Multiple myeloma (MM) is an incurable hematologic malignancy diagnosed primarily in older adults. As the population ages, myeloma incidence is expected to increase at a higher rate than many other malignancies.1 Approved chemotherapy and targeted regimens for older adults with MM are numerous and exhibit distinct toxicities. The physiological heterogeneity of older adults makes it challenging for physicians to identify the most effective, yet best tolerated regimen, for each MM patient. As such, use of a two-drug regimen, three-drug regimen, or intensive autologous hematopoietic stem cell transplant (AHSCT) is often subjective. Consequently, AHSCT eligibility is subjectively applied and more objective measures are warranted to better understand health status in older adults. We believe that objective markers of physiological age will improve treatment stratification and will be an additional tool in understanding how treatment and transplant have an impact on health status.
The molecular biomarker, p16INK4a (p16) is an established marker of systemic cellular senescence associated with physiological aging. p16 expression increases ~ 16-fold over an individual's lifetime and can be readily measured in peripheral blood T-lymphocytes (PBTLs).2 p16 originates from the INK4/ARF locus on human chromosome 9p21 and belongs to the INK4 family of cyclin-dependent kinase inhibitors (CDKis). These CDKis prevent cell cycle progression into S-phase by blocking phosphorylation of the retinoblastoma tumor suppressor by CDK4/6.3 On a cellular level, p16 expression increases with stress (for example, DNA-damaging stimuli, telomere erosion and oncogene expression) and, with prolonged induction, can promote an irreversible cell cycle arrest termed ‘cellular senescence.’ In humans, p16 rises exponentially with chronologic age and this rate of increase is further accelerated by physical inactivity, tobacco use, chronic HIV infection and cytotoxic chemotherapy.2,4 The regulation of p16 expression is also linked to age-related conditions (that is, cardiovascular disease, diabetes and decreased physical function) through single-nucleotide polymorphisms located near the INK4/ARF locus.5–7 To date, p16 expression has not been examined as a surrogate for biologic age in MM, a disease where treatment stratification is often based on chronologic age.
We hypothesized that p16 levels, a marker of cellular senescence in T cells, would impart knowledge of a patient's biological age pre- and post treatment, thus improving future therapeutic decision-making and patient outcome. We performed a pilot study to preliminarily determine the effects of therapy and/or intensive transplant (AHSCT) on biological aging using p16 levels in PBTL as a surrogate marker. Fifty-two peripheral blood samples were collected for evaluation divided into three cohorts; healthy control (n = 17), newly diagnosed (ND) MM (n = 11) and relapsed refractory (RR) MM (n = 24). Median age and ranges for healthy control, ND MM and RR MM were 60 (range 35–82), 70 (range 51–84) and 61 (range 40–70), respectively. Complete clinical data were available for 19 of 24 RR MM patients and 11 ND MM patients. RR MM patients were mostly of early stage, who underwent AHSCT (n = 23) and median two lines of chemotherapy (range 1–8), and who were never smokers (n = 12) (Supplementary Table 1).
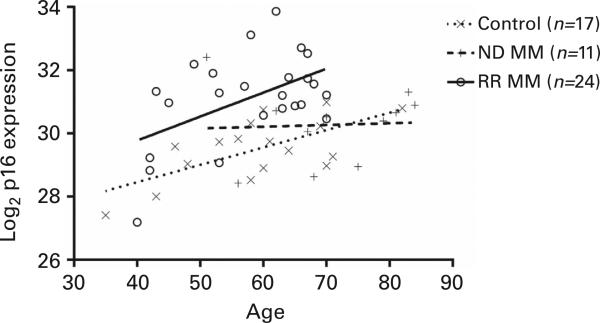
First, we determined whether MM patients have intrinsically higher p16 levels than the general aged-matched population. PBTLs were isolated from each population and assessed for p16 expression using a previously validated quantitative reverse-transcription PCR (qRT-PCR) protocol.2 Multivariate linear regression, showed a correlation between age and p16 expression, in healthy controls, which was consistent with prior publications (+0.05 Ct per year; P = 0.001). Controlling for age, p16 expression in RR MM patients was significantly higher than in healthy controls (1.685 Ct; P ≤ 0.0001). By contrast, p16 levels were only modestly increased in ND, chemotherapy naive, MM patients compared with healthy controls (0.165 Ct; P = 0.73) (Figure 1). Therefore, the diagnosis of MM does not, in itself, increase p16 expression; however, treated RR MM patients have increased p16 expression most likely as a consequence of prior cytotoxic therapy (see below).
Figure 1.
Age-matched p16 mRNA expression profiles in ND and RR MM contrasted with healthy controls. PBTL p16 mRNA levels were measured by qRT-PCR (n = 35 individual patients) relative to PBTL of a healthy control population (n = 17). Using multivariate linear regression, age correlates with p16 expression (+0.05 Ct per year; P = 0.001). p16 levels in RR MM patients was significantly higher than in healthy controls (1.685 Ct; P < 0.0001) and not significantly increased in ND MM (0.165 Ct; P = 0.73).
We next explored the association between ImiDs (immunomodulatory drugs) and p16 levels, as a marker of T-cell senescence. To do this, p16 was measured serially in the same patient, at two separate time points. In MM patients receiving no treatment during the assessment window (n = 8), median p16 expression levels and range did not change over time (first sample = 30.94 (range 27.19–32.41); second sample = 30.365 (range 27.42–33.37); P = 0.3828), thus demonstrating the reproducibility of our assay. Similarly, in patients treated with ImiDs during the assessment window (n = 8), no changes in p16 expression were detected (first sample = 30.985 (range 30.06–33.86); second sample = 31.945 (range 29.87–33.69); P = 0.1094). These data indicate that ImiD therapy does not augment expression of senescence markers, a finding we believe to be consistent with using a targeted, nongenotoxic therapy.
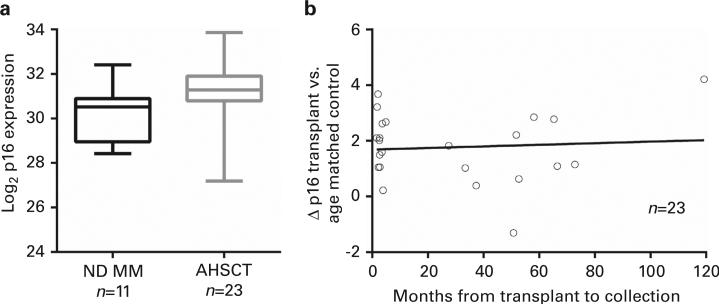
In contrast, we compared PBTL p16 expression of ND MM with RR MM patient to determine whether treatment, including AHSCT, influenced markers of T-cell senescence. Median time from AHSCT to p16 analysis was 4.9 months (range 1.6–119.3). Using Wilcoxon rank-sum test, p16 expression was significantly higher in patients who underwent AHSCT (n = 22, median 31.3) than those who did not undergo AHSCT (n = 11, median 30.46; P = 0.01) (Figure 2a). After controlling for age, no significant differences exists for p16 expression if samples were collected early after transplant compared with those collected late after transplant (Figure 2b), where the difference in p16 expression is plotted by an age-matched theoretical normal compared with months after AHSCT. For a limited number of patients (n = 7), we analyzed p16 expression before and after AHSCT. Samples were collected ~ 3 months after AHSCT (median = 84 days post transplant). p16 expression increased in all samples that ranged from 1.17 to 5.03 Ct's, 2.25- to 32.2-fold increase.
Figure 2.
p16 strongly correlates with MM transplant. p16 mRNA levels measured by qRT-PCR were plotted using Wilcoxon rank-sum test. (a) AHSCT significantly increases p16 levels in comparison with those who did not undergo AHSCT. (b) Increases in p16 appear durable both early and late after transplant, where the difference in p16 expression is plotted by an aged-matched theoretical normal compared with months after AHSCT.
Therefore, treatment and AHSCT appear to increase markers of T-cell senescence in MM patients as measured by elevated p16 levels. Many oncology treatment decisions are shaped by a patient's age. However; chronologic age may not reflect physiological fitness. Physiological aging is a complex process, associated with chronologic aging, but is also the result of chronic inflammation and internal stresses.8 p16 expression is valuable to describe how the basic biological process of senescence is impacted by stem cell transplant. p16 increases with age in humans and mouse models, and is associated with known mediators of inflammation such as interleukin (IL)-6.2,9 Recently, Sanoff et al.4 prospectively reported on a breast cancer population where adjuvant chemotherapy increases p16 expression to levels equivalent to 14.7 years of chronologic aging. This chemotherapy-induced aging was detected independent of other cellular senescence markers such as telomere length and was associated with adverse events such as hematologic toxicity. Here we examined the impact of MM diagnosis and treatment on p16 expression.
The process of stem cell mobilization and expansion of hematopoietic stem cells could contribute to T-cell stress and induce cellular senescence. It is postulated that T-cell senescence is secondary to melphalan-based myeloablative therapy, but G-CSF mobilization may also play a role in this process. G-CSF stimulation results in a twofold increase in circulating CD3+ T cells.10 G-CSF also has pleiotropic effects on T-cell subtypes, promoting Th2/Treg differentiation while limiting proliferation in the Th1/T17 subtypes.11 Still, the duration of T-cell responses to G-CSF is largely unknown. We conclude that CD3+ T-cell populations recover in number and p16 expression increases post transplant.
IMiDs serve as a backbone for MM therapy. The exact mechanism of IMiD efficacy in MM is uncertain, but is thought to both arrest myeloma cell growth and exhibit collateral effects on the immune system by inhibiting TNFα, upregulating IL-2 and increasing regulatory T cells.12 Therefore, our observation that ImiDs do not decrease total T-cell numbers or p16 expression suggests that short-term use of ImiD therapy is not detrimental to T-cell growth and division.
To our knowledge, this is the first report describing T-cell senescence post transplant. The increase in PBTL p16 expression associated with treatment/AHSCT is equivalent to 33.7 years of chronological aging (1.685/0.05 Ct; where 0.05 is the expected change in p16 per year2), with healthy control PBTLs exhibiting a mean increase in p16 of 0.05 Ct per year. This finding has significant implications for MM patients. Treatment of MM is challenging given the rapidly evolving therapeutic strategies and host factors that contribute to AHSCT eligibility. As such, markers of cellular senescence represent an attractive proxy for physiologic age, both before and after treatment. Although it is widely accepted that age alone is not the sole factor in determining AHSCT eligibility, the clinical need for objective quantitative measures of physiological fitness remains unmet. Therefore, many investigations are focusing on biomarkers of age-to-weight treatment options and gauge toxicity.13 We know that disparities exist in MM treatment, where older individuals are less likely to undergo transplant.14 The need for objective biomarkers of aging is especially important in the field of myeloma due to the aged population affected, heterogeneity in older adult fitness and diverse treatment strategies that are available. It is our hope that this report will stimulate interest in aging research to capture physiological fitness and with future investigations, and be an additional tool in the clinical evaluation.
Supplementary Material
ACKNOWLEDGEMENTS
This study was supported by D Warren Brown Foundation and OSUCCC Comprehensive CCC grant P30 CA016058-39.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supplementary Information accompanies this paper on Bone Marrow Transplantation website (http://www.nature.com/bmt)
REFERENCES
- 1.Smith BD, Smith GL, Hurria A, Hortobagyi GN, Buchholz TA. Future of cancer incidence in the United States: burdens upon an aging, changing nation. J Clin Oncol. 2009;27:2758–2765. doi: 10.1200/JCO.2008.20.8983. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y, Sanoff HK, Cho H, Burd CE, Torrice C, Ibrahim JG, et al. Expression of p16 (INK4a) in peripheral blood T-cells is a biomarker of human aging. Aging Cell. 2009;8:439–448. doi: 10.1111/j.1474-9726.2009.00489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romagosa C, Simonetti S, Lopez-Vicente L, Mazo A, Lleonart ME, Castellvi J, et al. p16(Ink4a) overexpression in cancer: a tumor suppressor gene associated with senescence and high-grade tumors. Oncogene. 2011;30:2087–2097. doi: 10.1038/onc.2010.614. [DOI] [PubMed] [Google Scholar]
- 4.Sanoff HK, Deal AM, Krishnamurthy J, Torrice C, Dillon P, Sorrentino J, et al. Effect of cytotoxic chemotherapy on markers of molecular age in patients with breast cancer. J Natl Cancer Inst. 2014;106:dju057. doi: 10.1093/jnci/dju057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jeck WR, Siebold AP, Sharpless NE. Review: a meta-analysis of GWAS and age-associated diseases. Aging Cell. 2012;11:727–731. doi: 10.1111/j.1474-9726.2012.00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melzer D, Frayling TM, Murray A, Hurst AJ, Harries LW, Song H, et al. A common variant of the p16(INK4a) genetic region is associated with physical function in older people. Mech Ageing Dev. 2007;128:370–377. doi: 10.1016/j.mad.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helgadottir A, Thorleifsson G, Manolescu A, Gretarsdottir S, Blondal T, Jonasdottir A, et al. A common variant on chromosome 9p21 affects the risk of myocardial infarction. Science. 2007;316:1491–1493. doi: 10.1126/science.1142842. [DOI] [PubMed] [Google Scholar]
- 8.Hubbard JM, Jatoi A. Incorporating biomarkers of frailty and senescence in cancer therapeutic trials. J Gerontol A Biol Sci Med Sci. 2014;70:722–728. doi: 10.1093/gerona/glu046. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Johnson SM, Fedoriw Y, Rogers AB, Yuan H, Krishnamurthy J, et al. Expression of p16(INK4a) prevents cancer and promotes aging in lymphocytes. Blood. 2011;117:3257–3267. doi: 10.1182/blood-2010-09-304402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tayebi H, Kuttler F, Saas P, Lienard A, Petracca B, Lapierre V, et al. Effect of granulocyte colony-stimulating factor mobilization on phenotypical and functional properties of immune cells. Exp Hematol. 2001;29:458–470. doi: 10.1016/s0301-472x(01)00613-0. [DOI] [PubMed] [Google Scholar]
- 11.Bunse CE, Borchers S, Varanasi PR, Tischer S, Figueiredo C, Immenschuh S, et al. Impaired functionality of antiviral T cells in G-CSF mobilized stem cell donors: implications for the selection of CTL donor. PLoS One. 2013;8:e77925. doi: 10.1371/journal.pone.0077925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clave E, Douay C, Coman T, Busson M, Bompoint C, Moins-Teisserenc H, et al. Lenalidomide consolidation and maintenance therapy after autologous stem cell transplant for multiple myeloma induces persistent changes in T-cell homeostasis. Leuk Lymphoma. 2013;55:1788–1795. doi: 10.3109/10428194.2013.865182. [DOI] [PubMed] [Google Scholar]
- 13.Hubbard JM, Cohen HJ, Muss HB. Incorporating biomarkers into cancer and aging research. J Clin Oncol. 2014;32:2611–2616. doi: 10.1200/JCO.2014.55.4261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Hamadani M, Hashmi SK, Go RS. Use of autologous hematopoietic cell transplantation as initial therapy in multiple myeloma and the impact of socio-geo-demographic factors in the era of novel agents. Am J Hematol. 2014;89:825–830. doi: 10.1002/ajh.23753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.