Summary
This study examined the relationship between children’s hair cortisol and socioeconomic status of the family, as measured by parental education and income. Low family socioeconomic status has traditionally been considered a long-term environmental stressor. Measurement of hair cortisol provides an integrated index of cumulative stress exposure across an extended period of time. The present study is the first to examine the relationship between hair cortisol and parental education as well as parental income in a representative sample of preschoolers.
Data on hair cortisol, family income, and parental education were collected for a representative sample of 339 children (Mean age = 4.6 years; SD = .5 years) from across 23 neighbourhoods of the city of Vancouver, Canada. As maternal education was shown previously to be associated with hair zinc level, hair zinc measurements were included as well in order to explore potential relationships between hair zinc and hair cortisol. The relationship between hair cortisol and parental education was examined using hierarchical regression, with hair zinc, gender, age, and single parenthood included as covariates.
Maternal and paternal education both were correlated significantly with hair cortisol (r = −0.18; p = .001). The relationship remained statistically significant even after controlling for all demographic covariates as well as for hair zinc and after taking the neighbourhood-level clustering of the data into account. Parental income, on the other hand, was not related significantly to children’s hair cortisol.
This study provides evidence that lower maternal and paternal education are associated with higher hair cortisol levels. As hair cortisol provides an integrated index of cortisol exposure over an extended time period, these findings suggest a possibly stable influence of SES on the function of the hypothalamic–pituitary–adrenal (HPA) axis. Cumulative exposure to cortisol during early childhood may be greater in children from low socio-economic backgrounds, possibly through increased exposure to environmental stressors.
Keywords: Hair cortisol, Preschoolers, Parental education, Socioeconomic, Stress
1. Introduction
Childhood experiences may have a life-long influence on the function of the hypothalamic–pituitary–adrenal (HPA) axis and thus on the regulation of cortisol. In children, differences in cortisol levels have been reported in relation to mother’s depressive symptoms (Lupien et al., 2001; Essex et al., 2002), childhood adversity (Carlson and Earls, 1997; Gunnar et al., 2001), and stressful neonatal (Grunau et al., 2007) and childhood (Flinn and England, 1997; Gunnar and Vazquez, 2001) environments. Moreover, socioeconomic status (SES) in childhood has been shown to influence cortisol secretion patterns (Lupien et al., 2000, 2005; Li et al., 2007). Both direct and indirect effects of factors such as pre- or postnatal nutrition, stress, early life adversity, maternal depression and/or environmental (school, home, neighbourhood) variables on HPA development could mediate the association between childhood SES and cortisol. Several studies have shown that cortisol levels are also associated with adult SES (Brandtstädter et al., 1991; Steptoe et al., 2003; Kristenson et al., 2004; Kunz-Ebrecht et al., 2004; Cohen et al., 2006a,b) and, in fact, gradients in cortisol levels have been shown according to income, education, and occupational status in adult life (Kunz-Ebrecht et al., 2004; Cohen et al., 2006b). Interestingly, one life course study suggested that associations between cortisol secretion patterns and SES in adulthood can be attributed, at least in part, to SES gradients in cortisol in childhood (Li et al., 2007).
Overall cortisol levels or secretion patterns in childhood are important because they are related to trajectories of physical, cognitive and socio-emotional development. Although the direction of association may vary, it has been demonstrated that growth and adiposity trajectories from age 7 to 45 years are associated with cortisol secretion patterns (Power et al., 2006). Moreover, associations between cortisol and childhood cognition have been observed in several studies including the 1958 British Birth Cohort (Power et al., 2008) and in a cross-sectional sample of 53 Spanish children aged 9–12 years (Maldonado et al., 2008), reflecting the fact that the brain is a major target for the glucocorticoid hormones. Importantly, the relationship is bidirectional, with effects of cortisol on cognitive function and, conversely, effects of cognitive processing on cortisol secretion (Lupien et al., 2002, 2005). The HPA axis is also thought to play a role in socio-emotional development and psychological state (Goodyer et al., 2001; Herbert et al., 2006). Associations between emotional state and cortisol have been observed in infancy (Gunnar and Nelson, 1994; van Bakel and Riksen-Walraven, 2004; Lewis and Ramsay, 2005) and childhood (McBurnett et al., 2000; Shirtcliff et al., 2005; Herbert et al., 2006; Brummelte et al., 2010). One longitudinal study found that preschoolers exposed to high levels of maternal stress had elevated cortisol levels and, in turn, exhibited greater mental health symptoms in first grade (Essex et al., 2002). Over the life course, the HPA axis may become dysregulated due to social stressors (Power et al., 2010), as suggested by studies of low SES and of maltreatment in childhood (Carlson and Earls, 1997; Lupien et al., 2000; Cicchetti and Rogosch, 2001; Gunnar et al., 2001; Gunnar and Vazquez, 2001; Ranjit et al., 2005; O’Connor et al., 2009). In turn, dysregulation of the HPA axis may lead to either hypo- or hyper-secretion of cortisol, carrying increased risk of depression and other mental health problems (Bremmer et al., 2007).
Taken together, this research on the determinants of HPA axis function, cortisol secretion patterns, and life course health and development has generated a tantalizing glimpse of the ‘life’ of a key stress response pathway in human society. However, our understanding of the significance of the HPA axis for human health and development has been hindered to some extent because of limitations in the available methods of cortisol measurement. Accordingly, a principal challenge for studies that seek to understand the relationships among HPA axis function, social factors, life course and health is the method of estimation of cortisol secretion patterns. Cortisol levels in biological samples such as plasma and especially, saliva, have commonly been used as markers of daily HPA axis function and, also, of the HPA response to experimental stressors. However, in the life course context, even multi-time-point, multi-day sampling of saliva or plasma cannot provide a full picture of long-term HPA axis function and cortisol secretion.
In recent years, methods to assay cortisol in hair have been developed that provide an integrated index of cumulative cortisol exposure over an extended period of time. Raul et al. (2004), in the context of tracking illegal use of performance-enhancing steroids by athletes, demonstrated that physiological concentrations of cortisol and cortisone could be detected in human hair. Further studies demonstrated that hair cortisol levels correlate significantly with cortisol in a 24-hr urine sample (Sauvé et al., 2007) and with contemporaneously collected salivary cortisol (D’Anna-Hernandez et al., 2011). Moreover, hair cortisol was shown to correlate positively with waist-to-hip ratio, suggesting that hair cortisol reflects cortisol exposure over the long term at tissue levels (Manenschijn et al., 2011). Subsequent data have supported the use of hair cortisol as an integrated measure of long-term HPA activity. To cite just a few examples, adults with severe chronic non-malignant pain syndromes were found to have increased subjectively perceived stress, as measured by the Perceived Stress Scale questionnaire, and concomitantly, had elevated hair cortisol levels compared to control subjects, suggesting that hair cortisol levels could be a novel biomarker for long-term stress exposure (Van Uum et al., 2008). Steudte et al. (2011) showed that hair cortisol levels were significantly higher in traumatized (from a civil war area of Northern Uganda) individuals with post-traumatic stress disorder (PTSD) than in traumatized individuals without PTSD, suggesting that PTSD in traumatized individuals who continue to live under stressful conditions might reflect general hypercortisolism. Yamada et al. (2007) showed that whereas hair cortisol levels did not distinguish between preterm and term neonates in neonatal intensive care units, they did correlate with number of days on a ventilator in the term born infants, and thus might be a marker of chronic neonatal stress. Hair cortisol levels were also shown to be a useful marker of hypercortisolism in alcohol-dependent individuals (Stalder et al., 2010). Three- to four-fold higher cortisol levels were seen in hair samples from alcoholics during acute withdrawal than in those of abstinent alcoholics or controls. These findings paralleled those of previous work using well-established measures of systemic cortisol secretion. Thus, in a variety of settings, and in both adults and infants, hair cortisol appears to provide an ideal measure to monitor cumulative cortisol release over an extended period of time; providing an integrated measure of exposure to stress and, also, to longer-term HPA axis function. Moreover, hair requires no special handling or storage. Therefore hair provides an ideal tissue for large-scale surveys of prolonged stress levels.
In the literature, stress is also commonly associated with socioeconomic status background. Studies have repeatedly found a correlation between SES (income, education, occupational status) and developmental health outcomes, and researchers therefore speak of the ‘SES gradient of developmental health’ (Keating and Hertzman, 1999). Multiple studies have described the underlying causal mechanisms that associate low SES – poverty in particular – with poor developmental outcomes (e.g., Berliner, 2005). In the context of this study, it is important to note that income and education have been found to be correlated with stress-preventing or -buffering family mechanisms and parenting processes, such as social support, warmth/caring combined with appropriate control/supervision, providing healthy nutrition and regular routines, family violence, unclear and/or age-inappropriate expectations (Dornbusch et al., 1987). These family mechanisms and parenting processes, in turn, are known to have direct impact on the developmental health and well-being of children (Baumrind, 1966; Bronfenbrenner, 2005; Luthar, 2006). Parental education and income may therefore be considered indicator variables of environmental stressors, as they gauge the availability of resources in a family context (e.g., time, money, access to information), and some of these resources represent necessary, though not sufficient, prerequisites for a number of positive family mechanisms and parenting practices (e.g., time to be with children, money to provide safe housing and healthy nutrition and activities, knowledge about child development/parenting).
The aim of the present study was to examine whether in young children, hair cortisol, as an integrated measure of cortisol exposure across an extended period of time is associated with parental SES. Hair cortisol was assessed in relation to gender, parental income and education, family structure, ethnicity, and socioeconomic characteristics of the neighbourhood of residence. Our previous community-based survey of hair zinc, conducted among 342 children age 4–6 years in an ethnically and socioeconomically diverse urban environment (Vaghri et al., 2008, 2010), demonstrated an association between maternal education and hair zinc. Hair zinc is considered a biological marker of long-term adequate zinc nutrition; that is, children who have an inadequate zinc intake have lower hair zinc levels than children who have adequate zinc intake (Prasad, 1988). Given the relationship between nutrition and SES (Sandstead, 1973), hair zinc provided an index of adequacy of zinc nutrition to complement the environmental factors described above and to explore the possible relationships between hair zinc and hair cortisol.
2. Methods
2.1. Sample
The final sample consisted of 339 children (49% boys; 51% girls; mean age = 4.6 years, SD = 0.5 years) who were recruited from child care centres located in the city of Vancouver, British Columbia, Canada. Below, we provide details on recruitment, inclusion, and exclusion criteria.
In Section 3, we present descriptive statistics for the full sample. For the multivariate analyses, we only included cases with complete data on all variables (n = 273 for the analysis for maternal education; n = 269 for the analysis with paternal education). The cases with missing data did not differ statistically from the cases with complete data on any predictor or outcome variable.
2.2. Study design
The study was designed as a citywide cross-sectional survey, representing all 23 social planning neighbourhoods of the city of Vancouver, BC, Canada. A comprehensive list of licensed child-care and preschool centres was the sampling frame and source of study subjects. Data from the national census showed that there were wide variations in SES both between and within social planning neighbourhoods. Accordingly, neighbourhoods were broken down into smaller units referred to as dissemination areas (DA), the basic unit of Canada’s census geography composed of one or more neighbouring blocks having a population of 400–700 people. Maps of each neighbourhood were produced with the DAs colour-coded to indicate their SES, such that the potential participating centres (child-care and preschool centres) could be accounted for by both neighbourhood and DA. Quota sampling was then used to achieve representation of each colour (geographic SES) when selecting centres for participation.
Centres were contacted by telephone and were asked about their willingness to participate in the survey. In total, 55 centres agreed to participate (55/68 = 81% response rate), while 13 refused. With each refusal a new centre, within the same neighbourhood DA or within another DA of that neighbourhood with similar SES, was selected for contact. Following initial contact, survey information packages were mailed to the centres, containing a letter from the principal investigator explaining the project, along with a schematic presentation outlining the sequence of the survey events and their allotted time intervals. Subsequently, meetings were held with each participating centre, during which the survey and the survey team were introduced to the child-care professionals, the time line for the survey and the activities involved were discussed, and the initial letter of contact was given to the centres to be sent out to the parents in one week’s time. One to two weeks after the letter of initial contact, survey packages containing a subject information letter and a consent form, as well as the survey questionnaire were sent to the children’s homes. During the next two weeks, parents returned completed survey packages to the centre. Response rate of parents varied from one neighbourhood to another within the range of 16–80% with the overall response rate of 30.3% for the survey. Following this, visits to the centres were scheduled during which anthropometric measurements and hair samples were obtained from the consenting children. The survey was conducted over a period of six weeks, between March and June.
The study was approved by the ethics board on human research of the University of British Columbia. Participating parents signed a consent form following an information session during which the study procedures and objectives were explained.
Inclusion criteria
The inclusion criteria for our survey were: (1) residency in the city of Vancouver, (2) being in generally good health, and (3) child aged 41–71 months.
Exclusion criteria
If the child had been diagnosed with any serious or chronic disease, and/or major sensory/cognitive/motor developmental disability, and/or was currently suffering or recovering from common infections such as flu, cough, or diarrhoea, the child was excluded.
2.3. Measures
2.3.1. Hair sample collection
Hair samples were originally collected for a study of zinc levels, using methods described previously in Vaghri et al. (2008). Hair samples were taken with stainless steel scissors, which were wiped with ethanol swabs between subjects to avoid cross contamination. Following the protocol proposed by the International Atomic Energy Agency (Deppisch et al., 1999), which is adopted by the Center of Disease Control and the US Environmental Protection Agency (EPA), hair samples were cut from three or four locations at the back of the head, and only the first 1–2 cm of hair proximal to the scalp were used for analysis.
2.4. Biochemical analyses
2.4.1. Hair cortisol
Hair samples were washed twice with 12 ml of isopropanol for two minutes and air-dried for 48 h. Each dried hair sample was pulverized using a ball mill (Retsch, MM301) at 25 Hz for 2 min. Approximately 30 mg of ground hair powder were transferred to a pre-weighed 2 ml micro-centrifuge tube and re-weighed. Methanol (1.5 ml) was added and the tube was rotated on the RPI Mix-All Laboratory Tube Mixer for 24 h at room temperature. The sample was then centrifuged at 3500 rpm for 15 min, and 1.4 ml of supernatant was aliquotted into a clean tube. Another 1.5 ml methanol was added to the sample tube and the whole extraction process was repeated. The supernatant was pooled and allowed to air dry for 48 h to ensure complete evaporation of the solvent. It was then reconstituted with 250 μL of the Salimetric salivary cortisol assay diluent and analysed in duplicate by ELISA using the High Sensitivity Salivary Cortisol Immunoassay Kit (Cat# 1–3002, Salimetrics, Pennsylvania), per the manufacturer’s instructions. The intra and inter-assay coefficients of variation were 3.65% and 6.41% respectively. Cortisol levels are expressed as ng/mg hair.
2.4.2. Hair zinc
Hair samples were collected into labelled coin-envelopes, and sent to JR Labs, Burnaby, British Columbia. The samples were processed and analysed for zinc content by inductively coupled plasma mass spectrometry (Puchyr et al., 1998). They were analysed as single samples, with a duplicate analysis for every 10th sample. The within assay CV for this method was 3.3% at a mean of 175.3 μg zinc/g hair, while the between assay CV was 6.0% at a mean of 157.7 μg zinc/g hair.
2.4.3. Demographic information
Information on children’s gender and age (in months), single parent status, neighbourhood of residence, ethnicity of parents, maternal and paternal education, and parental annual income in Canadian dollars was obtained via a survey filled out by the parent(s) of the child. In 94.5% of the cases, the person completing the questionnaire was the mother.
2.4.4. Neighbourhood-level socioeconomic status
SES for the 23 neighbourhoods of Vancouver was measured via an index based on information obtained from Statistics Canada’s 2006 census and 2004 tax-filer data. Although samples were collected at child-care centres, the children’s residential postal codes were used to place them in their neighbourhood of residence. The SES index represents a continuous composite calculated from the following variables: average wealth of families with children; employment rate; residential stability; poverty (average female only income for families with children under age 6 years); lone parent rate; housing density; population diversity (percentage of foreign language at home; percentage of population first generation in Canada); and percentage of women in manufacturing. This index was created by the Human Early Learning Partnership (2009) from those census and tax-filer variables that most strongly correlated with neighbourhood variations in early child development outcomes for children aged 5 years as measured by the Early Development Instrument (EDI) (Guhn et al., 2007; Janus and Offord, 2007; Human Early Learning Partnership, 2009; Guhn et al., 2011).
2.5. Statistical analyses
Data were analysed with the statistical software package PASW/SPSS, version 18 (SPSS Inc., Chicago, IL). First, the hair cortisol data were examined for outliers, defined as any value three standard deviations above the mean. Outlier values were winsorized following Tukey’s method (Tukey, 1977) and retained for data analysis. Winsorized cortisol values were log transformed for statistical analyses; however, actual cortisol values prior to log transformation are displayed graphically for ease of interpretation.
Mixed Model Analyses (using the procedure MIXED) were used to examine the relationship between hair cortisol (as the dependent variable) and the child-level (level 1) predictor variables maternal education (analysis 1) or paternal education (analysis 2), and hair zinc. Demographic child-level variables (age, gender, ethnicity, parental annual income, single parent status) were included as covariates at level 1 (child-level). The variable ‘Neighbourhood’ – indicating the neighbourhood of residence for each child – was used as the grouping variable to take into account the geographically nested structure of the data (i.e., taking into account the fact that children live in different neighbourhoods). In order to examine any potential neighbourhood effects of neighbourhood-level SES on children’s hair cortisol, the neighbourhood-level SES index variable was used as a predictor of the neighbourhood intercept at level 2 (neighbourhood-level).
3. Results
Table 1 shows the descriptive characteristics for the child-level factors age, gender, single parent status, maternal and paternal education, ethnicity, family income and hair zinc in relation to hair cortisol. Given its subsequent importance in the analysis, we additionally illustrate the relationship between the four categories of maternal education and the absolute cortisol values in Fig. 1 (as bar graph, mean ± standard error of the mean [SEM]). We note that the relationship between paternal education and children’s hair cortisol is very similar.
Table 1.
Descriptives of the child-level variables.
| Predictors/covariates | n | Cortisol (ng/mg hair) | SD | |
|---|---|---|---|---|
| Gender | Boys | 167 | 0.021 | 0.04 |
| Girls | 172 | 0.019 | 0.04 | |
| Age | <51 months | 85 | 0.024 | 0.04 |
| 51–55 months | 85 | 0.022 | 0.05 | |
| 56–60 months | 84 | 0.017 | 0.02 | |
| >60 months | 85 | 0.018 | 0.03 | |
| Ethnicity | Caucasian | 150 | 0.018 | 0.03 |
| Chinese | 98 | 0.022 | 0.03 | |
| East Indian | 25 | 0.024 | 0.06 | |
| Other ethnicities | 45 | 0.019 | 0.03 | |
| Mixed | 21 | 0.024 | 0.07 | |
| Maternal education | Less than high school | 25 | 0.050 | 0.09 |
| High school | 33 | 0.030 | 0.05 | |
| Some college or university | 90 | 0.017 | 0.02 | |
| University or college degree | 185 | 0.016 | 0.03 | |
| Missing | 6 | |||
| Paternal education | Less than high school | 31 | 0.037 | 0.06 |
| High school | 34 | 0.013 | 0.01 | |
| Some college or university | 81 | 0.025 | 0.04 | |
| University or college degree | 186 | 0.017 | 0.03 | |
| Missing | 7 | |||
| Parental annual income | <20,000 | 30 | 0.031 | 0.07 |
| 20,000–39,000 | 56 | 0.018 | 0.02 | |
| 40,000–60,000 | 46 | 0.019 | 0.03 | |
| >60,000 | 143 | 0.018 | 0.03 | |
| Missing | 64 | |||
| Parenthood | Two parents | 297 | 0.020 | 0.04 |
| Single parent | 42 | 0.020 | 0.05 | |
| Hair zinc | Below 110a | 126 | 0.019 | 0.03 |
| Above 110 | 213 | 0.021 | 0.04 |
Hair zinc of 110 μg/g (1.68 μmol/g) is the accepted cutoff for seasons with shortest daylight (Gibson et al., 1989).
Figure 1.
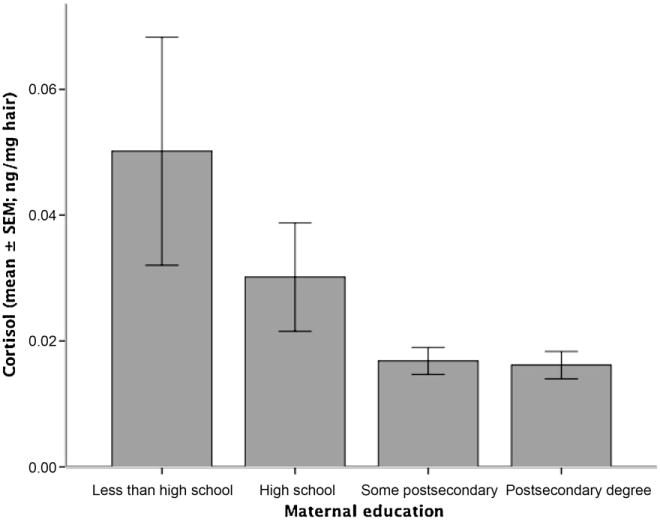
Mean absolute cortisol values for four categories of maternal education. Legend: nmol: nanomol. The width of the error bars reflects different group sizes (see Table 1).
3.1. Associations of hair cortisol with socioeconomic variables and hair zinc
Hair zinc, maternal education, and paternal education were significantly correlated with hair cortisol. No other variables were significantly correlated with hair cortisol. Given that some of the predictor variables and covariates correlated significantly amongst each other – that is, maternal and paternal education were correlated significantly with each other (p < 0.001), as well as with annual income (p < 0.001) and with single parent status (p = 0.02), and hair zinc was correlated significantly with age (p = 0.04) and single parent status (p = 0.001) – mixed model analyses were conducted to take into account the multi-collinearity and the (geographically) nested structure of the data (i.e., children living in different neighbourhoods). Table 2 shows the zero-order correlations between the winsorized and log-transformed cortisol variable with the predictor variables and the covariates included in the mixed model analyses.
Table 2.
Correlations of the log-transformed, winsorized cortisol variable with predictor variables and covariates.
| n | Correlation coefficients (p-values) | |
|---|---|---|
| Maternal educationb | 333 | −0.18 (0.001) |
| Paternal educationb | 332 | −0.18 (0.001) |
| Hair zinca | 339 | −0.15 (0.006) |
| Agea | 339 | −0.09 (0.098) |
| Genderc | 339 | −0.01 (0.790) |
| Parenthood statusc | 339 | 0.01 (0.920) |
| Annual family incomeb | 275 | −0.07 (0.235) |
Legend: n: number of children in the respective subgroups; p-values: values for statistical probability. The categories for the variables are shown in Table 1 (e.g., maternal education: Less than high school = 1; high school = 2; some college or university = 3; University or college degree = 4).
Bold font indicates significant p-values.
Pearson correlations were calculated for the continuous variables.
Polyserial correlations were calculated for the ordinal variables.
Biserial correlations were calculated for the dichotomous variables.
3.2. Mixed model analyses
3.2.1. Parental education, hair zinc, and covariates
Table 3 shows the results (raw coefficients and p-values) from the mixed model analyses. Results for analysis 1 with maternal education as the predictor variable are shown in the centre column, and for analysis 2 with paternal education as the predictor variable are shown on the right. As can be seen, parental education was a significant predictor of hair cortisol in both analyses, and the coefficients and p-values were equal, regardless of whether maternal or paternal education was used as the predictor variable. Also, in both analyses, hair zinc is significantly related with hair cortisol, with similar coefficients and p-values.
Table 3.
(Raw) coefficients and p-values from the mixed model analyses.
| Predictors/covariates | Analysis 1 (maternal education)
|
Analysis 1 (paternal education)
|
||
|---|---|---|---|---|
| (Raw) coefficient | p | (Raw) coefficient | p | |
| na | 273 | 269 | ||
| Parental education | −0.137 | 0.01 | −0.140 | 0.01 |
| Hair zinc | −0.003 | 0.02 | −0.003 | 0.01 |
| Age | −0.013 | 0.06 | −0.010 | 0.16 |
| Gender | −0.022 | 0.81 | −0.050 | 0.59 |
| Parenthood (single vs. two parents) | 0.163 | 0.28 | 0.185 | 0.22 |
| Parental annual income | 0.009 | 0.86 | 0.010 | 0.84 |
Legend: n: number of children in the respective subgroups; p: statistical probability value; coefficients and p-values below the statistical significance level of p = 0.05 are bolded.
Analysis 1: Multiple hierarchical regression analysis, with hair cortisol as dependent variable, and maternal education and other variables (i.e., hair zinc, age, etc.) as independent variables.
Analysis 2: Multiple hierarchical regression analysis, with hair cortisol as dependent variable, and paternal education and other variables (i.e., hair zinc, age, etc.) as independent variables.
The categories for the variables are shown in Table 1 (e.g., parental education: less than high school = 1; high school = 2; some college or university = 3; University or college degree = 4).
The multiple regression used only cases that had complete data (i.e., no missing data on any predictor variable).
3.2.2. Ethnicity
We ran the analyses with and without four dummy variables representing the five categories of ethnicity. The ethnicity variables did not significantly correlate with hair cortisol values and regardless of whether the ethnicity variables were included in the analyses or not, the coefficients for all other predictor variables and covariates remained of equal size. Therefore, we chose to show only the results from the analyses without the ethnicity variables, because the inclusion of the four dummy variables reduced the power – and thus affected the p-values – of our analyses.
3.2.3. Neighbourhood-level socioeconomic status
The neighbourhood-level SES index variable did not significantly covary with differences in neighbourhood-level intercepts (i.e., neighbourhood average values) of hair cortisol. We note that a neighbourhood sample of 23 represents a lower bound of the sample size range that is considered adequate for hierarchical analyses. A larger neighbourhood sample would have yielded higher statistical power, and would have resulted in a higher chance of detecting a neighbourhood effect. For the present analyses, it is important to highlight that the coefficients for the child-level predictors had equal size and significance levels regardless of whether a hierarchical linear regression or a (non-hierarchical) multiple regression was performed. In other words, the results remained robust across different analytic strategies.
4. Discussion
This study is the first to examine hair cortisol, as an integrated index of stress over an extended period of time, and its relationship to social determinants in a community sample of young children. The results indicate that parental education – as indicated by maternal or paternal education – and hair zinc both have significant inverse associations with hair cortisol. These relationships remained significant even after controlling for covariates, such as the child’s age, gender, ethnicity, parental income, and single parenthood. As noted, hair zinc is considered a biological marker of long-term adequate zinc nutrition (Prasad, 1988), and there is an established relationship between nutrition and SES (Sandstead, 1973). In this context, an inverse association of hair zinc and cortisol may reflect some level of chronic stress in these children. Parental education represents a proxy variable that has been found to correlate with family mechanisms and parenting practices (e.g., authoritative parenting versus laissez-faire or authoritarian parenting; see Dornbusch et al., 1987) that, in turn, have an impact on children’s developmental health (Baumrind, 1966). In particular, it has been found that parents with higher education backgrounds are more likely to use authoritative parenting (high warmth, paired with high expectations, support, and control), which is associated with higher social and cognitive problem solving skills in children, and with fewer stress-associated problem behaviours and mental disorders (Baumrind, 1966). These associations are compatible with our findings insofar as higher parental education is associated with lower cortisol levels, and this association may be due to an association between parental education and stress-buffering or stress-reducing parenting styles.
The association of hair cortisol with parental education and with hair zinc remained significant after taking into account the geographically nested structure of the data (i.e., children living in different neighbourhoods). This finding suggests that these relationships are not associated with, or caused by, any unmeasured neighbourhood-level variables. In other words, the relationships are most likely not spurious relationships of unknown geographically varying factors, because the geographical clustering in the statistical analyses did not account for a significant amount of variance.
During early development in humans, cortisol activity is sensitive to social regulation. Under conditions of sensitive and responsive care-giving, cortisol secretion patterns may be affected and shaped by children’s attachment behaviours and distress reactions to and from caregivers (Gunnar and Donzella, 2002). When young children are exposed to less sensitive and responsive care, cortisol secretion patterns may, instead, be exaggerated, usually presenting as hyperresponsiveness to stresses. However, other patterns, such as hypo-responsiveness, may also occur, especially under adverse conditions that persist over years or decades. Our results suggest that parental education, and in particular increasing maternal education, is associated with reduced cumulative cortisol secretion over the long-term (Davis-Kean, 2005). In support, the literature presents an abundance of evidence indicating the positive effect of maternal education on various aspects of children’s lives, including social and cognitive development (Kohen et al., 2002; Davis-Kean, 2005), emotional well-being and physical health (Zill et al., 1995; Zill, 1996), and nutrition (North and Emmett, 2000). Moreover, our results are consistent with two recent reviews suggesting that parental education is more important than income as a determinant of child development because it strongly predicts the quality of interaction between parents and children (Davis-Kean, 2005; Carneiro et al., 2007).
We found a significant relationship between long-term stress (hair cortisol) and zinc nutrition (hair zinc). Marginal zinc deficiency (MZD) as indicated by low hair zinc level is a very early stage in the spectrum of zinc deficiency when the body’s zinc supply is chronically and marginally short of meeting physiological needs (Prasad, 1988). MZD is a nutritional assault that, similar to many other nutritional deficiencies, can result in physiological stress in the human body. It would be of value to investigate to what extent this deficiency and its root causes (i.e., poor nutrition or malnutrition) are associated with – and thus indicative of – other environmental stressors. For example, in view of the fact that rich sources of zinc in the diet are animal-source foods and often much more expensive than plant-source foods, it may be the case that children who experience poor zinc nutrition are also more likely to live in family environments where the financial capacity of the family is limited. Caregivers’ financial hardship may also create an environment in which children may have less time with their parents and/or caregivers, and may even experience less caring or less interactive relationships with them. Such associations between MZD and family environment, which has been documented for other nutritional deficiencies (Drotar and Eckerle, 1989), remains to be further explored.
It is important to note that while statistically significant, the associations between hair cortisol and both zinc levels and socioeconomic factors, were relatively weak. This may reflect the relatively high living standards of the Canadian setting involved. We would expect these associations to be more pronounced in conditions of greater social adversity and/or a sample with greater variation in SES. This study, as the first conducted utilizing the hair cortisol status of a community-based sample of preschoolers, provides a first estimate of hair cortisol reference values, at least for healthy Canadian preschoolers. Due to its relatively large sample size, and given that the participants were healthy children, this study may also serve as a starting point for reference values for healthy preschoolers of other wealthy societies.
In generalizing the findings of this study, some limitations should be kept in mind. Firstly, by using the preschool and day-care centres as the source of recruitment, we have excluded children of stay-at-home mothers who are not utilizing these services. Secondly, the overall response rate was 30%, with 70% of the parents receiving our survey package choosing not to respond. We did not have any systematic way of comparing the characteristics of children of stay-at-home mothers with those in preschool and group child-care settings, or of non-respondents with respondents.
In summary, this work has reiterated the significance of parental education on child health, while reaffirming the role of parental education in attenuating or buffering long-term cumulative stress levels of the child. Associations observed between hair cortisol and hair zinc levels of healthy children in a community setting warrant further research to shed light on the mechanism of this relationship.
Acknowledgments
Supported by a grant from the Human Early Learning Partnership (HELP), The University of British Columbia, Vancouver, Canada. We are grateful to the children who participated in this study.
Role of the funding source
Research was supported by a grant from the Human Early Learning Partnership (HELP), The University of British Columbia, Vancouver, Canada.
Dr. Weinberg’s research is currently funded by grants from NIH/NIAAA, the Coast Capital Savings Depression Research Fund, and the Canadian Foundation for Fetal Alcohol Research. She receives honoraria from NIH for grant reviews. Dr. Grunau’s research is currently funded by grants from NIH/NICHD and CIHR. She receives salary support from the Child and Family Research Institute.
Footnotes
Endnote
As described in the text, the correlation between maternal education and the winsorized and log-transformed cortisol values is statistically significant. In the graph, we illustrate the group means for each for the four groups of maternal education, using the means of the (not-winsorized, not-log-transformed) cortisol values. We note that due to the small cell sizes of the ‘less than high school’ and ‘high school’ groups, a Bonferroni post hoc ANOVA analysis does not show statistical significance between the individual groups shown in the graph; however, if we combine the ‘less than high school’ and ‘high school’ groups into one group (n = 58), it is significantly different from the group ‘post-secondary degree’ (n = 185; p = 0.004).
Conflicts of interest
All the authors report no potential conflicts of interest.
References
- Baumrind D. Effects of authoritative parental control on child behavior. Child Dev. 1966;37:887–907. [Google Scholar]
- Berliner DC. Our impoverished view of educational reform. Teachers College Record. 2005 Retrieved from http://www.tcrecord.org/content.asp?contentid=12106.
- Brandtstädter J, Baltes-Götz B, Kirschbaum C, Hellhammer D. Developmental and personality correlates of adrenocortical activity as indexed by salivary cortisol, observations in the age range of 35–65 years. J Psychosom Res. 1991;35:173–185. doi: 10.1016/0022-3999(91)90072-v. [DOI] [PubMed] [Google Scholar]
- Bremmer MA, Deeg DJ, Beekman AT, Penninx BW, Lips P, Hoogendijk WJ. Major depression in late life is associated with both hypo- and hypercortisolemia. Biol Psychiatry. 2007;62:479–486. doi: 10.1016/j.biopsych.2006.11.033. [DOI] [PubMed] [Google Scholar]
- Bronfenbrenner U, editor. Making Human Beings Human Bioecological Perspectives on Human Development. Sage; Thousand Oaks, CA: 2005. [Google Scholar]
- Brummelte S, Grunau RE, Zaidman-Zait A, Weinberg J, Nordstokke D, Cepeda IL. Cortisol levels in relation to maternal interaction and child internalizing behavior in preterm and full term children at 18 months corrected age. Dev Psychobiol. 2010 doi: 10.1002/dev.20511. Published online 28/10/2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Ann NY Acad Sci. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Carneiro P, Meghir C, Parey M. Maternal Education, Home Environments and the Development of Children and Adolescents. The Institute for Fiscal Studies. 2007 WP15/07. [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Dev Psychopathol. 2001;13:783–804. [PubMed] [Google Scholar]
- Cohen S, Doyle WJ, Baum A. Socioeconomic status is associated with stress hormones. Psychosom Med. 2006a;68:414–420. doi: 10.1097/01.psy.0000221236.37158.b9. [DOI] [PubMed] [Google Scholar]
- Cohen S, Schwartz JE, Epel E, Kirschbaum C, Sidney S, Seeman T. Socioeconomic status, race, and diurnal cortisol decline in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2006b;68:41–50. doi: 10.1097/01.psy.0000195967.51768.ea. [DOI] [PubMed] [Google Scholar]
- D’Anna-Hernandez KL, Ross RG, Natvig CL, Laudenslager ML. Hair cortisol levels as a retrospective marker of hypothalamic-pituitary axis activity throughout pregnancy: comparison to salivary cortisol. Physiol Behav. 2011;104(2):348–353. doi: 10.1016/j.physbeh.2011.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Kean PA. The influence of parent education and family income on child achievement: the indirect role of parental expectations and the home environment. J Fam Psychol. 2005;19(2):294–304. doi: 10.1037/0893-3200.19.2.294. [DOI] [PubMed] [Google Scholar]
- Deppisch LM, Centeno JA, Gemmel DJ, Torres NL. Andrew Jackson’s exposure to mercury and lead: poisoned president? JAMA. 1999;282(6):569–571. doi: 10.1001/jama.282.6.569. [DOI] [PubMed] [Google Scholar]
- Dornbusch SM, Ritter PL, Leiderman H, Roberts DF, Fraleigh MJ. The relation of parenting style to adolescent school performance. Child Dev. 1987;58(5):1244–1257. doi: 10.1111/j.1467-8624.1987.tb01455.x. Special Issue on Schools and Development (October, 1987). [DOI] [PubMed] [Google Scholar]
- Drotar D, Eckerle D. The family environment in nonorganic failure to thrive: a controlled study. J Pediatr Psychol. 1989;14(2):245–257. doi: 10.1093/jpepsy/14.2.245. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biol Psych. 2002;52(8):776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Flinn MV, England BG. Social economics of childhood glucocorticoid stress response and health. Am J Phys Anthropol. 1997;102:33–53. doi: 10.1002/(SICI)1096-8644(199701)102:1<33::AID-AJPA4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Gibson RS, Ferguson EF, Vanderkooy PD, MacDonald AC. Seasonal variations in hair zinc concentrations in Canadian and African children. Sci Total Environ. 1989;84:291–298. doi: 10.1016/0048-9697(89)90391-4. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Park RJ, Netherton CM, Herbert J. Possible role of cortisol and dehydroepiandrosterone in human development and psychopathology. Br J Psychiatry. 2001;179:243–249. doi: 10.1192/bjp.179.3.243. [DOI] [PubMed] [Google Scholar]
- Grunau RE, Haley DW, Whitfield MF, Weinberg J, Yu W, Thiessen P. Altered basal cortisol levels at 3, 6, 8 and 18 months in infants born extremely low gestational age. J Pediatr. 2007;150(2):151–156. doi: 10.1016/j.jpeds.2006.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guhn Janus M, Hertzman M, Eds C. Special issue: the early development instrument. Early Educ Dev. 2007;17:369–570. [Google Scholar]
- Guhn Zumbo M, Janus BD, Hertzman M, Eds C. Special issue: validation theory and research for a population-level measure of children’s development, wellbeing, and school readiness. Soc Indicators Res. 2011;103:179–325. [Google Scholar]
- Gunnar MR, Nelson CA. Event-related potentials in year-old infants: relations with emotionality and cortisol. Child Dev. 1994;65:80–94. [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisholm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Dev Psychopathol. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Donzella B. Social regulation of the cortisol levels in early human development. Psychoneuroendocrinology. 2002;27:199–220. doi: 10.1016/s0306-4530(01)00045-2. [DOI] [PubMed] [Google Scholar]
- Herbert J, Goodyer IM, Grossman AB, Hastings MH, de Kloet ER, Lightman SL, Lupien SJ, Roozendaal B, Seckl JR. Do corticosteroids damage the brain? J Neuroendocrinol. 2006;18:393–411. doi: 10.1111/j.1365-2826.2006.01429.x. [DOI] [PubMed] [Google Scholar]
- Human Early Learning Partnership. SES mapping package. 2009 Retrieved on October 2009. http://earlylearning.ubc.ca/media/uploads/mapsets/ses_t1t2/BC_SES/BC_SES_T1T2.pdf.
- Janus M, Offord D. Psychometric properties of the Early Development Instrument (EDI): a teacher-completed measure of children’s readiness to learn at school entry. Can J Behav Sci. 2007;39(1):1–22. [Google Scholar]
- Keating DP, Hertzman C, editors. Developmental Health and Wealth of Nations. Guilford; New York: 1999. [Google Scholar]
- Kohen DE, Brooks-Gunn J, Leventhal T, Hertzman C. Neighbourhood income and physical and social disorder in Canada: associations with young children’s competencies. Child Dev. 2002;73:1844–1860. doi: 10.1111/1467-8624.t01-1-00510. [DOI] [PubMed] [Google Scholar]
- Kristenson M, Eriksen HR, Sluiter JK, Starke D, Ursin H. Psychobiological mechanisms of socioeconomic differences in health. Soc Sci Med. 2004;58:1511–1522. doi: 10.1016/S0277-9536(03)00353-8. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Kirschbaum C, Steptoe A. Work stress, socioeconomic status and neuroendocrine activation over the working day. Soc Sci Med. 2004;58:1523–1530. doi: 10.1016/S0277-9536(03)00347-2. [DOI] [PubMed] [Google Scholar]
- Lewis M, Ramsay D. Infant emotional and cortisol responses to goal blockage. Child Dev. 2005;76:518–530. doi: 10.1111/j.1467-8624.2005.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Power C, Kelly S, Kirschbaum C, Hertzman C. Lifetime socioeconomic position and cortisol patterns in mid-life. Psychoneuroendocrinology. 2007;32:824–833. doi: 10.1016/j.psyneuen.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, King S, Meaney MJ, McEwen BS. Child’s stress hormone levels correlate with mother’s socioeconomic status and depressive state. Biol Psychiatry. 2000;48:976–980. doi: 10.1016/s0006-3223(00)00965-3. [DOI] [PubMed] [Google Scholar]
- Lupien S, King S, Meaney MJ, McEwen BS. Can poverty get under your skin?: basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol. 2001;13:651–674. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Wilkinson CW, Brière S, Ménard C, Ng Ying Kin NM, Nair NP. The modulatory effects of corticosteroids on cognition: studies in young human populations. Psychoneuroendocrinology. 2002;27(3):401–416. doi: 10.1016/s0306-4530(01)00061-0. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, Fiocco A, Wan N, Maheu F, Lord C, Schramek T, Tu MT. Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology. 2005;30:225–242. doi: 10.1016/j.psyneuen.2004.08.003. [DOI] [PubMed] [Google Scholar]
- Luthar SS. Resilience in development: a synthesis of research across five decades. In: Cicchetti D, Cohen DJ, editors. Developmental Psychopathology: Risk, Disorder, and Adaptation. Wiley; New York: 2006. pp. 740–795. [Google Scholar]
- Maldonado E, Fernandez FJ, Trianes MV, Wesnes K, Petrini O, Zangara A, Enguix A, Ambrosetti L. Cognitive performance and morning levels of salivary cortisol and alpha-amylase in children reporting high vs. low daily stress perception. Span J Psychol. 2008;11:3–15. doi: 10.1017/s1138741600004066. [DOI] [PubMed] [Google Scholar]
- Manenschijn L, van Kruysbergen Rulanda GPM, de Jong FH, Koper JW, van Rossum EFC. Shift work at young age is associated with elevated long-term cortisol levels and body mass index. J Clin Endocrin Metab. 2011 doi: 10.1210/jc.2011-1551. First published ahead of print August 31, 2011 as. [DOI] [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Arch Gen Psychiatry. 2000;57:38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- North K, Emmett P. Multivariate analysis of diet among three-year-old children and associations with socio-demographic characteristics. The Avon Longitudinal Study of Pregnancy and Childhood (ALSPAC) Study Team. Eur J Clin Nutr. 2000;54(1):73–80. doi: 10.1038/sj.ejcn.1600896. [DOI] [PubMed] [Google Scholar]
- O’Connor DB, Hendrickx H, Dadd T, Elliman TD, Willis TA, Talbot D, Mayes AE, Thethi K, Powell J, Dye L. Cortisol awakening rise in middle-aged women in relation to psychological stress. Psychoneuroendocrinology. 2009;34:1486–1494. doi: 10.1016/j.psyneuen.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Power C, Li L, Hertzman C. Associations of early growth and adult adiposity with patterns of salivary cortisol in adulthood. J Clin Endocrinol Metab. 2006;91:4264–4270. doi: 10.1210/jc.2006-0625. [DOI] [PubMed] [Google Scholar]
- Power C, Li L, Hertzman C. Cognitive development and cortisol patterns in mid-life: findings from a British birth cohort. Psychoneuroendocrinology. 2008;33(4):530–539. doi: 10.1016/j.psyneuen.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Power C, Li L, Atherton K, Hertzman C. Psychological health throughout life and adult cortisol patterns at age 45 yr. Psychoneuroendocrinology. 2010;36(1):87–97. doi: 10.1016/j.psyneuen.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Prasad A. Zinc in growth and development and spectrum of human zinc deficiency. J Am Coll Nutr. 1988;7:377–384. doi: 10.1080/07315724.1988.10720255. [DOI] [PubMed] [Google Scholar]
- Puchyr RF, Bass DA, Gajewski R, Calvin M, Marquardt W, Urek K, Druyan ME, Quig D. Preparation of hair for measurement of elements by inductively coupled plasma-mass spectrometry (ICP-MS) Biol Trace Element Res. 1998;62:167–182. doi: 10.1007/BF02783969. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Young EA, Kaplan GA. Material hardship alters the diurnal rhythm of salivary cortisol. Int J Epidemiol. 2005;34:1138–1143. doi: 10.1093/ije/dyi120. [DOI] [PubMed] [Google Scholar]
- Raul JS, Cirimele V, Ludes B, Kintz P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin Biochem. 2004;37:1105–1111. doi: 10.1016/j.clinbiochem.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Sandstead HH. Zinc nutrition in the United States. Am J Clin Nutr. 1973;26:1251–1260. doi: 10.1093/ajcn/26.11.1251. [DOI] [PubMed] [Google Scholar]
- Sauvé B, Koren G, Walsh G, Tokmakejian S, Van Uum SHM. Measurement of cortisol in human hair as a biomarker of systemic exposure. Clin Invest Med. 2007;30(5):E183–E191. doi: 10.25011/cim.v30i5.2894. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Dev Psychopathol. 2005;17:167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Kunz-Ebrecht S, Owen N, Feldman PJ, Willemsen G, Kirschbaum C, Marmot M. Socioeconomic status and stress-related biological responses over the working day. Psychosom Med. 2003;65:461–470. doi: 10.1097/01.psy.0000035717.78650.a1. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Heinze K, Steudte S, Foley P, Tietze A, Dettenborn L, et al. Use of hair cortisol analysis to detect hypercortisolism during active drinking phases in alcohol-dependent individuals. Biol Psychol. 2010 doi: 10.1016/j.biopsycho.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Steudte S, Kolassa IT, Stalder T, Pfeiffer A, Kirschbaum C, Elbert T. Increased cortisol concentrations in hair of severely traumatized Ugandan individuals with PTSD. Psychoneuroendocrinology. 2011;36(8):1193–1200. doi: 10.1016/j.psyneuen.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Tukey JW. Exploratory Data Analysis. Addison-Wesley; Don Mills, ON: 1977. [Google Scholar]
- Vaghri Z, Barr S, Wong H, Chapman G, Hertzman C. Age-based differences in hair zinc of Vancouver preschoolers. Biol Trace Elem Res. 2008;126:21–30. doi: 10.1007/s12011-008-8215-7. [DOI] [PubMed] [Google Scholar]
- Vaghri Z, Barr S, Wong H, Chapman G, Hertzman C. Hair zinc status and determinants of hair zinc of Vancouver preschoolers and the usefulness of these determinants as a screening tool for marginal zinc deficiency. In: Berhardt LV, editor. Advances in Medicine and Biology. Vol. 1. Nova Science Publishers, Inc; 2010. pp. 1–48. Expiry date of publication: 2010 2nd Quarter. [Google Scholar]
- van Bakel HJ, Riksen-Walraven JM. Stress reactivity in 15-month-old infants: links with infant temperament, cognitive competence, and attachment security. Dev Psychobiol. 2004;44:157–167. doi: 10.1002/dev.20001. [DOI] [PubMed] [Google Scholar]
- Van Uum SH, Sauvé B, Fraser LA, Morley-Forster P, Paul TL, Koren G. Elevated content of cortisol in hair of patients with severe chronic pain: a novel biomarker for stress. Stress. 2008;11(6):483–488. doi: 10.1080/10253890801887388. [DOI] [PubMed] [Google Scholar]
- Yamada J, Stevens B, de Silva N, Gibbins S, Beyene J, Taddio A, Newman C, Koren G. Hair cortisol as a potential biologic marker of chronic stress in hospitalized neonates. Neonatology. 2007;92(1):42–49. doi: 10.1159/000100085. Epub 2007 March 14. [DOI] [PubMed] [Google Scholar]
- Zill N. Parental schooling and children’s health. Public Health Rep. 1996;111:34–43. [PMC free article] [PubMed] [Google Scholar]
- Zill N, Coffins M, West J, Germino-Hausken E. National Center for Education Statistics. Washington, DC: 1995. Approaching Kindergarten: a Look at Preschoolers in the US Department of Education. [Google Scholar]


