine, citalopram, sertraline, and venlafaxine concentrations were determined by LC/MS using a single combination assay developed by Cantest Biopharma Services (Vancouver, BC, Canada). Briefly 100 μl of internal standard solution (d5-fentanyl and d3-sertraline) was added to individual tubes containing 100 μl of study sample (serum, breast milk), calibration standards and QC samples. This was followed by the addition of 0.2 ml of 0.1 M tetraborate buffer solution and 3 ml of chlorobutane. Samples were vortex mixed, subsequently centrifuged and the tubes placed in a rack and frozen at −70 °C for 30 min. The upper organic layer was then removed and evaporated to dryness under a gentle stream of nitrogen (at 40 °C). Dried residues were reconstituted using 500 μl of 10 mM NH4OAc: acetonitrile 6:4 containing 0.5% formic acid. Extracts were then transferred to amber glass vials for LC/MS analysis (10 μl injection volume). Chromatography was performed using an Agilent 1100 HPLC with a CTC-PAL HTS autosampler and an Agilent Zorbax Eclipse XDB-C8 column (4.6×50 mm). Mobile phases were: A=0.5% formic acid in 10 mM NH4OAc and B=0.1% formic acid in acetonitrile. The analytes were eluted using the following gradient: A: B=60:40 for 0.5 min, A:B=40:60 at 1.0 minute and A:B: 5:95 at 2.0 min and held at this value until 3.0 min. Analyte concentrations were determined by mass spectrometry (API 5000; Applied Biosystems, Foster City, CA) using the following MRM transitions: fluoxetine, m/z 310.2 to m/z 148.2; norfluoxetine, m/z 296.1 to m/z 134.2; paroxetine, m/z 330.2 to 192.2; sertraline, m/z 306.2 to m/z 159.1; d3-sertraline, m/z 311.2 to m/z 161.2; citalopram, m/z 325.1 to 262.1; venlafaxine, m/z 278.2 to m/z 121.2; d5-fentanyl, m/z 342.2 to m/z 188.2. The lower limit of quantitation (LLOQ) was 0.1 ng/ml for all analytes. Coefficients of variation were less than 25% at the LLOQ and less than 20% at all other standard curve concentrations (0.1–100 ng/ml).
2.6. Data analysis
Cortisol means, SD and cortisol change scores (Baseline–Stress1) were calculated and for analytic purposes values were log transformed to normalize distributions, however, non-log transformed values were used for display purposes. Cortisol area under the curve (AUC) was calculated as described by Sephton [26] using the 3 cortisol values, and as well, a cortisol reactivity slope (CRS) was calculated using Graphpad Prism(v3) to assess cortisol change across the three study time periods. A group (exposed vs. non-exposed)×feeding condition (breast vs. non-breastfed)×event (Baseline, S1, Recovery) mixed model analysis of covariance (ANCOVA) was carried out to assess reactivity, with feeding mode, maternal mood during pregnancy and at 3 months as covariates. Univariate ANCOVAs were used to examine basal cortisol group differences, using the same covariates. To compare levels across different drugs, 3-month drug levels for each medication were converted to a standardized z-score and used in a regression model. To examine the relationships between infant drug and cortisol levels, linear regression models were used with key maternal mood and infant covariates.
3. Results
Maternal and infant demographic characteristics are shown in Table 1. With the exception of mood, antidepressants used and parity, maternal characteristics did not differ significantly between groups. All infants were born at term, with the exception of two SSRI exposed infants (36.85 weeks) and one non-SSRI exposed infant (36.57 weeks). Infants in the SSRI exposed group were older than non-SSRI exposed infants by a mean of 8 days (3.3 [.44] vs. 3.1 [.35] months; F[1,79]= 7.2; p=0.009). At the time of the 3 month visit, 75% of our cohort infants were considered breastfeeding, i.e., breast milk constituted at least 75% of daily diet. Among non-breastfeeders, 13 (68.4%) had never breastfed, and the remainder had stopped by 3 months or were obtaining <25% of their diet from breast milk. There was a trend toward group differences in feeding, which approached significance (breastfeeding occurred in 62.2% of the SSRI exposed infants vs. 82.2% of the non-SSRI exposed infants; p=.070) (Table 2). Mothers in the SSRI group had fewer years of education than the control mothers (p<0.05).
Table 2.
Neonatal characteristics
| SSRI exposed (n = 31) (mean (SD)) | Non-exposed (n = 45) (mean (SD)) | |
|---|---|---|
| Prenatal characteristics | ||
| Gestational age at birth (weeks) | 39.5 (1.41) | 40.1 (1.3) |
| Birth Weight (g) | 3435 (422) | 3600 (512) |
| Sex (M:F) | 13:18 | 21:24 |
| SSRI exposure during pregnancy (# days) | 230.2 (72.7) | N/A |
| 3 months | ||
| • Feeding (% Breastfeeding (i.e. providing >75% infant diet) | 70.1 | 80.1 |
| • % not breast feeding at all | 26 | 9 |
| • Primary caregiver (birth mothers) (%) | 100 | 100 |
| • Age at study visit (months) | 3.3 (.44) | 3.1 (.36) * |
| • Weight (kg) | 6.2 (0.85) | 7.6 (9.3) |
| • Length (cm) | 61.2 (3.0) | 61.2 (2.3) |
| • Head circumference (cm) | 41.0 (1.1) | 41.0 (1.7) |
p < 0.05 for differences between groups.
3.1. Cortisol stress reactivity
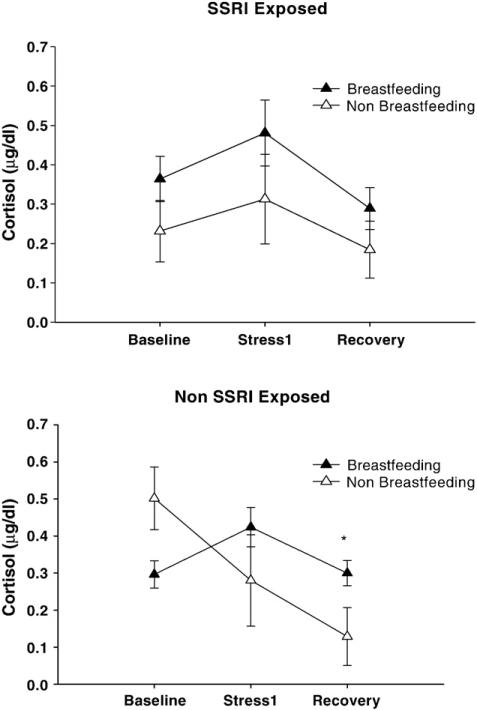
The 3-way ANCOVA model (time/event [Baseline, S1, S2])×feeding condition (breastfeeding/non-breastfed×prenatal medication exposure), revealed differential responses to the stress of the habituation procedure depending on medication exposure and mode of feeding (F[2,104]=4.124, p=.026, partial η2 =.074), controlling for maternal depressed mood during pregnancy (HAM-D3rd trimester) and 3 month maternal depression (HAM-D). Specifically, in the non-SSRI exposed group, there was a significant interaction between time and feeding mode (F[2,68]=7.314, p=.003, partial η2 =.177) that was not present in the SSRI exposed group. Non-SSRI exposed infants who were non-breastfed had lower levels post-stress (S2) cortisol levels than non-SSRI exposed infants who were breastfed (F[1,41]=7.154; p=.011, partial η2 =.149) (Fig. 1, bottom panel). Similar patterns were observed in separate ANCOVAs using pre and postnatal maternal anxiety (HAM-A) and self-rated depressed mood measures (EPDS). Controlling for maternal years of education did not contribute to these findings. In contrast, SSRI exposed infants showed similar patterns of stress reactivity regardless of mode of feeding; there was no significant difference in cortisol levels between breastfed and non-breastfed infants in this group (Fig. 1, top panel).
Figure 1.
Cortisol Stress Reactivity at 3 months as a function of feeding condition (μg/dl±SEM) * p>0.05 for difference between breast feeding vs. non-breast fed in non-exposed infants.
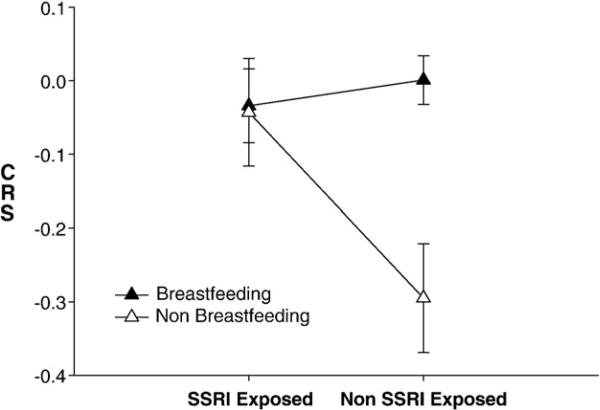
The cortisol reactivity slope (CRS) also reflected these differences and was significantly lower among non-SSRI exposed non-breastfed infants than among non-SSRI exposed breastfed infants (F[1,51]=13.056, p<0.001, partial η2=.266), controlling for maternal mood during and following pregnancy (Fig. 2). Importantly, maternal mood varied by feeding mode, namely HAM-A scores were higher in the non-SSRI exposed, non-breastfeeding group at 3 months compared with anxiety ratings among non-SSRI exposed breastfeeding mothers. (Table 3). However, when HAM-A at 3 months was added to the reactivity ANCOVA or the CRS models as a covariate, the results remained unchanged. AUC did not differ significantly between groups or vary with maternal mood.
Figure 2.
Cortisol Stress Reactivity (CSR) Slope: SSRI exposure and feeding group * F[1,51]=13.056,p<0.001, partial η2=.266), for CRS differences between exposed/non-exposed breast feeding vs. non-breast feeding infants (controlling for maternal mood (EPDS) during 3rd trimester and parental stress report (PSI) at 3 months).
Table 3.
Maternal mood for prenatal exposure groups and feeding condition (mean, SD)
| SSRI Exposed |
Non-exposed |
|||
|---|---|---|---|---|
| Breast feeding at 3 months (n = 20) | Non-breast Feeding at 3 months (n = 11) | Breast feeding at 3 months (n = 37) | Non-breast feeding at 3 months (n = 8) | |
| Maternal age at study time (years) | 32.6 (5.2) | 31.45 (3.9) | 32.8 (4.5) | 37.38 (4.3) * |
| Maternal mood 2nd trimester | ||||
| Ham-A | 13.31(8.7) | 17.7 (5.8) | 6.9 (7.9) | 8.4 (7.5) |
| Ham-D | 12.1 (8.2) | 14.1 (2.8) | 5.5 (6.6) | 8.1 (10.9) |
| EPDS | 7.5 (4.9) | 16.1 (3.4) * | 4.7 (7.4) | 7.4 (8.0) |
| Maternal mood 3rd trimester | ||||
| Ham-A | 10.0 (6.6) | 15.3 (8.0) | 5.5 (4.7) | 5.3 (5.1) |
| Ham-D | 8.3 (5.5) | 12.6 (7.0) | 2.7 (4.7) | 3.3 (4.4) |
| EPDS | 7.0 (3.5) | 10.4 (6.4) | 4.4 (4.4) | 4.1 (3.3) |
| Maternal mood 3 months | ||||
| Ham-A | 9.5 (7.8) | 12.1 (6.4) | 3.3 (3.4) | 6.4 (5.0) * |
| Ham-D | 6.8 (5.5) | 7.9 (6.0) | 2.8 (3.5) | 4.8 (5.8) |
| EPDS | 6.7 (5.7) | 8.9 (4.6) | 3.5 (3.7) | 4.8 (4.8) |
HAM-A: Hamilton Rating Scale for Anxiety.
HAM-D: Hamilton Rating Scale for Depression.
EPDS: Edinburgh Postnatal Depression Scale.
SD: Standard Deviation.
3.2. Basal evening cortisol
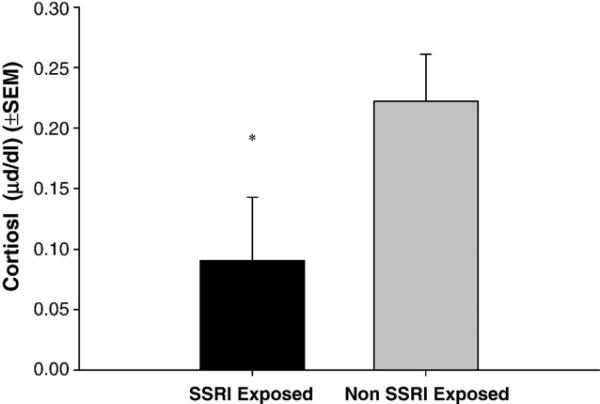
Late afternoon/early evening cortisol levels (Fig. 3) were significantly lower at 3 months in the SSRI exposed infants compared with the non-SSRI exposed infants, controlling for maternal mood and feeding condition (F[1,55] = 4.002; p=.050, partial η2 =.068).
Figure 3.
Late early evening salivary cortisol at 3 months: SSRI exposed vs. non-exposed infants * (F[1,55]=4.585; p=.037, partial η2=.077), controlling for 3rd trimester maternal mood and PSI score at 3 months and breast feeding at 3 months.
3.3. Pharmacological variables and cortisol levels
To examine the impact of postnatal drug exposure on cortisol reactivity and basal patterns, drug levels were obtained from the mother's plasma and breast milk, and from infants who continued to breastfeed (Table 4). Neither length of prenatal medication exposure (days), maternal plasma or breast milk, nor infant drug levels at 3 months were associated with mean overall cortisol levels (Baseline, S1 or S2), cortisol change scores (baseline to stress) or evening basal levels (p>0.05).
Table 4.
Postnatal drug exposure at 3 months: maternal, breast milk and infant drug levels (citalopram and venlafaxine levels were not obtained)
| Drug | Drug Levels (n, mean ng/dl) |
|---|---|
| Maternal | |
| • Fluoxetine (4) | 313.4(245.6) |
| • Norfluoxetine (4) | 301.8 (202.1) |
| • Paroxetine (7) | 135.96 (204.1) |
| • Sertraline (3) | 110.6(124.5) |
| Breast milk | |
| • Fluoxetine (4) | 136.6 (95.7) |
| • Norfluoxetine (4) | 89.8 (57.9) |
| • Paroxetine (5) (1 = <LLOQ) | 139.6 (141.3) |
| • Sertraline (3) | 191.9 (224.4) |
| Infant | |
| • Fluoxetine (3) | 1.69 (2.00) |
| • Norfluoxetine (3) | 13.09 (11.1) |
| • Paroxetine(6) | All <LLOQ |
| • Sertraline (2) (1 = <LLOQ) | 2.41 |
* LLOQ = below the Lower Limit of Quantitation (0.1 ng/ml).
4. Discussion
At 3 months of age, early evening basal cortisol levels were significantly lower in infants with prenatal SSRI exposure compared to non-SSRI exposed infants, even when controlling for feeding mode and maternal mood. Prenatal SSRI exposure altered HPA stress response patterns, but this only became apparent when infant feeding mode was accounted for. Namely, non-SSRI exposed, non-breastfed infants had significantly lower post-stress cortisol levels than non-SSRI exposed breastfed infants. Importantly, differences in cortisol stress-reactivity patterns emerged in non-SSRI exposed infants depending on breastfeeding condition, whereas among SSRI exposed infants cortisol response patterns were similar to that of non-SSRI exposed breastfed infants, regardless of feeding mode. Similarly, exposure and feeding group differences were also observed in the CRS slope measure, with significantly lower scores among non-breastfed infants compared with breastfed infants, but only in the non-SSRI exposed condition. While SSRI drug levels were detected at 3 months of age in the blood of a few breastfeeding infants, reflecting postnatal medication exposure, (especially in infants whose mothers were taking fluoxetine), infant drug levels were not associated with cortisol stress responses or basal cortisol levels, making it unlikely that the HPA patterns were a direct pharmacological effect. Total cortisol, reflected by the AUC measure, did not differ significantly between exposure groups. Pre and post-natal maternal mood alone, length of drug exposure, or drug levels did not directly influence HPA function. Moreover, while differences in mood during or following pregnancy were observed between breastfeeding and non-breastfeeding mothers, clinician-rated maternal anxiety (HAM-A) at 3 months did not appear to influence infant cortisol reactivity patterns.
In an animal model analogous to our study, prenatal SSRI exposure has been shown to influence HPA function via early alterations in 5HT levels. Ishiwata et al [17] reported that prenatally stressed mice treated during postnatal weeks 1–3 with fluoxetine showed increased 5HT turnover in the hippocampus during the prepubertal period and increased or “normalized” corticosterone responses to a subsequent stressor. Such exposure also restored the ability to learn spatial information compared with the effects of exposure to prenatal stress alone. This might be analogous to the increased HPA activity that is associated with depression, and that is “normalized” following SSRI antidepressant treatment [16]. Similarly, given the neurotrophic role of 5HT in the development of hippocampal GRs, the lower early evening cortisol levels in SSRI exposed infants may reflect an upregulation of GRs, leading to increased negative feedback later in the day. Conceivably, altered number or function of GRs following SSRI exposure, resulting in altered HPA feedback activity, could also account for differences in stress response patterns among the exposed non-breastfeeding infants. When compared to the cortisol response of non-SSRI exposed infants, it is possible that stress reactivity was “normalized” by prenatal SSRI exposure and in this way SSRIs might “buffer” the effects of environmental influences inherent to care-giving in the non-breastfed group. In other words, postnatal care-giving from a depressed mother, in combination with prenatal SSRI exposure (i.e. increased prenatal 5HT), might have altered GR function and buffered infant HPA activity, even in a stressful postnatal environment. Such a ‘correction’ may reflect the SSRI-induced hippocampal GR changes, as have been observed using a prenatal stress model in animals [17]. Furthermore, given that lower basal evening cortisol is typical of the daily circadian cortisol pattern (rise in morning and fall in afternoon), the lower evening cortisol observed in the SSRI exposed group might suggest an early emergence of the diurnal cortisol pattern which becomes established over the first 3–6 months of life [27].
Whether breastfeeding itself influences cortisol patterns, or whether breastfeeding is actually a proxy measure that reflects an unmeasured aspect inherent to the postnatal environment, such as parental responsiveness, remains to be determined. The HPA system is sensitive to variations in early care giving [28] and social regulation [29], and thus differences in cortisol stress reactivity between feeding groups in non-SSRI exposed infants may reflect the impact of early social experience, inherent to environmental differences between breastfeeding and non-breastfeeding practices. In rodent models, prenatal stress reduces hippocampal GRs and attenuates negative feedback [30], leading to an elevation in plasma corticosterone. Conversely, early ‘neonatal handling’ increases levels of early maternal licking and grooming, which in turn, increases hippocampal GR gene expression [31]. These changes are mediated via increased 5HT, and lead to reduced HPA stress reactivity (i.e. enhanced glucocorticoid negative feed back sensitivity) in infancy [7,31] that persists into adulthood [32,33]. In humans, neonates of women experiencing high levels of anger, depression or anxiety during pregnancy had increased urinary cortisol and decreased dopamine levels within 24 h of delivery [4,8].
Breastfeeding was accounted for in this study as a way to control for postnatal drug exposure. While this study was not specifically designed to examine effects of social environment on HPA development, feeding practice may be what Hofer has termed a “hidden regulator” of psychobiological development [34]. The impact of breastfeeding on human infant HPA function has, to the best of our knowledge, not been previously reported. Further study is required to examine the impact of care giving and feeding (handling, carrying, maternal interaction or dietary factors), as well as daily patterns of sleep and maternal mood on the developmental ontogeny of HPA function in early infancy.
In sum, our findings may suggest an early “programming” effect that reflects a combination of factors related to maternal mood, prenatal SSRI exposure and postnatal maternal care giving that appears to influence the developing HPA stress regulation system. Relationships between these factors are a complex and dynamic interplay that requires further study. SSRI antidepressants may have a role in managing antenatal maternal mood disorders and determining the benefits and risks for mothers and their offspring are beyond the scope of this study. As the HPA system plays a key role in emerging learning, behavior, cardiovascular, metabolic and immune functions across the lifespan, the long term functional implications of altered HPA function in early infancy need to be evaluated in a broader context. This initial report of the impact of prenatal SSRI exposure may offer insight into early programming of the HPA system, but these findings need to be replicated with a larger sample size, accounting for the impact of prenatal and postnatal care giving social environment, and examining outcomes beyond 3 months of age.
Acknowledgements
We are grateful to the mothers and their infants who participated and contributed to this work, as well as to Colleen Fitzgerald and Ursula Brain for their contributions in organizing and facilitating this research program. We also gratefully acknowledge the thoughtful comments provided by Ursula Brain, Jodi Pawluski, and Tracey Weir.
References
- 1.Laplante P, Diorio J, Meaney MJ. Serotonin regulates hippo-campal glucocorticoid receptor expression via a 5-HT7 receptor. Brain Res Dev Brain Res. 2002;139:199–203. doi: 10.1016/s0165-3806(02)00550-3. [DOI] [PubMed] [Google Scholar]
- 2.Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65:427–51. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- 3.Welberg LA, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13:113–28. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- 4.Field T, Diego M, Dieter J, Hernandez-Reif M, Schanberg S, Kuhn C, et al. Prenatal depression effects on the fetus and the newborn. Infant Behav Dev. 2004;27:216–29. doi: 10.1016/j.infbeh.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Van den Bergh BR, Van CB, Smits T, Van HS, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsycho-pharmacology. 2007 doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- 6.King JA. Perinatal stress and impairment of the stress response. Possible link to nonoptimal behavior. Ann N Y Acad Sci. 1996;794:104–12. doi: 10.1111/j.1749-6632.1996.tb32514.x. [DOI] [PubMed] [Google Scholar]
- 7.Francis D, Diorio J, Laplante P, Weaver S, Seckl JR, Meaney MJ. The role of early environmental events in regulating neuroendocrine development. Moms, pups, stress, and glucocorticoid receptors. Ann N Y Acad Sci. 1996;794:136–52. doi: 10.1111/j.1749-6632.1996.tb32517.x. [DOI] [PubMed] [Google Scholar]
- 8.Field T, Diego M, Hernandez-Reif M, Vera Y, Gil K, Schanberg S, et al. Prenatal maternal biochemistry predicts neonatal biochemistry. Int J Neurosci. 2004;114:933–45. doi: 10.1080/00207450490461305. [DOI] [PubMed] [Google Scholar]
- 9.Fuller RW. Mechanisms and functions of serotonin neuronal systems: opportunities for neuropeptide interactions. Ann N Y Acad Sci. 1996;780:176–84. doi: 10.1111/j.1749-6632.1996.tb15122.x. [DOI] [PubMed] [Google Scholar]
- 10.Lefebvre H, Contesse V, Delarue C, Feuilloley M, Hery F, Grise P, et al. Serotonin-induced stimulation of cortisol secretion from human adrenocortical tissue is mediated through activation of a serotonin4 receptor subtype. Neuroscience. 1992;47:999–1007. doi: 10.1016/0306-4522(92)90047-6. [DOI] [PubMed] [Google Scholar]
- 11.Peters DA. Maternal stress increases fetal brain and neonatal cerebral cortex 5-hydroxytryptamine synthesis in rats: a possible mechanism by which stress influences brain development. Pharmacol Biochem Behav. 1990;35:943–7. doi: 10.1016/0091-3057(90)90383-s. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi A, Nagaoka M, Yamada K, Ichitani Y, Miake Y, Okado N. Maternal stress induces synaptic loss and developmental disabilities of offspring. Int J Dev Neurosci. 1998;16:209–16. doi: 10.1016/s0736-5748(98)00028-8. [DOI] [PubMed] [Google Scholar]
- 13.Seckl JR, Fink G. Use of in situ hybridization to investigate the regulation of hippocampal corticosteroid receptors by monoamines. J Steroid Biochem Mol Biol. 1991;40:685–8. doi: 10.1016/0960-0760(91)90291-c. [DOI] [PubMed] [Google Scholar]
- 14.Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry. 2006;63:898–906. doi: 10.1001/archpsyc.63.8.898. [DOI] [PubMed] [Google Scholar]
- 15.Gillespie CF, Nemeroff CB. Hypercortisolemia and depression. Psychosom Med. 2005;67(Suppl 1):S26–8. doi: 10.1097/01.psy.0000163456.22154.d2. [DOI] [PubMed] [Google Scholar]
- 16.Barden N, Reul JM, Holsboer F. Do antidepressants stabilize mood through actions on the hypothalamic–pituitary–adreno-cortical system? Trends Neurosci. 1995;18:6–11. doi: 10.1016/0166-2236(95)93942-q. [DOI] [PubMed] [Google Scholar]
- 17.Ishiwata H, Shiga T, Okado N. Selective serotonin reuptake inhibitor treatment of early postnatal mice reverses their prenatal stress-induced brain dysfunction. Neuroscience. 2005;133:893–901. doi: 10.1016/j.neuroscience.2005.03.048. [DOI] [PubMed] [Google Scholar]
- 18.Whitaker-Azmitia PM, Druse M, Walker P, Lauder JM. Serotonin as a developmental signal. Behav Brain Res. 1996;73:19–29. doi: 10.1016/0166-4328(96)00071-x. [DOI] [PubMed] [Google Scholar]
- 19.Gunnar MR, Brodersen L, Krueger K, Rigatuso J. Dampening of adrenocortical responses during infancy: normative changes and individual differences. Child Dev. 1996;67:877–89. [PubMed] [Google Scholar]
- 20.Mayes LC, Bornstein MH, Chawarska K, Granger RH. Information processing and developmental assessments in 3-month-old infants exposed prenatally to cocaine. Pediatrics. 1995;95:539–45. [PubMed] [Google Scholar]
- 21.Mayes LC, Bornstein MH, Chawarska K, Haynes OM, Granger RH. Impaired regulation of arousal in 3-month-old infants exposed prenatally to cocaine and other drugs. Dev Psychopathol. 1996;8:29–42. [Google Scholar]
- 22.Prechtl HF. The behavioural states of the newborn infant (a review). Brain Res. 1974;76:185–212. doi: 10.1016/0006-8993(74)90454-5. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 25.Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. Development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1987;150:782–6. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 26.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 27.Spangler G. The emergence of adrenocortical circadian function in newborns and infants and its relationship to sleep, feeding and maternal adrenocortical activity. Early Hum Dev. 1991;25:197–208. doi: 10.1016/0378-3782(91)90116-k. [DOI] [PubMed] [Google Scholar]
- 28.Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- 29.Levine S. The ontogeny of the hypothalamic–pituitary–adrenal axis. The influence of maternal factors. Ann N Y Acad Sci. 1994;746:275–88. doi: 10.1111/j.1749-6632.1994.tb39245.x. [DOI] [PubMed] [Google Scholar]
- 30.Szuran TF, Pliska V, Pokorny J, Welzl H. Prenatal stress in rats: effects on plasma corticosterone, hippocampal glucocorticoid receptors, and maze performance. Physiol Behav. 2000;71:353–62. doi: 10.1016/s0031-9384(00)00351-6. [DOI] [PubMed] [Google Scholar]
- 31.Meaney MJ, Aitken DH, Vanberkel C, Bhatnagar S, Sapolsky RM. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–8. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- 32.Ladd CO, Owens MJ, Nemeroff CB. Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology. 1996;137:1212–8. doi: 10.1210/endo.137.4.8625891. [DOI] [PubMed] [Google Scholar]
- 33.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic–pituitary–adrenal responses to stress. Science. 1997;277:1659–62. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- 34.Hofer MA. Hidden regulatory processes in early social relationships. In: Bateson PPG, Klopfer PH, editors. Perspectives in Ethology: Social Behavior. Plenum Press; New York: 1978. pp. 135–66. [Google Scholar]