Abstract
Alcohol dependence leads to persistent neuroadaptations, potentially related to structural plasticity. Previous work has shown that hippocampal neurogenesis is modulated by alcohol, but effects of chronic alcohol on neurogenesis in the forebrain subventricular zone (SVZ) have not been reported. Effects in this region may be relevant for the impairments in olfactory discrimination present in alcoholism. Here, we examined the effects of prolonged alcohol dependence on neurogenesis. Rats were sacrificed directly after 7 wk of intermittent alcohol vapour exposure, or 3, 7 or 21 d into abstinence. Proliferation was assessed using BrdU and Ki67 immunoreactivity, newly differentiated neurons (neurogenesis) as doublecortin-immunoreactivity (DCX-IR), and neural stem cells using the SOX2 marker. In the dentate gyrus, chronic dependence resulted in a pattern similar to that previously reported for acute alcohol exposure : proliferation and neurogenesis were suppressed by the end of exposure, rebounded on day 3 of abstinence, and returned to control levels by days 7 and 21. In the SVZ, proliferation was also suppressed at the end of alcohol exposure, followed by a proliferation burst 3 d into abstinence. However, in this area, there was a trend for reduced proliferation on days 7 and 21 of abstinence, and this was accompanied by significant suppression of DCX-IR, indicating a long-term suppression of forebrain neurogenesis. Finally, a decrease in the SOX2 stem cell marker was detected at days 7 and 21, suggesting long-term reduction of the SVZ stem cell pool. While suppression of hippocampal neurogenesis by alcohol dependence is transient, the suppression in the forebrain SVZ appears long-lasting.
Keywords: Adult neurogenesis, animal model, alcoholism, immunohistochemistry, plasticity
Introduction
Alcoholism develops following repeated and prolonged episodes of brain exposure to intoxicating levels of alcohol. Accordingly, prolonged brain alcohol exposure in rodents triggers lasting behavioural changes that parallel features of the clinical syndrome, including a persistent escalation of alcohol self-administration (Rimondini et al. 2002; Roberts et al. 2000). Models using repeated cycles of intoxication and withdrawal mimic the course of the clinical condition, and are most effective for inducing escalation of alcohol intake (O’Dell et al. 2004; Rimondini et al. 2002). Alcohol intake in post-dependent animals is sensitive to acamprosate, an approved alcoholism treatment, while alcohol intake of non-dependent rats is unaffected by this medication (Rimondini et al. 2002). Moreover, similar to the clinical condition (Gilman & Hommer, 2008), the post-dependent state is characterized by a persistently up-regulated behavioural sensitivity to stress (Sommer et al. 2008), while basal levels of circulating glucocorticoids are normal (Rimondini et al. 2002). Thus, neuroadaptive processes induced by prolonged exposure to cycles of intoxication and withdrawal parallel those in human alcoholism, and might be informative for human pathophysiology.
Adult neurogenesis is a mechanism of neuronal plasticity (Alvarez-Buylla & Lim, 2004; Curtis et al. 2007; Eriksson et al. 1998; Gould, 2007). A population of neural precursors is found in the dentate gyrus subgranular zone (SGZ). Neurogenesis in this region has been implicated in regulation of stress reactivity, and is itself suppressed by stress (Gould et al. 1997; Mirescu & Gould, 2006). Similar to stress, chronic alcohol self-administration by mice reduces hippocampal neurogenesis and induces depression-like behaviour, consequences that are reversed by the antidepressant fluoxetine (Stevenson et al. 2009). However, effects of alcohol on hippocampal neurogenesis are complex, and different effects have been reported depending on species, dose, pattern of intake and time following brain exposure (Aberg et al. 2005; Nixon, 2006; Nixon & Crews, 2004). The other major source of neuronal progenitors in the adult central nervous system is the forebrain subventricular zone (SVZ). Both in rodents and humans, cells that originate from progenitors in the SVZ migrate to become GABAergic and dopaminergic neurons in the olfactory bulb, where they contribute to learning, associating, and discriminating odours (Curtis et al. 2007; Wilson et al. 2004). Impaired olfactory discrimination has been found in human alcoholics (Rupp et al. 2003), but long-term effects of chronic alcohol on neurogenesis in the SVZ have to our knowledge not been reported previously.
Here, we enquired whether adult neurogenesis in the SGZ and the SVZ is altered following a prolonged history of dependence, and whether any changes persist into protracted abstinence. We exposed rats to 7 wk of daily intermittent cycles of alcohol vapour intoxication and withdrawal using a model that reliably produces alcohol dependence and a lasting post-dependent state (Heilig et al. 2009; Heilig & Koob, 2007). Cell proliferation, differentiation of new cells into neurons, and stem cell populations were studied at a series of time-points, covering a period from intoxication to 3 wk of abstinence.
Methods
Animals
Male Wistar rats (Møllegård, Denmark) weighing 220–250 g at the beginning of the experiment, were housed four per cage under a reversed 12-h light/dark cycle (lights on 23:00 hours) with free access to food and water. All experiments were approved by the Stockholm South Animal Ethics Committee (permits S84/98).
Alcohol vapour exposure
Vapour exposure was used because it allows a high degree of control over brain alcohol exposure at pharmacologically active levels, and induces behavioural and molecular changes relevant for the pathophysiology of alcoholism (Heilig & Koob, 2007). A total of 53 exposed animals and 24 matched controls were used. Exposure was done as described previously (Rimondini et al. 2002). Briefly, stainless-steel and glass chambers were used, and alcohol was pumped into heated stainless-steel coils connected to the airflow. Final alcohol concentration was adjusted by changing the pump flow, and was monitored via a spectrometer. Exposure was for 17 h during each 24-h period (on 16:00 hours, off 09:00 hours). Rats were allowed to habituate to the chambers for 1 wk, then exposed to a low alcohol concentration for 1 wk, and finally exposed to alcohol vapour to induce dependence for 7 wk. Control animals were kept with normal airflow. Each week all rats were weighed, and random subjects (n=6–8) were tested for blood alcohol concentration. Blood was collected from the lateral tail vein, and serum assayed for alcohol using an NAD/NADPH-spectrophotometric assay kit (Sigma Aldrich Inc., USA) according to the manufacturer’s instructions.
To confirm behavioural consequences of exposure, 10 exposed and eight control animals were randomly selected from the respective batch, and tested for voluntary alcohol consumption 3 wk after exposure. Two-bottle, free-choice, continuous access alcohol in 0.2% saccharin vs. 0.2% saccharin-only was assessed after a 3-wk resting period that followed the last exposure cycle as described. One week was allowed to gradually increase the alcohol concentration to 6% (w/v), i.e. 3 d with 2% and 4 d with 4% alcohol, followed by a 2-wk testing period. These animals were not used for the histological analysis because of the possibility that their differential voluntary alcohol consumption may confound estimates of proliferation and neurogenesis. The consumption data have constituted a part of a previous publication (Sommer et al. 2008).
To determine the effects of chronic alcohol dependence and abstinence, multiple time-points were studied: at the end of 7 wk daily alcohol exposure, and after 3, 7 and 21 d of abstinence.
5′-bromo-2-deoxyuridine (BrdU) labelling
To allow comparison to previously published work (Nixon & Crews, 2002) while maintaining temporal specificity needed to interpret effects on cell proliferation, rats were injected intraperitoneally (i.p.) with a single dose of BrdU nucleotide (200 mg in 0.9% saline/kg; Sigma, USA) immediately upon completion of the 7-wk exposure (day 0) or on days 3, 7 or 21 of abstinence (n=3–9/time-point). BrdU injections were performed starting at 09:00 hours (light off). Non-exposed controls (n=3–4/time-point) were injected in parallel. Animals were sacrificed 5 h after BrdU injection.
Immunohistochemistry
Rats were anaesthetized with a lethal dose of pentobarbital (100 mg/kg i.p.), and perfused intracardially with 0.1 M phosphate-buffered saline (PBS) followed by 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4). Brains were removed, post-fixed for 48 h in the same fixative and transferred to 0.1 M PBS solution. Forty μm coronal vibratome forebrain sections (Bregma +2.5 mm to −7.5 mm) were collected in cryoprotectant solution (30% ethylene glycol, 30% glycerol in 40 mM PB; pH 7.4) in 1:12 series and stored at −20 °C. Every sixth section/animal (each section 240 μm apart) was kept for analysis of BrdU immunoreactivity (IR), an established marker of cell proliferation. Because BrdU staining may be affected by factors other than proliferation rates, such as differences in BrdU availability caused by altered blood flow or in blood–brain barrier permeability, we also used the endogenous marker of the cell cycle, Ki67 (Gerdes et al. 1984) on adjacent sections. Every twelfth section/animal (each section 480 μm apart) was kept for DCX immunohistochemistry to detect newly differentiated neurons (Brown et al. 2003; Gleeson et al. 1999; Rao & Shetty, 2004), and immunohistochemistry for the high mobility group transcription factor SOX2, to label neuronal stem cells. SOX2 expression is largely restricted to neural stem cells, is known to be expressed within neural progenitors throughout adulthood (Brazel et al. 2005) and is necessary for neural stem cell maintenance and survival (Episkopou, 2005). SOX2 may be expressed in GFAP-positive astroglia ; however, the majority of SVZ progenitors are negative for GFAP (Komitova & Eriksson, 2004).
Immunohistochemistry of free-floating brain sections was performed as described previously (Nixon & Crews, 2004), using monoclonal anti-mouse BrdU antibody (1 :2000, MAB3424, Chemicon, USA), monoclonal mouse anti-Ki67 [1 :200; Novocastra Laboratories, UK (no. NCL-Ki67-MM1)], polyclonal goat anti-doublecortin antibody (anti-DCX, 1: 400, SC8066, Santa Cruz Biotechnology, USA) and polyclonal rabbit anti-Sry-related high-mobility group box 2 antibody (anti-SOX2, 1: 200, AB5770, Chemicon). To control for day-to-day variability, controls and alcohol-exposed animals for their respective time-point were always processed in parallel.
Quantification of BrdU, Ki67, DCX and SOX2 immunoreactivities
In the SGZ (Bregma −1.8 to −5.6 mm), BrdU-IR-positive cells were counted at 60× (oil immersion objective), while Ki67-IR and DCX-IR were counted at 40× magnification. Cell counts were obtained as number of IR positive cells/mm2 using the Bioquant Life Science Image Analysis System (USA). It has previously been established under similar conditions that cell counts obtained by this methodology result in estimates that are highly concordant with those obtained using stereology (Crews et al. 2004). In the SVZ (Bregma +1.0 to +0.2 mm), cell counts were obtained for Ki67-IR cells, while the densities of BrdU-, DCX- and SOX2-positive cells were too high to lend themselves to cell counting. We therefore first established, in a subset of sections, that optical density measurements were highly correlated with cell counts (r=0.92, p<0.0001, n=24), in agreement with a published comparison of these two measures within the dentate gyrus (Crews et al. 2004). Densitometry was then performed bilaterally, in 40× magnification, in 2×6 squares (25×25 μm) per section over Bregma levels +1.6 to −0.4 mm, and yielded mean integrated optical densities (IOD). Exposed and control animals sacrificed at their respective time-points were always processed in parallel.
Statistical analysis
The data met assumptions of normality and homogeneity of variances. For each marker within each region, unexposed controls from the respective time-points were first compared for possible differences due to batch variation of staining efficiency. When no differences were found (p>0.10), controls were pooled, and data were analysed by one-way ANOVA, and the respective alcohol-exposed group was compared to the pooled control group using Dunnett’s post-hoc test. This was true for all markers within the dentate gyrus, and BrdU, Ki67 and SOX2 in the SVZ. DCX-IR within the SVZ showed significant batch-to-batch variation. We approached this in two different ways. A widely used method to overcome batch variation is to normalize experimental values to control from the same batch, which allows for valid comparisons between batches (Walker, 2006). Following this approach, data from each batch were normalized to their respective controls, after which analysis proceeded as described above. To examine the robustness of this approach, we also analysed the data using a two-way ANOVA of raw, non-normalized optical densities, with time (0, 3, 7, 21 d) and treatment (alcohol or air) as factors, followed by post-hoc comparison of the respective alcohol-exposed group and its corresponding control at each time-point using Newman–Keuls test. The results of the two approaches were highly concordant. Both sets are provided in the Results section, and the normalized data are shown in Fig. 4a. In all analyses, p<0.05 was considered to be significant.
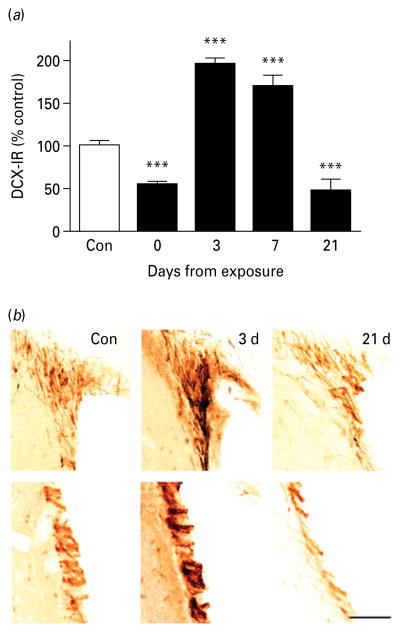
Fig. 4.
Neurogenesis in the subventricular zone (SVZ) during protracted abstinence. (a) Data showing doublecortin-immunoreactivity (DCX-IR) as normalized mean integrated optical density (IOD; mean±S.E.M.) in the SVZ at varying intervals after the last intoxication cycle (■) and in controls (□; absolute value 306±46, n=15). DCX-IR varied as a function of time (p<0.00001). It was suppressed immediately following exposure (day 0, n=8), rebounded to elevated levels on day 3 (n=8) and day 7 (n=8), and was again suppressed on day 21 (n=3). *** p<0.001 vs. controls. (For detailed statistics, see Results section.) (b) Illustrative brightfield photomicrographs of SVZ DCX-IR neurons at varying intervals following exposure. Scale bar, 80 μm; Bregma level=+1.0 to +0.2 mm.
Results
Dependence induction
Similar to our previous experiments (Rimondini et al. 2002), exposure induced blood alcohol concentrations (BACs) in the range of 150–300 mg/dl, which fell to undetectable levels within 5 h during the alcohol-off period. Signs of mild withdrawal, such as tail stiffness and piloerection, were observed during the off intervals by the end of the 7-wk exposure period, but withdrawal intensity never reached seizure levels. As reported previously (Sommer et al. 2008), exposed animals showed a more than 2-fold increase of voluntary alcohol intake compared to controls (3.8±0.35 vs. 1.44±0.28 g/kg.d, mean±S.E.M. ; F1,20=12.7, p<0.001).
Dentate gyrus
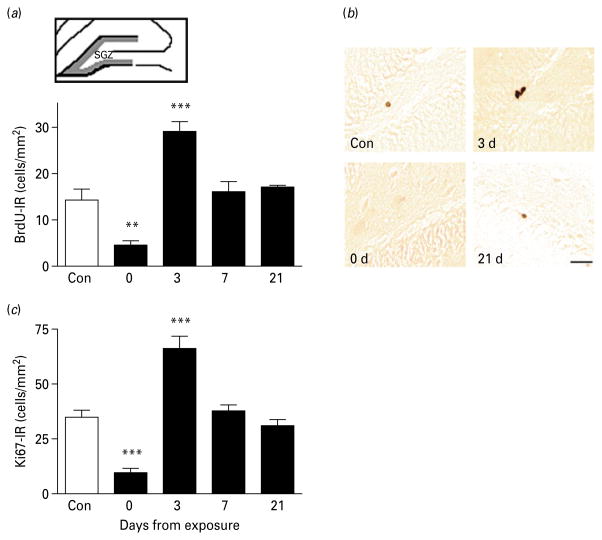
In the dentate gyrus, the number of BrdU-IR cells varied as a function of time from exposure (Fig. 1; main effect: F4,38=11.8, p<0.0001). Post-hoc analysis showed that numbers of BrdU-IR cells were decreased at the end of alcohol exposure (day 0, p<0.01). This was followed by a burst of proliferation on day 3 (p<0.001). Proliferating cells returned to control levels on days 7 and 21 of abstinence (Fig. 1a, b). The correlation of BrdU-IR and Ki67-IR was high (R=0.61, p<0.0001), and Ki67-IR followed a temporal pattern that closely paralleled that of BrdU-IR (Fig. 1c ; main effect of time: F4,39=25.3, p<0.0001).
Fig. 1.
Hippocampal cell proliferation during protracted abstinence. (a) Top: schematic illustration of the sampled area (grey outline) for BrdU-immunoreactive (IR) cell counting of the dentate gyrus subgranular zone (SGZ) in a coronal rat section (adapted from Vaidya et al. 2007). Bottom: data showing the number of BrdU-IR positive cells/mm2 (mean±S.E.M.) in the SGZ at various time-points after the last intoxication cycle (■) and in alcohol-naive controls (□, n=16). The number of BrdU-positive cells varied as a function of time (p<0.0001), and was decreased immediately after alcohol exposure (day 0, n=9), followed by a rebound burst on day 3 (n=8), returning to normal levels on day 7 (n=8) and day 21 (n=3). (b) Illustrative brightfield photomicrographs showing clusters of BrdU-positive cells in the SGZ at the different time-intervals. Scale bar, 30 μm; Bregma level=−1.8 to −5.6 mm according to Paxinos & Watson (2005). (c) Data showing the number of Ki67-IR positive cells/mm2 (mean±S.E.M.) at the respective time-point after the last intoxication cycle [■, n=9 (day 0), n=8 (day 3), n=8 (day 7), n=3 (day 21)] and in alcohol-naive controls (□, n=16). ** p<0.01, *** p<0.001 vs. controls. (For detailed statistics, see Results section.)
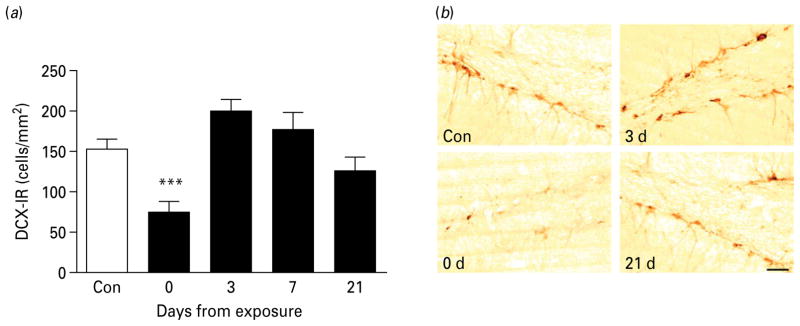
DCX-IR also varied as a function of time (Fig. 2; main effect: F4,40=8.5, p<0.0001). Similar to its effect on proliferation, chronic alcohol exposure decreased DCX-IR cells by the end of the exposure (day 0, p<0.001). There was a statistically non-significant trend (p=0.09) towards an increase on day 3. DCX-IR returned to control level after 7 and 21 d of abstinence.
Fig. 2.
Hippocampal neurogenesis during protracted abstinence. (a) Data showing the number of newly differentiated, doublecortin-immunoreactive (DCX-IR) cells/mm2 (mean±S.E.M.) in the subgranular zone (SGZ) at varying intervals after the last intoxication cycle (■) and alcohol-naive controls (□, n=16). The number of positive cells varied as a function of time (p<0.0001). DCX-IR cells were markedly decreased immediately following exposure (day 0, n=9), showed a trend level rebound (day 3, n=8), and then returned to levels that did not differ from controls (day 7, n=8; day 21, n=3). *** p<0.001 vs. controls. (For detailed statistics, see Results section.) (b) Illustrative brightfield photomicrographs of DCX-IR-labelled cells within the SGZ at the different time-intervals. Scale bar, 30 μm; Bregma level=−1.8 to −5.6 mm.
SVZ
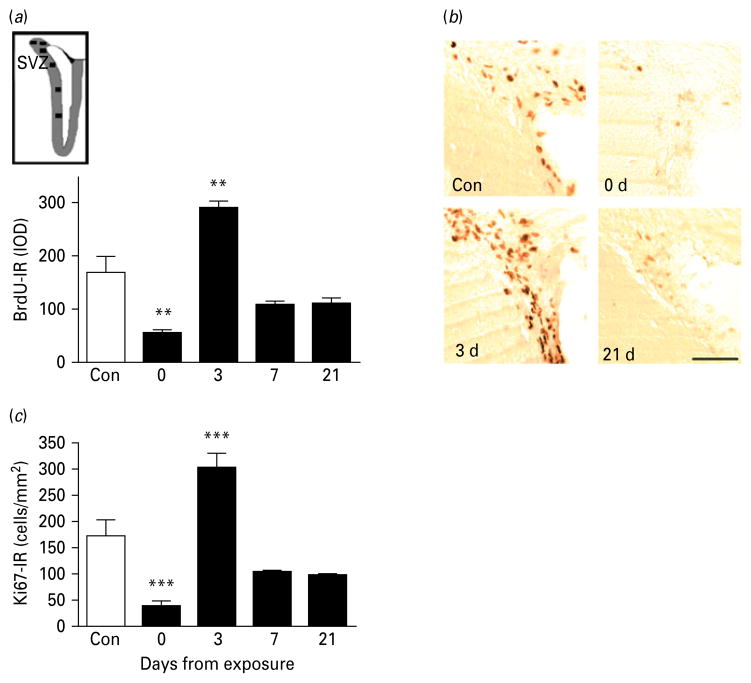
Proliferating cells within the forebrain SVZ were detected as a band of BrdU-IR cells outlining the frontal aspect of the lateral ventricles [Fig. 3a (inset), and b]. Proliferation was potently and biphasically modulated by chronic alcohol exposure (Fig. 3a ; main effect of time: F4,38=12.0, p<0.0001). Controls showed large numbers of BrdU-IR cells along the ventricles, that were markedly reduced by the end of alcohol exposure (p<0.001). A pronounced peak of proliferation compared to control levels was found 3 d into abstinence (p<0.001), followed by a decrease to levels that were numerically lower, but did not significantly differ from controls after 7 d as well as 21 d. The correlation of BrdU-IR and Ki67-IR was high also in this region (R=0.60, p<0.0001), and Ki67-IR followed a temporal pattern that closely paralleled that of BrdU-IR (Fig. 3c ; main effect of time: F4,40=4.5, p=0.00002).
Fig. 3.
Cell proliferation in the subventricular zone (SVZ) during protracted abstinence. (a) Top: schematic illustration representing the sampled areas (■) within the SVZ (grey outline) for densitometric evaluation of 5′-bromo-2-deoxyuridine-immunoreactivity (BrdU-IR) in coronal rat sections (adapted from Vaidya et al. 2007). Bottom: data showing mean integrated optical densities (IOD; mean±S.E.M.) of BrdU-IR-positive cells in relation to alcohol-naive controls (□, n=16) at different time-points after the last intoxication cycle (■). BrdU-IR varied as a function of time (p<0.001). BrdU-IR cells were markedly suppressed immediately following exposure (day 0, n=8), followed by a rebound burst on day 3 (n=8), and finally a return to levels on day 7 (n=8) and 21 (n=3) that were numerically lower than controls, although the individual post-hoc comparisons failed to reach significance. ** p<0.01 vs. controls. (For detailed statistics, see Results section.) (b) Illustrative brightfield photomicrographs showing clusters of BrdU-IR-positive proliferating cells in the SVZ at the different time-points. Scale bar, 80 μm; Bregma level=+1.0 to +0.2 mm. (c) Data showing mean integrated optical densities (IOD; mean±S.E.M.) from Ki67-IR at various time-points after the last intoxication cycle (■, n=9 (day 0), n=8 (day 3), n=8 (day 7), n=3 (day 21)] and in alcohol-naive controls (□, n=16).
One-way ANOVA of normalized DCX-IR values in the SVZ showed a biphasic variation as a function of time (Fig. 4; main effect: F4,37=57.2, p<0.00001), with chronic alcohol reducing DCX-IR at the end of exposure (p<0.001), followed by a doubling of DCX-IR at 3 d (p<0.001), with an increase still present on day 7 (p<0.001). In contrast, DXC-IR was reduced to levels below those of controls on day 21 (p<0.001, Fig. 4). Because this analysis used normalized optical densities to address batch variation in staining intensity, we also carried out a two-way ANOVA with time following exposure, and treatment as factors, on raw optical density data not subjected to normalization. Using this approach, there was also a significant effect of time (F3,34=18.5, p<0.00001), and a significant time×treatment interaction (F3,34=32.0, p<0.00001). Once again, post-hoc analysis showed that DCX-IR levels in alcohol-exposed animals were lower than their respective controls upon termination of exposure (p<0.001), higher than controls on day 3 (p<0.001) and day 7 (p<0.01), and finally decreased below control values on day 21 (p<0.01). Thus, analysis of normalized and raw optical density data yielded essentially identical results.
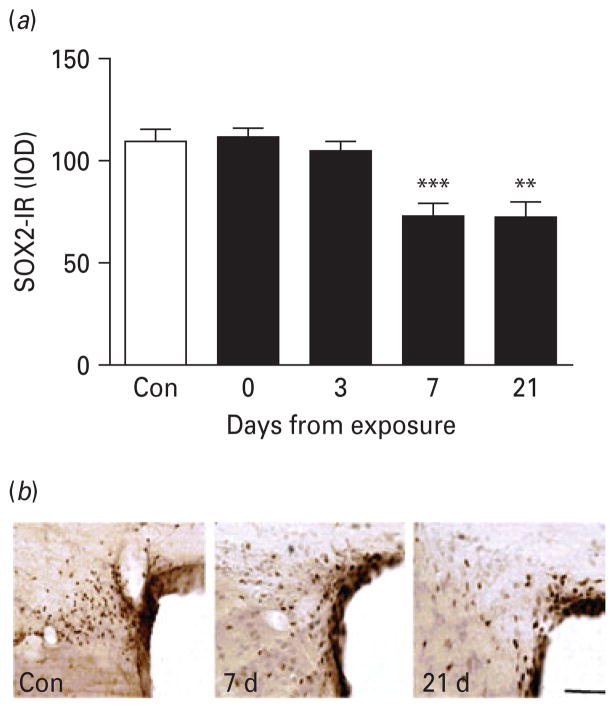
Because of the trend for decrease in proliferation rates, and the robust decrease in neurogenesis observed 3 wk after exposure, we assessed whether a loss of forebrain stem cells might underlie the impairment of SVZ neurogenesis. To this end expression of SOX2-IR was determined. SOX2-IR within the SVZ also varied as a function of time (Fig. 5; main effect of time: F4,38=8.8, p<0.0001). SOX2-IR in the SVZ was not altered during 7 wk of daily alcohol vapour exposure, or after 3 d of abstinence following dependence. However, on day 7 of abstinence, SOX2-IR was reduced 25–35% (p<0.001) and remained reduced on day 21 (p<0.01). The long-lasting reduction in SOX2 is consistent with a loss of neural stem cells in the forebrain.
Fig. 5.
Loss of SOX2-immunoreactive (IR)-labelled neural stem cells in the subventricular zone (SVZ) during protracted abstinence. (a) Data show mean integrated optical densities (IOD; mean±S.E.M.) of SOX2-IR-labelled neural stem cells at varying intervals following the last intoxication cycle (■) compared to control rats (□, n=16). SOX2-IR varied as a function of time (p<0.0001). Levels did not differ from controls on day 0 (n=8) and 3 (n=8), but were suppressed on day 7 (n=8), and remained suppressed on day 21 (n=3). ** p<0.01, *** p<0.001. (For detailed statistics, see Results section). (b) Illustrative brightfield photomicrographs of SOX2-IR-labelled neural stem cells in the SVZ at the different time-points. Scale bar, 80 μm; Bregma level=+1.0 to +0.
Discussion
We found that a prolonged history of alcohol dependence has pronounced effects on neurogenesis in the adult rat brain that vary with time and brain region.
Effects of prolonged alcohol dependence on dentate gyrus neurogenesis
In the hippocampal dentate gyrus, chronic intermittent alcohol exposure markedly reduced proliferation, as detected both by BrdU-IR and Ki67-IR. Because Ki67 measures were highly correlated with those for BrdU and produced the same temporal profile, it is unlikely that effects on circulation or integrity of the blood–brain barrier, that could otherwise affect BrdU incorporation, could confound our proliferation estimates. Furthermore, neurogenesis, detected as DCX-IR, was also suppressed at the end of alcohol exposure. These data are in agreement with published reports, which consistently find that hippocampal neurogenesis in rats is reduced by alcohol upon voluntary self-administration (Crews et al. 2004; Stevenson et al. 2009), combined vapour inhalation and self-administration (Richardson et al. 2009), and after a 4-d binge-like dependence model (Nixon & Crews, 2002). Our present findings demonstrate that suppression of hippocampal neurogenesis occurs with prolonged dependence, and does not recover as long as intoxicating blood alcohol levels are maintained.
Three days into abstinence following prolonged dependence, we observed a rebound burst of proliferation in the dentate gyrus. This was accompanied by a trend for increased neurogenesis. These findings are consistent with and expand on results from previous studies. In the 4-d binge intoxication model, initial depression of dentate gyrus neurogenesis was also followed by a rebound burst of proliferation some days into abstinence (Nixon et al. 2008; Nixon & Crews, 2004). This resulted in increased neurogenesis, as well as an increase in microglia that persisted in the brain for long periods, mimicking the increase in microglia found in brains of human alcoholics (He et al. 2007). Following the prolonged brain alcohol dependence in the present study, a similar burst in hippocampal proliferation was observed, although we did not detect a subsequent significant increase in newly differentiated neurons. It is possible that a burst in neurogenesis was missed by the time-points chosen (Brown et al. 2003). Alternatively, following prolonged rather than acute brain alcohol exposure, increased proliferation may to a lesser extent result in formation of new neurons that differentiate and survive. This could be caused by decreased survival of newly generated cells, as previously shown with prolonged brain alcohol exposure (Herrera et al. 2003), or decreased differentiation of progenitors into neuronal phenotypes following prolonged dependence. Finally, 1 and 3 wk into abstinence, both proliferation rates and neurogenesis in the dentate gyrus had returned to normal, indicating that effects of alcohol dependence in this region are transient and ultimately reversible.
A consistent picture thus emerges for the effects of prolonged brain exposure to intoxicating alcohol levels on hippocampal neurogenesis. During chronic intoxication, the rate of new neuron formation is markedly suppressed, presumably in part secondary to suppressed proliferation of neural precursor cells. During protracted abstinence, hippocampal rates of new neuron formation return to normal, but do not significantly rebound to compensate for the deficit that has accumulated during prolonged intoxication. The contribution of new dentate neurons has been postulated to be important for restraining stress reactivity (Mirescu & Gould, 2006), and its deletion through disruption of the gene encoding TrkB results in a highly anxious phenotype (Bergami et al. 2008). This predicts that a deficit in new dentate neurons following prolonged alcohol dependence might contribute to exaggerated stress reactivity and elevated anxiety in the post-dependent state, a behavioural phenotype that is in fact consistently observed (Heilig & Koob, 2007).
Loss of hippocampal neurogenesis due to prolonged alcohol exposure may contribute to depression-like behaviour observed in mice during protracted alcohol abstinence, and reversed by antidepressants (Stevenson et al. 2009). Alcohol-use disorders are frequently comorbid with depression and anxiety (Grant et al. 2004), and loss of hippocampal neurogenesis during prolonged alcohol dependence could also contribute to the high clinical comorbidity of depression and alcoholism. Finally, stimulation of hippocampal neurogenesis has been postulated as a therapeutic mechanism behind antidepressant actions (Duman & Monteggia, 2006; Malberg et al. 2000; Pittenger & Duman, 2008; Santarelli et al. 2003). Inhibition of hippocampal neurogenesis by chronic intoxication may therefore be speculated to prevent successful treatment of comorbid depression in patients with alcohol-use disorders, although antidepressant treatments may act through both neurogenesis-dependent and -independent mechanisms (Bjornebekk et al. 2009; David et al. 2009).
Effects of prolonged alcohol dependence on SVZ neurogenesis
Our report is the first to follow neurogenesis in forebrain SVZ during prolonged alcohol dependence and abstinence. Similar to the dentate gyrus, prolonged alcohol exposure suppressed SVZ neurogenesis, in the absence of effects on SOX2-expressing cells. The rodent and human SVZ are thought to contain true neuronal stem cells, with greater pluripotency than the neural precursor cells of the hippocampus (Quinones-Hinojosa et al. 2006; Seaberg & van der Kooy, 2002). The SVZ stem cells are heterogeneous, and encompass rapidly dividing cells, early neuroblasts and migrating neuroprogenitors (Doetsch et al. 1997). SOX2 is a stem cell transcription factor that identifies a subset of stem cells likely to represent the most pluripotent stem cells of the adult brain (Brazel et al. 2005; Ellis et al. 2004; Episkopou, 2005). Previous observations in the dentate gyrus have suggested that chronic alcohol exposure can disrupt neurogenesis by decreasing neural precursor cell proliferation, inhibiting cell survival and altering morphological maturation of newborn neurons (He et al. 2005). The initial suppression of SVZ neurogenesis following alcohol exposure occurred in the absence of effects on SOX2-positive cells, and may therefore be accounted for by similar mechanisms, while the subset of pluripotent SVZ stem cells expressing SOX2 is initially spared. Here, the initial suppression of SVZ neurogenesis following alcohol exposure occurred in the absence of effects on SOX2-positive cells, suggesting that SOX2-expressing stem cells are not sensitive to direct toxicity from alcohol, while more differentiated neural progenitors are.
Similar to the dentate gyrus, the initial suppression of SVZ neurogenesis was followed by a burst of proliferation 3 d into abstinence, accompanied by a corresponding peak of new neuron formation of a similar magnitude. Most importantly, the peaks of proliferation and neurogenesis in the SVZ were followed by trend-level reductions in the proliferation markers, and a robust decrease in the neurogenesis marker by the end of the 3-wk abstinence interval studied. This late phase decrease in neurogenesis was accompanied by reduced numbers of SOX2-expressing cells. There are only two plausible mechanisms to account for reduced neurogenesis that result from decreased progenitor cell proliferation : a loss of progenitor cells, or interference with their progression through the cell cycle. The closely correlated decrease in both BrdU-IR and Ki67-IR at day 21 is not consistent with cell-cycle effects. Against this background, the most parsimonious interpretation of the data is that forebrain SVZ neurogenesis is long-term impaired following prolonged alcohol dependence through a loss of SOX2-expressing progenitor cells. These data complement a recent study showing permanent impairments in cell proliferation in the prefrontal cortex in a model of alcohol dependence (Richardson et al. 2009), and provide a potential mechanism for this observation.
In rodents and humans, neurons newly formed from stem cells in the SVZ migrate to the olfactory bulb and contribute to olfactory function (Alvarez-Buylla & Lim, 2004; Curtis et al. 2007). Innate alcohol preference in mice seems to be strongly dependent on genes with localized expression in the olfactory system (Tabakoff et al. 2008). Deficits in olfactory sensitivity and discrimination specifically related to alcohol dependence have been described repeatedly in humans (Potter & Butters, 1979; Rupp et al. 2003; Rupp et al. 2004). It has recently been found that these impairments correlate with the degree of impairment for executive cognitive function (Rupp et al. 2006), a category of deficit that also predicts relapse in recently detoxified severe alcoholics (Wicks et al. 2001). The biological mechanisms linking SVZ neurogenesis, alcohol preference, olfactory bulb function and executive deficits are presently unknown. However, alcoholics have cognitive deficits that improve in abstinence, but do not completely return to control levels (Crews et al. 2005), consistent with partial but incomplete recovery of neurogenesis. The loss of SVZ progenitors following prolonged alcohol dependence could thus contribute to long-term changes in cognitive function and risk for relapse in abstinent alcoholics.
Conclusions
In summary, we report here that prolonged brain alcohol exposure results in a reversible suppression of proliferation and neurogenesis in the hippocampal dentate gyrus, in a manner that may contribute to increased behavioural stress reactivity and negative affect. We further report that SVZ neurogenesis is long-term impaired following a prolonged history of alcohol dependence, in a manner that might contribute to cognitive deficits and other long-term neurobiological changes commonly observed in alcoholism. Whether these alterations in neurogenesis are directly involved in excessive alcohol consumption in post-dependent animals remains to be determined.
Acknowledgments
Supported by NIAAA Intramural Program.
Footnotes
Statement of Interest
None.
References
- Aberg E, Hofstetter CP, Olson L, Brene S. Moderate ethanol consumption increases hippocampal cell proliferation and neurogenesis in the adult mouse. International Journal of Neuropsychopharmacology. 2005;8:557–567. doi: 10.1017/S1461145705005286. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron. 2004;41:683–686. doi: 10.1016/s0896-6273(04)00111-4. [DOI] [PubMed] [Google Scholar]
- Bergami M, Rimondini R, Santi S, Blum R, et al. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proceedings of the National Academy of Sciences USA. 2008;105:15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornebekk A, Mathe AA, Brene S. The antidepressant effects of running and escitalopram are associated with levels of hippocampal NPY and Y1 receptor but not cell proliferation in a rat model of depression. Hippocampus. 2009 doi: 10.1002/hipo.20683. Published online : 21 July 2009. [DOI] [PubMed] [Google Scholar]
- Brazel CY, Limke TL, Osborne JK, Miura T, et al. Sox2 expression defines a heterogeneous population of neurosphere-forming cells in the adult murine brain. Aging Cell. 2005;4:197–207. doi: 10.1111/j.1474-9726.2005.00158.x. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, et al. Transient expression of doublecortin during adult neurogenesis. Journal of Comparative Neurology. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Crews FT, Buckley T, Dodd PR, Ende G, et al. Alcoholic neurobiology: changes in dependence and recovery. Alcoholism : Clinical and Experimental Research. 2005;29:1504–1513. doi: 10.1097/01.alc.0000175013.50644.61. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K, Wilkie ME. Exercise reverses ethanol inhibition of neural stem cell proliferation. Alcohol. 2004;33:63–71. doi: 10.1016/j.alcohol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Curtis MA, Kam M, Nannmark U, Anderson MF, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang JW, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch F, GarciaVerdugo JM, AlvarezBuylla A. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. Journal of Neuroscience. 1997;17:5046–5061. doi: 10.1523/JNEUROSCI.17-13-05046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biological Psychiatry. 2006;59:1116–1127. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Ellis P, Fagan BM, Magness ST, Hutton S, et al. SOX2, a persistent marker for multipotential neural stem cells derived from embryonic stem cells, the embryo or the adult. Developmental Neuroscience. 2004;26:148–165. doi: 10.1159/000082134. [DOI] [PubMed] [Google Scholar]
- Episkopou V. SOX2 functions in adult neural stem cells. Trends in Neurosciences. 2005;28:219–221. doi: 10.1016/j.tins.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, et al. Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4:1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baisch H, Wacker HH, et al. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. Journal of Immunology. 1984;133:1710–1715. [PubMed] [Google Scholar]
- Gilman JM, Hommer DW. Modulation of brain response to emotional images by alcohol cues in alcohol-dependent patients. Addiction Biology. 2008;13:423–434. doi: 10.1111/j.1369-1600.2008.00111.x. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Gould E. Opinion – how widespread is adult neurogenesis in mammals? Nature Reviews Neuroscience. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Gould E, McEwen BS, Tanapat P, Galea LAM, et al. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. Journal of Neuroscience. 1997;17:2492–2498. doi: 10.1523/JNEUROSCI.17-07-02492.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Stinson FS, Dawson DA, Chou SP, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders : results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2004;61:807–816. doi: 10.1001/archpsyc.61.8.807. [DOI] [PubMed] [Google Scholar]
- He J, Nixon K, Shetty AK, Crews FT. Chronic alcohol exposure reduces hippocampal neurogenesis and dendritic growth of newborn neurons. European Journal of Neuroscience. 2005;21:2711–2720. doi: 10.1111/j.1460-9568.2005.04120.x. [DOI] [PubMed] [Google Scholar]
- He L, Marecki JC, Serrero G, Simmen FA, et al. Dose-dependent effects of alcohol on insulin signaling: partial explanation for biphasic alcohol impact on human health. Molecular Endocrinology. 2007;21:2541–2550. doi: 10.1210/me.2007-0036. [DOI] [PubMed] [Google Scholar]
- Heilig M, Koob GF. A key role for corticotropin-releasing factor in alcohol dependence. Trends in Neurosciences. 2007;30:399–406. doi: 10.1016/j.tins.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig M, Thorsell A, Sommer WH, Hansson AC, et al. Translating the neuroscience of alcoholism into clinical treatments: from blocking the buzz to curing the blues. Neuroscience & Biobehavioral Reviews. 2009 doi: 10.1016/j.neubiorev.2009.11.018. Published online : 24 November 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera DG, Yague AG, Johnsen-Soriano S, Bosch-Morell F, et al. Selective impairment of hippocampal neurogenesis by chronic alcoholism: protective effects of an antioxidant. Proceedings of the National Academy of Sciences USA. 2003;100:7919–7924. doi: 10.1073/pnas.1230907100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komitova M, Eriksson PS. Sox-2 is expressed by neural progenitors and astroglia in the adult rat brain. Neuroscience Letters. 2004;369:24–27. doi: 10.1016/j.neulet.2004.07.035. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. Journal of Neuroscience (Online) 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirescu C, Gould E. Stress and adult neurogenesis. Hippocampus. 2006;16:233–238. doi: 10.1002/hipo.20155. [DOI] [PubMed] [Google Scholar]
- Nixon K. Alcohol and adult neurogenesis : roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus. 2006;16:287–295. doi: 10.1002/hipo.20162. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. Journal of Neurochemistry. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. Journal of Neuroscience. 2004;24:9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Kim DH, Potts EN, He J, et al. Distinct cell proliferation events during abstinence after alcohol dependence: microglia proliferation precedes neurogenesis. Neurobiology of Disease. 2008;31:218–229. doi: 10.1016/j.nbd.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcoholism : Clinical and Experimental Research. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Amsterdam: Elsevier Academic Press; 2005. [Google Scholar]
- Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- Potter H, Butters NM. Continuities in the olfactory deficits of chronic-alcoholics and alcoholics with the korsakoff syndrome. Alcoholism: Clinical and Experimental Research. 1979;3:190. [PubMed] [Google Scholar]
- Quinones-Hinojosa A, Sanai N, Soriano-Navarro M, Gonzalez-Perez O, et al. Cellular composition and cytoarchitecture of the adult human subventricular zone: a niche of neural stem cells. Journal of Comparative Neurology. 2006;494:415–434. doi: 10.1002/cne.20798. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. European Journal of Neuroscience. 2004;19:234–246. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Richardson HN, Chan SH, Crawford EF, Lee YK, et al. Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiological Disorders. 2009;36:1–10. doi: 10.1016/j.nbd.2009.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB Journal. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- Roberts AJ, Heyser CJ, Cole M, Griffin P, et al. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- Rupp CI, Fleischhacker WW, Drexler A, Hausmann A, et al. Executive function and memory in relation to olfactory deficits in alcohol-dependent patients. Alcoholism: Clinical and Experimental Research. 2006;30:1355–1362. doi: 10.1111/j.1530-0277.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- Rupp CI, Fleischhacker WW, Hausmann A, Mair D, et al. Olfactory functioning in patients with alcohol dependence: impairments in odor judgements. Alcohol and Alcoholism. 2004;39:514–519. doi: 10.1093/alcalc/agh100. [DOI] [PubMed] [Google Scholar]
- Rupp CI, Kurz M, Kemmler G, Mair D, et al. Reduced olfactory sensitivity, discrimination, and identification in patients with alcohol dependence. Alcoholism : Clinical and Experimental Research. 2003;27:432–439. doi: 10.1097/01.ALC.0000057945.57330.2C. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Seaberg RM, van der Kooy D. Adult rodent neurogenic regions : the ventricular subependyma contains neural stem cells, but the dentate gyrus contains restricted progenitors. Journal of Neuroscience. 2002;22:1784–1793. doi: 10.1523/JNEUROSCI.22-05-01784.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, et al. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biological Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Stevenson JR, Schroeder JP, Nixon K, Besheer J, et al. Abstinence following alcohol drinking produces depression-like behavior and reduced hippocampal neurogenesis in mice. Neuropsychopharmacology. 2009;34:1209–1222. doi: 10.1038/npp.2008.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakoff B, Saba L, Kechris K, Hu W, et al. The genomic determinants of alcohol preference in mice. Mammalian Genome. 2008;19:352–365. doi: 10.1007/s00335-008-9115-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya VA, Vadodaria KC, Jha S. Neurotransmitter regulation of adult neurogenesis : putative therapeutic targets. CNS & Neurological Disorders Drug Targets. 2007;6:358–374. doi: 10.2174/187152707783220910. [DOI] [PubMed] [Google Scholar]
- Walker RA. Quantification of immunohistochemistry – issues concerning methods, utility and semiquantitative assessment I. Histopathology. 2006;49:406–410. doi: 10.1111/j.1365-2559.2006.02514.x. [DOI] [PubMed] [Google Scholar]
- Wicks S, Hammar J, Heilig M, Wisen O. Factors affecting the short-term prognosis of alcohol dependent patients undergoing inpatient detoxification. Substance Abuse. 2001;22:235–245. doi: 10.1080/08897070109511465. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Best AR, Sullivan RM. Plasticity in the olfactory system: lessons for the neurobiology of memory. Neuroscientist. 2004;10:513–524. doi: 10.1177/1073858404267048. [DOI] [PMC free article] [PubMed] [Google Scholar]