Abstract
Various lines of evidence indicate the presence of progressive pathophysiological processes occurring within the brains of patients with schizophrenia. By modulating chemical neurotransmission, anti-psychotic drugs may influence a variety of functions regulating neuronal resilience and viability and have the potential for neuroprotection. This article reviews the current literature describing preclinical and clinical studies that evaluate the efficacy of antipsychotic drugs, their mechanism of action and the potential of first- and second-generation antipsychotic drugs to exert effects on cellular processes that may be neuroprotective in schizophrenia. The evidence to date suggests that although all antipsychotic drugs have the ability to reduce psychotic symptoms via D2 receptor antagonism, some antipsychotics may differ in other pharmacological properties and their capacities to mitigate and possibly reverse cellular processes that may underlie the pathophysiology of schizophrenia.
I. Introduction
Our understanding of the pathophysiology of schizophrenia has increased as knowledge of the molecular, cellular, and systems biology of brain function has advanced. Beginning with the dopamine (DA1) hypothesis of schizophrenia, we now have more sophisticated and powerful ways of modeling the pathophysiology of schizophrenia. With this enhanced capacity to conceptualize the disease, we have acquired the ability to examine the actions of therapeutic agents at a variety of levels and to discern any differences that may exist among them. Ultimately, in controlled clinical trials, the clinical relevance of such differences can be tested.
Although some debate exists as to whether schizophrenia is wholly neurodevelopmental in nature, there is evidence supporting a progressive and possibly neurodegenerative process as well. “Neuroprotection” refers to therapies that help to maintain the structural integrity and normal functioning of the central nervous system in response to a pathological process and consequent neurobiological stress. Therapies that may be neuroprotective will probably encompass the mitigation and/or possible reversal of a broad range of anatomical, physiological, and molecular processes thought to underlie the pathophysiology of schizophrenia.
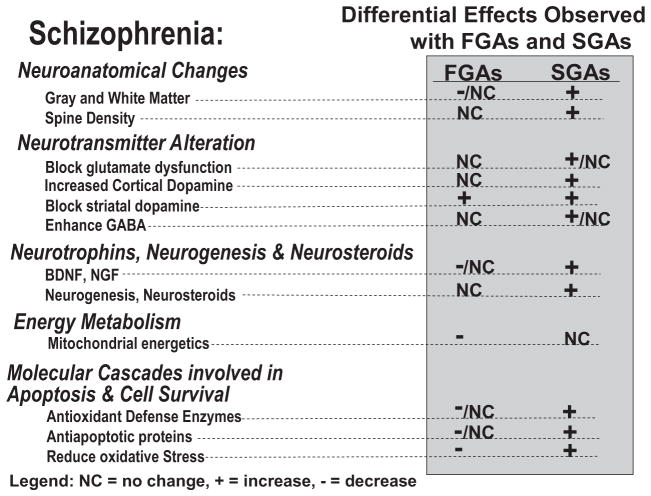
There is increasing interest in understanding not only the manner through which antipsychotic drugs (APDs) are believed to play an important role in modulating dysfunction in chemical neurotransmission to control the symptoms of schizophrenia but also their potential role for neuroprotection. The first-generation antipsychotic drugs (FGAs) treat some of the symptoms of schizophrenia including delusions and hallucinations but, depending on their potency and the dose used, can have substantial side effects, including effects on the extrapyramidal system in the form of extrapyramidal signs (EPS) and tardive dyskinesia (TD) and hyperprolactinemia. The second-generation antipsychotic drugs (SGAs) also reduce the positive symptoms of schizophrenia, but with less EPS and TD and, in general, reduced hyperprolactinemia as well. However, most SGAs tend to cause weight gain and disturbances in glucose and lipid metabolism.
Research also suggests that some of the SGAs may have additional therapeutic properties including cognitive enhancement, reduction of negative symptoms, enhanced relapse prevention, and prevention of disease progression and clinical deterioration, although these effects have not been consistently or definitively demonstrated. Presumably, the differential therapeutic effects of SGAs are due to some distinct pharmacological properties. Heretofore, theories of the mechanism of action of APDs have focused on drug effects on dopamine receptors and to a lesser extent on other neuroreceptors including those for serotonin (5-HT1A,2A,2C,3,6,7) and norepinephrine (α1,2) (Miyamoto et al., 2005).
Recently, a growing body of evidence derived from nontraditional assays and paradigms used to study APDs has demonstrated that specific SGAs induce effects in a range of cellular and molecular assays that suggest unique therapeutic targets (not shared by all SGAs and FGAs) beyond the antagonism of DA neurotransmission. These include in vitro and whole animal studies, which show that some SGAs may increase or preserve neurotrophic factor levels, neurogenesis, neuronal plasticity, mitochondrial biogenesis, cell energetics, and antioxidant defense enzymes. Furthermore, some SGAs may uniquely protect against N-methyl-D-aspartic acid (NMDA) antagonist-induced neurotoxicity and the consequent behavioral effects. Recent findings of the ability of specific SGAs to ameliorate the loss of gray matter in patients in the early stages of schizophrenia further support the hypothesis of unique pharmacological properties and therapeutic benefits (Lieberman et al., 2005b; van Haren et al., 2007).
These putative properties of select SGAs have become more relevant in light of the increasing acceptance by the field of a progressive pathophysiological process and possibly neurodegenerative process coincident with (or shortly before) the onset of the illness that may underlie the clinical deterioration that occurs in many patients with schizophrenia (Wyatt, 1991; DeLisi et al., 1997; Csernansky and Bardgett, 1998; Woods, 1998; Lieberman, 1999). In this article we will critically review studies of the effects of FGAs and SGAs on a number of processes pertinent to the neurobiology and pharmacotherapy of schizophrenia.
II. Pathophysiology of Schizophrenia
Schizophrenia has been characterized as both a neurodegenerative and neurodevelopmental disorder. Kraepelin proposed in the early 1900s that schizophrenia was a degenerative disease in which a patient’s deterioration occurred after the onset of the illness marked by mental symptoms after what seemed to be a relatively normal childhood. However, more recent research has emphasized the role of genes and their effects, along with environmental factors, on neurodevelopment as producing the diathesis from which schizophrenia arises. Numerous genetic association and linkage studies have implicated genetic variants within many components of each neurotransmitter system in the pathophysiology of schizophrenia, although not without controversy (for review, see Riley and Kendler, 2006; Catapano and Manji, 2007; Eisener et al., 2007; Lang et al., 2007), leading to compensatory changes and alterations in brain development. However, it has been proposed that distinct pathological processes may underlie the various clinical stages of the illness with neurodevelopmental mechanisms underlying the premorbid phase of the illness and a progressive pathophysiological process beginning with neurochemical dysregulation that can lead to neurodegeneration occurring after the formal onset of the illness and possibly beginning in its prodromal stage (for review, see Wyatt, 1991; DeLisi et al., 1997; Csernansky and Bardgett, 1998; Woods, 1998; Lieberman, 1999; Lieberman et al., 2001b, 2006) (Fig. 1). It seems likely that if there are distinct pathophysiological stages of schizophrenia, the clinical manifestations of the illness derive from some process involving dysregulation in chemical neurotransmission of genetically susceptible neural pathways (Lieberman et al., 2001b).
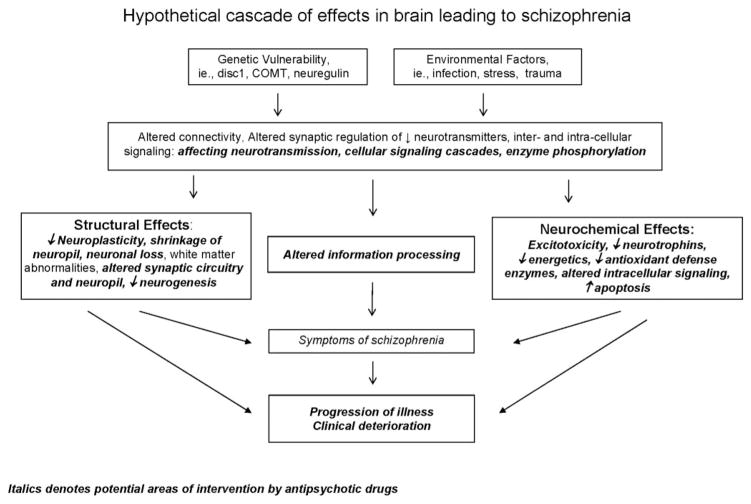
Fig. 1.
Overview of the possible mechanisms of neurodegeneration occurring in schizophrenia. Italic denotes potential areas of intervention by antipsychotic drugs.
A. Neurotransmitter Dysregulation
Although dopamine has been predominant, schizophrenia has been associated with dysregulation of many neurotransmitter systems including GABA, glutamate, serotonin, noradrenaline, and acetylcholine in addition to dopamine. Dysregulation has been observed at many different levels including neurotransmitter synthesis, storage, release, reuptake and inactivation, metabolism, number and structure of presynaptic/postsynaptic receptors, functioning of receptors as high or low affinity, number of transporters, and alterations at the level of postreceptor signaling pathways.
There are two major categories of neurotransmitter receptors: 1) iontropic receptors and 2) G-protein-coupled or metabotropic receptors. Iontropic receptors are ligand-gated ion channels that regulate ionic currents and membrane potential and include glutamatergic receptors as the predominant excitatory receptors and GABAergic receptors as the predominant inhibitory receptors within the brain. Additional iontropic excitatory receptors include the nicotinic acetylcholine receptor and the serotonergic 5-HT3 receptor. Metabotropic receptors involve coupling to various G-proteins leading to the regulation of cAMP and inositol triphosphate (IP3) second messengers and subsequent downstream signaling systems including kinase cascades and transcriptional factors. The metabotropic receptors include members of the dopamine, glutamate, serotonin, acetylcholine, and noradrenaline neurotransmitter systems. Ligand-gated ion channels are thought to reflect fast synaptic neurotransmission and to account for quasi-instantaneous functioning within the brain, whereas metabotropic receptors are slower-acting and are thought to be essential for neuromodulation and long-term regulation (Girault and Greengard, 2004).
The molecular changes observed in each of these neurotransmitter systems occur within discrete neurocircuits within the brain thought to underlie the various symptom profiles observed in patients with schizophrenia, including positive symptoms, negative symptoms, cognitive dysfunction, anxiety, depression, and agitation. Although discussion of the specific details of these neurocircuits is out of the scope of this review, dysfunction of neurotransmitter regulation in schizophrenia has been observed across multiple brain regions, reflecting distinct neurocircuits [reviews of specific brain circuits: basal ganglia-thalamo-cortical loops (Alexander et al., 1986), amygdalo-entorrhinal inputs to hippocampus (Benes and Berretta, 2000), and basal ganglia and cerebellar loops (Middleton and Strick, 2000); reviews of specific brain regions: basal ganglia (Tisch et al., 2004) and thalamus (Clinton and Meador-Woodruff, 2004)].
In addition, altered regulation at the level of molecules and neurocircuits is thought to underlie alterations in complex brain processes. In this regard, schizophrenia has been conceptualized as a disease characterized by abnormal information processing that occurs within subcortical and cortical regions, including sensory gating deficits at the level of the thalamus and altered desynchronization of modal or supramodal cortical associative functions (for review, see Braus et al., 2002). Related to the “abnormal information processing” concept, schizophrenia has been associated with abnormalities in neural oscillations, an “emergent property” of neural networks arising from temporal synchrony between synaptic transmission and the firing of distinct neuronal populations (Ford et al., 2007).
We will focus primarily on the dopaminergic, GABAergic, and glutamatergic neurotransmitter systems, although references to other systems will be made. As a review, several of the molecules involved in signaling cascades associated with neurotransmitter-receptor interactions and that of other molecules are summarized in Fig. 2.
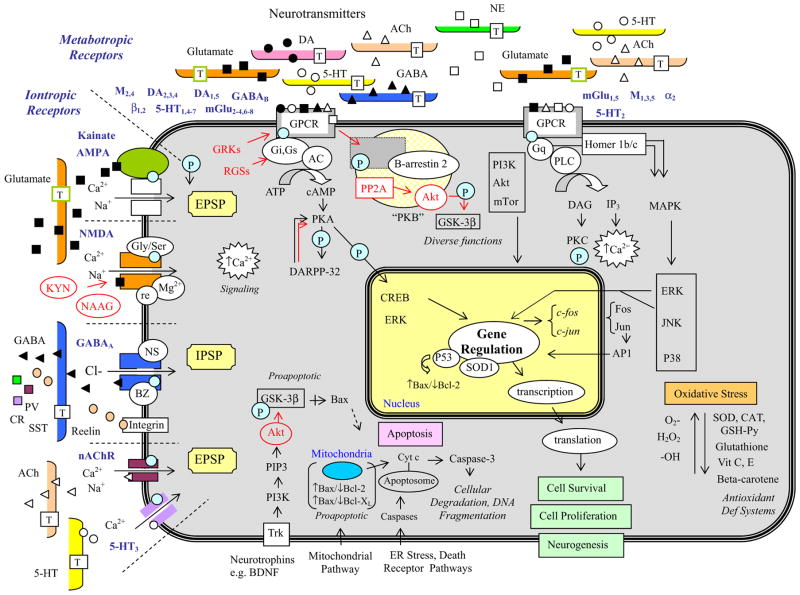
Fig. 2.
A summary of the intracellular signaling cascades that occur within neurons and glia within the brain. Schizophrenia has been associated with dysregulation at a number of loci along these signaling pathways, and antipsychotic drugs may act to reverse some of the pathological changes that have been observed. This slide provides a summary of signaling cascades that occur within neurons and glial cells in the brain that may contribute to schizophrenia, although not all of the cascades shown will be found in a given cell or pathway. The left side summarizes the excitatory and inhibitory iontropic receptors. The top illustrates components of the two key signaling cascades associated with G-protein-coupled metabotropic receptors including adenylyl cyclase and phospholipase C activation. The bottom demonstrates the apoptosis cascade and specific neurotrophic factor-receptor interactions. The right side summarizes some of the key molecules involved in oxidative stress. AP-1, activator protein-1 complex; BZ, benzodiazepines; CAT, catalase; Cl−, chloride; CR, calretinin; Cyt c, cytochrome c; DAG, diacylglycerol; ER, endoplasmic reticulum; GRK, G-protein-coupled receptor kinases; GSH-Px, glutathione peroxidase; H2O2, hydrogen peroxide; IPSP, inhibitory postsynaptic potential; KYN, kynurenic acid; M, muscarinic acetylcholine receptors; MAPK, mitogen-activated protein kinase; mTOR, mammalian target of rapamycin; nAChR, nicotinic acetylcholine receptor; NE, norepinephrine; NS, neuroactive steroids; O2-, superoxide radical; -OH, hydroxyl anion; P, phosphorylation; PI3K, phosphatidylinositol 3-kinase; PIP3, phosphatidylinositol triphosphate; PP2A, protein phosphatase 2A; T, transporter protein; Vit, vitamin.
1. Dopamine
One of the most popular theories underlying the pathophysiology of schizophrenia involves increased dopaminergic activity within the mesolimbic dopamine system thought to underlie the positive or psychotic symptoms of schizophrenia and decreased dopaminergic activity within the mesocortical dopamine system thought to reflect negative symptoms and cognitive dysfunction also seen in schizophrenia (for reviews, see Abi-Dargham and Moore, 2003; Guillin et al., 2007; Meisenzahl et al., 2007). In line with this theory, all currently available APDs reduce psychotic symptoms via blockade of dopamine neurotransmission within the striatal complex of the mesolimbic pathways.
Dopamine is synthesized from tyrosine to DOPA via tyrosine hydroxylase and then to dopamine via DOPA decarboxylase, reactions occurring within two major cell groups, substantia nigra pars compacta projecting to the striatum and the ventral tegmental area projecting to the ventral striatum and cerebral cortex. There are five metabotropic dopamine receptors divided into two major classes: D1-like receptors (D1 and D5) and D2-like receptors (D2–4). Two enzymes are responsible for the catabolic inactivation of dopamine, different isozymes of monoamine oxidase (MAO-A and MAO-B) and catechol-O-methyltransferase. In addition, dopamine released from presynaptic terminals is recaptured into presynaptic terminals via the dopamine transporter.
All antipsychotics to date act as antagonists (or partial agonists) at the D2 receptor, and most show a dose-dependent threshold of D2 receptor occupancy for their therapeutic effects (Kapur and Mamo, 2003). Although individual studies report contradictory findings, a meta-analysis of 13 in vivo studies demonstrated a 12% increase in D2 receptor binding in drug-naive and in drug-free patients with schizophrenia, providing limited support for D2 receptor up-regulation and supersensitivity in schizophrenia (Laruelle, 1998). Seeman et al. (2006) have suggested that elevations in the high-affinity state of dopamine D2 receptors (D2High receptors) may reflect a common point of convergence among the various pathways for eliciting psychosis. They indicated that many of the causes of psychosis in adult humans such as drugs, steroids, ethanol, and brain lesions lead to dopamine supersensitivity in rats and to an increase in the high-affinity state of dopamine D2High receptors in striata (Seeman et al., 2006). Other proposed links between dopamine D2 neurotransmission and schizophrenia include polymorphisms in the D2 receptor gene (for review, see Lang et al., 2007) and alterations in the components of the post-dopamine D2 receptor signaling cascade discussed in section II.A.5. However, because APDs can up-regulate D2 receptors, the possibility of a treatment effect and drug artifact must be considered.
Whereas the D2 receptor plays a predominant role in the current treatment of psychotic symptoms, other components of the dopamine neurotransmitter system have been implicated in schizophrenia. These include the D1 receptor (Abi-Dargham and Moore, 2003; Goldman-Rakic et al., 2004), D3 receptor (Micheli and Heidbreder, 2006), D4 receptor (Krämer et al., 2007), catechol-O-methyltransferase (Krämer et al., 2007; Lewandowski, 2007), dopamine transporter (Schmitt et al., 2006; Mateos et al., 2007), dopamine receptor-interacting proteins calcyon and neuronal Ca2+ sensor 1 (Bergson et al., 2003), and dopamine receptor-adenosine receptor interactions (Fuxe et al., 2007).
2. GABA
GABAergic synapses are the key inhibitory synapses within the brain, and decreased GABAergic neurotransmission has been implicated in the pathophysiology of schizophrenia (for review, see Benes and Berretta, 2001; Blum and Mann, 2002; Wassef et al., 2003; Lewis et al., 2004; Guidotti et al., 2005). It has been proposed that deficits in GABAergic neurotransmission may result in an imbalance between excitatory and inhibitory neurotransmission, favoring excitation and possible excitotoxicity. Olney et al. (1999) suggested that a developmental deficit of inhibitory GABA interneurons may set the stage for ongoing neurodegeneration through the uncontrolled activation of glutamatergic neurons. In addition, GABAergic interneurons play an important role in regulating pyramidal neuron firing rates (McBain and Fisahn, 2001), and, as a result, reduced GABAergic function would alter the synchronous firing patterns of cortical neurons, which may underlie information-processing deficits known to be present in patients with schizophrenia (Hajós, 2006).
GABA is synthesized from glutamate via two molecular forms of glutamic acid decarboxylase (GAD67 and GAD65). GABAergic neurons (or interneurons) coexpress specific proteins and can be classified by location within specific neuronal circuits based on the expression of these proteins—reelin, parvalbumin (PV), and calretinin. Reelin is an extracellular matrix protein constitutively released from GABAergic terminals that binds to integrin receptors to regulate synaptic plasticity (e.g., long-term potentiation) and protein synthesis within neuronal dendrites and spines. PV and calretinin are calcium-binding proteins that probably contribute to intracellular Ca2+ signaling cascades. Three GABA receptors have been identified thus far: GABAA and GABAC receptors are iontropic receptors, whereas the GABAB receptor is metabotropic and coupled to a GTP-binding protein. The GABAA receptor is a heteropentameric structure consisting of various subtypes composed of at least 16 different GABAA receptor subunits—six α, four β, three γ, one δ, one ε, and one θ (Mohler et al., 1995; Whiting, 2003). The α subunits of the GABAA receptor confer different affinities for GABA, and these subunits show a very specialized regional cellular and subcellular distribution. In addition, a subset of GABAA receptors contain a binding site for benzodiazepines. The benzodiazepine binding site on the GABAA receptor is allosterically coupled to the GABA binding site, resulting in increased receptor occupancy at low GABA concentrations that increases the frequency of channel openings (Pritchett et al., 1989). GABA neurotransmission is terminated via reuptake by GABA transporter proteins.
GABAergic dysfunction in schizophrenia has been characterized as a reduction in the availability of GABA and related proteins presynaptically and compensatory up-regulation of GABA receptors postsynaptically. Most studies have reported low GABA levels in at least some brain regions in patients with schizophrenia, although there is no clear consensus on the specific brain loci affected with the exception of the amygdala. At the presynaptic level, down-regulation of mRNA and/or protein for GAD67, reelin, and PV has been observed in postmortem brain tissues of patients with schizophrenia. Lower levels of the GABA transporter 1 (GAT1) have also been observed, which may reflect a compensatory change in response to low GABA levels.
Within the cerebral cortex and hippocampus, there is evidence for fewer GABAergic interneurons, although this reduction is localized primarily to cortical layer II. Several authors have suggested that the loss of this subset of GABAergic neurons is probably not sufficient to support the reductions observed in GAD67, reelin, and GAT1 (for review, see Guidotti et al., 2005), implying that other mechanisms such as promoter-related down-regulation of gene expression must be involved. Recent work has demonstrated an increase in DNA-methyltransferase-1 expression within select GABAergic interneurons in postmortem schizophrenia brains that could underlie down-regulation of gene expression (Veldic et al., 2004, 2005). Other work focusing on the chandelier class of GABAergic neurons that form distinctive vertical arrays called “cartridges” of synaptic terminals along the axon initial segments of pyramidal neurons found no differences in the relative density, laminar distribution, or size of parvalbumin-containing neurons (Lewis, 2000). However, the density of GAT1-immunoreactive chandelier neuronal axon cartridges was decreased by 40% in subjects with schizophrenia compared with healthy control subjects and subjects with other psychiatric disorders (Lewis, 2000).
At the postsynaptic level, the majority of data support increased expression of GABAA receptors in schizophrenia. The numbers of GABAA receptors labeled by [3H]muscimol (which labels all GABA receptors) in the prefrontal cortex (Hanada et al., 1987; Benes et al., 1996b), superior temporal gyrus (Deng and Huang, 2006), and hippocampus (Benes et al., 1996a; Benes, 1997) are increased in postmortem brain tissue of patients with schizophrenia. In contrast, the numbers of GABAA receptors with benzodiazepine-binding sites labeled by [3H]flunitrazepam are reduced or unchanged in prefrontal cortex (Pandey et al., 1997) and hippocampus (Squires et al., 1993; Benes et al., 1996a) of schizophrenia brains. Subsequent work has demonstrated up-regulation of mRNAs and proteins for α1 and α5 subunits within the prefrontal cortex. The α5 subunit confers a 3-to 10-fold higher affinity for GABA than that observed for the α1-containing receptor, suggesting increases in GABAA receptors with a higher affinity for GABA.
The data implicating the GABAB receptor in the pathophysiology of schizophrenia are more limited. There is evidence for a reduction in GABAB receptor immunoreactivity in the entorhinal cortex and inferior temporal cortex of the brain in schizophrenia (Mizukami et al., 2002). In addition, baclofen, a GABAB agonist, can reverse spontaneous gating deficits in animal models of schizophrenia (Bortolato et al., 2007).
Somatostatin (SST) is a neuropeptide present in a subpopulation of GABA neurons, and a reduction in the density of neurons positive for SST as well as expression of SST mRNA per neuron is seen in dorsolateral prefrontal cortex in schizophrenia (Morris et al., 2008). There is evidence that neuroregulin-1 (NRG1) may regulate GABAergic neurotransmission via binding to presynaptic ErbB4 receptors (Woo et al., 2007). NRG1 is a regulator of neural development, and NRG1 and ErbB4 have been identified as susceptibility genes for schizophrenia (Britsch, 2007).
Preliminary data using real-time quantitative polymerase chain reaction demonstrated that several of these molecular changes (i.e., decreased transcripts for SST, PV, GAD67, GAT1, and the α1 and δ subunits of GABAA receptors) are observed within four cortical areas (dorsolateral prefrontal cortex, anterior cingulate cortex, and primary motor and visual cortices). This finding suggests that a conserved set of molecular alterations in GABA neurotransmission may contribute to the pathophysiology of schizophrenia (Hashimoto et al., 2008).
3. Glutamate
Glutamatergic synapses are the key excitatory synapses within the brain, and mechanisms of both hyperglutamatergic and hypoglutamatergic functioning have been implicated in the pathophysiology of schizophrenia (for review, see Olney et al., 1999; Deutsch et al., 2001; Coyle, 2006). It has been proposed that NMDA receptor hypofunction may lead to excessive stimulation of other iontropic receptors, causing a cascade of excitotoxic events including oxidative stress and apoptosis (for review, see Deutsch et al., 2001). Dysregulation of glutamateric functioning has been observed across many components of the glutamate neurotransmission system.
Glutamatergic receptors include both iontropic and metabotropic receptor subtypes. The iontropic receptors include NMDA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), and kainate receptors. Binding of glutamate to these receptors causes Ca2+ and Na+ entry into neurons, resulting in excitatory postsynaptic potentials and membrane depolarization. In addition, increased intracellular Ca2+ levels activate a number of signaling cascades (Berridge, 1998). The NMDA receptor forms a channel allowing for ion influx, whereas the AMPA and kainate receptors open voltage-sensitive ion channels on the cell membrane. The NMDA receptor is voltage-gated and is blocked by magnesium and modulated by two coagonists, glycine and D-serine, as well as by several intracellular and extracellular mediators (for review, see Millan, 2005). The NMDA receptor is a heteromeric assembly of an obligatory NR1 subunit (eight distinct isoforms) and a combination of NR2A, NR2B, NR2C, NR2D, NR3A, and NR3B subunits (Dingledine et al., 1999; Millan, 2005). The properties of the NMDA receptor depend on the composition of subunits. In the human cortex, NR1, NR2A, and NR2B are the predominant subunits found (Cull-Candy et al., 2001). NR2 is the binding site for glutamate and other mediators, and NR1 is the binding site for glycine and D-serine (glycine modulatory site) (Johnson and Ascher, 1987). Eight metabotropic glutamate receptors, termed mGlu1–8, have been cloned and are classified into three groups based on sequence, identity, and transduction mechanisms: group I, mGlu1,5, are coupled to Gq protein, leading to an increase in PLC; group II, mGlu2,3, are coupled to Gi and Go, leading to a decrease in AC; and group III, mGlu4,6,7,8, are coupled to Gi and Go, leading to a decrease in AC. Glutamate neurotransmission is terminated via excitatory amino acid transporters (EAATs) expressed on astrocytes, Bergmann glia, and neurons throughout the brain, and several EAAT-interacting proteins can regulate EAAT activity (for review, see Huerta et al., 2006).
The NMDA receptor hypofunction hypothesis of schizophrenia is based on the observation that phencyclidine (PCP), an NMDA antagonist, can induce a spectrum of behavioral effects in humans that resemble the positive, negative, and cognitive symptoms seen in schizophrenia (Deutsch et al., 1989; Javitt and Zukin, 1991; Coyle, 1996; Tamminga, 1998). All NMDA antagonists [including ketamine and MK-801 (dizocilpine)] tested in humans can trigger a florid psychotic response similar to that with PCP (for review, see Olney et al., 1999). In addition, ketamine can precipitate psychoses in patients with schizophrenia (Lahti et al., 1995, 2001; Malhotra et al., 1996).
There are numerous indications that NMDA receptor functioning is reduced in patients with schizophrenia (for review, see Millan, 2005). Endogenous antagonists of the NMDA receptor, kynurenic acid and N-acetyl-aspartyl-glutamate (NAAG), are elevated within the cerebrospinal fluid and/or brain of patients with schizophrenia (Tsai et al., 1995; Coyle, 1996; Schwarcz et al., 2001; Erhardt et al., 2007). NAAG is also a potent selective agonist of the mGluR3 metabotropic receptor, which inhibits glutamate release (Wroblewska et al., 1997), further limiting NMDA receptor function. Indices of oxidative stress are elevated in schizophrenia, which could lead to reduced activation of the NMDA receptor via oxidation of the redox-sensitivity site (Smythies, 1999). Of particular note, the levels of glutathione, an endogenous redox regulator, are reduced in the cerebrospinal fluid and prefrontal cortex of patients with schizophrenia (Do et al., 2000), and expression of two genes responsible for glutathione synthesis is decreased in fibroblasts of subjects with schizophrenia compared with that in control subjects (Tosic et al., 2004). Phosphorylation of the NR1 or NR2 subunits by protein kinases can dramatically affect NMDA receptor activity (Dingledine et al., 1999; Yamakura and Shimoji, 1999; Cull-Candy et al., 2001), and there is evidence for decreased phosphorylation of the NMDA receptor type 1 subunit at serine 897, a target of protein kinase A, in the brains of patients with schizophrenia (Emamian et al., 2004).
NMDA receptor activity requires the binding of coagonists glycine or D-serine, and alterations in glycine and D-serine metabolism have been reported in schizophrenia (for review, see Boks et al., 2007). Low glycine levels and low glycine/serine ratios but elevated levels of serine were observed in medication-free patients with schizophrenia compared with those in healthy control subjects (Sumiyoshi et al., 2004). Likewise, Neeman et al. (2005) reported lower glycine levels and glycine/serine ratios in chronically ill patients with schizophrenia treated with FGAs or SGAs (Neeman et al., 2005). Of interest, in both studies, low glycine levels correlated with greater negative symptomatology (Sumiyoshi et al., 2004; Neeman et al., 2005). Increased binding to the glycine binding site has been reported in several cortical regions in schizophrenia (Ishimaru et al., 1992). Grimwood et al. (1999) reported an increase in the number of glycine binding sites per NMDA receptor subunits in patients with schizophrenia. Burnet et al. (2008) reported a reduction in sodium-coupled neutral amino acid transporter 2, a possible transporter of glycine, within the dorsolateral prefrontal cortex and cerebellum of patients with schizophrenia, although no change was observed for the glycine transporter GlyT1 mRNA or protein. Low levels of D-serine have been observed in patients with schizophrenia (Hashimoto et al., 2003, 2005; Yamada et al., 2005), along with select increases in postmortem tissue in the activity and/or expression of mRNA for D-amino acid oxidase (DAAO), the enzyme that degrades D-serine (Verrall et al., 2007; Madeira et al., 2008). The D-serine transporter in neurons and glia, Asc-1 protein, was found to be reduced within the dorsolateral prefrontal cortex and cerebellum of subjects with schizophrenia (Burnet et al., 2008). There is evidence for increased levels of serine racemase, the enzyme that synthesizes D-serine from L-serine, within the dorsolateral prefrontal cortex of patients with schizophrenia (Verrall et al., 2007). Glycine, D-serine, other glycine modulatory site agonists, and glycine transport inhibitors show benefit in treating symptoms in schizophrenia and in animal models of schizophrenia (for review, see Boks et al., 2007, Shim et al., 2008).
Alterations in NMDA subunit receptor mRNA expression have been observed in the brains of patients with schizophrenia (for review, see Millan, 2005). However, there is considerable inconsistency in the observations that have been made, possibly reflecting variations in treatment, disease status, outcome measurements, age, and brain region examined. The majority of findings suggest a reduction in mRNA for the NR1 subunit within the thalamus, hippocampus, and cortex, which would be associated with reduced NMDA receptor function. Alterations have also been observed in NMDA receptor binding and in expression of NR2A, NR2B, NR2C, and NR2D subunits. Of interest, an increase in the levels of NR1 subunits was observed in the substantia nigra in schizophrenia (Mueller et al., 2004). Cortical and subcortical glutamatergic pathways send glutamatergic afferents to the substantia nigra and ventroteg-mental area. The increase in NR1 subunit expression within the substantia nigra could reflect increased activity at NMDA receptors on subcortical dopaminergic cell bodies that may contribute to the dopaminergic hypersensitivity/hyperactivity seen in schizophrenia (for review, see Millan, 2005).
In animal testing, administration of NMDA antagonists results in a number of behavioral, metabolic, and electrophysiological changes thought to model various symptoms occurring in patients with schizophrenia (for review, see Morris et al., 2005; Rujescu et al., 2006; Mouri et al., 2007). In addition, administration of NMDA antagonists has been linked to neurodegenerative changes associated with excitotoxicity (Olney et al., 1999; Deutsch et al., 2001) and apoptosis (Griffiths et al., 2000; Wang et al., 2000, 2003). Excitotoxicity is thought to reflect excessive synaptic release of glutamate, over-stimulation of glutamatergic iontropic receptors leading to dysregulation of Ca2+ homeostasis and subsequent cell damage (Arundine and Tymianski, 2003). Indeed, postmortem studies have revealed a number of pathological changes occurring within the brains of patients with schizophrenia as reviewed in section II.B.
In rodents, blocking of NMDA receptors is associated with increased release of glutamate within the cerebral cortex (Moghaddam et al., 1997; Adams and Moghaddam, 1998) and nucleus accumbens (Razoux et al., 2007). However, elevations in glutamate within the prefrontal cortex of rodents occurs during short-term administration of NMDA antagonists, whereas long-term administration over 7 consecutive days actually results in a trend for lower basal levels and lower dialysate levels of glutamate upon challenge (Zuo et al., 2006). Thus, excitotoxic events associated with NMDA antagonists may be reflected by initial increases in glutamatergic neurotransmission that are followed subsequently and chronically by lower levels.
Studies measuring glutamate levels within patients with schizophrenia compared with healthy control subjects have shown variable results. In cerebrospinal fluid (CSF), a reduction in glutamate has been reported (Kim et al., 1980), although a number of other studies have reported no change (Perry, 1982; Gattaz et al., 1985; Tsai et al., 1995; Korpi et al., 1987; Deutsch et al., 1989; Faustman et al., 1999) in patients with schizophrenia compared with control subjects. In one of these studies, cluster analysis had revealed one subgroup of patients with schizophrenia characterized by low CSF glutamate, enlarged ventricles, and higher thought disorder, whereas another was characterized by high CSF glutamate, normal brain structure, and less thought disorder (Tsai et al., 1995). In another study, ratings of positive symptoms were inversely correlated with glutamate concentrations (Faustman et al., 1999). These two studies suggest that lower glutamate levels may be associated with greater severity of positive symptoms and possibly also degenerative changes within the brain. In postmortem brain tissue, Perry (1982) reported no change in glutamate levels relative to those of controls, whereas Tsai et al. (1995) reported a reduction. In blood, no difference in glutamate levels (Alfredsson and Wiesel, 1989), increased levels of glutamate (Macciardi et al., 1990; van der Heijden et al., 2004), and reduced levels of glutamate (Palomino et al., 2007) have been reported.
Studies using short-echo proton magnetic resonance spectroscopy (1H-MRS) to examine brain glutamate/glutamine levels in vivo revealed significantly higher levels of glutamine in the left anterior cingulate cortex and thalamus of neuroleptic-naive patients experiencing their first episode of schizophrenia compared with those in healthy control subjects (Théberge et al., 2002). With use of this “in vivo” approach, significantly lower levels of glutamine and glutamate were found in the left anterior cingulate cortex of patients with chronic schizophrenia than in healthy volunteers, whereas glutamine levels in the left thalamus were higher (Théberge et al., 2002). Another study using 3-T 1H-MRS reported significant elevations of glutamate/glutamine levels in the medial prefrontal cortex of nonpsychotic adolescents at high genetic risk for schizophrenia compared with those in low-risk offspring. These subsequent studies provide tentative support for the proposition that higher levels of glutamate may be present during the early stages of the illness followed by lower levels subsequently. However, many different factors could affect the measurement of glutamate and other excitatory amino acids in schizophrenia notwithstanding the type of assessment (i.e., CSF, postmortem tissue, blood, or 3-T 1H-MRS) and brain region, including the likelihood of compensatory changes in glutamate and related neurotransmitter systems over time, effects of medication, response to treatment, active psychosis, subtypes of schizophrenia, and patients’ current symptom profile.
There is some evidence for regionally selective increases in the density of kainate and AMPA binding sites in the postmortem brains of patients with schizophrenia (Nishikawa et al., 1983; Toru et al., 1988; Noga et al., 1997), although not all studies have shown increased binding (Kurumaji et al., 1992; Healy et al., 1998). In addition, there is evidence for decreased expression of the neuronal transporter (EAAT3) in schizophrenia (McCullumsmith and Meador-Woodruff, 2002), but increased levels of expression of the glial EAAT transporter in medication-free patients (Matute et al., 2005). Increased expression of EAAT-interacting proteins has been observed within the thalamus (Huerta et al., 2006). There is evidence for dysfunction of the astrocytic neuropeptidase glutamate carboxypeptidase II, the dipeptidase that hydrolyzes NAAG into glutamate and N-acetylaspartate, which could contribute to NMDA receptor hypoactivity (Carlsson and Carlsson, 1990; Olney and Farber, 1995; Coyle, 1996). These collective findings suggest that glutamate signaling is impaired in schizophrenia, although the mechanisms of regulation are complex.
Several authors have proposed a model of the neuroanatomical circuitry within the cerebral cortex that may be altered in the brains of patients with schizophrenia (for review, see Olney et al., 1999): Stimulation of NMDA receptors on the GABAergic inhibitory interneurons within the cortex leads to the release of GABA, which acts upon GABA-gated chloride ion channels (GABAA receptor complex) to inhibit glutamatergic neurons and the release of glutamate. Blockade of NMDA receptors would therefore decrease GABAergic inhibitory tone and result in heightened activity of glutamatergic neurons within the cortex and at their terminal fields. In rat, administration of dizocilpine, a selective NMDA antagonist, can decrease the amplitude and frequency of excitatory postsynaptic currents in GABAergic interneurons and inhibitory postsynaptic currents in pyramidal neurons and from the rat cerebral cortex (Li et al., 2002), a finding consistent with reduced GABAergic inhibitory tone.
NMDA antagonists can also up-regulate dopamine neurotransmission. In addition, blocking of NMDA receptors is associated with dopamine release within the cerebral cortex in rodents (Moghaddam et al., 1997; Adams and Moghaddam, 1998). Increased mesolimbic dopaminergic responsivity and stress- and psychostimulant-induced hyperlocomotion have been observed after subchronic PCP administration (Jentsch et al., 1998). It has been suggested that NMDA receptor hypofunction may actually precede the dopaminergic alterations observed in schizophrenia.
4. Other—Serotonin, Acetylcholine, Norepinephrine
Whereas dopamine, GABA, and glutamate are three key neurotransmitter systems implicated in the pathophysiology of schizophrenia, alterations in other neurotransmitter systems have been suggested and include serotonin (Abi-Dargham, 2007), acetylcholine (Sarter et al., 2005) [muscarinic receptors (Raedler et al., 2007; Langmead et al., 2008) and nicotinic receptors (Woodruff-Pak and Gould, 2002; Levin and Rezvani, 2007)], norepinephrine (Friedman et al., 1999; Yamamoto and Hornykiewicz, 2004), and numerous neuropeptides [neuropeptide Y (Eaton et al., 2007), tachykinins (Chahl, 2006), neurotensin (Cáceda et al., 2006), and orexins/hypocretins (Deutch and Bubser, 2007)].
5. Intracellular Signaling Cascades
As mentioned in section II.A, metabotropic receptors involve coupling to various G-proteins, leading to the regulation of cAMP and IP3 second messengers and subsequent downstream signaling systems including kinase cascades and transcriptional factors. One key regulatory aspect of the kinase signaling cascades is protein phosphorylation, with protein kinases resulting in phosphorylation of proteins, which alters their regulation and downstream effects, and protein phosphatases reversing the phosphorylation reactions providing for finely tuned regulation. As a model, we will briefly review the intracellular signaling underlying the actions of dopamine (for review, see Girault and Greengard, 2004; Beaulieu et al., 2005, 2007). However, many metabotropic receptors and even iontropic receptors can interact with these and other effector molecules. There is evidence for dysregulation within these signaling cascades in patients with schizophrenia.
As reviewed by Beaulieu et al. (2007), the stimulation of dopamine receptors leads to a conformation change in the receptor and activation of G-proteins that either activate or inhibit adenylyl cyclase, thereby modulating the activity of cAMP-dependent protein kinase (PK) A. The D1 class receptors activate adenylyl cyclase, whereas the D2 class receptors inhibit adenylyl cyclase. PKA phosphorylates a number of downstream protein targets including DARPP-32, cAMP-response element-binding protein (CREB), and extracellular signal-regulated kinase (ERK). This initial wave of responses reflects G-protein-mediated signaling and is thought to be relatively rapid and transient in nature. After stimulation, dopamine receptors are phosphorylated by G-protein-coupled receptor kinases and β-arrestins are recruited, leading to termination of G-protein-dependent signaling and internalization of the receptor (desensitization). In addition, the D2 class receptors are associated with cAMP-independent signaling involving formation of a signaling complex comprising β-arrestin 2, protein phosphatase 2A, and Akt (protein kinase B). The formation of this signaling complex leads to inactivation of Akt by protein phosphatase 2A, and subsequent activation of glycogen synthase kinase-3 (GSK-3)-mediated signaling. This second wave reflects β-arrestin 2-mediated signaling and is thought to be a more progressive and longer-lasting response. These signaling cascades control protein phosphorylation, resulting in the regulation of ligand- and voltage-gated ion channels, as well as production of transcription factors that regulate the subsequent expression of specific genes.
In addition to adenylyl cyclase regulation, several neurotransmitter receptors interact with G-proteins to regulate PLC and subsequent signaling via IP3 and intracellular Ca2+ release and diacylglycerol. IP3 interacts with receptors on the endoplasmic reticulum, leading to increased Ca2+ levels within the cytosol and increased Ca2+ signaling. Diacylglycerol activates protein kinase C, leading to the phosphorylation of a number of proteins. Whereas dopamine receptors and the GABAB receptor (Bowery, 2006) regulate adenylyl cyclase, select metabotropic receptors within the other neurotransmitter systems interact with both signaling cascades: glutamatergic receptors (AC: mGlu2–4,6–8; PLC: mGlu1,5) (Gerber et al., 2007, Moghaddam, 2004), muscarinic acetylcholine (ACh) receptors (AC: M2,4; PLC: M1,3,5) (Raedler et al., 2007; Langmead et al., 2008), 5-HT receptors (AC: 5-HT1A,B,D,4,5A,B,6,7; PLC: 5-HT2A,B,C) (Barnes and Sharp, 1999; Hoyer et al., 2002), and adrenergic receptors (PLC: α1,2; AC: β1,2) (Ramos and Arnsten, 2007).
Regulators of G-protein signaling (RGS4) (28 RGS proteins) function as GTPase-activator proteins for heteromeric G-protein α (Gα) subunits and accelerate the hydrolysis of Gα-bound GTP, shortening the duration of intracellular G-protein-coupled receptor signaling and thereby modulating the intracellular effects of G-protein-coupled neurotransmitters (for review, see Lang et al., 2007). RGS4 mRNA levels were significantly lower in postmortem samples of the dorsolateral prefrontal cortex of subjects with schizophrenia compared with those of matched control subjects (Mirnics et al., 2001b). RGS9-2 expression was reduced in schizophrenia hippocampi compared with control tissue and in amphetamine-sensitized rat striatum as an animal model of schizophrenia (Seeman et al., 2007).
DARPP-32 is a key regulator of kinase phosphatase signaling cascades and is modulated by dopaminergic, serotonergic, and glutamateric neurotransmission (Svenningsson et al., 2003). DARPP-32 can be phosphorylated at four distinct sites, the location of phosphorylation influencing its function as an amplifier or inhibitor of PKA (or PKG)-mediated signaling. A significant reduction in DARPP-32 expression has been observed postmortem in the dorsolateral prefrontal cortex of patients with schizophrenia (Albert et al., 2002).
Akt is a serine/threonine protein kinase regulated by both G-protein-coupled receptors (GPCRs) and a number of neurotrophic receptors. Akt is involved in a range of diverse cellular processes including neuronal cell proliferation, survival, apoptosis, differentiation, neurotrophin secretion, and synaptic plasticity (Dudek et al., 1997; Lawlor and Alessi, 2001; Ciani et al., 2002; Brazil et al., 2004; Sweatt, 2004). Akt is modulated by phosphorylation at different residues after dopamine receptor activation or NMDA receptor potentiation (for review, see Lei et al., 2008). GSK-3β is constitutively active and is involved in a number of diverse functions including glycogen synthesis, cell growth and differentiation, amyloid β metabolism, and phosphorylation of tau (Gould and Manji, 2005).
GSK-3 is a central component of the developmentally important wingless signaling and insulin signaling pathways, and both pathways have been implicated in schizophrenia (for review, see Lovestone et al., 2007). Akt-GSK-3β signaling has also been implicated in PCP-induced neurodegeneration (Lei et al., 2008). Of interest, heightened GSK-3β activity is proapoptotic via activation of the Bcl-2 family member BAX. Akt is the principal kinase to phosphorylate and inhibit GSK-3β activity, a regulatory pathway that may facilitate neuronal survival. A decrease in AKT1 protein levels and decreased phosphorylation of GSK-3β at Ser-9 were observed in peripheral lymphocytes and postmortem brain tissue from patients with schizophrenia, suggestive of a proapoptotic state (Emamian et al., 2004). Emamian et al. (2004) and others (Bajestan et al., 2006; Kalkman, 2006) have implicated the Akt1 gene as a potential susceptibility gene for schizophrenia. Likewise, Zhao et al. (2006) reported decreases in Akt content and activity in the dorsolateral prefrontal cortex that were accompanied by an elevated content of GSK-3α and GSK-3β but without changes in phospho-Ser(21/9) GSK-3α/β levels in postmortem tissue of medicated patients with schizophrenia (relative to those of control patients).
In contrast, others have observed a reduction in GSK-3β protein levels and GSK-3 activity in frontal cortex (Kozlovsky et al., 2000, 2001) and decreased GSK-3β mRNA in postmortem dorsolateral prefrontal cortex of patients with schizophrenia compared with that of patients with bipolar and unipolar disorders and healthy control subjects (Kozlovsky et al., 2004). Reductions in GSK-3β may result in an imbalance in the rate and timing of apoptosis during neurodevelopment (Kozlovsky et al., 2004).
Mitogen-activated protein (MAP) kinases are a family of serine/threonine kinases that regulate neuronal survival, differentiation, and plasticity and are activated after ligand binding to NMDA, muscarinic, acetylcholine, serotonin, and dopamine receptors (for review, see Schaeffer and Weber, 1999; Einat et al., 2003; Kyosseva, 2004). MAP kinases include ERK1 and ERK2, c-Jun NH2-terminal kinase/stress-activated protein kinase, and p38 MAP kinase. When activated, the MAP kinases are translocated to the nucleus and activate transcription of immediate early genes c-fos and c-jun, leading to increased translation of the Fos and Jun families of proteins, which heterodimerize to form the activator protein-1 complex that controls subsequent transcription of neuronal genes encoding neuropeptides and neurotransmitter receptors (for review, see Kyosseva, 2004). Increased expression of several intermediates of the ERK cascade and downstream transcript targets was observed in the cerebellar vermis of patients with schizophrenia (Kyosseva et al., 1999). In addition, selective increases in ERK2, c-fos, and c-jun protein and mRNA levels were observed within the thalamus of patients with schizophrenia relative to levels in control subjects (Kyosseva, 2004). Finally, given the pervasiveness of Ca2+ signaling motifs, it has been argued that many of the changes observed in schizophrenia may be associated with dysfunction in calcium signaling (Lidow, 2003).
In summary, schizophrenia has been associated with dysfunction in many neurotransmitter systems and at many different levels. The current view emphasizes NMDA receptor hypofunction as an underlying mechanism that may lead to both reduced GABAergic tone and increased dopaminergic tone. However, this basic tenet rests upon a plethora of molecular changes that have been observed across many brain pathways, for which there exists an intricate balance of interactions among several of the neurotransmitter systems. In addition, differences in gene expression and the experience of environmental “insults” may underlie the variability that is seen in the risk of developing this mental illness.
B. Neuroanatomical Pathology
Numerous studies have documented the presence of structural changes in the brains of patients with schizophrenia including loss of cortical volume (gray matter and white matter), increased ventricular volume, increased neuronal density, reduction of neuropil, damage to myelinated fiber tracts (white matter), and alterations in the number and distribution of supporting glia. Collectively, these changes reflect alterations in the structure and connections of neurons, a finding that underscores disruption in the communication between brain regions.
A large number of studies have suggested that there is a loss of cortical volume in schizophrenia, particularly in prefrontal and temporal cortical areas (for review, see Harrison, 1999; Convit et al., 2001; Narr et al., 2005, Steen et al., 2006). Despite a decrease in the volume of the prefrontal cortex (PFC) in schizophrenia, a significant decrease in neuronal number has not been found, giving rise to the “reduced neuropil hypothesis” (Selemon and Goldman-Rakic, 1999). Postmortem studies suggest that decreases in axon terminals, preterminals (presynaptic elements), and dendrites, albeit to varying degrees, contribute to the loss of cortical volume (Harrison, 1999; Glantz and Lewis, 2000; Mirnics et al., 2001a). However, these observations do not preclude the loss of selective groups of neurons, and several studies have described reduced numbers of neurons in several cortical and subcortical regions and within specific neurochemically defined neuronal cell groups (for review, see Pérez-Neri et al., 2006). Longitudinal studies have suggested that there is progressive volume loss in first-episode schizophrenia (Steen et al., 2006) in several cortical regions/DeLisi et al., 1997), total cerebral gray matter (Cahn et al., 2002), frontal cortex (Gur et al., 1998), and superior temporal gyrus (Kasai et al., 2003). As a corollary, there is evidence for increased ventricle volume in patients with schizophrenia during the course of the disease and/or during a psychotic episode (DeLisi et al., 1997; Nair et al., 1997; Rapoport et al., 1997; Davis et al., 1998; Lieberman et al., 2001b; Mathalon et al., 2001).
Although the majority of studies have reported decreases in cortical gray matter volume, an increase in the volume of the caudate nucleus has been observed in patients with schizophrenia. Caudate hypertrophy, earlier thought to be a pathological feature of schizophrenia (Heckers et al., 1991; Swayze et al., 1992), has more recently been shown to be a side effect of antipsychotic treatment (Chakos et al., 1994; Hokama et al., 1995).
Several lines of evidence suggest a compromise in the integrity of white matter tracts providing anatomical and functional connections between brain regions (for review, see Davis et al., 2003, Walterfang et al., 2006). Decreased global white matter volume has been observed in patients with schizophrenia (Cannon et al., 1998; Wright et al., 2000), with reductions revealed in comparison with both unaffected siblings and healthy control subjects (Cannon et al., 1998), an important finding as white matter volumes also decrease with age in healthy individuals (Bartzokis et al., 2001). Volume reductions have also been observed specifically within the white matter of the PFC (Breier et al., 1992; Buchanan et al., 1998; Sanfilipo et al., 2000; Sigmundsson et al., 2001), frontal cortex (Ho et al., 2003), temporal cortex (Okugawa et al., 2002; Mitelman et al., 2003), and parietal and occipital cortices (Milev et al., 2003; Mitelman et al., 2003). In some studies, a reduction in white matter volume has been associated with negative symptoms (Sanfilipo et al., 2000; Sigmundsson et al., 2001; Ho et al., 2003). Numerous studies provide evidence of focal damage occurring and accumulating along white matter tracts within the brains of patients with schizophrenia, including white matter hyperintensities, reductions in myelin or axonal membrane integrity, and decreased anisotrophy (or decreased coherence) within white matter (for details, see Davis et al., 2003; Walterfang et al., 2006).
Again, although the majority of studies indicate decreases in white matter volume in patients with schizophrenia, at least one study has reported an increase in white matter in the temporal lobes in childhood-onset schizophrenia (Taylor et al., 2005). Recently, Federspiel et al. (2006) found evidence for both reduced and elevated anisotrophy (connectivity in white matter bundles) in patients with schizophrenia compared with that in control subjects. Increases in white matter volume have been observed during exacerbation of psychosis with decreases occurring upon symptom remission (Christensen et al., 2004), and increased anisotrophy has been reported in hallucinating patients compared with control subjects and patients without hallucinations (Hubl et al., 2004). These disparate findings as a whole may point to dynamic changes taking place within the brain wherein increases in white matter volume might reflect active processes of disease (i.e., swelling of myelin, necrosis and apoptosis of oligodendroglia, or remodeling of connections associated with psychosis) or possibly compensatory, restorative changes, whereas loss of white matter might reflect a more refractory state.
At the cellular level, morphological abnormalities and density changes have been observed in neurons and in glia, including the oligodendroglia, which provide and maintain the myelin sheath surrounding neuronal axons (for review, see Walterfang et al., 2006). Among the most intriguing of the pathological features of schizophrenia is a decrease in dendritic spine density in PFC neurons. Postmortem studies have shown a decrease in basal dendritic spine density of layer III and V pyramidal cells in the PFC (Garey et al., 1998; Glantz and Lewis, 2000; Kalus et al., 2000; Broadbelt et al., 2002; Black et al., 2004; Kolluri et al., 2005). Because the dendritic spines of pyramidal cells receive inputs from DA axons (Sesack et al., 2003) and DA receptors are expressed on spines, it is possible that changes in DA transmission may lead to structural changes in the dendrites of PFC pyramidal cells. Specifically, because DA axons synapse predominantly on spine necks, with an excitatory input synapsing with spine heads (Sesack et al., 2003), a loss of cortical DA would be predicted to decrease the capacity of pyramidal cells to gate excitatory input onto dendritic spines, which in turn would lead to hyperexcitability of the cell and a (slow) excitotoxic process. This seems to be the case in striatal medium spiny neurons, which share with cortical pyramidal cells the triadic arrangement of DA axons terminating on the spine neck and a corticostriatal glutamatergic axon that synapses onto the spine head. Thus, medium spiny neurons in the striatum of animals with lesions of the nigrostriatal DA neurons or humans with Parkinson’s disease have a decrease in overall dendritic length and spine density (Zaja-Milatovic et al., 2005).
Following this reasoning, Wang and Deutch (2008) recently examined the effects of lesions disrupting the DA innervation of the PFC on pyramidal cells. They found that layer V pyramidal cells had a decrease in total dendritic length, dendritic spine density, and dendritic complexity (branching). Thus, DA denervation of the PFC resulted in dystrophic changes of pyramidal cell dendrites in the PFC, recapitulating a key pathological feature of schizophrenia (Glantz and Lewis, 2000).
In summary, many studies have shown neuropathological changes within the brains of patients with schizophrenia. Altered brain structure and function are evident during the first episode of schizophrenia, and there is evidence (at least for some patients) of progressive loss of tissue volume and cellular elements over time. Several of the changes seem to reflect active states of psychosis, illustrating the dynamic state of morphological changes occurring within the brain and the potential for compensatory changes to occur at least early in the stages of the illness.
C. Apoptosis and N-Methyl-D-aspartate Antagonist-Induced Neurodegeneration
As noted in the preceding section, the cortical neuropathology observed in schizophrenia predominantly includes neuronal atrophy, decreased neuropil, and alterations in neuronal density suggesting that the connections between neurons, synaptic circuitry, is altered. Dysregulation of neuronal apoptosis has been implicated in the pathophysiology of schizophrenia, and most recently sublethal apoptotic activity has been proposed, resulting in the loss of synapses without cell death (for review, see Jarskog et al., 2005; Glantz et al., 2006).
Apoptosis or programmed cell death is a process normally associated with the elimination of redundant neurons during neurodevelopment (Johnson et al., 1995). Apoptosis involves the regulation of a complex molecular cascade controlling the activation of a family of cysteine proteases known as caspase proteins (for review, see Glantz et al., 2006). Caspases are responsible for breaking down important structural and functional proteins, leading to cellular degradation and eventually death. Apoptosis results from a cascade of gene activation and involves genes that both promote (i.e., Bax) (Schlesinger et al., 1997; Gross et al., 1998) and oppose the process (i.e., Bcl-2) (Craig, 1995; Schlesinger et al., 1997; Adams and Cory, 1998).
Although widespread neuronal loss is not observed within the brains of patients with schizophrenia, the anterior cingulate cortex is one area in which layer-specific reductions in subtypes of neurons have been identified (Benes et al., 1991, 2001). Using the Klenow method to identify apoptotic-positive neurons, subjects with chronic schizophrenia actually demonstrated a decrease in a distinct subset of Klenow-positive neurons compared with that in matched control subjects and subjects with bipolar disorder (Benes et al., 2003). Benes et al. suggested that the reduction in apoptotic-positive neurons represented either a compensatory down-regulation to promote cell survival or a failure to mount an appropriate apoptotic response to an oxidative challenge.
Caspase activity has also been localized to dendrites, dendritic spines, and axonal terminals (Yan et al., 2001), and synaptic apoptotic activity has been implicated in adaptive plasticity and neurodegenerative disorders (Mattson and Duan, 1999). Two reports have described alterations in apoptotic regulatory proteins in patients with schizophrenia. In one study, a 50% increase in the Bax/Bcl-2 ratio was observed in the temporal cortex of patients with schizophrenia compared with the ratio in matched control subjects (Jarskog et al., 2004). An elevated ratio of proapoptotic (i.e., Bax) to antiapoptotic (e.g., Bcl-2) protein levels may up-regulate cytochrome c release from mitochondria and subsequent caspase activation [for review (Glantz et al., 2006). In a second study, Bcl-2 levels were reported to be 30% lower in the temporal cortex in patients with schizophrenia than in control subjects (Jarskog et al., 2000). Bcl-2 levels can exert neuroprotective and neurotrophic effects, and the lower levels suggest less neuroprotection.
A vast array of stimuli can activate apoptosis in neurons (Sastry and Rao, 2000). Many of these stimuli have been implicated in the pathophysiology of schizophrenia including glutamate excitotoxicity, increased calcium flux, mitochondria dysfunction, oxidative stress, and decreased neurotrophic levels. Given the importance of NMDA receptor hypofunction to schizophrenia, it is important to note that the administration of NMDA antagonists is associated with apoptotic neurodegeneration. Early work identified vacuolated neurons as injured or dying neurons within posterior cingulate and retrosplenial cortices after the administration of NMDA antagonists, with additional regions being affected, depending on dose and duration of exposure (Farber et al., 1995). Subsequent work demonstrated NMDA antagonist-induced apoptotic neurons via electron microscopy or terminal dUTP nick-end labeling (Johnson et al., 1998) or silver staining (Griffiths et al., 2000). The mechanism of NMDA antagonist (PCP)-induced apoptosis was shown to involve increased expression of Bax and decreased expression of Bcl-XL, with a decrease in the Bcl-XL/Bax ratio that could be prevented by the addition of superoxide dismutase or catalase (Wang et al., 2000). Additional studies have supported and extended these initial findings (Wang et al., 2003, 2004a, 2005a, 2008; Wang and Johnson, 2005). Recent studies have demonstrated a role for caspase-3 (Wang and Johnson, 2007) and Akt-GSK-3β signaling (Lei et al., 2008) in PCP-induced neurodegeneration.
In summary, schizophrenia is not associated with widespread neuronal cell loss but rather with a selective reduction in the number of specific cell types, as well as changes in the morphology of neurons including reductions in dendritic length and spine density. Apoptotic mechanisms may underlie both the loss of specific groups of neurons and changes in neuronal morphology. Of interest, in some instances, there is evidence for reduced apoptotic activity. Given that schizophrenia reflects impaired information processing, an inability to reduce neuronal number and/or connections seen normally in development may be as relevant to schizophrenia as a reduction in dendritic processes and spine density or loss of specific cell groups.
D. Altered Levels of Neuroactive Steroids
Neuroactive steroids are endogenous neuromodulators synthesized either within the brain (neurosteroids) or in the periphery by the adrenal glands and gonads. Neuroactive steroids can alter neuronal excitability via nongenomic effects by acting at inhibitory GABAA receptors and/or excitatory NMDA receptors, among others (for review, see Paul and Purdy, 1992; Belelli and Lambert, 2005). There is also evidence for a potential role of these neurosteroids in controlling GABA and glutamate release. Neuroactive steroids/neurosteroids have also been implicated in neuroprotection, myelination, and modulation of the stress response (for review, see Marx et al., 2006b).
A number of neuroactive steroids are present in human postmortem brain at physiologically relevant nanomolar concentrations (Marx et al., 2006b) and serve as allosteric modulators of the GABAA receptor. Allopregnanolone (ALLO) potentiates the GABAA receptor response more potently than benzodiazepines or barbiturates (Majewska et al., 1986; Morrow et al., 1987, 1990). ALLO levels are lower in postmortem brain tissue from parietal cortex in subjects with schizophrenia, suggesting that an ALLO deficit is potentially present in this disorder (Marx et al., 2006b).
Pregnenolone sulfate and dehydroepiandrosterone (DHEA) are positive modulators of NMDA receptors (Wu et al., 1991; Irwin et al., 1994; Bergeron et al., 1996; Debonnel et al., 1996; Compagnone and Mellon, 1998) and negative modulators of GABAA receptors (Majewska et al., 1988, 1990; Imamura and Prasad, 1998; Park-Chung et al., 1999). Pregnenolone and DHEA levels are elevated postmortem in subjects with schizophrenia in the posterior cingulate and parietal cortex compared with levels in control subjects (Marx et al., 2006b).
A number of studies have reported altered levels of neuroactive steroids in patients with schizophrenia (for review, see Shulman and Tibbo, 2005), although variations in the results observed clearly exist. Recent findings have described significant elevations of plasma levels of DHEA in patients with schizophrenia compared with control subjects regardless of gender (di Michele et al., 2005). There is evidence that DHEA can improve some of the symptoms of schizophrenia (Strauss et al., 1952; Strous et al., 2003).
In summary, given the complexity of the regulation of neuroactive steroids and neurosteroids and the range of changes observed in patients with schizophrenia, it is difficult to assimilate all of the current information into a single, explanatory model. Given that both pregnenolone sulfate and DHEA are positive modulators of excitatory NMDA receptors and allopregnanolone is a positive modulator of inhibitory GABAA receptors, the neuroactive steroid milieu in subjects with schizophrenia may reflect a net increase in neuronal excitation (Marx et al., 2006b). Alternatively, the NMDA receptor hypofunction theory of schizophrenia suggests that elevations in pregnenolone and DHEA may be beneficial (Shulman and Tibbo, 2005), which is consistent with some of these data.
E. Decreased Mitochondrial Function
Mitochondrial insufficiency during brain development has been suggested as a cause of reduced synaptic plasticity that eventually contributes to the pathogenesis of schizophrenia (Ben-Shachar and Laifenfeld, 2004). Impairment of oxidative energy metabolism has been shown to potentiate NMDA receptor-mediated excitotoxicity, and it has been proposed that decreases in ATP synthesis can impair the function of the Na+/K+-AT-Pase pump (Simpson and Isacson, 1993; Weller and Paul, 1993; Greene and Greenamyre, 1995), thereby decreasing plasma membrane potential, relieving the voltage-dependent Mg2+ blockade of NMDA-receptor, and resulting in hypersensitivity to glutamate (Greene and Greenamyre, 1996). Several independent lines of evidence support a role for mitochondrial insufficiency in the pathogenesis of schizophrenia.
Brain imaging studies have revealed decreased energy metabolism in the frontal lobes of patients with schizophrenia compared with that in healthy control subjects (for review, see Ben-Shachar and Laifenfeld, 2004). Analysis of mitochondrial respiratory enzymes in postmortem brain samples indicates a decrease in the activity of respiratory complex IV (cytochrome c oxidase) in the frontal cortex and temporal cortex and a decrease in activity of respiratory enzyme complexes I and III in the temporal cortex and basal ganglia of patients with schizophrenia compared with that in healthy control subjects (Maurer et al., 2001). A reduction in the number of mitochondria throughout the neuropil in both the caudate and putamen of postmortem samples of patients with schizophrenia versus control subjects has also been observed (Kung and Roberts, 1999).
More recently, altered gene expression of mitochondrial proteins has been demonstrated in patients with schizophrenia, including a reduction in mRNA and protein levels of the 24- and 51-kDa subunits of complex I in the prefrontal cortex, consistent with diminished respiratory capacity (Karry et al., 2004). More extensive proteomic analysis has revealed that nearly half of all protein differences detected between patients with schizophrenia and healthy control subjects are associated with mitochondrial function and oxidative stress (Prabakaran et al., 2004). Large-scale DNA microarray analysis of postmortem brains of patients with schizophrenia has demonstrated a global down-regulation of mitochondrial genes, although medication effects could not be ruled out (Iwamoto et al., 2005).
In summary, mitochondrial functioning is essential for normal brain development and the maintenance of normal brain function. Evidence has shown impaired mitochondrial functioning within the brains of patients with schizophrenia. Possible links between impaired mitochondrial functioning and glutamate-induced neurotoxicity have been proposed.
F. Dysfunction of Glucose Metabolism
The notion of a possible relationship between glucose metabolism and psychiatric illness is more than 100 years old and was first articulated by Maudsley who observed that diabetes and insanity are often coexpressed in families (as quoted by Mukherjee et al., 1989). Insulin-shock therapy was subsequently shown to be successful in treating some patients with longstanding psychosis (Sakel, 1994). Since these early observations, evidence has accumulated to support a relationship between glucose metabolism dysfunction and schizophrenia.
Ben-Shachar (2002), Karry et al. (2004), and Maurer et al. (2001) have reported generalized mitochondrial (energy) dysfunction in schizophrenia, whereas Blass (2002) has emphasized a more selective defect in glucose metabolism as a contributory factor in psychosis. These findings are consistent with the hypofrontality or decreased cerebral blood flow and glucose metabolic rate in the frontal cortex of patients with untreated schizophrenia detected in most brain imaging studies (Ingvar and Franzén, 1974; Wolkin et al., 1985; Weinberger et al., 1986; Buchsbaum et al., 1990; Andreasen et al., 1992), but not all (Mathew et al., 1982; Gur et al., 1987). Moreover, lower rates of glucose metabolism (especially in prefrontal areas) are correlated with negative symptoms (Andreasen et al., 1992) and poorer cognitive performance (Weinberger et al., 1986; Buchsbaum et al., 1990) in patients with schizophrenia.
Glucose is the required energetic fuel for the mammalian brain, with glucose transporter (GLUT) proteins delivering glucose from the circulation to the brain: GLUT1 found in the microvascular endothelial cells of the blood-brain barrier and glia and GLUT3 found in neurons (for review, see Simpson et al., 2007). Lactate is the glycolytic product of glucose metabolism and is transported into and out of neural cells by monocarboxylate transporters (MCTs): MCT1 in the blood-brain barrier and astrocytes and MCT2 in neurons. McDermott and de Silva (2005) postulated that impaired neuronal glucose uptake via GLUT 1 and GLUT 3 may explain the imaging, postmortem, and pharmacological findings in schizophrenia. They have suggested that reduced glucose availability in situations of high demand may produce acute symptoms of misperceptions, misinterpretations, anxiety, and irritability that are features similar to those seen in prodromal and first-onset schizophrenia. In addition, reduced glucose uptake would reduce the production of glutamate, resulting in a state functionally similar to those produced by NMDA antagonists. It is also possible that abnormalities in insulin signaling may contribute to deficiencies in glucose metabolism in neurons and limit optimal brain development and brain function (Bondy and Cheng, 2002).
In summary, decreased cerebral blood flow and glucose metabolic rate in the frontal cortex of patients with untreated schizophrenia have been detected in most brain imaging studies. Lower rates of glucose metabolism have been correlated with negative symptoms and poorer cognitive performance. And it has been postulated that reduced availability of glucose via fewer glucose transporters or decreased functional capacity could explain the imaging, postmortem and pharmacological findings reported in schizophrenia, although supporting data for this theory are needed. Dwyer et al. (2003b) have suggested that it may be possible to improve functional activity in patients with schizophrenia by enhancing glucose metabolism and related signaling pathways (e.g., insulin-like growth factor and Akt/protein kinase B) in the brain with small-molecule drugs. Girgis et al. (2008) suggested that this may be a mechanism by which clozapine-like SGAs exert their therapeutic effects.
G. Elevated Levels of Oxidative Stress
Oxidative stress occurs when there is dysequilibrium between prooxidant and antioxidant processes in favor of the former and generally occurs as a consequence of increased production of free radicals when the antioxidant defense system is inefficient or as a combination of both events. Free radicals (superoxide radical, hydrogen peroxide, and hydroxyl anion) are formed during many biochemical reactions involving oxygen including the mitochondrial respiratory process. These reactive oxygen species are generally kept in check by an antioxidant defense system comprising a series of enzymatic [superoxide dismutase (SOD), catalase, and glutathione peroxidase] and nonenzymatic [glutathione, α-tocopherol (vitamin E), ascorbic acid (vitamin C), and β-carotene] components (for review, see Reddy and Yao, 1996). Oxidative stress can initiate a number of pathophysiological processes, leading to cellular toxicity and is a mechanism that is common to many neurodegenerative diseases (Reddy and Yao, 1996).
A number of studies provided evidence for an elevation in oxidative stress and a reduction in antioxidant capacity in patients with schizophrenia. Markers of lipid peroxidation, thiobarbituric acid reactive species and malondialdehyde, are elevated in patients with schizophrenia (Dietrich-Muszalska et al., 2005; Zhang et al., 2006). An increase in the oxidative metabolites of bilirubin (i.e., biopyrrins) has been observed in urine of patients with schizophrenia (Miyaoka et al., 2005). Postmortem studies have provided evidence for oxidative DNA damage in the hippocampus of elderly patients with schizophrenia (Nishioka and Arnold, 2004) and for elevated levels of nitric oxide in brains of patients with schizophrenia (Yao et al., 2004). In addition, there is evidence for lower levels of antioxidants and/or antioxidant activity in patients with schizophrenia (Ranjekar et al., 2003; Dietrich-Muszalska et al., 2005; Yao et al., 2006; Zhang et al., 2006).
Because lower antioxidant enzyme activity has been observed in patients with schizophrenia, it has been proposed that oxidative stress-mediated cell damage may underlie development of schizophrenia (Ranjekar et al., 2003). However, some studies have actually reported increased levels of antioxidants and/or antioxidant enzymes in patients with schizophrenia (Zhang et al., 2003; Michel et al., 2004). This latter finding might suggest the presence of a compensatory process designed to maintain homeostasis (Michel et al., 2004), whereby up-regulation of some antioxidants such as SOD may occur in response to and coincide with elevations in oxidative stress.
In summary, markers of oxidative stress are elevated in schizophrenia, and there is evidence for both up- and down-regulation of antioxidants and/or antioxidant enzymes. Similar to changes occurring in neurotransmitter regulation and neuronal morphology, the brain may try to compensate for ongoing neurodegenerative changes (i.e., increased oxidative stress). The increases in oxidative stress parameters may reflect changes co-occurring in other systems such as free radical production that occurs in mitochondria during oxidative phosphorylation.
H. Reduced Neurotrophic Factor Expression
Neurotrophic factors are critical for normal brain development and maintenance throughout the life of the organism (Levi-Montalcini, 1987; Barde, 1994; Dono, 2003; Rosenstein and Krum, 2004). The neurotrophic factors include nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), basic fibroblast growth factor (FGF), neurotrophin-3 (NT-3), epidermal growth factor, and vascular endothelial growth factor. Their role has generally been to enhance neuroplasticity (i.e., regulation of apoptosis and increased cell survival) and to promote regrowth (dendritic sprouting and synaptogenesis) and new growth (neurogenesis) (Thoenen, 1995; Cameron et al., 1998; Sofroniew et al., 2001; Sun et al., 2003; Radecki et al., 2005). Because abnormal neurodevelopment and a variety of pathophysiological processes after the onset of symptoms are considered to contribute to the complex neuropathophysiology of schizophrenia, neurotrophic factors may play a pivotal role in improved neuroplasticity and thereby long-term clinical outcome.
Altered expression of neurotrophic factors has been implicated in the neuropathophysiology of schizophrenia and bipolar disorders (Shoval and Weizman, 2005; Buckley et al., 2007a). In postmortem brain tissue from patients with schizophrenia, a significant increase in BDNF concentrations and a decrease in NT-3 concentrations were observed in cortical areas and a significant decrease of BDNF levels was observed in the hippocampus (Durany et al., 2001). Significant reductions in BDNF levels have been reported in the serum of patients with chronic schizophrenia (Toyooka et al., 2002; Tan et al., 2005) and in drug-naive, first-episode psychotic patients (Buckley et al., 2007b). Pillai et al. (2007) also reported significant reductions of BDNF levels in the plasma and cerebrospinal fluid CSF of drug-naive, first-episode patients. Likewise, significant reductions in plasma NGF have been observed in never-medicated, first-episode psychotic patients and in chronically medicated patients with schizophrenia (Parikh et al., 2003).
In summary, neurotrophic factors play an important role in neuroplasticity, promotion of regrowth and new growth, and the general resilience of cells. The majority of findings have demonstrated a reduction in neurotrophic factors BDNF, NT-3, and NGF. These observations suggest that the brains of patients with schizophrenia may be disadvantaged in their ability to maintain adequate connections between neurons, to effectively control programmed cell death and cell proliferation, and to adapt to changes in their environment and defend against various physiological insults.
III. Comparison of Antipsychotic Drugs in Animal Models of Antipsychotic Efficacy, Neurotransmitter Regulation, and Neuroprotection
A. Traditional Animal Models of Antipsychotic Activity
All marketed APDs to date fundamentally share some degree of interaction with DA D2 receptors acting either as DA antagonists or as weak partial agonists (as in the case of aripiprazole). The clinical effects of APDs are well correlated with DA D2 receptor affinity in binding assays (Seeman et al., 1976; Creese et al., 1996). However, most SGAs have been shown to have lower affinity for DA D2 receptors in binding assays than FGAs and to have high affinity for 5-HT2A receptors relative to DA D2 receptor affinity (Meltzer et al., 1989; Bymaster et al., 1996; Schotte et al., 1996). Both lower affinity for DA D2 receptors and higher affinity for 5-HT2A receptors have been proposed to contribute to the novel profile of the SGAs (Meltzer et al., 1989; Kapur and Seeman, 2001). Particular SGAs also may have affinity for a number of other neuronal receptors possibly including α-adrenergic, histamine H1, serotonergic receptors other than 5-HT2A, and muscarinic receptors, and this may affect their efficacy and side effect profile (Bymaster et al., 1996; Schotte et al., 1996). Thus, in vitro binding assays have demonstrated that SGAs have a different and more variable receptor binding profile than FGAs.
Traditional animal models of schizophrenia have focused on finding drugs that selectively block limbic DA D2 receptors. Models include dopaminomimetic-induced motor hyperactivity, conditioned avoidance behavior, electrophysiological, brain regional selectivity, neurochemical, and neuroendocrine paradigms. Drug-induced catalepsy (freezing behaviors), which is the animal homolog of EPS, is often evaluated to determine the potential for this adverse event. We highlight the comparative effects of FGAs and SGAs in these models.
1. Dopamine Stimulant-Induced Hyperactivity
A widely used screening test for in vivo DA D2 antagonist activity is blockade of DA stimulant-induced (i.e., d-amphetamine) hyperactivity, which proceeds to stereotypy at higher doses. The hyperactivity is considered to be due predominantly to activation of limbic (i.e., nucleus accumbens) DA D2 receptors, whereas the stereotypy is caused by stimulation of DA D2 receptors in the dorsal striatal brain regions (Ellenbroek, 1993). Thus, the greater potency of a drug to block agonist-induced hyperactivity versus agonist-induced stereotypy is considered an indication of antipsychotic efficacy with reduced propensity to induce EPS. In this model, SGAs more potently block hyperactivity than stereotypy, consistent with the low level of EPS observed with SGAs (for review, see Arnt and Skarsfeldt, 1998).
2. Conditioned Avoidance Responding
Dopamine D2 antagonists inhibit conditioned avoidance responding at doses lower than those required to inhibit escape responding, and the doses of the various drugs are correlated with their antipsychotic activity (Arnt, 1982). However, most FGAs produce escape failures indicative of motoric effects only slightly above the doses required to block conditioned avoidance responses. In contrast, SGAs effectively inhibit conditioned avoidance responding at doses that do not cause significant escape failures, suggesting reduced propensity to produce EPS (Moore et al., 1992, 1993; Seeger et al., 1995). Consistent with this observation, SGAs compared with FGAs are more potent in blocking agonist-induced hyperactivity or conditioned avoidance responding than in causing catalepsy (Moore et al., 1992; Arnt and Skarsfeldt, 1998). Furthermore, long-term treatment of rats with SGAs did not produce chronic jaw movements thought to model tardive dyskinesia, unlike treatment with FGAs (Gao et al., 1998; Rosengarten and Quartermain, 2002).
3. Forelimb and Hind Limb Retraction Time (Paw Test)
The paw test is a paradigm established in rats that distinguishes between EPS and the therapeutic effects of APDs based on forelimb (FRT) and hind limb retraction time (HRT) (Ellenbroek et al., 1987; Ellenbroek, 1993). After receiving an injection of an APD, the rats are placed on a platform that has four holes (two holes for the forelimbs and two holes for the hind limbs). The retraction times for the forelimbs and hind limbs seem to be predictive of the EPS liability and therapeutic efficacy (respectively) of APDs. FGAs such as haloperidol and chlorpromazine increase HRT and FRT at equipotent doses, whereas SGAs such as clozapine and olanzapine are more potent in increasing HRT than FRT. Thus, it seems that the FRT is predictive of EPS effects, whereas the HRT is predictive of treatment efficacy. The paw test has been extensively characterized, and more than 25 APDs have been shown to reliably increase HRT. In a recent review, Geyer and Ellenbroek (2003) concluded that the paw test has a high degree of predictive validity and is effective in assessing antipsychotic effects and EPS liability.
4. Drug Discrimination
Drug discrimination (DD) has been used both to classify drugs in terms of their subjective effects and to identify in vivo pharmacological properties and mechanisms of drug action. DD with clozapine as the training drug has proven to be useful as a preclinical screen in the development of putative APDs (Millan et al., 1999b; Tang et al., 1997). The majority of clozapine DD studies have been conducted with rats trained to discriminate 5.0 mg/kg clozapine from vehicle (Goudie and Taylor, 1998; Goudie and Smith, 1999; Kelley and Porter, 1997; Wiley and Porter, 1992); however, recent studies suggest that a lower training dose of clozapine may provide a more sensitive preclinical assay for screening SGAs from FGAs (Goudie and Taylor, 1998; Goudie et al., 1998; Porter et al., 2000; Prus et al., 2004, 2005). For example, Porter et al. (2000) trained rats to discriminate 1.25 mg/kg clozapine from vehicle and found that the SGAs olanzapine, risperidone, and sertindole fully substituted for clozapine. These SGAs had previously been shown not to substitute for clozapine when the clozapine training dose was 5.0 mg/kg (Goudie and Taylor, 1998). More SGAs seem to be “clozapine-like” when a lower training dose is used, although it should be noted that quetiapine produces only partial substitution for clozapine at 1.25 mg/kg (Porter et al., 2000), but substitutes fully for clozapine when a 5.0 mg/kg training dose is used (Goudie and Taylor, 1998). Thus, the clozapine DD model can provide valuable information about the similarities and differences among FGAs and SGAs that is useful for the development of new drugs.
5. Electrophysiology and Brain Activation Patterns
Electrophysiological studies have shown that long-term treatment with FGAs reduces the number of spontaneously firing ventral tegmental area (A10) DA neurons that project to limbic and cortical areas and of nigrostriatal (A9) DA neurons that project to the striatum (Chiodo and Bunney, 1983; White and Wang, 1983). In contrast, SGAs reduce the number of spontaneously firing DA neurons in the A10 area but not the A9 area, consistent with their antipsychotic activity and reduced EPS potential (Goldstein et al., 1993; Skarsfeldt, 1995; Stockton and Rasmussen, 1996). Activation of the immediate early gene c-fos and its protein product Fos has been shown to be associated with increases in neuronal activity including those induced by APDs (Robertson et al., 1994; Deutch and Duman, 1996; Deutch et al., 1996; Robertson and Fibiger, 1996). However, the pattern of Fos expression differs, depending on the type of drug. Fos expression is increased by FGAs and SGAs in the nucleus accumbens, but expression is increased to greater degree in the striatum by FGAs than SGAs. Furthermore, SGAs uniquely increase Fos expression in the prefrontal cortex.
In summary, numerous differences have been observed between SGAs and FGAs in traditional animal models of antipsychotic activity. These differences probably reflect the more variable receptor binding profile of SGAs compared with FGAs and other potential as yet unidentified mechanisms of action such as effects on insulin signaling and glucose metabolism.
B. Neurotransmitter Regulation via Antipsychotic Drugs
1. Dopamine and Antipsychotic Drugs
APDs act as antagonists at the dopamine D2 receptor, reducing the hypothesized overactivity in dopamine neurotransmission and, consequently, the positive symptoms of schizophrenia. Whereas the FGAs are effective in alleviating the positive symptoms of schizophrenia, they are less effective or ineffective in reducing negative symptoms and cognitive deficits, and they have been associated with a number of side effects including extrapyramidal symptoms (i.e., dystonia, akathisia, parkinsonism, and tardive dyskinesia), hyperprolactemia, and weight and metabolic effects (Lieberman et al., 2005a).
The SGAs are also effective in reducing the positive symptoms of schizophrenia, and a meta-analysis of FGAs and SGAs by Davis et al. (2003) suggested that some SGAs (amisulpride, clozapine, olanzapine and risperidone) may be clinically superior to FGAs. However, not all studies or reviews support this position (Lieberman et al., 2005a; Jones et al., 2006; Lewis and Lieberman, 2008).
The proposal of clinical superiority of SGAs over the FGAs are based on a number of observations (for review, see Abi-Dargham and Laruelle, 2005) as follows:
Improvements are seen in symptom domains other than psychosis—negative symptoms, depression, and anxiety.
A lower incidence of extrapyramidal symptoms has been observed in patients treated with SGAs than with FGAs.
Most SGAs share with clozapine a high 5-HT2A/D2 affinity ratio, which is thought to provide protection against EPS and superiority in terms of negative symptoms; 5-HT2A and D2 receptor antagonism may act synergistically to increase prefrontal DA, an effect not observed with selective D2 or 5-HT2A receptor antagonists administered alone.
SGAs show greater selectivity for the mesolimbic DA system more than for the nigrostriatal DA system: 1) SGAs show a dose-related selectivity for affecting the firing of A10 versus A9 neurons and for inducing gene expression in the nucleus accumbens versus the corpus striatum, 2) imaging studies have shown that SGAs provide higher blockade of D2 receptors in temporal cortex compared with the striatum, whereas FGAs provide a similar level of D2 receptor occupancy in both, and 3) several imaging studies have reported higher extrastriatal occupancies compared with striatal occupancies for amisulpride, clozapine, olanzapine, quetiapine, risperidone, and sertindole.
Imagining studies show lower occupancies of striatal D2 receptors at therapeutic doses of SGAs that FGAs and suggest that clinical results obtained after moderate occupancies (50–75%) are better than those obtained after high occupancies (75–100%).
SGAs are typically associated with a faster dissociation from D2 receptors in rodents than FGAs, a finding that may allow for a physiological synaptic surge of DA to stimulate D2 receptors, supporting the proposal of moderate D2 receptor blockade.
Although the initial treatment of patients with schizophrenia may serve to decrease dopamine hyperactivity, the long-term therapeutic use of APDs is known to elicit dopamine supersensitivity (up-regulation of D2High receptors) (for review, see Seeman et al., 2006). However, not all antipsychotic drugs produce the same level of dopamine supersensitivity or elevation of D2High receptors. In this regard, clozapine and quetiapine induce the lowest elevation of D2High receptors, in contrast to the elevation elicited by haloperidol, risperidone, ziprasidone, and olanzapine. These differences probably reflect the important differences in how tightly an antipsychotic drug binds to the dopamine D2 receptor and the rate of dissociation in vitro or in vivo (i.e., the fast-off-D2 principle) (Seeman and Tallerico, 1999).
Recently a meta-analysis of the single photon emission computed tomography and positron emission tomography in vivo receptor imaging literature was published (Stone et al., 2008). Single photon emission computed tomography and positron emission tomography enable in vivo imaging of regional antipsychotic medication binding to receptor subtypes in living patients with schizophrenia. SPECT The results of this 15-study meta-analysis revealed that both FGAs and SGAs produce high temporal cortex D2/D3 receptor occupancy, whereas only FGAs produce high striatal D2/D3 receptor occupancy. The extrapyramidal side effects were related primarily to striatal D2/D3 receptor occupancy. The clinically effective dose correlated with doses inducing maximal dopamine D2/D3 receptor occupancy in both the striatum and temporal cortex, a strong correlation occurring in the temporal cortex. It was concluded that cortical dopamine D2/D3 receptor occupancy is involved in antipsychotic efficacy, striatal D2/D3 occupancy having a likely therapeutic role and also inducing EPS.
2. GABA and Antipsychotic Drugs
A variety of different responses on GABAA receptor binding have been observed in rodents treated with APDs or APDs combined with benzodiazepines. Early studies showed that haloperidol is associated with increases in the density of GABA receptors (Frey et al., 1987; Gale, 1980; Huffman and Ticku, 1983; See et al., 1989). In rats treated with APDs for 28 days, [3H]muscimol binding was shown to be decreased in the hippocampus and temporal regions after 28 days of treatment with clozapine or olanzapine but not after treatment with haloperidol or chlorpromazine (Farnbach-Pralong et al., 1998), a result that was possibly suggestive of increased GABAergic neurotransmission with SGAs. In contrast, Skilbeck et al. (2008) reported that after 7 days of administration of haloperidol or olanzapine, [3H]muscimol binding density was increased most prominently in the PFC after treatment with either drug, although larger and more prolonged effects were induced by olanzapine in subcortical regions. After 28 days, no changes were observed in [3H]muscimol binding in any region, although [3H]flunitrazepam binding density (Bmax) was increased for both antipsychotic treatments in the PFC. They argued that a subset of GABAA receptors sensitive to benzodiazepines are regulated differently from other GABAA receptor subtypes after antipsychotic drug administration in a time- and region-dependent manner (Skilbeck et al., 2008). It is possible that differences in dosing or brain region examined may explain some of the differences observed between these studies.
In rats treated with APDs for 6 months, Zink et al. (2004b) reported increased expression of GAD67 in the infralimbic cortex and anterior cingulate cortex and differential effects of haloperidol and clozapine on [3H]muscimol binding to GABAA receptors within cortical, limbic, and subcortical areas. Haloperidol strongly increased GABAA receptor binding in the striatum and nucleus accumbens with reduced binding in the parietal and temporal cortex. In contrast, clozapine had only small effects in the basal ganglia and failed to elicit major changes in these parts of the association cortex. However, clozapine led to an increase in GABAA receptor binding in limbic areas including the infralimbic cortex and anterior cingulate cortex, whereas haloperidol had a similar effect in the anterior cingulate cortex but a smaller one in the infralimbic cortex. As discussed by Zink et al. (2004b), the increased GABAA receptor binding in the basal ganglia seen with haloperidol and suggestive of reduced GABAergic tone may explain the greater association of haloperidol treatment with extra-pyramidal symptoms. In contrast, the increased GABAA receptor binding in the limbic cortical regions seen to the greatest extent with clozapine may reflect the positive effects of clozapine on negative symptoms and cognitive abilities.
Benzodiazepines can be also be used in conjunction with atypical antipsychotics to treat schizophrenia, and benzodiazepines can alter GABAA receptor density in rat brain (Wu et al., 1994; Hutchinson et al., 1996, Toki et al., 1996). McLeod et al. (2008) assessed the effects of treatment with diazepam, haloperidol, or the combination of diazepam and haloperidol on GABAA binding sites and found regionally selective increases in GABA binding sites with diazepam or the combination of diazepam and haloperidol. However, treatment with haloperidol alone decreased GABA binding sites in the thalamus and increased these sites in the hypothalamus. By contrast, treatment with diazepam, haloperidol, and a combination of the two drugs resulted in widespread decreases in the number of benzodiazepine binding sites in the rat central nervous system, the notable exception being increased numbers of benzodiazepine binding sites in frontal cortex of rats that received diazepam (McLeod et al., 2008).
Long-term treatment with clozapine or haloperidol has also been shown to affect GABA transporter expression (Zink et al., 2004a). In adult male rats, clozapine or haloperidol treatment resulted in up-regulation of GAT1 mRNA expression, whereas vesicular GABA transporter expression declined in cortical and limbic brain regions, and haloperidol showed a greater effect than clozapine. GAT3 mRNA expression was suppressed in parietal and temporal cortices.
3. Glutamate and Antipsychotic Drugs
APDs can play a role in facilitating glutamatergic neurotransmission and lessening NMDA receptor hypofunction. In this regard, there is evidence that long-term treatment with haloperidol, clozapine, or raclopride can significantly reduce levels of the NMDA receptor endogenous antagonist kynurenic acid in the striatum, hippocampus, and frontal cortex of rat brain (Ceresoli-Borroni et al., 2006). APDs (e.g., haloperidol and clozapine) can increase phosphorylation of the NR1 subunit of the NMDA receptor (Leveque et al., 2000). In striatal culture, activation of the cAMP pathway led to the phosphorylation of 897Ser-NR1 in a PKA-dependent manner. Thus, D2 antagonists probably activate the NMDA receptor via PKA-mediated phosphorylation of 897Ser-NR1. Haloperidol has been shown to increase NR1 phosphorylation levels at S897 in vivo in the striatum as well as in a neuronal culture system (Leveque et al., 2000). APDs can also reduce oxidative stress in a number of neurotoxic models (see section III.H), resulting in a possible reduction in the oxidative inhibition of the NMDA receptor. Of particular note, Steullet et al. (2006) demonstrated that decreasing glutathione levels in slices of rat hippocampus results in hypofunction of NMDA receptors (for review, see Steullet et al., 2006). In PC12 cells, SGAs olanzapine and quetiapine were able to restore reductions in glutathione peroxidase activity observed after exposure to β-amyloid peptide (Aβ25–35) (Wang et al., 2005b). A number of studies have shown facilitatory effects of clozapine on glutamate neurotransmission and NMDA functioning primarily in cortex, whereas haloperidol seems to enhance glutamate levels and activity at NMDA receptors primarily in the striatum (for review, see Millan, 2005).
There is also evidence that haloperidol may inhibit NMDA receptor function. Long-term treatment with haloperidol but not with clozapine decreased NMDA NR1 subunit expression within the primate dorsolateral prefrontal cortex, a region involved in cognition and negative symptoms (O’Connor et al., 2006). Similar findings were observed in rat prefrontal cortex wherein prolonged treatment with haloperidol but not with olanzapine reduced the synaptic level of the obligatory NMDA subunit NR1 and the regulatory NMDA subunit NR2A and its scaffolding protein PSD95 and reduced trafficking of GluR1 to the membrane (Fumagalli et al., 2008). In addition, haloperidol altered the total and phosphorylated levels of calcium calmodulin kinase type II at synaptic sites and its interaction with regulatory NMDA subunit NR2B (Fumagalli et al., 2008).
APDs as a whole do not seem to regulate D-serine or its metabolic enzymes or glycine. Administration of haloperidol to rats did not significantly affect serine race-mase or degrading enzyme DAAO (Verrall et al., 2007). Previous work had shown a 2-fold elevation in DAAO activity in patients with schizophrenia, the levels of DAAO activity being the highest in patients with prior antipsychotic drug use (Madeira et al., 2008). However, long-term administration of haloperidol or clozapine for 21 days to mice did not alter DAAO activity, suggesting that antipsychotic drug administration was not responsible for the higher levels of DAAO activity seen in patients with schizophrenia (Madeira et al., 2008). Of interest, in chronically ill patients who were treated with FGAs or SGAs, the glycine/serine ratio was significantly higher in patients treated with clozapine than in those treated with FGAs or other SGAs and not different from that in healthy subjects (Neeman et al., 2005). Glycine agonists and transporter inhibitors have been shown to potentiate the ability of FGAs and most SGAs but not of clozapine to improve negative and cognitive symptoms. This finding may reflect the fact that clozapine itself can enhance activity at NMDA receptors (for review, see Millan, 2005).
Several of the early studies assessing changes in glutamate levels in the CSF, postmortem brain tissue, or blood in patients with schizophrenia indicated increased glutamate levels with antipsychotic drug therapy (Gattaz et al., 1985; Tsai et al., 1995; Tortorella et al., 2001; van der Heijden et al., 2004), although not all (Korpi et al., 1987; Alfredsson and Wiesel, 1989; Faustman et al., 1999). Some studies have shown increases in glutamate and/or aspartate levels in patients switched from FGAs to SGAs (Evins et al., 1997; Goff et al., 2002), although in at least one study a reduction was observed. In this latter study, serum levels of aspartate, glutamate, and other amino acids were elevated in patients with neuroleptic-resistant schizophrenia before clozapine treatment, and 12 weeks after clozapine treatment a significant reduction in serum levels of glutamate was observed (Tortorella et al., 2001). However, in the study of Tortorella et al. (2001), glutamate levels were already elevated and increased further after exposure to SGAs. In patients diagnosed with schizophrenia, bipolar disorder, or nonspecified psychosis at their first psychotic episode, the observed decrease in plasma glutamate levels was restored after treatment with APDs or APDs combined with lithium or other mood stabilizers (Palomino et al., 2007). Of interest, although the majority of patients were treated with atypical antipsychotics, 7 to 11% were treated with typical antipsychotics and showed similar increases in plasma glutamate levels (Palomino et al., 2007).
The mechanism of how APDs elevate glutamate levels is not known, although a reduction in glutamate transporter expression has been demonstrated in astrocytic cultures treated with clozapine (Vallejo-Illarramendi et al., 2005) and in rat brain after treatment with clozapine or haloperidol (Schneider et al., 1998; Melone et al., 2001; Schmitt et al., 2003). Interestingly, although both clozapine and haloperidol decreased glutamate transporter GLT-1 in the striatum, haloperidol resulted in significantly greater reductions than those with clozapine, a finding that may have relevance in neuroleptic-induced movement disorders (Schneider et al., 1998). APDs, and the SGAs in particular, can also up-regulate the levels of neurotrophin BDNF (see section III.I), and the time course of the medication-induced restoration of plasma glutamate levels in patients who experienced their first psychotic episode seemed to parallel that of BDNF (Palomino et al., 2007).
a. N-Methyl-D-aspartate Antagonists in Animal Models
NMDA antagonists induce a number of changes in animal models that resemble schizophrenia. In this section, we review data from animal models assessing the effects of SGAs and FGAs on regulation of NMDA antagonists in behavioral and electrophysiological studies.
i. Behavioral activation
In rodents, NMDA antagonists induce behavioral activation that is characterized by increased locomotor activity, ataxia, and stereotypic head weaving. Both SGAs and FGAs can block the behavioral activation induced by NMDA antagonists, but the SGAs are more selective. Corbett et al. (1995) found that clozapine and olanzapine were substantially more potent in blocking MK-801-induced behavioral activation than apomorphine-induced climbing. In contrast, haloperidol was more potent in blocking effects of apomorphine than those of MK-801, and risperidone was almost equipotent in blocking effects of both drugs. Clozapine and olanzapine block the effects of PCP at doses having no effect on baseline locomotor activity, whereas haloperidol is effective only at doses that suppress normal activity (Gleason and Shannon, 1997). The differential effects of FGAs and SGAs on NMDA antagonist-induced behavioral activation may be due to the 5-HT2 receptor-blocking properties of the SGAs, as similar effects are induced by selective 5-HT2 antagonists (Gleason and Shannon, 1997). The PCP-induced locomotor hyperactivity was reversed after short-term administration of olanzapine or clozapine and after long-term 10-month administration of olanzapine but not of haloperidol (Moy et al., 2004).
ii. Prepulse inhibition
Patients with schizophrenia exhibit deficits in sensorimotor gating as indicated by reduced prepulse inhibition (PPI) of startle responses (Braff et al., 2001). In animal studies using PPI procedures, deficits can be induced by DA agonists, 5-HT agonists, and noncompetitive NMDA antagonists. Although both FGAs and SGAs can block the disruptive effects of dopaminergic agonists on PPI, most studies showed that SGAs but not FGAs block the effects of NMDA antagonists on PPI (Geyer et al., 2001). Clozapine is more effective than haloperidol in blocking the consequence of this experimentally induced NMDA receptor hypofunction (Keith et al., 1991; Bakshi et al., 1994). Some of the newer SGAs (e.g., olanzapine, quetiapine, and ziprasidone) are also effective in blocking PPI deficits induced by NMDA antagonists (Bakshi and Geyer, 1995; Swerdlow et al., 1996; Mansbach et al., 2001). Postnatal administration of PCP produced a deficit in PPI that was reversed by either pretreatment or post-treatment with olanzapine (Wang et al., 2001). Furthermore, similar to the rodent findings, clozapine blocked PCP-induced disruption of PPI in monkeys, whereas haloperidol did not (Linn et al., 2003).
iii. Social interactions
PCP can also disrupt normal social interactions in rats. Pretreatment with clozapine or olanzapine before injection of PCP reduced the disruption in social behavior induced by PCP (Corbett et al., 1995). However, haloperidol and risperidone were not effective in altering the effects of PCP in the social interaction test (Corbett et al., 1995).
iv. Ketamine-induced activation of brain metabolism
Subanesthetic doses of NMDA antagonists induce robust increases in regional 2-deoxyglucose uptake (Kurumaji et al., 1989; Duncan et al., 1998b) presumably by disinhibiting neural circuits (Grunze et al., 1996; Greene, 2001). The striking alterations in brain metabolic activity patterns induced by subanesthetic doses of ketamine are almost identical to those induced by the selective NMDA antagonist MK-801 (Duncan et al., 1999) indicating that the neuroanatomically selective effects of ketamine result from reduced NMDA receptor function. Pretreatment of rats with clozapine but not with haloperidol blocked the brain metabolic activation induced by ketamine (Duncan et al., 1998a). Clozapine and olanzapine but not risperidone or haloperidol also blocked ketamine-induced brain metabolic activation (Duncan et al., 2000). Thus, the profile of the different FGAs and SGAs in the ketamine-induced brain metabolic activation model is similar to that reported for the behavioral models described in section III.B.3.a.i.
v. Electrophysiological responses to N-methyl-D-aspartate antagonists
Electrophysiological studies in vitro indicate that SGAs can modulate responses to glutamate and reverse the inhibitory effects of PCP on glutamate-induced excitation. Haloperidol and clozapine modulate the glutamate-mediated neurotransmission in the rat medial prefrontal cortical slice with application of both drugs markedly facilitating NMDA-evoked responses (Arvanov et al., 1997). Clozapine, but not haloperidol, produced bursts of excitatory postsynaptic potentials (EPSPs) that were blocked by glutamate receptor antagonists, suggesting that clozapine increased release of excitatory amino acids in the preparation (Arvanov et al., 1997). This latter finding is consistent with in vivo work showing that short-term clozapine but not haloperidol increases extracellular concentrations of glutamate in the medial PFC of rats (Daly and Moghaddam, 1993; Yamamoto et al., 1994).
Clozapine also increased the amplitude of EPSPs produced by stimulation of the corpus callosum, whereas haloperidol decreased EPSP amplitude in this test system (Arvanov et al., 1997). Clozapine preferentially potentiated NMDA receptor-mediated transmission and haloperidol depressed non-NMDA-mediated responses. Robust electrophysiological enhancement of NMDA responses after corpus callosum stimulation was also found for olanzapine, risperidone, and quetiapine but not for chlorpromazine or loxapine (Ninan et al., 2003a). These results suggest that the SGAs, in comparison with the FGAs, could have very different effects on glutamate-mediated transmission in vivo. However, there are no studies available to demonstrate the effects of APDs on NMDA receptor function in vivo.
Consistent with the findings of differential effects of haloperidol and clozapine on NMDA receptor function, clozapine, but not haloperidol, can prevent PCP-induced blockade of NMDA responses in vitro (Wang and Liang, 1998). Furthermore, subchronic (7-day) treatment of rats with PCP induces electrophysiological hypersensitivity that is blocked by clozapine or olanzapine but not haloperidol (Ninan et al., 2003b).
In vivo work has shown that administration of the NMDA antagonist PCP can induce marked disruption of the activity of pyramidal neurons in the rat PFC, increasing the activity of 45% of pyramidal neurons recorded and decreasing the activity of 33%. PCP administration also markedly reduced cortical synchrony in the delta frequency range (0.3–4Hz) as assessed by recording local field potentials. The subsequent administration of haloperidol or clozapine reversed PCP effects on pyramidal cell firing and cortical synchronization (Kargieman et al., 2007).
4. Other—Peptides and Antipsychotic Drugs
Administration of MK-801, a noncompetitive NMDA receptor antagonist, increased levels of the neuropeptide-degrading enzymes, prolyl oligopeptidase and thimet oligopeptidase, in the posterior cingulate/retrospelenial cortices. Clozapine but not haloperidol significantly attenuated MK-801-induced changes in the levels of the neuropeptide-degrading enzymes in rat brain (Arif et al., 2007).
5. Intracellular Signaling Cascades and Antipsychotic Drugs
The effects of FGAs and SGAs on neuronal signaling systems are complex. FGAs and particularly SGAs may interact with a number of cell surface receptors including GPCR and some iontropic receptors either directly or indirectly through neurotransmitter release (Bymaster et al., 1996). Thus, the drugs may interact with GPCRs and alter cAMP and IP3 second messengers, depending on the receptors involved. In addition, SGAs and FGAs may interact with GPCRs as antagonists, partial agonists, or inverse agonists, thereby adding to their signaling complexity (Zorn et al., 1994: Weiner et al., 2001; Bymaster et al., 1999; Olianas et al., 1999; Shapiro et al., 2003). The process whereby the interaction of FGAs and SGAs with cell surface receptors or intracellular proteins results in long-term adaptive processes that produces their antipsychotic effects is unknown. These long-term adaptive processes seem to be important as antipsychotics take several weeks or more for full effectiveness (Lieberman et al., 1993). Recently, a number of studies have focused on the effects of FGAs and SGAs on downstream signaling systems including kinase cascades and transcriptional factors (Pozzi et al., 2003; Browning et al., 2005; Lu and Dwyer, 2005).
In vitro and in vivo studies have shown that FGAs and SGAs modulate mitogen-activated protein kinase signaling cascades including Akt (protein kinase B) and ERK (also called MAP kinase) signal transduction pathways. In vitro studies in hippocampal neuron cultures at 25 days demonstrated that 50 nM haloperidol and risperidone significantly increased the levels of ERK phosphorylation (Yang et al., 2004). Clozapine, quetiapine, and olanzapine significantly enhanced neurite outgrowth and Akt phosphorylation induced by NGF in PC12 cells (Lu and Dwyer, 2005). In contrast, the FGAs haloperidol, fluphenazine, and chlorpromazine reduced neurite outgrowth and AKT phosphorylation induced by NGF. In primary cultured rat cortical neurons, haloperidol induced apoptotic injury and reduced phosphorylation levels of Akt and activated caspase-3 (Ukai et al., 2004).
In vivo studies showed that short-term administration of haloperidol stimulated the phosphorylation of ERK1/2 in mouse dorsal striatum, whereas clozapine reduced ERK1/2 phosphorylation (Pozzi et al., 2003). FGAs caused mild activation of ERK in dorsal striatum and superficial prefrontal cortex, whereas clozapine had no effect in the striatum, but more widespread effects in cortex (Valjent et al., 2004). In a short-term study 30 min after administration of clozapine, there was a selective increase in phosphorylation of prefrontal cortical mitogen-activated protein kinase kinase 1/2 and ERK, which was reversed by administration of a 5-HT2A receptor agonist (Browning et al., 2005). Short-term treatment with haloperidol and olanzapine produced a general reduction in ERK1/2 phosphorylation in rat prefrontal cortex in the nuclear and cytosolic compartments, an effect Browning et al. (2005) suggested may be the result of blockade of dopaminergic and serotonergic receptors. However, olanzapine treatment for 14 days resulted in an increase in ERK1/2 phosphorylation in the nuclear and membrane compartments at various time points after sacrifice. Long-term haloperidol administration did not alter ERK1/2 phosphorylation. Thus, FGAs and SGAs can modulate ERK phosphorylation and activity and thus modulate neuronal vitality, survival, and plasticity and some, but not all, studies suggest that there are differential effects between the two groups of drugs.
Long-term administration of haloperidol to rats significantly reduced phosphorylated GSK-3β protein levels in frontal cortex, whereas long-term administration of clozapine caused significant elevation of protein levels (Kozlovsky et al., 2006). In another study, significant increases in the levels of total protein were identified after administration of clozapine, haloperidol, or risperidone (Alimohamad et al., 2005). Short-term treatment of mice with SGAs including risperidone, clozapine, olanzapine, quetiapine, and ziprasidone rapidly increased the level of brain phosphorylated GSK-3 in the cortex, hippocampus, striatum, and cerebellum in a dose-dependent manner (Li et al., 2007). Haloperidol and clozapine increased phosphorylation of GSK-3α/β in rat frontal cortex, whereas clozapine increased phosphorylation of Akt for 1 h, and the response to haloperidol was transient (Roh et al., 2007). Overall, SGAs increase phosphorylation of GSK-3β, resulting in inhibition of its activity, consistent with increased activity of Akt. The effects of FGAs on GSK3β are controversial.
Antipsychotic treatment has been suggested to require cAMP-induced PKA activation and subsequent phosphorylation of nuclear proteins. PKA-deficient mice do not demonstrate haloperidol-induced catalepsy and fail to induce Fos in striatal regions (Adams et al., 1997). CREB is a downstream target of PKA, and short-term haloperidol treatment in rats enhanced phosphorylation of the transcription factor CREB in striatum (Konradi et al., 1993). Short-term administration of haloperidol or olanzapine to mice increased PKA activity in dorsal striatum and had no effect in nucleus accumbens, whereas olanzapine decreased PKA activity in medial prefrontal cortex (Turalba et al., 2004). Olanzapine reduced phosphorylated CREB immunoreactivity in medial prefrontal cortex, consistent with reduced activity of PKA, but haloperidol and olanzapine were without effect in nucleus accumbens and striatum. A short-term study showed that haloperidol stimulated the phosphorylation of CREB in mouse dorsal striatum, but, in contrast, clozapine reduced CREB phosphorylation (Pozzi et al., 2003). Long-term, but not short-term, clozapine increased PKA activity in rat cortex, hippocampus, and striatum, whereas long-term, but not short-term, haloperidol increased activity of PKA only in striatal areas (Dwivedi et al., 2002.). However, a PKA inhibitor did not block the dopamine D2 antagonist eticlopride-induced Fos expression (Adams and Keefe, 2001), raising questions about the role of phosphorylation of CREB in the effects of haloperidol. Long-term treatment of mice with haloperidol produced increases in the guanosine 5′-O-(3-thio)triphosphate-mediated adenylyl cyclase activity in mouse frontal cortex, whereas olanzapine caused reduced activity. In striatum, olanzapine treatment significantly reduced the activity, whereas the effect of haloperidol treatment was not significantly different from the control (Kaplan et al., 1999).
6. Effects of Antipsychotic Drugs on Monoamine and Amino Acid Neurotransmitter Efflux
The differential release of monoamines and amino acids by the SGAs and FGAs has been suggested to account for some of the clinically relevant differences observed between these two classes of drugs, as well as differences among drugs within the class of SGAs. Because of the likely effects of neurotransmitters on neuronal activation and metabolism, altered efflux of monoamines and amino acids may be involved in the neuroprotective properties of SGAs.
a. Dopamine and Norepinephrine Extracellular Concentrations
Decreased dopaminergic activity in the cortex of patients with schizophrenia has been inferred on the basis of cerebrospinal fluid and imaging studies (Weinberger et al., 1988), but no direct evidence for this inference has been offered. However, altered DA D1 receptor density has been related to impaired working memory in patients with schizophrenia (Okubo et al., 1997; Abi-Dargham et al., 2002). Thus, it has been suggested that cognitive deficits in schizophrenia may be related, in part, to diminished cortical dopaminergic or noradrenergic activity or both (Meltzer and McGurk, 1999).
In microdialysis studies, Invernizzi et al. (1990) first reported the ability of clozapine, the prototypical SGA, to increase the efflux of cortical DA in the rat. Subsequently, many studies have shown that clozapine preferentially increases DA efflux in the cortex compared with that observed in the dorsal and ventral striatum (Moghaddam and Bunney, 1990; Kuroki et al., 1999). Other SGAs including olanzapine, quetiapine, risperidone, ziprasidone, and zotepine have been shown to have a preferential ability to increase cortical DA efflux (Volonté et al., 1997; Li et al., 1998; Kuroki et al., 1999; Rowley et al., 2000; Zhang et al., 2000), and for clozapine, risperidone, olanzapine, the increase in DA release occurs after long- and short-term administration. SGAs, but not FGAs, also increase cortical norepinephrine efflux in prefrontal cortex (Li et al., 1998; Rowley et al., 1998; Zhang et al., 2000). The SGAs have also been shown to increase the efflux of both neurotransmitters in the hippocampus (Chung et al., 2004). Aripiprazole, a partial DA D2/D3 agonist, which is also a serotonergic 5-HT2A inverse agonist and 5-HT1A partial agonist, has been shown to increase DA release in the medial prefrontal cortex and hippocampus after short- and long-term administration (Li et al., 2004). However, one study showed that aripiprazole produced no change in prefrontal cortical DA efflux (Jordan et al., 2004). Recently, an increase in DA efflux in cortical regions by aripiprazole has been reported in the mouse (Zocchi et al., 2005).
The ability of the SGAs to increase cortical DA release is correlated with their affinity for 5-HT2A receptors (Kuroki et al., 1999). However, 5-HT2A inverse agonists such as M100907 increase cortical DA efflux only when combined with low doses of D2 blockers such as haloperidol (Liégeois et al., 2002). Moreover, M100907 is ineffective in increasing DA efflux in the cortex in the presence of high-dose (1.0 mg/kg) haloperidol. Other 5-HT2A inverse agonists such as SR46349B increase DA efflux in the cortex when combined with haloperidol (Rinaldi-Carmona et al., 1992; Bonaccorso et al., 2002), and α2A receptor blockade can also potentiate the ability of the D2 antagonist, raclopride, to increase cortical DA release (Hertel et al., 1999).
b. Serotonin Extracellular Concentrations
The SGAs, with the exception of aripiprazole, all share 5-HT2A receptor antagonism but have a varied effect on other 5-HT receptors. Some are 5-HT2C, 5-HT6, and 5-HT7 antagonists, as well as 5-HT1A partial agonists. All have been shown to enhance DA efflux by a 5-HT1A-dependent mechanism (Ichikawa et al., 2001). 5-HT1A agonism may inhibit the firing of 5-HT neurons in the raphe and, thus, decrease 5-HT release in terminal regions. Risperidone (1 or 2 mg/kg) and clozapine (20 mg/kg) significantly increased extracellular 5-HT levels in the medial prefrontal cortex and the nucleus accumbens of rats, respectively (Hertel et al., 1996; Ichikawa et al., 1998). Olanzapine (1 and 10 mg/kg), S(−)-sulpiride (10 and 25 mg/kg), haloperidol (0.1 and 1 mg/kg), and the selective 5-HT2A receptor antagonist M100907 (1 mg/kg) had no significant effect on extracellular 5-HT levels in either region (Ichikawa et al., 1998). Thus, the ability to increase extracellular 5-HT levels in the medial prefrontal cortex and the nucleus accumbens by these APDs is not directly related to their affinity for 5-HT2A receptors, as olanzapine and M100907 had no significant effect on extracellular 5-HT levels.
c. Acetylcholine Extracellular Concentrations
SGAs, with the exception of aripiprazole, have been shown to increase the efflux of ACh in the rat medial prefrontal cortex and hippocampus (Parada et al., 1997; Ichikawa et al., 2002; Shirazi-Southall et al., 2002; Chung et al., 2004). This effect is blocked by the cholinergic M1 receptor antagonist telenzepine (Li et al., 2005), suggesting that the ACh efflux is due to activation of cholinergic cortical inputs. Given the observed effects of muscarinic agonists on cognitive behaviors in a variety of animal models, it is highly likely that the increased efflux of ACh is relevant to the ability of the SGAs to improve cognition.
d. Glutamate and GABA Extracellular Concentrations
There have been numerous in vivo microdialysis studies on the effect of haloperidol and clozapine, as well as other APDs, in modulating the efflux of glutamate or aspartate in the cortex or striatum. The results are mixed, reflecting differences in dosages, duration of treatment, use of anesthesia, and the difficulty of separating glutamate efflux that is neuronally based from that of the amino acid compartment (Yamamoto et al., 1994). Pietraszek et al. (2002) reported that 6 weeks of treatment with clozapine, followed by its withdrawal for 4 days, lowered both the basal and the stimulated levels of glutamate and aspartate. In contrast, a 6-week treatment with haloperidol, followed by withdrawal, elevated the basal but not the veratridine-stimulated extracellular levels of glutamate and aspartate. Haloperidol, but not clozapine, enhanced the activity of cortical neurons. In a study of the effect of these two APDs on glutamate release from nerve terminals isolated from rat prefrontal cortex, Yang and Wang (2005) found that both haloperidol and clozapine inhibited glutamate release by ion channel activities that influence nerve terminal excitability. At the present time, the effects of APDs on glutamate release cannot be related to their therapeutic actions with any degree of confidence. Short-term systemic administration of clozapine markedly and administration of haloperidol to a much lesser extent reduced extracellular GABA levels in prefrontal cortex in awake rats without altering striatal GABA efflux (Bourdelais and Deutch, 1994). Thus, release of GABA from interneurons in the prefrontal cortex is inhibited by both FGAs and SGAs.
In summary, although variations do exist across studies and in the plethora of findings reported, compared with most SGAs, FGAs, as represented by haloperidol, show specific pharmacological differences including 1) the highest occupancy of striatal D2 receptors with a slow dissociation rate from the receptor indicating greater overall blockade, 2) regionally selective increased efflux in neurotransmitter release of DA and acetylcholine particularly after long-term administration, 3) evidence for decreasing expression of the NR1 subunit of the NMDA receptor, which would reduce NMDA receptor function, and 4) greater selectively for increased glutamatergic neurotransmission within the basal ganglia. In addition, FGAs and SGAs in several instances had distinctly different effects on intracellular signaling cascades in brain. Of particular note, SGAs had more effects on cortical signaling cascades than FGAs, consistent with their effects in many systems in cortical areas.
C. Neuroanatomical Plasticity after Treatment with Antipsychotic Drugs
In a longitudinal, controlled, double-blind clinical trial, Lieberman et al. (2005b) reported significant reductions in gray matter volume in first-episode patients who were treated with haloperidol, but only a slight and nonstatistically significant reduction in patients who were treated with olanzapine. This finding suggested that olanzapine seemed to ameliorate neurodegenerative changes in the brain occurring either as a progression of the disease and/or exposure to the FGA haloperidol. Reductions in gray matter have been reported previously in first-episode schizophrenia patients who received primarily FGAs (DeLisi et al., 1997; Gur et al., 1998; Lieberman et al., 2001a; Cahn et al., 2002). In addition, van Haren et al. (2007) found that treatment with clozapine and olanzapine was associated with less reduction in gray matter signal intensity than treatment with haloperidol in patients with schizophrenia followed longitudinally for up to 5 years and undergoing serial magnetic resonance imaging scans.
It is noteworthy that caudate enlargement has been encountered primarily with FGAs and not with SGAs (Chakos et al., 1994; Corson et al., 1999; Andersson et al., 2002). The functional significance of caudate hypertrophy is presently unclear, but it may be related to the induction of EPS by the FGAs (Keshavan et al., 1994). In rats, striatal enlargement was most evident in the animals that developed high vacuous chewing movements, a potential animal model of TD (Chakos et al., 1998). In patients with schizophrenia, larger caudate volumes have been associated with poor performance on neuropsychological tests (Hokama et al., 1995), the deficit syndrome (Buchanan et al., 1993), and greater severity of symptoms (Gur et al., 1998).
Clozapine has been reported to reverse the increases in basal ganglia volume that are associated with FGA drug therapy (Chakos et al., 1995; Scheepers et al., 2001a). In treatment-resistant patients who responded to clozapine treatment, the degree of reduction in the left caudate volume was significantly related to the extent of improvement in positive and general symptoms (Scheepers et al., 2001b). In a study of first-episode patients, caudate volume increases were observed after treatment with haloperidol but not after treatment with olanzapine (Lieberman et al., 2005b). This finding confirms results of an earlier study suggesting that SGAs do not produce an increase in basal ganglia volume (Corson et al., 1999). However, a recent study of patients with neuroleptic-naive, first-episode schizophrenia reported an increase in basal ganglia volume in patients treated with risperidone (Massana et al., 2005). In addition, Molina et al. (2005) found increases in gray matter volume in neuroleptic-naive patients treated with risperidone and neuroleptic-resistant patients treated with clozapine (Molina et al., 2005).
In an attempt to define the mechanism underlying the ability of SGAs such as olanzapine and clozapine to prevent volume loss in the PFC in schizophrenia, Wang and Deutch (2008) subjected rats to lesions disrupting the DA innervation of the PFC and then 3 weeks later, a time point when a large decrease in dendritic spine density and length was observed, assigned animals randomly to receive haloperidol, olanzapine, or vehicle administration for 3 weeks (Wang and Deutch, 2008). The animals were then sacrificed, and layer V pyramidal cells in the prelimbic (area 32) PFC were reconstructed from Golgi-impregnated material. Strikingly, olanzapine reversed the dystrophic changes in dendrites of layer V pyramidal cells, whereas haloperidol did not provide any benefit. Neither olanzapine nor haloperidol treatment of sham-lesioned animals changed the dendritic trees of pyramidal cells, suggesting that there is not a direct neurotoxic effect of haloperidol.
The ability of the SGA olanzapine to reverse dystrophic changes in pyramidal neuron dendrites suggests that dystrophic changes in dendrites of pyramidal cells may be a factor responsible for the decrease in cortical volume in patients with schizophrenia. One possible reason for this is that some SGAs increase DA tone in the PFC, which may slow progressive morphological changes in schizophrenia. Consistent with this speculation, SGAs (e.g., clozapine and olanzapine) but not haloperidol increase extracellular DA concentrations in the PFC (Li et al., 1998). The loss of dendritic spines also suggests that the presynaptic elements that normally form synapses with spines either retract or reroute to synapse onto a different site, possibly causing the changes in cortical connectivity seen in schizophrenia. A retraction of presynaptic elements that pairs with spines may account for the decreased expression of a group of genes associated with presynaptic elements in schizophrenia, as uncovered in gene array studies (Mirnics et al., 2001a).
The data of Wang and Deutch (2008) on dendritic spine recovery in response to treatment with an SGA are also striking because the response to the SGA was complete recovery of dendritic elements rather than some attenuation of the loss of spines or dendritic length. It is not yet known whether starting an SGA at a time point later than 3 weeks after cortical DA denervation will attenuate but not completely reverse the changes in pyramidal cell dendrites. However, the complete reversal in animal studies offers the exciting possibility that intervention with a suitable APD at an appropriate time point may not only prevent further volume changes in schizophrenia but also actually reverse dystrophic neuronal changes, including those in patients with a long duration of illness.
In summary, recent evidence suggests that SGAs can, to varying degrees, mitigate and in some cases reverse some of the morphological changes observed in patients with schizophrenia, including gray matter volume reductions, caudate hypertrophy, white matter volume increases, and decreases in dendritic spine density and length observed within the prefrontal cortex. The mechanisms underlying these changes need to be defined but may reflect important differences between SGAs and FGAs in receptor binding profiles, oxidative stress parameters, regulation of neuromodulators, and neurotrophic factors.
D. Apoptosis and N-Methyl-D-aspartate Antagonist-Induced Neurodegeneration
In this section, we review data from animal models assessing the effects of APDs on the effects of NMDA antagonist-induced glutamate release and neurodegeneration and in other animal models of apoptosis.
NMDA antagonists stimulate the release of several neurotransmitters within the brain. In the rat prefrontal cortex, short-term administration of NMDA antagonists stimulates the release of glutamate (Moghaddam et al., 1997; Adams and Moghaddam, 2001; Lorrain et al., 2003), dopamine (Schmidt and Fadayel, 1996; Moghaddam and Adams, 1998; Mathé et al., 1999), and serotonin (Martin et al., 1998; Millan et al., 1999a; Adams and Moghaddam, 2001; Amargós-Bosch et al., 2006). Clozapine and haloperidol both blocked the MK-801-induced increase in glutamate in rat medial prefrontal cortex, whereas only clozapine was able to block the increased efflux of serotonin (López-Gil et al., 2007). Only systemic administration of MK-801 and not local administration within the prefrontal cortex resulted in an increased efflux of glutamate and serotonin, suggesting that NMDA receptor blockade was occurring distal to the prefrontal cortex. In contrast, both systemic and local administration of clozapine or haloperidol could block the effects of MK-801, indicating that the medial prefrontal cortex was a site of action of these two APDs. Abekawa et al. (2006) also reported that clozapine and haloperidol dose relatedly attenuated PCP-induced hyperlocomotion, and concentration dependently blocked PCP-induced short-term increases in glutamate levels in the medial PFC but with locomotor activity in the saline-treated group reduced to a lesser extent by clozapine than haloperidol. In contrast, Adams and Moghaddam (2001) reported no effect of haloperidol, clozapine, or the 5-HT2A antagonist, M100907, on the hyperglutamatergic effects of PCP. Overall, these findings suggest that both FGAs and SGAs can reverse some of the effects of NMDA receptor antagonists (i.e., short-term increase in glutamate release).
Early work demonstrated efficacy for both FGAs and SGAs in preventing NMDA antagonist neurotoxicity (Farber et al., 1993). However, a subsequent study showed differences among APDs on the basis of their potency in blocking MK-801 neurotoxicity, demonstrating that olanzapine, clozapine, and fluperlapine segregated into a high-potency group for blocking neurotoxic effects, followed by the three typical antipsychotics (haloperidol, loxapine, and thioridazine) as a moderate potency group, the antidepressant amoxapine being the least effective of all (Farber et al., 1996). Fujimura et al. (2000) reported similar findings showing that pretreatment (15 min) with clozapine or olanzapine but not with risperidone or haloperidol blocked the neuronal vacuolization produced by dizocilpine and significantly attenuated the expression of Fos-like protein in the retrosplenial cortex of rats. These findings suggest that some antipsychotics and in particular olanzapine and clozapine may be more effective than other APDs in reducing neurodegenerative changes associated with the administration of an NMDA antagonist.
Similar results have been more recently obtained in neonatal rats administered olanzapine in conjunction with repeated doses of PCP (Wang et al., 2001). In this study, the characteristics of neuronal loss, as well as Western blot analysis of Bac and Bcl-2 expression, suggest that PCP induced progressive apoptotic neuronal death in the cortex and olanzapine ameliorated these effects. In addition, in neonatal rats exposed to intraventricular kainic acid (Humphrey et al., 2002), administration of olanzapine and the antioxidant, melatonin, but not haloperidol ameliorated apoptotic neuronal loss in the hippocampus (Csernansky et al., 2006).
Long-term administration of methamphetamine triggers a mitochondria-dependent induction of apoptotic cascades and altered Bcl-2 expression that results in selected brain lesions. Long-term olanzapine exposure reduces methamphetamine-induced mortality and tyrosine hydroxylase immunoreactivity and prevents Bcl-2 decreases (He et al., 2004). Likewise, quetiapine counteracts anxiety-like behavioral changes that occur after long-term methamphetamine exposure (He et al., 2005a).
Okadaic acid is a protein phosphatase-2A inhibitor used to increase tau phosphorylation and induce neuronal death in models of Alzheimer’s disease and has been shown to result in neurodegeneration and a spatial memory deficit after injections into the hippocampus of rats (He et al., 2001). Olanzapine pretreatment was able to block both the okadaic acid-induced hippocampal cell death and impairment in spatial memory (He et al., 2005b). Repeated stress also leads to a reduction in the hippocampal expression of Bcl-2 in addition to a reduction in BDNF levels, and these effects can be blocked by long-term olanzapine treatment (Luo et al., 2004).
Not all SGAs have the ability to block apoptotic neurodegeneration. For example, the catechol and hydroquinone metabolites of remoxipride, a substituted benzamide-type APD, has been shown to induce apoptosis in HL60 cells and human bone marrow progenitor cells (McGuinness et al., 1999).
Recently, olanzapine and quetiapine were observed to block the activation of caspase-3, an enzyme involved in apoptosis (Wang et al., 2005b). In addition, these drugs blocked overproduction of intracellular free radicals in PC12 cells exposed to Aβ, which is the major constituent of amyloid plaques found in Alzheimer’s disease and known to be toxic in a variety of cell cultures (Wang et al., 2005b). In addition, clozapine or olanzapine administration for 28 days has been shown to up-regulate Bcl-2 gene expression in the frontal cortex and hippocampus (Bai et al., 2004).
In summary, although both FGAs and SGAs can mitigate increases in glutamate release associated with short-term administration of NMDA antagonists, SGAs seem to enhance cell survival to a greater extent than FGAs. Beyond the familiar effects of SGAs on monoaminergic and cholinergic neurotransmitter receptors and possible links against excitotoxic damage (Farber et al., 1998; Olney et al., 1999), SGAs in varying degrees may have properties that promote cell survival via antiapoptotic effects on the molecular cascade associated with apoptosis as shown here. In addition, some SGAs may facilitate cell survival via an ability to increase neuroactive steroids, reduce oxidative stress, and to facilitate neurotrophic factor expression as discussed in the next section.
E. Second-Generation Antipsychotic Drugs Increase Neuroactive Steroids in Animal Models
In animal models, olanzapine (Marx et al., 2000, 2003) and clozapine (Barbaccia et al., 2001; Marx et al., 2003) but not haloperidol dose dependently increase ALLO levels in rodent cerebral cortex. Olanzapine also increases ALLO levels in rodent hippocampus (Marx et al., 2006a). It has been hypothesized that olanzapine- and clozapine-induced elevations in ALLO may represent a potential mechanism contributing to the therapeutic efficacy of these agents (Marx et al., 2003, 2005). Recent reports that ALLO administration potentiates the actions of olanzapine on DA-mediated rodent behaviors further support the possibility that ALLO induction may be relevant to antipsychotic therapeutic mechanisms (Ugale et al., 2004).
In addition to a potential impact on GABAergic neurotransmission, clozapine- and olanzapine-induced elevations in ALLO may also result in neuroprotective and neurotrophic effects. For example, ALLO is neuroprotective against kainic acid- and NMDA-induced excitotoxicity (Lockhart et al., 2002; Ciriza et al., 2004) and contusion injury (Djebaili et al., 2004) and demonstrates anticonvulsant actions in a number of rodent seizure models (Belelli et al., 1989; Kokate et al., 1994, 1996; Devaud et al., 1995). ALLO administration doubles the lifespan of Niemann-Pick type C mice and delays the onset of neurological symptoms in this model (Griffin et al., 2004). ALLO also increases proliferation in human and rodent neuronal stem cells (Wang et al., 2005c). Antipsychotic-induced changes in ALLO may thus play a role in the regulation of a number of functionally relevant central nervous system events.
Neuroactive steroids also affect myelination. For example, ALLO administration in vitro increases myelin basic protein expression (Ghoumari et al., 2003), and the ALLO precursor progesterone, a steroid that can be synthesized in the brain and in the periphery, also affects myelination (Schumacher et al., 2000; Ibanez et al., 2003). Both clozapine and olanzapine dose dependently increase progesterone in rodents (Barbaccia et al., 2001; Marx et al., 2003, 2006a). If alterations in ALLO and progesterone also occur in patients with schizophrenia after antipsychotic drug administration, these changes could be relevant to myelin regulation, a process that seems to be dysregulated in subjects with schizophrenia (Hakak et al., 2001; Tkachev et al., 2003). Progesterone is also neuroprotective against cerebral contusion injury (Roof et al., 1994; Djebaili et al., 2004) and ischemia (Jiang et al., 1996; Gibson et al., 2005; Moralí et al., 2005).
Finally, recent data suggest that clozapine and olanzapine also elevate the neuroactive steroid pregnenolone in rodent hippocampus (Marx et al., 2006a), a potential precursor to many other neuroactive steroids. Pregnenolone (Flood et al., 1992) and pregnenolone sulfate (Flood et al., 1992, 1995; Vallée et al., 1997, 2003; Akwa et al., 2001) demonstrate pronounced effects on learning and memory in rodents and deficits in this neuroactive steroid have been linked to depression in humans (George et al., 1994). Clozapine- and olanzapine-induced elevations in pregnenolone could be relevant to cognitive deficits and depressive symptoms in patients with schizophrenia.
In subjects with schizophrenia or bipolar disorder, median ALLO levels were 67% higher in the posterior cingulate in subjects who were receiving clozapine at the time of death compared with subjects with these disorders who were not receiving clozapine at this time point, although these results did not achieve statistical significance (Marx et al., 2006b). Given the small sample sizes in human postmortem tissue studies, however, type II error cannot be excluded. Earlier work demonstrated a role for ALLO in antidepressant-like (Khisti and Chopde, 2000; Khisti et al., 2000; Pinna et al., 2003) and anxiolytic-like (Crawley et al., 1986; Wieland et al., 1991; Brot et al., 1997) effects in rodent models, and low ALLO levels have been linked to depressive symptoms in humans (Uzunova et al., 1998).
In summary, the SGAs olanzapine and clozapine can increase ALLO levels in rodent brain, and ALLO has been shown to be neuroprotective in models of excitotoxicity, to increase proliferation of human and rodent neuronal stem cells, and to potentially regulate myelination. In fact, both olanzapine and clozapine can elevate other neurosteroids including progesterone and pregnenolone. Only one study to date has reported a trend for increases in ALLO levels in patients with schizophrenia who were receiving clozapine at the time of their death. Additional studies are necessary in patients with schizophrenia to understand how neurosteroids and their metabolic pathways may be dysregulated in schizophrenia and the role that SGAs can play in restoring neurosteroid levels.
F. Effects of Antipsychotic Drugs on Mitochondria and Oxidative Phosphorylation
APDs have a negative impact on energy metabolism via inhibition of the electron transport system, although some drugs show significantly greater inhibition than others. Alterations in energy metabolism have been suggested to reflect a greater risk for development of tardive dyskinesia.
1. Impaired Mitochondrial Function and Risk for Tardive Dyskinesia
Because schizophrenia has historically been associated with alterations in the dopamine system, the development of APDs has been guided by the ability of potential new drugs to bind DA receptors. Thus, both FGAs and SGAs ameliorate schizophrenic symptoms most certainly by acting as DA receptor blockers. Over time, however, enthusiasm for the FGAs as a treatment for schizophrenia has been tempered by the fact that extended use of FGAs is correlated with the onset of TD. Some evidence suggests that impairment of mitochondrial function may be related to FGA-induced extrapyramidal symptoms such as TD, for example, 1) alterations in brain energy metabolism have been demonstrated in human patients symptomatic for TD (Goff et al., 1995) and 2) vacuous chewing movements, the rodent equivalent of TD, can be induced by long-term treatment with haloperidol (Andreassen and Jørgensen, 2000; Rosengarten and Quartermain, 2002) or the mitochondrial toxin, 3-nitropropionic acid (Andreassen and Jørgensen, 1995).
2. Antipsychotic Drugs Differentially Inhibit Complex I Activity
In vitro, several FGAs have been shown to interfere with the enzymes of the electron transport pathway in isolated mitochondrial membranes and brain homogenates (Burkhardt et al., 1993; Maurer and Möller, 1997; Modica-Napolitano et al., 2003). The site of the greatest inhibitory effect of FGAs is respiratory complex I, the NADH-oxidizing enzyme first in the electron transport sequence. Strong inhibition of complex I activity is observed in isolated mitochondria after the administration of the FGAs, chlorpromazine, thioridazine, and haloperidol. In contrast, risperidone and quetiapine have only mild inhibitory effects, and olanzapine and clozapine have barely measurable effects on complex I activity (Modica-Napolitano et al., 2003). Long-term administration of haloperidol or fluphenazine results in a generalized reduction in complex I activity in rat brain tissue, whereas long-term administration of clozapine has no effect on complex I activity (Prince et al., 1997). Altogether, in vivo and in vitro studies consistently demonstrate greater potency of FGAs versus SGAs as inhibitors of complex I activity.
Although separate assays have traditionally been used for each step in the electron transport pathway, an integrated assay has been developed to assess the relative ability of FGAs and SGAs to inhibit the entire electron transport pathway in intact mitochondria. This is an in vitro assay that includes the sequential activities of respiratory complexes I, III, and IV, comprising the complete sequence for stepwise oxidative transfer of electrons from NADH to oxygen. This integrated assay may be a better indication of any possible rate-limiting effect of site-specific drug inhibition on respiration overall (Modica-Napolitano et al., 2003). The drug potency for respiratory inhibition measured in the integrated assay was chlorpromazine > risperidone > haloperidol > quetiapine. Clozapine and olanzapine did not inhibit at all, and the insolubility of thioridazine limited its testing in this assay (see Fig. 3 in Modica-Napolitano et al., 2003). Except for risperidone and possibly thioridazine, the order of potency for inhibition of electron transport was well correlated with the relative risk of these drugs for causing TD.
3. Compensatory Changes in Mitochondrial Function with Antipsychotic Drug Treatment
We were surprised to find that some studies suggest that neuroleptics may reverse mitochondrial insufficiency in the frontal cortex of patients with schizophrenia. This suggestion may seem puzzling given the aforementioned ability of the FGAs to inhibit mitochondrial respiration but can be reconciled by distinguishing separate effects of drug treatment on mitochondrial biogenesis versus a direct effect on mitochondrial enzyme activity. Ultrastructural studies of postmortem brain samples revealed a reduction in the number of mitochondria in the caudate and putamen of patients with schizophrenia off drugs compared with control subjects and near-normal numbers of mitochondria in the striatum of a subset of patients with schizophrenia taking drugs (Kung and Roberts, 1999). In rats, long-term treatment with anti-psychotics (haloperidol, fluphenazine, and clozapine) caused a significant increase in mitochondrial cytochrome c oxidase activity in the frontal cortex (Prince et al., 1998). Mitochondrial cytochrome c oxidase activity was also significantly increased in postmortem samples from several regions of brain tissue from medicated patients with chronic schizophrenia (Prince et al., 2000). These studies suggest that antipsychotic treatment may normalize mitochondrial density in frontal cortex, possibly contributing to a reversal of schizophrenic symptoms by restoration of the capacity for oxidative energy metabolism. This finding is a likely corollary of DA receptor blockade, as cells can adapt to altered energy demands by adjusting the number and subcellular localization of their mitochondria (Ben-Shachar and Laifenfeld, 2004).
In summary, APDs show differential effects on inhibition of the electron transport system, with FGAs consistently showing greater inhibition of the electron transport system compared with SGAs. The greater impairment of mitochondrial function by FGAs has been implicated in the development of extrapyramidal symptoms, such as tardive dyskinesia. Interestingly, the negative impact that some of the APDs may have on energy metabolism seems to lead to compensatory changes in activity of mitochondrial enzymes and the number and location of mitochondria that may serve at least temporally to restore the capacity for oxidative energy metabolism.
G. Glucose Transport and Mechanism of Neuroprotection
Many FGAs and SGAs inhibit glucose transport, albeit to varying degrees, but paradoxically SGAs have been shown to stimulate neuronal growth and survival. The activation of Akt and ERK signaling pathways may play an important role in stimulation of neuronal growth and survival.
1. Antipsychotic Drugs Inhibit Glucose Transport
Many APDs produce significant inhibition of glucose transport in erythrocytes (Baker and Rogers, 1972) and cultured neuronal and muscle cell lines (Dwyer et al., 1999b, 2002). The FGAs, chlorpromazine and fluphenazine, are potent inhibitors of glucose transport, whereas haloperidol and sulpiride are essentially inactive (Table 1). Among the SGAs, risperidone, ziprasidone, and clozapine effectively block glucose transport, whereas olanzapine and quetiapine are somewhat less effective (Ardizzone et al., 2001; Dwyer et al., 2002, 2003b); aripiprazole has not been evaluated to date. The drugs seem to act by binding directly to the glucose transporter and affect transport via GLUT1 and GLUT3 isoforms. With longer incubation periods, the drugs significantly increase the expression of GLUT1 and GLUT3 in cells, perhaps as a consequence of relative glucose deprivation (Dwyer et al., 1999b). Furthermore, olanzapine has been shown to enhance cellular uptake of glucose (Dwyer et al., 2003a).
TABLE 1.
Biological profile of antipsychotic drugs
| Inhibition of Glucose Transporta | Calmodulin Antagonismb | Activation of Akt/Erkc | Neuroprotectiond | Enhancement of Neurite Outgrowthe | |
|---|---|---|---|---|---|
| Chlorpromazine | +++ | ++ | − | − | − |
| Fluphenazine | +++ | +++ | − | − | − |
| Haloperidol | − | + | ± | − | − |
| Clozapine | +++ | _ | + | ++ | ++ |
| Olanzapine | +++ | N.R. | ++ | ++ | ++ |
| Quetiapine | + | N.R. | + | + | ++ |
| Risperidone | ++ | N.R. | ± | ± | − |
| Ziprasidone | ++ | N.R. | ± | N.E. | + |
+, intensity of drug activity; −, no effect; N.R., not reported; N.E., not established.
Compiled from:
2. SGAs Promote Neurite Outgrowth and Cell Survival—Role of Akt
In light of these effects on glucose transport, the effects of APDs on cell growth are paradoxical and reflect the dual nature of many of these drugs. Thus, at low concentrations (10–50 μM), clozapine and quetiapine produce positive effects on neuronal growth (Bai et al., 2002; Lu and Dwyer, 2005), whereas at slightly higher concentrations, they adversely affect cell viability. Chlorpromazine and fluphenazine are uniformly toxic for cells over the same concentration range. On the other hand, olanzapine is mitogenic and neuroprotective over a wide range of concentrations (Dwyer et al., 2003a; Lu et al., 2004). Recently, some APDs have been reported to protect neuronal cells against a variety of insults (Bai et al., 2002; Qing et al., 2003; Lu et al., 2004) and to promote neurite outgrowth in the PC12 cell line (Lu and Dwyer, 2005). Other APDs produce the opposite effects: inhibition of cell growth and neurite extension (Dwyer et al., 2003a; Lu and Dwyer, 2005). The nature of the cellular response to a particular drug seems to depend on its overall biochemical profile, including the degree of glucose transport inhibition, affinity for calmodulin, mitochondrial toxicity, and ability to activate signaling pathways (Akt and Src) that provide trophic influences (Table 1).
It is not known how the specific APDs, particularly the SGAs, produce these neurotrophic effects; however, clues are beginning to emerge. Optimization of glucose metabolism in cells or preferential use of glucose rather than glutamine for energy is associated with decreased susceptibility to harmful insults (Kan et al., 1994; Goossens et al., 1996; Dwyer et al., 1999a; Moley and Mueckler, 2000). Moreover, the SGAs, olanzapine, quetiapine, and clozapine, stimulate phosphorylation (activation) of the serine/threonine kinases, Akt and ERK (Lu et al., 2004; Lu and Dwyer, 2005). Inhibition of Akt with a selective antagonist abrogates the protective effects of olanzapine on PC12 cells (Lu et al., 2004). In addition, inhibition of Akt and ERK activation with LY294002 and PD98059, respectively, blocks the induction of neurite outgrowth by olanzapine, quetiapine, and clozapine (Lu and Dwyer, 2005). In contrast, fluphenazine, chlorpromazine, and selective calmodulin antagonists inhibit activation of Akt by olanzapine and nerve growth factor (Lu and Dwyer, 2005), suggesting that calmodulin plays a significant role in the trophic effects of this SGA. Thus, with respect to activation of key signaling pathways (Akt and ERK), the SGAs seem to have a more favorable profile than the FGAs.
The role of Akt in the neuroenhancement produced by SGAs is intriguing for several reasons. First, Akt is downstream of the insulin receptor and regulates recruitment of GLUTs to the cell surface (Hajduch et al., 2001; Lawlor and Alessi, 2001). It also regulates the production of glucose-6-phosphate dehydrogenase, the rate-limiting step in the pentose phosphate pathway (Hajduch et al., 2001; Lawlor and Alessi, 2001). The pentose phosphate pathway provides NADPH to defend the cell against oxidative stress (neuroprotection) and for the synthesis of fatty acids (in support of neurite outgrowth), nitric oxide, and neurotransmitters (Baquer et al., 1988; Biagiotti et al., 2001). Second, Akt is broadly involved in the regulation of cell growth, differentiation, and survival (Hajduch et al., 2001; Lawlor and Alessi, 2001). Finally, Emamian et al. (2004) recently reported an association between a particular haplotype of the Akt1 gene and schizophrenia and found reduced levels of Akt1 in the brain and peripheral blood lymphocytes of patients with schizophrenia compared with control subjects. Thus, therapeutic strategies with the aim of enhancing the activity of Akt or its downstream targets may provide clinical benefits in schizophrenia.
In summary, APDs are associated with inhibition of glucose transport, albeit to varying degrees. Somewhat paradoxically, APDs can promote neurite outgrowth and cell survival in cell cultures, a finding that is dependent on drug and dose, degree of glucose transport inhibition, affinity for calmodulin, mitochondrial toxicity, and the ability to active the Akt and Src pathways. Overall, SGAs seem to have a more favorable profile than FGAs.
H. Second-Generation Antipsychotic Drugs Demonstrate Antioxidant Properties
The rat pheochromocytoma (PC12) cell line possesses properties associated with neuroblasts and neurons and is a well established model for studying the cellular biology of neurons, including the mechanisms involved in neurotoxicity, neuroprotection, and neuronal repair. Exposure of cells to hydrogen peroxide induces a concentration-dependent decrease in cell viability that was attenuated by olanzapine treatment (Wei et al., 2003a). β-amyloid(25–35) peptide induces oxidative stress and apoptosis in PC12 cultures, which are prevented by olanzapine and quetiapine (Wei et al., 2003b; Wang et al., 2005b). These findings suggest that some SGAs may act as antioxidants and that this mechanism may be the basis for part of their neuroprotective effects.
SOD1 reduces cellular oxidative stress and neuronal damage by inactivation of oxygen free radicals (Fridovich, 1986). The enzyme occurs in the large pyramidal neurons of the hippocampus and cortex (Delacourte et al., 1988; Ceballos et al., 1991) and its long-term inhibition produces apoptotic cell death of spinal neurons (Rothstein et al., 1994). It has been shown that PC12 cultures treated with olanzapine show increased SOD1 gene expression (Li et al., 1999). Other SGAs, such as clozapine, quetiapine, and risperidone were found to modulate the expression of SOD1 in a similar fashion (Bai et al., 2002). Inhibition of the mitochondrial oxidative processes by the neurotoxin 1-methyl-4-phenylpyridinium ion (MPP+) reduces SOD1 gene expression, thus increasing cell death, whereas SGAs protect the cultures against MPP+-induced cell death (Qing et al., 2003). This effect was observed for the SGAs clozapine, olanzapine, quetiapine, and risperidone but not for the FGA haloperidol.
A reduction in oxidative stress has also been observed in patients undergoing APD therapy. Dakhale et al. (2004) reported a significant increase in serum SOD and serum malondialdehyde and a decrease in plasma ascorbic acid in patients with schizophrenia, and treatment with SGAs significantly decreased serum malondialdehyde and increased plasma ascorbic acid levels. In addition, the levels of oxidative stress may differ in patients being treated with FGAs versus SGAs. Kropp et al. (2005) compared the levels of malondialdehyde in patients taking antipsychotic medications and found significantly lowers levels of this marker of lipid peroxidation in patients being treated with SGAs (clozapine, quetiapine, amisulpride, and risperidone) compared with treatment with FGAs (Kropp et al., 2005). However, not all studies have found differences between FGAs and SGAs in oxidative stress parameters (Zhang et al., 2006).
In summary, some of the SGAs have been shown to increase cell viability in a number of experimental conditions that are associated with cell death such as exposure to hydrogen peroxide, β-amyloid, or a mitochondrial neurotoxin. SGAs also lead to a reduction in measures of oxidative stress in patients with schizophrenia that tend to be greater than that observed with FGAs.
I. Regulation of Neurogenesis and Neurotrophic Factor Expression
In animal studies, APDs can modulate the levels and expression of specific neurotrophin factors and modulate neurogenesis and cell proliferation, with a number of differences noted between FGAs and SGAs. There is evidence for regulation of neurotrophin factors in patients treated with antipsychotic agents, although not without controversy. Recent work in rodents has also demonstrated a role for APDs in stimulating neurogenesis and cell proliferation.
1. Regulation of Neurotrophic Factor Expression
In the rat, differential treatment effects of APDs (primarily haloperidol, olanzapine, risperidone, and quetiapine) have resulted in brain region-specific changes in the levels of BDNF protein, BDNF mRNA, or the BDNF receptor, TrkB (Table 2) (Angelucci et al., 2000b, 2005; Dawson et al., 2001; Chlan-Fourney et al., 2002; Bai et al., 2003; Fumagalli et al., 2003a; Parikh et al., 2004a). Changes in the levels of NGF (Angelucci et al., 2000a, 2005) and basic FGF (Fumagalli et al., 2004) have also been reported in rat brain with APD treatment. Data indicate that less than 3 days of treatment with either a FGA or SGA had little or no effect on the levels of any neurotrophic factor, whereas 21 to 28 days of treatment with haloperidol generally decreased the levels. In contrast, SGAs showed either no effect or a slight increase in neurotrophic factor levels.
TABLE 2.
Regulation of neurotrophic factors by antipsychotic drug treatments in animals and schizophrenic patients
| Treatment | Factor/Region | Effect | Reference |
|---|---|---|---|
| Atypical | |||
| Clozapine (10 mg/kg, 28 days) | BDNF/HP | Decreased | Lipska et al., 2001 |
| Clozapine (20 mg/kg, 19 days) | BDNF/HP | No Effect | Chlan-Fourney et al., 2002 |
| Clozapine (27 mg/kg, 28 days) | BDNF/HP | Decreased | Bai et al., 2003 |
| Olanzapine (2.7 mg/kg, 28 days) | BDNF/HP | Increased | Bai et al., 2003 |
| Olanzapine (15 mg/kg, 30 days) | BDNF/HP | Decreased | Angelucci et al., 2005 |
| Olanzapine (10 mg/kg, 45 days) | BDNF/HP | No effect | Parikh et al., 2004a |
| Risperidone (2.3 mg/100g food, 19 days) | BDNF/HP, FrCtx | Decreased | Angelucci et al., 2000b |
| Risperidone (1 mg/kg, 19 days) | BDNF/HP | No effect | Chlan-Fourney et al., 2002 |
| Risperidone (4 mg/kg, 19 days) | BDNF/HP | Decreased | Chlan-Fourney et al., 2002 |
| Olanzapine (15 mg/kg) | NGF/HP, OcCtx | Increased | Angelucci et al., 2005 |
| Risperidone (2.3 mg/100g food, 19 days) | NGF/HP, Striatum | Decreased | Angelucci et al., 2000a |
| Clozapine (10 mg/kg, 21 days) | FGF2/PfrCtx, Striatum | Increased | Riva et al., 1999; Maragnoli et al., 2004 |
| Typical | |||
| Haloperidol (1.15 mg/100g food, 19 days) | BDNF/HP, FrCtx | Decreased | Angelucci et al., 2000b |
| Haloperidol (1 mg/kg, 28 days) | BDNF/HP | Decreased | Lipska et al., 2001 |
| Haloperidol (2 mg/kg, 21 days) | BDNF/HP, FrCtx | Decreased | Nibuya et al., 1995 |
| Haloperidol (1 mg/kg, 28 days) | BDNF/HP | Decreased | Bai et al., 2003 |
| Haloperidol (1 mg/kg, 19 days) | BDNF/HP | Decreased | Chlan-Fourney et al., 2002 |
| Haloperidol (2 mg/kg) | BDNF/HP | Decreased | Parikh et al., 2004a |
| Haloperidol (1 mg/kg, 3 days) | BDNF/Amy, Ctx | Decreased | Meredith et al., 2004 |
| Ritanserin (2 mg/kg, 19 days) | BDNF/HP (CA1) | Decreased | Chlan-Fourney et al., 2002 |
| Eticlopride (3 mg/kg, 3 days) | BDNF/Amy, Ctx | Decreased | Meredith et al., 2004 |
| Haloperidol (1.15 mg/100g food, 19 days) | NGF/HP, striatum | Decreased | Angelucci et al., 2000a |
| Haloperidol (1 mg/kg, 21 days) | FGF2/striatum | No effect | Riva et al., 1999 |
| Schizophrenia models | |||
| Ibotinic acid lesion | BDNF/HP, PfrCtx | Decreased | Lipska et al., 2001; Ashe et al., 2002 |
| MK-801 | BDNF/HP | Decreased | Fumagalli et al., 2003b |
| MK-801 + quetiapine | BDNF/HP | Normalized | Fumagalli et al., 2003b |
| MK-801 + haloperidol | BDNF/HP | Decreased | Fumagalli et al., 2003b |
| Stress | BDNF/HP | Decreased | Smith et al. 1995; Luo et al, 2004 |
| Stress + olanzapine | BDNF/HP | Increased | Luo et al., 2004 |
| Postmortem | |||
| Schizophrenic | BDNF/Ctx | Decreased | Durany et al., 2001 |
| Schizophrenic | BDNF/PfrCtx | Decreased | Weickert et al., 2003, 2005 |
| Schizophrenic | BDNF/Serum | Decreased | Toyooka et al., 2002 |
| Schizophrenic | BDNF/Serum | No effect | Shimizu et al., 2003 |
| Schizophrenic | BDNF/Serum | Decreased | Tan et al., 2005 |
HP, hippocampus; OcCtx, occipital cortex; FrCtx, frontal cortex; Amy, amygdala; CTx, cortex; PFRCtx, prefrontal cortex.
There has been a systematic investigation over the last few years of the effects of APDs on the levels of NGF, BDNF, and their respective receptors, TrkA and TrkB, in several regions of rat brain after 7, 14, 45, 90, and 180 days of treatment. The NGF and BDNF levels in rat hippocampus, striatum, and sensory motor cortex were significantly higher after a 7- or 14-day treatment with haloperidol, olanzapine, or risperidone compared with vehicle controls (S. Mahadik, unpublished observations). The data from a 45- and 90-day treatment indicated that haloperidol significantly reduced the NGF levels in rat septum, hippocampus, nucleus basalis, and cortex, but no change was found with risperidone, olanzapine, and clozapine (Parikh et al., 2004b,c). Likewise, a 45-day treatment with haloperidol, but not with olanzapine, reduced hippocampal BDNF and its receptor TrkB levels (Parikh et al., 2004a). Furthermore, switching rats after 45 days of treatment with haloperidol to risperidone or clozapine for the next 45 days increased the NGF levels from 25 to >70% (Parikh et al., 2004b), and switching rats after 45 days of treatment with haloperidol to olanzapine increased BDNF levels comparable with the levels observed in 90-day, vehicle-treated controls (Parikh et al., 2004a). Finally, because NGF primarily regulates cholinergic activity, the effects of antipsychotics on NGF levels paralleled the levels of the cholinergic marker, choline acetyltransferase (ChAT) (Parikh et al., 2004b,c). The representative immunohistochemical data from these studies are shown in Fig. 3.
Fig. 3.
Differential effects of 90-day treatment with FGAs [haloperidol (HAL)] and SGAs [risperidone (RISP) and clozapine (CLOZ)] on cerebral cortical NGF and ChAT. Animals received each drug (HAL = 2 mg/kg/day; RISP = 2.5 mg/kg/day; CLOZ = 20 mg/kg/day) in drinking water continuously for 90 days. Some animals treated with HAL for 45 days were switched to either RISP (HAL/RISP) or CLOZ (HAL/CLOZ) administration, and some animals with RISP and CLOZ administration for 45 days were switched to HAL treatment (RISP/HAL and CLOZ/HAL, respectively) for the next 45 days to investigate the restoration or prevention, respectively, of HAL-induced reduction of NGF and ChAT. Plasma levels of drugs were similar to plasma drug levels reported in patients with schizophrenia at therapeutic doses, and all the immunohistochemical procedures were done as described previously (Parikh et al., 2004a). Immunohistograms show NGF (red) in cortical neuronal cell bodies that are surrounded by cholinergic projections (ChAT, green) of cholinergic neurons from nucleus basalis. CO (vehicle-treated) shows dense localization of NGF and ChAT. HAL shows very significant reductions in both NGF and ChAT. However, RISP shows a slight reduction, whereas no reduction was found with CLOZ. Furthermore, post-treatment with RISP or CLOZ shows significant restoration (HAL/RISP < HAL/CLOZ) of HAL-induced reduction of NGF and ChAT. Likewise, pretreatment with RISP or CLOZ shows significant prevention (RISP/HAL < CLOZ/HAL) of HAL-induced reduction of NGF and ChAT. The detailed quantitative data were reported earlier (Parikh et al., 2004a,b).
Recently, Terry et al. (2006) reported that exposure of rats to oral haloperidol or ziprasidone for 7 or 14 days resulted in significant increases in NGF and ChAT immunoreactivity in the hippocampus. At 45 days, NGF and ChAT immunoreactivity had decreased to control levels in the ziprasidone-treated group but was markedly reduced in rats treated with haloperidol. After 90 days, NGF and ChAT levels were substantially lower than those of controls in both groups. Although exposure to ziprasidone had less deleterious effects on NGF and ChAT levels at 45 days, this beneficial effect was not evident at 90 days.
Data from 90 and 180 days of antipsychotic treatment in rats indicated that rat hippocampal BDNF levels were not altered by treatment with olanzapine but were significantly reduced by treatment with haloperidol (40 and 60% after 90 and 180 days, respectively), chlorpromazine (50% at both time points), and risperidone (20 and 40% after 90 and 180 days, respectively) (Pillai et al., 2006). Moreover, hippocampal NGF levels were significantly reduced (>60%) with haloperidol and chlorpromazine, but only smaller reductions (20–30%) were found with olanzapine and risperidone. In contrast, levels of both striatal BDNF and NGF were very significantly reduced (75–90%) by treatment with all of the APDs after 90 and 180 days (90 < 180). Of interest, even though treatment with olanzapine or risperidone for 180 days very significantly reduced (>80–90% versus controls) the levels of striatal BDNF and NGF, these anti-psychotics very effectively restored the levels (60–70% of controls) in animals treated with haloperidol for 90 days when they were switched to olanzapine or risperidone for the next 90 days (total 180 days). However, the mechanisms involved in reducing neurotrophic levels and those involved in elevating neurotrophic levels after a switch from haloperidol to olanzapine or risperidone are not known.
Erythropoietin (EPO) and its receptor, EPOr, are highly expressed within neuronal, glial, and endothelial cells in the brain, and they have been thought to play a role in neuroprotection (Bernaudin et al., 1999, 2000; Brines et al., 2000; Sirén et al., 2001; Marti, 2004). In the rat brain, 14 days of exposure to haloperidol increased both EPO and EPOr in the hippocampus; however, after 45 days of exposure to haloperidol, the levels were decreased significantly relative to day 14 (Pillai and Mahadik, 2006). In contrast, olanzapine treatment for 14 and 45 days resulted in elevations in the levels of EPO and EPOr in both brain regions in the rat, and EPO levels in the hippocampus were significantly increased at day 45 compared with those at day 14.
SGAs can also ameliorate the reductions in neurotrophins observed after various experimental paradigms. For example, immobilization stress decreases BDNF protein levels and BDNF immunoreactivity in the rat hippocampus, and the stress-induced BDNF reductions were attenuated by long-term administration of quetiapine (Xu et al., 2002). In addition, quetiapine has been shown to up-regulate FGF-2 and BDNF expression in rat hippocampus when NMDA receptors were blocked (Fumagalli et al., 2004). Similar effects were observed with olanzapine, but not with haloperidol, providing a further link between SGAs and neurotrophic responses (Fumagalli et al., 2003b).
How APDs affect neurotrophin levels is not well understood. For example, DA D2 receptor activation has been shown to up-regulate FGF-2 expression within the rat brain (Heckers et al., 1991; Swayze et al., 1992). Likewise, in cell cultures, dopamine and dopamine receptor agonists increased neurotrophic factor expression (Küppers and Beyer, 2001; Guo et al., 2002; Ohta et al., 2003). These observations suggest that APDs by virtue of their DA D2 receptor antagonism would inhibit neurotrophin expression albeit to varying degrees, reflecting their binding affinities for the DA D2 receptor, dissociation rates, and doses used. There is evidence that haloperidol can block the expression of BDNF by DA D2 agonists (Okazawa et al., 1992). In addition, high, but not low, doses of clozapine or risperidone that are known to markedly block DA D2 receptors also decrease the expression of BDNF in the hippocampus (Chlan-Fourney et al., 2002). Another factor may involve the interaction of the SGAs with a number of other neuronal receptors including 5-HT2 receptors, as the selective 5-HT2 receptor antagonist ritanserin increases the expression of hippocampal BDNF (Chlan-Fourney et al., 2002).
A reduction in neurotrophin levels has been observed in patients with schizophrenia, but only a few studies to date have demonstrated a selective role for SGAs in ameliorating these changes. For example, plasma NGF levels in patients with chronic schizophrenia treated with atypical antipsychotics were significantly higher than the levels observed in patients treated with FGAs (Parikh et al., 2003). Another study reported that serum BDNF levels in patients treated with clozapine were not significantly higher than values from patients treated with typical antipsychotics (Grillo et al., 2007), although serum BDNF levels were strongly and positively correlated with the dose of clozapine (Grillo et al., 2007). These findings are consistent with an earlier study reporting reductions in basal NGF levels in neuroleptic-free patients with schizophrenia subsequently treated with haloperidol (Aloe et al., 1997). Other studies, though, have not shown increases in neurotrophin levels after treatment with SGAs (Pirildar et al., 2004; Tan et al., 2005). Of interest, there is evidence for elevation of NGF serum concentrations in APD-naive patients with schizophrenia who were and were not substance abusers. Furthermore, reductions (or normalization) in NGF levels has been reported with APD treatment (Jockers-Scherübl et al., 2006). These disparate findings may reflect to some degree complexities surrounding the presence of comorbid substance abuse, environmental factors such as stress, and the potential for endogenous compensatory changes within the brain.
2. Regulation of Neurogenesis and Cell Proliferation
One mechanism that has generated a great deal of interest is the regulation of cell proliferation and neurogenesis in the adult brain. Recent studies demonstrate that neural progenitor cells continue to divide and give rise to new neurons in the subgranular zone of the hippocampus and the subventricular zone. Increased neurogenesis, cell migration to target sites, and differentiation into mature neuronal and glial phenotypes have been reported in the adult rodent brain under a variety of pathophysiological conditions (Dawirs et al., 1998; Gould et al., 1999; Nilsson et al., 1999; Madsen et al., 2000; Malberg et al., 2000; Wakade et al., 2002; Halim et al., 2004; Kodama et al., 2004; Wang et al., 2004b; Kippin et al., 2005). The rate of proliferation and survival of new neurons is a dynamic process regulated by neuronal activity and environmental and endocrine factors (Duman, 2004). In addition to neurogenesis, proliferation of glia, including astrocytes and oligodendrocytes and endothelial cells, takes place throughout the adult brain.
Recent studies demonstrate that neurogenesis and cell proliferation in the rodent brain are regulated by various psychotropic drugs, including APDs (Duman, 2004; Kodama et al., 2004), although the findings for FGAs have been controversial. After 4 days of treatment with haloperidol (5 mg/kg) followed by labeling of newborn cells for 7 days with bromodeoxyuridine (BrdU), the marker for cell proliferation, an increase in dentate granule cell proliferation was seen in gerbil hippocampus (Dawirs et al., 1998). Likewise, 3 days of haloperidol (2 mg/kg) administration followed by labeling of newborn cells with BrdU and subsequently analyzing labeled cells surviving after 28 days with continuous exposure to haloperidol was found to increase neural stem cell proliferation in rat brain (Kippin et al., 2005). In contrast, a 28-day study of haloperidol (2.0 mg/kg/day) followed by labeling of proliferating cells with BrdU and analyzing them either immediately or 21 days later with continuing drug exposure for determination of cell survival showed no increased proliferation or survival in hippocampal dentate gyrus (Halim et al., 2004). Another study with 21 days of exposure to haloperidol (2.0 mg/kg/day) followed by labeling of proliferating cells with BrdU and analyzing immediately or analyzing for cell survival 14 days later with or without haloperidol administration also demonstrated no increased proliferation or survival (Wang et al., 2004b). More recently, the temporal course of cell proliferation was investigated after continuous exposure to haloperidol for 7, 14, 21, and 45 days at doses 0.05 and 2.0 mg/kg/day (S. Mahadik, unpublished observations). Compared with vehicle treatment, haloperidol (2.0 mg/kg/day group) had increased the number of proliferating cells at the 7th day, which reached a maximum (2- to 3-fold) at the 14th day. However, cell proliferation returned to control levels at the 21st day and was significantly reduced (haloperidol 0.5 mg/kg = <25% and haloperidol 2 mg/kg = <5% of controls) at the 45th day. Data on the survival of the 14th day BrdU-labeled cells for the next 28 days on continuous exposure to haloperidol indicated that these cells do not survive.
The effects of SGAs on cell proliferation in the adult rodent brain have been more consistent. Both olanzapine and risperidone significantly increased cell proliferation in the subventricular zone and hippocampus after 21 days of treatment (Wakade et al., 2002). In other studies, olanzapine treatment for 21 days was also reported to cause 2- to 4-fold increases in cell proliferation, depending on the rat brain region examined (Kodama et al., 2004; Wang et al., 2004b; Green et al., 2006). Representative data on the effects of haloperidol and olanzapine on cell proliferation are shown in Fig. 4.
Fig. 4.
Differential temporal effects of haloperidol (HAL) and olanzapine (OLZ) administration on cell proliferation in hippocampus of adult rat brain. Animals were treated with vehicle, HAL or OLZ, as described in Fig. 4 for 14 and 45 days. All procedures were as described in Wakade et al. (2002). Newly born cells were labeled with bromodeoxyuridine (BrdU) and visualized with DAB immunostaining staining (brown dots), and then stained cells were counted. A, top shows the representative immunohistograms of control-14 day, HAL-14 day, and olanzapine-14 day; bottom shows the control-45 day, HAL-45 day, and olanzapine-45 day. Most of the proliferating cells are in the hilus and subgranular zone of the dentate gyrus. The inset in olanzapine-45 day shows a higher magnification of a group of BrdU-positive cells. B, differential temporal effects on the numbers of proliferating cells (*, p < 0.001 versus vehicle).
The formation of new neurons in the hippocampus is significant as adult rat brain neurogenesis has been consistently observed with both pharmacological and nonpharmacological antidepressant therapies (Madsen et al., 2000; Malberg et al., 2000) and seems to be functionally important in the behavioral effects of antidepressant drugs observed in mice (Santarelli et al., 2003). APDs seem to produce similar effects to varying degrees. The increase in cell proliferation in the prefrontal cortex and striatum leads to increased numbers of endothelial cells and oligodendrocytes, as well as a subpopulation of unidentified cells (Kodama et al., 2004). The increase in oligodendrocytes could contribute to a reversal of white matter loss that has been reported in patients with schizophrenia (Hakak et al., 2001; Davis et al., 2003). In addition, increased striatal and cortical cell proliferation could contribute in part to increased gray matter and also reflects the ability of these antipsychotic agents to either protect against cell loss or to confer beneficial increases in endothelial cell number.
In summary, a number of studies have demonstrated differential effects of APDs on the level and expression of specific neurotrophin factors and their receptors within the rodent brain, and some evidence exists for regulation in patients with schizophrenia also. In addition, APDs can stimulate neurogenesis and cell proliferation in the adult rodent brain. However, to date the studies pointing to APD effects on neurogenesis have been performed predominantly in nonprimate species, and there are no data to support survival of newly generated neurons in the adult primate forebrain (Bhardwaj et al., 2006). Additional work will be necessary to determine whether the regulation of neurogenesis and cell proliferation with APDs occurs within the adult nonhuman primate forebrain and is a mechanism that exists in humans.
IV. Conclusions
Schizophrenia entails a progressive pathophysiological process that possibly involves a limited neurodegenerative component, which causes the clinical deterioration that historically has been the hallmark of the illness. In this article, we reviewed studies of the effects of APDs, including FGAs and SGAs, on a number of assays and mechanisms pertinent to the pharmacotherapy of schizophrenia (Fig. 5) in an attempt to understand the clinical implications of the range of pharmacological effects demonstrated by these various paradigms. Many studies have now shown that some APDs, particularly those of the SGA class, can enhance neural cell functions, resilience, and plasticity. These observations may have potential clinical relevance, because they suggest the different cellular mechanisms by which some APDs may exert effects beyond those that are traditionally measured through neuroreceptor binding or neurotransmitter release and immediately observable behaviorally, which may be able to ameliorate the pathophysiological progression of this illness.
Fig. 5.
Generalization of comparative changes of FGAs and SGAs on physiological processes thought to be dysfunctional in schizophrenia. These comparative observations are based on either changes found in patients with schizophrenia or inferred from in vitro or animal studies cited in this review. In some instances, the effects of the drugs within the class differ, and this is denoted by the use of two symbols. NC, no change;+, increase or improvement; −, decrease or decline.
With some variation, SGAs display unique pharmacological actions that may distinguish them from FGAs. In both traditional animal models and neurochemical models and with regard to receptor binding profiles, SGAs display a pattern of activity that predicts antipsychotic activity with a reduced liability to produce EPS. This pattern has been consistently associated with a greater potential for pharmacological activity in novel brain regions such as temporolimbic and prefrontal cortices. In addition, the SGAs variably enhance levels of DA, norepinephrine, 5-HT, ACh, and glutamate (aspartate), whereas they decrease efflux of GABA. Enhancement of monoamine levels has been shown to increase neurotrophic factors.
SGAs can reduce caudate hypertrophy observed in patients treated with FGAs, and recent data indicate a significant role of SGAs in limiting the loss of gray matter and dendritic remodeling. The molecular underpinning of dendritic remodeling is the subject of considerable scrutiny, with different players such as receptors, growth factors, and GTPases being examined. It will important to determine the cytoskeletal structure of neurons in future studies of how APDs influence these factors.
In a variety of model systems, some of the SGAs are more effective than the FGAs in attenuating the effects of noncompetitive NMDA receptor antagonists. Some SGAs induce neuroactive steroids that may result in actions that enhance GABAergic neurotransmission and offer neuroprotective and neurotrophic effects. Some SGAs can reduce oxidative stress in animal models of neurotoxicity and potentially in patients with schizophrenia. Some SGAs but not FGAs show the ability to block and, in some instances, reverse neurodegenerative processes associated with apoptosis and excitotoxicity in animal models of neurodegeneration. It is clear that FGAs have much greater potential than do SGAs to inhibit oxidative phosphorylation directly in vitro. Some SGAs, by virtue of their effects on glucose metabolism, Akt, and neurite outgrowth, may also offer a new mechanism for therapeutic effects and prevent the neurodegenerative effects that seem in the course of the illness.
The possibility that some SGAs might limit neurodegenerative processes and effects that occur in the brains of patients with schizophrenia is exciting and offers hope in limiting the cumulative morbidity of patients with schizophrenia and reducing the burden of disease for patients and their families. Furthermore, studies of the neuroprotective effects of APDs may reflect another mechanism of action that APDs can act through that is clinically relevant and should stimulate the search for new drugs for schizophrenia with novel mechanisms beyond the more familiar effects current drug treatments have on the monoaminergic neurotransmitter systems.
As pointed out in this critical review of the literature on the effects of APDs, there is inconsistency in the results of these different studies and many instances of interstudy variability of the biological effects between the FGA and SGA drug classes and also variation within each of the two drug classes. Research is new and limited in some of the areas reviewed, and technical procedures have not been standardized, which contributes to variability. In addition, many studies use only haloperidol as the FGA and not all of the SGAs have been fully evaluated. It is difficult in animal studies to be sure that clinically equivalent doses of the APDs are administered. Many studies were short-term in nature, and often subchronic studies were relatively short-term. These factors make extrapolation of these results to the effects of APDs in humans after weeks or months of treatment difficult at best. Furthermore, many of the biological processes evaluated in cell lines and in animals have not been fully characterized in humans, and their clinical relevance is not known. Consequently, the impact of alterations of these processes in patients is unknown. Clearly, more work is needed, but the bulk of the data supports our tentative conclusion that some APDs, mainly of the SGA group, may have effects that can be therapeutic on many biological processes and neuroprotective on the pathophysiology of schizophrenia, whereas the FGAs are neutral and in high doses may even promote neurodegeneration. Therefore, we believe that there may be clinical benefits in the use of selective SGAs versus FGAs for long-term treatment particularly in the early stages of schizophrenia and related psychotic disorders.
Acknowledgments
We acknowledge the many contributions of our colleagues to this review, Dr. Sara Kollack-Walker for editorial assistance, and Dr. Yvonne Cole for formatting and submitting the manuscript.
Footnotes
Abbreviations: DA, dopamine; 1H-MRS, proton magnetic resonance spectroscopy; 5-HT, serotonin, 5-hydroxytryptamine; A10, ventral teg-mental area; A9, nigrostriatal; AC, adenylyl cyclase; ACh, acetylcholine; ALLO, allopregnanolone, 3α-hydroxy-5α-pregnan-20-one; AMPA, α-amino3-hydroxy-5-methyl-4-isoxazole propionic acid; APD, antipsychotic drug; BrdU, bromodeoxyuridine; ChAT, choline acetyltransferase; CREB, cAMP-response element-binding protein; CSF, cerebrospinal fluid; DAAO, D-amino acid oxidase; DARPP-32, dopamine- and an adenosine 3′,5′-monophosphate-regulated phospho-protein of 32 kDa; DD, drug discrimination; DHEA, dehydroepiandrosterone; DOPA, 3,4-dihydroxyphenylalanine; EAAT, excitatory amino acid transporter; EPO, erythropoietin; EPOr, erythropoietin receptor; EPS, extrapyramidal symptoms; EPSPs, excitatory postsynaptic potentials; ERK, extracellular-regulated kinase; FGA, first-generation antipsychotic drug; FRT, forelimb retraction time; GAD, glutamic acid decarboxylase; GAT, GABA transporter; GLUT, glucose transporter; GPCR, G-protein-coupled receptor; GSK-3, glycogen synthase kinase-3; HRT, hind limb retraction time; IP3, inositol triphosphate; LY294002, 2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride; M100907, [R-(+)-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine-methanol]; MAP, mitogen-activated protein; MCT, monocarboxylate transporter; mGlu, metabotropic glutamate receptors; MK-801, dizocilpine, 5H-dibenzo[a,d]cyclohepten-5,10-imine (dizocilpine maleate); MPP+, 1-methyl-4-phenylpyridinium ion; NAAG, N-acetylaspartylglutamate; NGF, nerve growth factor, BDNF, brain-derived neurotrophic factor, FGF, fibroblast growth factor; NMDA, N-methyl-D-aspartic acid; NRG1, neuroregulin-1; NT-3, neurotrophin-3; PCP, phencyclidine; PD98059, 2′-amino-3′-methoxyflavone; PFC, prefrontal cortex; PK, protein kinase; PLC, phospholipase C; PPI, prepulse inhibition; PV, parvalbumin; RGS, regulators of G-protein signaling; SGA, second-generation antipsychotic drug; SOD, superoxide dismutase; SR46349B, eplivanserin; SST, somatostatin; TD, tardive dyskinesia.
C.E.M. is a coapplicant on a pending U.S. patent application for the use of neurosteroids to treat central nervous system disorders.
References
- Abekawa T, Ito K, Koyama T. Role of the simultaneous enhancement ofNMDA and dopamine D1 receptor-mediated neurotransmission in the effects of clozapine on phencyclidine-induced acute increases in glutamate levels in the rat medial prefrontal cortex. Naunyn Schmiedebergs Arch Pharmacol. 2006;374:177–193. doi: 10.1007/s00210-006-0115-9. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A. Alterations of serotonin transmission in schizophrenia. Int Rev Neurobiol. 2007;78:133–164. doi: 10.1016/S0074-7742(06)78005-9. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Laruelle M. Mechanisms of action of second generation antipsychotic drugs in schizophrenia: insights from brain imaging studies. Eur Psychiatry. 2005;20:15–27. doi: 10.1016/j.eurpsy.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang DR, Keilp J, Kochan L, Van Heertum R, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abi-Dargham A, Moore H. Prefrontal DA transmission at D1 receptors and the pathology of schizophrenia. Neuroscientist. 2003;9:404–416. doi: 10.1177/1073858403252674. [DOI] [PubMed] [Google Scholar]
- Adams AC, Keefe KA. Examination of the involvement of protein kinase A in D2 dopamine receptor antagonist-induced immediate early gene expression. J Neurochem. 2001;77:326–335. doi: 10.1046/j.1471-4159.2001.t01-1-00247.x. [DOI] [PubMed] [Google Scholar]
- Adams B, Moghaddam B. Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci. 1998;18:5545–5554. doi: 10.1523/JNEUROSCI.18-14-05545.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams BW, Moghaddam B. Effect of clozapine, haloperidol, or M100907 on phencyclidine-activated glutamate efflux in the prefrontal cortex. Biol Psychiatry. 2001;50:750–757. doi: 10.1016/s0006-3223(01)01195-7. [DOI] [PubMed] [Google Scholar]
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Adams MR, Brandon EP, Chartoff EH, Idzerda RL, Dorsa DM, McKnight GS. Loss of haloperidol induced gene expression and catalepsy in protein kinase A-deficient mice. Proc Natl Acad Sci U S A. 1997;94:12157–12161. doi: 10.1073/pnas.94.22.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akwa Y, Ladurelle N, Covey DF, Baulieu EE. The synthetic enantiomer of pregnenolone sulfate is very active on memory in rats and mice, even more so than its physiological neurosteroid counterpart: distinct mechanisms? Proc Natl Acad Sci U S A. 2001;98:14033–14037. doi: 10.1073/pnas.241503698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert KA, Hemmings HC, Jr, Adamo AI, Potkin SG, Akbarian S, Sandman CA, Cotman CW, Bunney WE, Jr, Greengard P. Evidence for decreased DARPP-32 in the prefrontal cortex of patients with schizophrenia. Arch Gen Psychiatry. 2002;59:705–712. doi: 10.1001/archpsyc.59.8.705. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Alfredsson G, Wiesel FA. Monoamine metabolites and amino acids in serum from schizophrenic patients before and during sulpiride treatment. Psychopharmacology (Berl) 1989;99:322–327. doi: 10.1007/BF00445551. [DOI] [PubMed] [Google Scholar]
- Alimohamad H, Rajakumar N, Seah YH, Rushlow W. Antipsychotics alter the protein expression levels of beta-catenin and GSK-3 in the rat medial prefrontal cortex and striatum. Biol Psychiatry. 2005;57:533–542. doi: 10.1016/j.biopsych.2004.11.036. [DOI] [PubMed] [Google Scholar]
- Aloe L, Iannitelli A, Bersani G, Alleva E, Angelucci F, Maselli P, Manni L. Haloperidol administration in humans lowers plasma nerve growth factor level: evidence that sedation induces opposite effects to arousal. Neuropsychobiology. 1997;36:65–68. doi: 10.1159/000119364. [DOI] [PubMed] [Google Scholar]
- Amargós-Bosch M, López-Gil X, Artigas F, Adell A. Clozapine and olanzapine, but not haloperidol, suppress serotonin efflux in the medial prefrontal cortex elicited by phencyclidine and ketamine. Int J Neuropsychopharmacol. 2006;9:565–573. doi: 10.1017/S1461145705005900. [DOI] [PubMed] [Google Scholar]
- Andersson C, Hamer RM, Lawler CP, Mailman RB, Lieberman JA. Striatal volume changes in the rat following long-term administration of typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2002;27:143–151. doi: 10.1016/S0893-133X(02)00287-7. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Rezai K, Alliger R, Swayze VW, 2nd, Flaum M, Kirchner P, Cohen G, O’Leary DS. Hypofrontality in neuroleptic-naïve patients and in patients with chronic schizophrenia. Assessment with xenon 133 single-photon emission computed tomography and the Tower of London. Arch Gen Psychiatry. 1992;49:943–958. doi: 10.1001/archpsyc.1992.01820120031006. [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Jørgensen HA. The mitochondrial toxin 3-nitropropionic acid induces vacuous chewing movements in rats. Implications for tardive dyskinesia? Psychopharmacology (Berl) 1995;119:474–476. doi: 10.1007/BF02245864. [DOI] [PubMed] [Google Scholar]
- Andreassen OA, Jørgensen HA. Neurotoxicity associated with neuroleptic-induced oral dyskinesias in rats: implications for tardive dyskinesia. Prog Neurobiol. 2000;61:525–541. doi: 10.1016/s0301-0082(99)00064-7. [DOI] [PubMed] [Google Scholar]
- Angelucci F, Aloe L, Gruber SH, Fiore M, Mathé AA. Chronic antipsychotic treatment selectively alters nerve growth factor and neuropeptide Y immunoreactivity and the distribution of choline acetyl transferase in rat brain regions. Int J Neuropsychopharmacol. 2000a;3:13–25. doi: 10.1017/S1461145700001759. [DOI] [PubMed] [Google Scholar]
- Angelucci F, Aloe L, Iannitelli A, Gruber SH, Mathé AA. Effect of chronic olanzapine treatment on nerve growth factor and brain-derived neurotrophic factor in the rat brain. Eur Neuropsychopharmacol. 2005;15:311–317. doi: 10.1016/j.euroneuro.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Angelucci F, Mathé AA, Aloe L. Brain-derived neurotrophic factor and tyrosine kinase receptor TrkB in rat brain are significantly altered after haloperidol and risperidone administration. J Neurosci Res. 2000b;60:783–794. doi: 10.1002/1097-4547(20000615)60:6<783::AID-JNR11>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Ardizzone TD, Bradley RJ, Freeman AM, 3rd, Dwyer DS. Inhibition of glucose transport in PC12 cells by the atypical antipsychotic drugs risperidone and clozapine, and structural analogs of clozapine. Brain Res. 2001;923:82–90. doi: 10.1016/s0006-8993(01)03026-8. [DOI] [PubMed] [Google Scholar]
- Arif M, Chikuma T, Ahmed MM, Yoshida S, Kato T. Suppressive effect of clozapine but not haloperidol on the increases of neuropeptide-degrading enzymes and glial cells in MK-801-treated rat brain regions. Neurosci Res. 2007;57:248–258. doi: 10.1016/j.neures.2006.10.021. [DOI] [PubMed] [Google Scholar]
- Arnt J. Pharmacological specificity of conditioned avoidance response inhibition in rats: inhibition by neuroleptics and correlation to dopamine receptor blockade. Acta Pharmacol Toxicol (Copenh) 1982;51:321–329. doi: 10.1111/j.1600-0773.1982.tb01032.x. [DOI] [PubMed] [Google Scholar]
- Arnt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology. 1998;18:63–101. doi: 10.1016/S0893-133X(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Arundine M, Tymianski M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium. 2003;34:325–337. doi: 10.1016/s0143-4160(03)00141-6. [DOI] [PubMed] [Google Scholar]
- Arvanov VL, Liang X, Schwartz J, Grossman S, Wang RY. Clozapine and haloperidol modulate N-methyl-D-aspartate- and non-N-methyl-D-aspartate receptor-mediated neurotransmission in rat prefrontal cortical neurons in vitro. J Pharmacol Exp Ther. 1997;283:226–234. [PubMed] [Google Scholar]
- Ashe PC, Chlan-Fourney J, Juorio AV, Li XM. Brain-derived neurotrophic factor (BDNF) mRNA in rats with neonatal ibotenic acid lesions of the ventral hippocampus. Brain Res. 2002;956:126–135. doi: 10.1016/s0006-8993(02)03176-1. [DOI] [PubMed] [Google Scholar]
- Bai O, Chlan-Fourney J, Bowen R, Keegan D, Li XM. Expression of brain-derived neurotrophic factor mRNA in rat hippocampus after treatment with antipsychotic drugs. J Neurosci Res. 2003;71:127–131. doi: 10.1002/jnr.10440. [DOI] [PubMed] [Google Scholar]
- Bai O, Wei Z, Lu W, Bowen R, Keegan D, Li XM. Protective effects of atypical antipsychotic drugs on PC12 cells after serum withdrawal. J Neurosci Res. 2002;69:278–283. doi: 10.1002/jnr.10290. [DOI] [PubMed] [Google Scholar]
- Bai O, Zhang H, Li XM. Antipsychotic drugs clozapine and olanzapine upregulate bcl-2 mRNA and protein in rat frontal cortex and hippocampus. Brain Res. 2004;1010:81–86. doi: 10.1016/j.brainres.2004.02.064. [DOI] [PubMed] [Google Scholar]
- Bajestan SN, Sabouri AH, Nakamura M, Takashima H, Keikhaee MR, Behdani F, Fayyazi MR, Sargolzaee MR, Bajestan MN, Sabouri Z, et al. Association of AKT1 haplotype with the risk of schizophrenia in Iranian population. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:383–386. doi: 10.1002/ajmg.b.30291. [DOI] [PubMed] [Google Scholar]
- Baker GF, Rogers HJ. Effects of psychotropic drugs on the erythrocyte permeability to glucose and ethylidene glucose. Biochem Pharmacol. 1972;21:1871–1878. doi: 10.1016/0006-2952(72)90183-9. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Geyer MA. Antagonism of phencyclidine-induced deficits in prepulse inhibition by the putative atypical antipsychotic olanzapine. Psychopharmacology (Berl) 1995;122:198–201. doi: 10.1007/BF02246096. [DOI] [PubMed] [Google Scholar]
- Bakshi VP, Swerdlow NR, Geyer MA. Clozapine antagonizes phencyclidine-induced deficits in sensorimotor gating of the startle response. J Pharmacol Exp Ther. 1994;271:787–794. [PubMed] [Google Scholar]
- Baquer NZ, Hothersall JS, McLean P. Function and regulation of the pentose phosphate pathway in brain. Curr Top Cell Regul. 1988;29:265–289. [PubMed] [Google Scholar]
- Barbaccia ML, Affricano D, Purdy RH, Maciocco E, Spiga F, Biggio G. Clozapine, but not haloperidol, increases brain concentrations of neuroactive steroids in the rat. Neuropsychopharmacology. 2001;25:489–497. doi: 10.1016/S0893-133X(01)00254-8. [DOI] [PubMed] [Google Scholar]
- Barde YA. Neurotrophins: a family of proteins supporting the survival of neurons. Prog Clin Biol Res. 1994;390:45–56. [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men: a magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/β-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the progesterone metabolite 5 α-pregnan-3 α-ol-20-one. Eur J Pharmacol. 1989;166:325–329. doi: 10.1016/0014-2999(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar D. Mitochondrial dysfunction in schizophrenia: a possible linkage to dopamine. J Neurochem. 2002;83:1241–1251. doi: 10.1046/j.1471-4159.2002.01263.x. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar D, Laifenfeld D. Mitochondria, synaptic plasticity, and schizophrenia. Int Rev Neurobiol. 2004;59:273–296. doi: 10.1016/S0074-7742(04)59011-6. [DOI] [PubMed] [Google Scholar]
- Benes FM. The role of stress and dopamine-GABA interactions in the vulnerability for schizophrenia. J Psychiatr Res. 1997;31:257–275. doi: 10.1016/s0022-3956(96)00044-1. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. Amygdalo-entorhinal inputs to the hippocampal formation in relation to schizophrenia. Ann N Y Acad Sci. 2000;911:293–304. doi: 10.1111/j.1749-6632.2000.tb06733.x. [DOI] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Benes FM, Khan Y, Vincent SL, Wickramasinghe R. Differences in the subregional and cellular distribution of GABAA receptor binding in the hippocampal formation of schizophrenic brain. Synapse. 1996a;22:338–349. doi: 10.1002/(SICI)1098-2396(199604)22:4<338::AID-SYN5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Benes FM, McSparren J, Bird ED, SanGiovanni JP, Vincent SL. Deficits in small interneurons in prefrontal and cingulated cortices of schizophrenic and schizoaffective patients. Arch Gen Psychiatry. 1991;48:996–1001. doi: 10.1001/archpsyc.1991.01810350036005. [DOI] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Marie A, Khan Y. Increased GABAA receptor binding on neurons of prefrontal cortex in schizophrenic subjects. Neuroscience. 1996b;75:1021–1031. doi: 10.1016/0306-4522(96)00328-4. [DOI] [PubMed] [Google Scholar]
- Benes FM, Vincent SL, Todtenkopf M. The density of pyramidal and nonpyramidal neurons in anterior cingulate cortex of schizophrenic and bipolar subjects. Biol Psychiatry. 2001;50:395–406. doi: 10.1016/s0006-3223(01)01084-8. [DOI] [PubMed] [Google Scholar]
- Benes FM, Walsh J, Bhattacharyya S, Sheth A, Berretta S. DNA fragmentation decreased in schizophrenia but not bipolar disorder. Arch Gen Psychiatry. 2003;60:359–364. doi: 10.1001/archpsyc.60.4.359. [DOI] [PubMed] [Google Scholar]
- Bergeron R, de Montigny C, Debonnel G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: effects mediated via sigma receptors. J Neurosci. 1996;16:1193–1202. doi: 10.1523/JNEUROSCI.16-03-01193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergson C, Levenson R, Goldman-Rakic PS, Lidow MS. Dopamine receptor-interacting proteins: the Ca2+ connection in dopamine signaling. Trends Pharmacol Sci. 2003;24:486–492. doi: 10.1016/S0165-6147(03)00232-3. [DOI] [PubMed] [Google Scholar]
- Bernaudin M, Bellail A, Marti HH, Yvon A, Vivien D, Duchatelle I, Mackenzie ET, Petit E. Neurons and astrocytes express EPO mRNA: oxygen-sensing mechanisms that involve the redox-state of the brain. Glia. 2000;30:271–278. [PubMed] [Google Scholar]
- Bernaudin M, Marti HH, Roussel S, Divoux D, Nouvelot A, MacKenzie ET, Petit E. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J Cereb Blood Flow Metab. 1999;19:643–651. doi: 10.1097/00004647-199906000-00007. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Björk-Eriksson T, Nordborg C, Gage FH, Druid H, Eriksson PS, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A. 2006;103:12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagiotti E, Guidi L, Capellacci S, Ambrogini P, Papa S, Del Grande P, Ninfali P. Glucose-6-phosphate dehydrogenase supports the functioning of the synapses in rat cerebellar cortex. Brain Res. 2001;911:152–157. doi: 10.1016/s0006-8993(01)02615-4. [DOI] [PubMed] [Google Scholar]
- Black JE, Kodish IM, Grossman AW, Klintsova AY, Orlovskaya D, Vostrikov V, Uranova N, Greenough WT. Pathology of layer V pyramidal neurons in the prefrontal cortex of patients with schizophrenia. Am J Psychiatry. 2004;161:742–744. doi: 10.1176/appi.ajp.161.4.742. [DOI] [PubMed] [Google Scholar]
- Blass JP. Glucose/mitochondria in neurological conditions. Int Rev Neurobiol. 2002;51:325–376. doi: 10.1016/s0074-7742(02)51010-2. [DOI] [PubMed] [Google Scholar]
- Blum BP, Mann JJ. The GABAergic system in schizophrenia. Int J Neuropsychopharmacol. 2002;5:159–179. doi: 10.1017/S1461145702002894. [DOI] [PubMed] [Google Scholar]
- Boks MP, Rietkerk T, van de Beek MH, Sommer IE, de Koning TJ, Kahn RS. Reviewing the role of the genes G72 and DAAO in glutamate neurotransmission in schizophrenia. Eur Neuropsychopharmacol. 2007;17:567–572. doi: 10.1016/j.euroneuro.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Bonaccorso S, Meltzer HY, Li Z, Dai J, Alboszta AR, Ichikawa J. SR46349-B, a 5-HT2A/2C receptor antagonist, potentiates haloperidol-induced dopamine release in rat medial prefrontal cortex and nucleus accumbens. Neuropsychopharmacology. 2002;27:430–441. doi: 10.1016/S0893-133X(02)00311-1. [DOI] [PubMed] [Google Scholar]
- Bondy CA, Cheng CM. Insulin-like growth factor-1 promotes neuronal glucose utilization during brain development and repair processes. Int Rev Neurobiol. 2002;51:189–217. doi: 10.1016/s0074-7742(02)51006-0. [DOI] [PubMed] [Google Scholar]
- Bortolato M, Frau R, Orrù M, Piras AP, Fà M, Tuveri A, Puligheddu M, Gessa GL, Castelli MP, Mereu G, et al. Activation of GABAB receptors reverses spontaneous gating deficits in juvenile DBA/2J mice. Psychopharmacology (Berl) 2007;194:361–369. doi: 10.1007/s00213-007-0845-5. [DOI] [PubMed] [Google Scholar]
- Bourdelais AJ, Deutch AY. The effects of haloperidol and clozapine on extracellular GABA levels in the prefrontal cortex of the rat: an in vivo microdialysis study. Cereb Cortex. 1994;4:69–77. doi: 10.1093/cercor/4.1.69. [DOI] [PubMed] [Google Scholar]
- Bowery NG. GABAB receptor: a site of therapeutic benefit. Curr Opin Pharmacol. 2006;6:37–43. doi: 10.1016/j.coph.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology (Berl) 2001;156:234–258. doi: 10.1007/s002130100810. [DOI] [PubMed] [Google Scholar]
- Braus DF, Weber-Fahr W, Tost H, Ruf M, Henn FA. Sensory information processing in neuroleptic-naive first-episode schizophrenia patients: a functional magnetic resonance imaging study. Arch Gen Psychiatry. 2002;59:696–701. doi: 10.1001/archpsyc.59.8.696. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signaling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–242. doi: 10.1016/j.tibs.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Gellad F. Brain morphology and schizophrenia. A magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Arch Gen Psychiatry. 1992;49:921–926. doi: 10.1001/archpsyc.1992.01820120009003. [DOI] [PubMed] [Google Scholar]
- Britsch S. The neuregulin-I/ErbB signaling system in development and disease. Adv Anat Embryol Cell Biol. 2007;190:1–65. [PubMed] [Google Scholar]
- Brines ML, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, Itri LM, Cerami A. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadbelt K, Byne W, Jones LB. Evidence for a decrease in basilar dendrites of pyramidal cells in schizophrenic medial prefrontal cortex. Schizophr Res. 2002;58:75–81. doi: 10.1016/s0920-9964(02)00201-3. [DOI] [PubMed] [Google Scholar]
- Brot MD, Akwa Y, Purdy RH, Koob GF, Britton KT. The anxiolytic-like effects of the neurosteroid allopregnanolone: interactions with GABAA receptors. Eur J Pharmacol. 1997;325:1–7. doi: 10.1016/s0014-2999(97)00096-4. [DOI] [PubMed] [Google Scholar]
- Browning JL, Patel T, Brandt PC, Young KA, Holcomb LA, Hicks PB. Clozapine and the mitogen-activated protein kinase signal transduction pathway: implications for antipsychotic actions. Biol Psychiatry. 2005;57:617–623. doi: 10.1016/j.biopsych.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Breier A, Kirkpatrick B, Elkashef A, Munson RC, Gellad F, Carpenter WT., Jr Structural abnormalities in deficit and nondeficit schizophrenia. Am J Psychiatry. 1993;150:59–65. doi: 10.1176/ajp.150.1.59. [DOI] [PubMed] [Google Scholar]
- Buchanan RW, Vladar K, Barta PE, Pearlson GD. Structural evaluation of the prefrontal cortex in schizophrenia. Am J Psychiatry. 1998;155:1049–1055. doi: 10.1176/ajp.155.8.1049. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Nuechterlein KH, Haier RJ, Wu J, Sicotte N, Hazlett E, Asarnow R, Potkin S, Guich S. Glucose metabolic rate in normals and schizophrenics during the Continuous Performance Test assessed by positron emission tomography. Br J Psychiatry. 1990;156:216–227. doi: 10.1192/bjp.156.2.216. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Mahadik S, Pillai A, Terry A. Neurotrophins and schizophrenia. Schizophr Res. 2007a;84:1–11. doi: 10.1016/j.schres.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Buckley PF, Pillai A, Evans D, Stirewalt E, Mahadik S. Brain derived neurotrophic factor in first-episode psychosis. Schizophr Res. 2007b;91:1–5. doi: 10.1016/j.schres.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhardt C, Kelly JP, Lim YH, Filley CM, Parker WD., Jr Neuroleptic medications inhibit complex I of the electron transport chain. Ann Neurol. 1993;33:512–517. doi: 10.1002/ana.410330516. [DOI] [PubMed] [Google Scholar]
- Burnet PW, Hutchinson L, von Hesling M, Gilbert EJ, Brandon NJ, Rutter AR, Hutson PH, Harrison PJ. Expression of D-serine and glycine transporters in the prefrontal cortex and cerebellum in schizophrenia. Schizophr Res. 2008;102:283–294. doi: 10.1016/j.schres.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14:87–96. doi: 10.1016/0893-133X(94)00129-N. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Nelson DL, DeLapp NW, Falcone JF, Eckols K, Truex LL, Foreman MM, Lucaites VL, Calligaro DO. Antagonism by olanzapine of dopamine D1, serotonin2, muscarinic, histamine H1 and α1-adrenergic receptors in vitro. Schizophr Res. 1999;37:107–122. doi: 10.1016/s0920-9964(98)00146-7. [DOI] [PubMed] [Google Scholar]
- Cáceda R, Kinkead B, Nemeroff CB. Neurotensin: role psychiatric and neurological diseases. Peptides. 2006;27:2385–2404. doi: 10.1016/j.peptides.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Cahn W, Hulshoff Pol HE, Lems EB, van Haren NE, Schnack HG, van der Linden JA, Schothorst PF, van Engeland H, Kahn RS. Brain volume changes in first-episode schizophrenia: a 1-year follow-up study. Arch Gen Psychiatry. 2002;59:1002–1010. doi: 10.1001/archpsyc.59.11.1002. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Hazel TG, McKay RD. Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol. 1998;36:287–306. [PubMed] [Google Scholar]
- Cannon TD, van Erp TG, Huttunen M, Lönnqvist J, Salonen O, Valanne L, Poutanen VP, Standertskjöld-Nordenstam CG, Gur RE, Yan M. Regional gray matter, white matter, and cerebrospinal fluid distributions in schizophrenic patients, their siblings, and controls. Arch Gen Psychiatry. 1998;55:1084–1091. doi: 10.1001/archpsyc.55.12.1084. [DOI] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A. Interactions between glutamatergic and monoaminergic systems within the basal ganglia—implications for schizophrenia and Parkinson’s disease. Trends Neurosci. 1990;13:272–276. doi: 10.1016/0166-2236(90)90108-m. [DOI] [PubMed] [Google Scholar]
- Catapano LA, Manji HK. G protein-coupled receptors in major psychiatric disorders. Biochem Biophys Acta. 2007;1768:976–993. doi: 10.1016/j.bbamem.2006.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos I, Javoy-Agid F, Delacourte A, Defossez A, Lafon M, Hirsch E, Nicole A, Sinet PM, Agid Y. Neuronal localization of copper-zinc superoxide dismutase protein and mRNA within the human hippocampus from control and Alzheimer’s disease brains. Free Radic Res Commun. 1991;12–13:571–580. doi: 10.3109/10715769109145832. [DOI] [PubMed] [Google Scholar]
- Ceresoli-Borroni G, Rassoulpour A, Wu HQ, Guidetti P, Schwarcz R. Chronic neuroleptic treatment reduces endogenous kynurenic acid levels in rat brain. J Neural Transm. 2006;113:1355–1365. doi: 10.1007/s00702-005-0432-z. [DOI] [PubMed] [Google Scholar]
- Chahl LA. Tachykinins and neuropsychiatric disorders. Curr Drug Targets. 2006;7:993–1003. doi: 10.2174/138945006778019309. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Alvir J, Bilder R, Ashtari M. Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine. Lancet. 1995;345:456–457. doi: 10.1016/s0140-6736(95)90441-7. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry. 1994;151:1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Shirakawa O, Lieberman J, Lee H, Bilder R, Tamminga CA. Striatal enlargement in rats chronically treated with neuroleptic. Biol Psychiatry. 1998;44:675–684. doi: 10.1016/s0006-3223(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Bunney BS. Typical and atypical neuroleptics: differential effects of chronic administration on the activity of A9 and A10 midbrain dopaminergic neurons. J Neurosci. 1983;3:1607–1619. doi: 10.1523/JNEUROSCI.03-08-01607.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlan-Fourney J, Ashe P, Nylen K, Juorio AV, Li XM. Differential regulation of hippocampal BDNF mRNA by typical and atypical antipsychotic administration. Brain Res. 2002;954:11–20. doi: 10.1016/s0006-8993(02)03215-8. [DOI] [PubMed] [Google Scholar]
- Christensen J, Holcomb J, Garver DL. State-related changes in cerebral white matter may underlie psychosis exacerbation. Psychiatry Res. 2004;130:71–78. doi: 10.1016/j.pscychresns.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Chung YC, Li Z, Dai J, Meltzer HY, Ichikawa J. Clozapine increases both acetylcholine and dopamine release in rat ventral hippocampus: role of 5-HT1A receptor agonism. Brain Res. 2004;1023:54–63. doi: 10.1016/j.brainres.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Ciani E, Virgili M, Contestabile A. Akt pathway mediates a cGMP-dependent survival role of nitric oxide in cerebellar granule neurones. J Neurochem. 2002;81:218–228. doi: 10.1046/j.1471-4159.2002.00857.x. [DOI] [PubMed] [Google Scholar]
- Ciriza I, Azcoitia I, Garcia-Segura LM. Reduced progesterone metabolites protect rat hippocampal neurones from kainic acid excitotoxicity in vivo. J Neuroendocrinol. 2004;16:58–63. doi: 10.1111/j.1365-2826.2004.01121.x. [DOI] [PubMed] [Google Scholar]
- Clinton SM, Meador-Woodruff JH. Thalamic dysfunction in schizophrenia: neurochemical, neuropathological, and in vivo imaging abnormalities. Schizophr Res. 2004;69:237–253. doi: 10.1016/j.schres.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Compagnone NA, Mellon SH. Dehydroepiandrosterone: a potential signaling molecule for neocortical organization during development. Proc Natl Acad Sci U S A. 1998;95:4678–4683. doi: 10.1073/pnas.95.8.4678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convit A, Wolf OT, de Leon MJ, Patalinjug M, Kandil E, Caraos C, Scherer A, Saint Louis LA, Cancro R. Volumetric analysis of the prefrontal regions: findings in aging and schizophrenia. Psychiatry Res. 2001;107:61–73. doi: 10.1016/s0925-4927(01)00097-x. [DOI] [PubMed] [Google Scholar]
- Corbett R, Camacho F, Woods AT, Kerman LL, Fishkin RJ, Brooks K, Dunn RW. Antipsychotic agents antagonize non-competitive N-methyl-D-aspartate antagonist-induced behaviors. Psychopharmacology (Berl) 1995;120:67–74. doi: 10.1007/BF02246146. [DOI] [PubMed] [Google Scholar]
- Corson PW, Nopoulos P, Miller DD, Arndt S, Andreasen NC. Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics. Am J Psychiatry. 1999;156:1200–1204. doi: 10.1176/ajp.156.8.1200. [DOI] [PubMed] [Google Scholar]
- Coyle JT. The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry. 1996;3:241–253. doi: 10.3109/10673229609017192. [DOI] [PubMed] [Google Scholar]
- Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384. doi: 10.1007/s10571-006-9062-8. [DOI] [PubMed] [Google Scholar]
- Craig RW. The Bcl-2 gene family. Semin Cancer Biol. 1995;6:35–43. doi: 10.1006/scbi.1995.0005. [DOI] [PubMed] [Google Scholar]
- Crawley JN, Glowa JR, Majewska MD, Paul SM. Anxiolytic activity of an endogenous adrenal steroid. Brain Res. 1986;398:382–385. doi: 10.1016/0006-8993(86)91500-3. [DOI] [PubMed] [Google Scholar]
- Creese I, Burt DR, Snyder SH. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. J Neuropsychiatry Clin Neurosci. 1996;8:223–226. doi: 10.1176/jnp.8.2.223. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Bardgett ME. Limbic-cortical neuronal damage and the pathophysiology of schizophrenia. Schizophr Bull. 1998;24:231–248. doi: 10.1093/oxfordjournals.schbul.a033323. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Martin MV, Czeisler B, Meltzer MA, Ali Z, Dong H. Neuroprotective effects of olanzapine in a rat model of neurodevelopmental injury. Pharmacol Biochem Behav. 2006;83:208–213. doi: 10.1016/j.pbb.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Brickley S, Farrant M. NMDA receptor subunits: diversity, development and disease. Curr Opin Neurobiol. 2001;11:327–335. doi: 10.1016/s0959-4388(00)00215-4. [DOI] [PubMed] [Google Scholar]
- Dakhale G, Khanzode S, Khanzode S, Saoji A, Khobragade L, Turankar A. Oxidative damage and schizophrenia: the potential benefit by atypical antipsychotics. Neuropsychobiology. 2004;49:205–209. doi: 10.1159/000077368. [DOI] [PubMed] [Google Scholar]
- Daly DA, Moghaddam B. Actions of clozapine and haloperidol on the extracellular levels of excitatory amino acids in the prefrontal cortex and striatum of conscious rats. Neurosci Lett. 1993;152:61–64. doi: 10.1016/0304-3940(93)90483-2. [DOI] [PubMed] [Google Scholar]
- Davis KL, Buchsbaum MS, Shihabuddin L, Spiegel-Cohen J, Metzger M, Frecska E, Keefe RS, Powchik P. Ventricular enlargement in poor-outcome schizophrenia. Biol Psychiatry. 1998;43:783–793. doi: 10.1016/s0006-3223(97)00553-2. [DOI] [PubMed] [Google Scholar]
- Davis KL, Stewart DG, Friedman JI, Buchsbaum M, Harvey PD, Hof PR, Buxbaum J, Haroutunian V. White matter changes in schizophrenia: evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- Dawirs RR, Hildebrandt K, Teuchert-Noodt G. Adult treatment with haloperidol increases dentate granule cell proliferation in the gerbil hippocampus. J Neural Transm. 1998;105:317–327. doi: 10.1007/s007020050061. [DOI] [PubMed] [Google Scholar]
- Dawson NM, Hamid EH, Egan MF, Meredith GE. Changes in the pattern of brain-derived neurotrophic factor immunoreactivity in the rat brain after acute and subchronic haloperidol treatment. Synapse. 2001;39:70–81. doi: 10.1002/1098-2396(20010101)39:1<70::AID-SYN10>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Debonnel G, Bergeron R, de Montigny C. Potentiation by dehydroepiandrosterone of the neuronal response to N-methyl-D-aspartate in the CA3 region of the rat dorsal hippocampus: an effect mediated via sigma receptors. J Endocrinol. 1996;150(Suppl):S33–S42. [PubMed] [Google Scholar]
- Delacourte A, Defossez A, Ceballos I, Nicole A, Sinet PM. Preferential localization of copper zinc superoxide dismutase in the vulnerable cortical neurons in Alzheimer’s disease. Neurosci Lett. 1988;92:247–253. doi: 10.1016/0304-3940(88)90597-6. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Sakuma M, Tew W, Kushner M, Hoff AL, Grimson R. Schizophrenia as a chronic active brain process: a study of progressive brain structural change subsequent to the onset of schizophrenia. Psychiatry Res. 1997;74:129–140. doi: 10.1016/s0925-4927(97)00012-7. [DOI] [PubMed] [Google Scholar]
- Deng C, Huang XF. Increased density of GABAA receptors in the superior temporal gyrus in schizophrenia. Exp Brain Res. 2006;168:587–590. doi: 10.1007/s00221-005-0290-9. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Bubser M. The orexins/hypocretins and schizophrenia. Schizophr Bull. 2007;33:1277–1283. doi: 10.1093/schbul/sbm096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutch AY, Duman RS. The effects of antipsychotic drugs on Fos protein expression in the prefrontal cortex: cellular localization and pharmacological characterization. Neuroscience. 1996;70:377–389. doi: 10.1016/0306-4522(95)00357-6. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Lewis DA, Whitehead RE, Elsworth JD, Iadarola MJ, Redmond DE, Jr, Roth RH. Effects of D2 dopamine receptor antagonists on Fos protein expression in the striatal complex and entorhinal cortex of the nonhuman primate. Synapse. 1996;23:182–191. doi: 10.1002/(SICI)1098-2396(199607)23:3<182::AID-SYN7>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Mastropaolo J, Schwartz BL, Rosse RB, Morihisa JM. A “glutamatergic hypothesis” of schizophrenia: rationale for pharmacotherapy with glycine. Clin Neuropharmacol. 1989;12:1–13. [PubMed] [Google Scholar]
- Deutsch SI, Rosse RB, Schwartz BL, Mastropaolo J. A revised excitotoxic hypothesis of schizophrenia: therapeutic implications. Clin Neuropharmacol. 2001;24:43–49. doi: 10.1097/00002826-200101000-00008. [DOI] [PubMed] [Google Scholar]
- Devaud LL, Purdy RH, Morrow AL. The neurosteroid, 3α-hydroxy-5α-pregnan-20-one, protects against bicuculline-induced seizures during ethanol withdrawal in rats. Alcohol Clin Exp Res. 1995;19:350–355. doi: 10.1111/j.1530-0277.1995.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Dietrich-Muszalska A, Olas B, Rabe-Jablonska J. Oxidative stress in blood platelets from schizophrenic patients. Platelets. 2005;16:386–391. doi: 10.1080/09537100500128872. [DOI] [PubMed] [Google Scholar]
- di Michele F, Caltagirone C, Bonaviri G, Romeo E, Spalletta G. Plasma dehydroepiandrosterone levels are strongly increased in schizophrenia. J Psychiatr Res. 2005;39:267–273. doi: 10.1016/j.jpsychires.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Djebaili M, Hoffman SW, Stein DG. Allopregnanolone and progesterone decrease cell death and cognitive deficits after a contusion of the rat prefrontal cortex. Neuroscience. 2004;123:349–359. doi: 10.1016/j.neuroscience.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Do KQ, Trabesinger AH, Kirsten-Krüger M, Lauer CJ, Dydak U, Hell D, Holsboer F, Boesiger P, Cuénod M. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. Eur J Neurosci. 2000;12:3721–3728. 2000. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- Dono R. Fibroblast growth factors as regulators of central nervous system development and function. Am J Physiol Regul Integr Comp Physiol. 2003;284:R867–R881. doi: 10.1152/ajpregu.00533.2002. [DOI] [PubMed] [Google Scholar]
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- Duman RS. Depression: a case of neuronal life and death? Biol Psychiatry. 2004;56:140–145. doi: 10.1016/j.biopsych.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Leipzig JN, Mailman RB, Lieberman JA. Differential effects of clozapine and haloperidol on ketamine-induced brain metabolic activation. Brain Res. 1998a;812:65–75. doi: 10.1016/s0006-8993(98)00926-3. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Miyamoto S, Leipzig JN, Lieberman JA. Comparison of brain metabolic activity patterns induced by ketamine, MK-801 and amphetamine in rats: support for NMDA receptor involvement in responses to subanesthetic dose of ketamine. Brain Res. 1999;843:171–183. doi: 10.1016/s0006-8993(99)01776-x. [DOI] [PubMed] [Google Scholar]
- Duncan GE, Miyamoto S, Leipzig JN, Lieberman JA. Comparison of the effects of clozapine, risperidone, and olanzapine on ketamine-induced alterations in regional brain metabolism. J Pharmacol Exp Ther. 2000;293:8–14. [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Knapp DJ, Mueller RA, Breese GR. Metabolic mapping of the rat brain after subanesthetic doses of ketamine: potential relevance to schizophrenia. Brain Res. 1998b;787:181–190. doi: 10.1016/s0006-8993(97)01390-5. [DOI] [PubMed] [Google Scholar]
- Durany N, Michel T, Zöchling R, Boissl KW, Cruz-Sánchez FF, Riederer P, Thome J. Brain-derived neurotrophic factor and neurotrophin 3 in schizophrenic psychoses. Schizophr Res. 2001;52:79–86. doi: 10.1016/s0920-9964(00)00084-0. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Rizavi HS, Pandey GN. Differential effects of haloperidol and clozapine on [3H]cAMP binding, protein kinase A (PKA) activity, and mRNA and protein expression of selective regulatory and catalytic subunit isoforms of PKA in rat brain. J Pharmacol Exp Ther. 2002;301:197–209. doi: 10.1124/jpet.301.1.197. [DOI] [PubMed] [Google Scholar]
- Dwyer DS, Liu Y, Bradley RJ. An ethanol-sensitive variant of the PC12 neuronal cell line: sensitivity to alcohol is associated with increased cell adhesion and decreased glucose accumulation. J Cell Physiol. 1999a;178:93–101. doi: 10.1002/(SICI)1097-4652(199901)178:1<93::AID-JCP12>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Dwyer DS, Ardizzone TD, Bradley RJ. Psychoactive drugs affect glucose transport and the regulation of glucose metabolism. Int Rev Neurobiol. 2002;51:503–530. doi: 10.1016/s0074-7742(02)51015-1. [DOI] [PubMed] [Google Scholar]
- Dwyer DS, Dickson A. Neuroprotection and enhancement of neurite outgrowth with small molecular weight compounds from screens of chemical libraries. Int Rev Neurobiol. 2007;77:247–289. doi: 10.1016/S0074-7742(06)77008-8. [DOI] [PubMed] [Google Scholar]
- Dwyer DS, Lu XH, Bradley RJ. Cytotoxicity of conventional and atypical antipsychotic drugs in relation to glucose metabolism. Brain Res. 2003a;971:31–39. doi: 10.1016/s0006-8993(03)02351-5. [DOI] [PubMed] [Google Scholar]
- Dwyer DS, Lu XH, Freeman AM. Neuronal glucose metabolism and schizophrenia: therapeutic prospects? Expert Rev Neurother. 2003b;3:29–40. doi: 10.1002/pmic.200390005. [DOI] [PubMed] [Google Scholar]
- Dwyer DS, Pinkofsky HB, Liu Y, Bradley RJ. Antipsychotic drugs affect glucose uptake and the expression of glucose transporters in PC12 cells. Prog Neuropsychopharmacol Biol Psychiatry. 1999b;23:69–80. doi: 10.1016/s0278-5846(98)00092-x. [DOI] [PubMed] [Google Scholar]
- Eaton K, Sallee FR, Sah R. Relevance of neuropeptide Y (NPY) in psychiatry. Curr Top Med Chem. 2007;7:1645–1659. doi: 10.2174/156802607782341037. [DOI] [PubMed] [Google Scholar]
- Einat H, Yuan P, Gould TD, Li J, Du J, Zhang L, Manji HK, Chen G. The role of the extracellular signal-regulated kinase signaling pathway in mood modulation. J Neurosci. 2003;23:7311–7316. doi: 10.1523/JNEUROSCI.23-19-07311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisener A, Pato MT, Medeiros H, Carvalho C, Pato CN. Genetics of schizophrenia: recent advances. Psychopharmacol Bull. 2007;40:168–177. [PubMed] [Google Scholar]
- Ellenbroek BA. Treatment of schizophrenia: a clinical and preclinical evaluation of neuroleptic drugs. Pharmacol Ther. 1993;57:1–78. doi: 10.1016/0163-7258(93)90036-d. [DOI] [PubMed] [Google Scholar]
- Ellenbroek BA, Peeters BW, Honig WM, Cools AR. The paw test: a behavioural paradigm for differentiating between classical and atypical neuroleptic drugs. Psychopharmacology (Berl) 1987;93:343–348. doi: 10.1007/BF00187254. [DOI] [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- Emamian ES, Karayiorgou M, Gogos JA. Decreased phosphorylation of NMDA receptor type 1 at serine 897 in brains of patients with schizophrenia. J Neurosci. 2004;24:1561–1564. doi: 10.1523/JNEUROSCI.4650-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S, Schwieler L, Nilsson L, Linderholm K, Engberg G. The kynurenic acid hypothesis of schizophrenia. Physiol Behav. 2007;92:203–209. doi: 10.1016/j.physbeh.2007.05.025. [DOI] [PubMed] [Google Scholar]
- Evins AE, Amico ET, Shih V, Goff DC. Clozapine treatment increases serum glutamate and aspartate compared to conventional neuroleptics. J Neural Transm. 1997;104:761–766. doi: 10.1007/BF01291892. [DOI] [PubMed] [Google Scholar]
- Farber NB, Foster J, Duhan NL, Olney JW. Olanzapine and fluperlapine mimic clozapine in preventing MK-801 neurotoxicity. Schizophr Res. 1996;21:33–37. doi: 10.1016/0920-9964(96)00024-2. [DOI] [PubMed] [Google Scholar]
- Farber NB, Hanslick J, Kirby C, McWilliams L, Olney JW. Serotonergic agents that activate 5HT2A receptors prevent NMDA antagonist neurotoxicity. Neuropsychopharmacology. 1998;18:57–62. doi: 10.1016/S0893-133X(97)00127-9. [DOI] [PubMed] [Google Scholar]
- Farber NB, Price MT, Labruyere J, Nemnich J, St Peter H, Wozniak DF, Olney JW. Antipsychotic drugs block phencyclidine receptor-mediated neurotoxicity. Biol Psychiatry. 1993;34:119–121. doi: 10.1016/0006-3223(93)90265-f. [DOI] [PubMed] [Google Scholar]
- Farber NB, Wozniak DF, Price MT, Labruyere J, Huss J, St Peter H, Olney JW. Age-specific neurotoxicity in the rat associated with NMDA receptor blockade: potential relevance to schizophrenia? Biol Psychiatry. 1995;38:788–796. doi: 10.1016/0006-3223(95)00046-1. [DOI] [PubMed] [Google Scholar]
- Farnbach-Pralong D, Bradbury R, Copolov D, Dean B. Clozapine and olanzapine treatment decreases rat cortical and limbic GABAA receptors. Eur J Pharmacol. 1998;349:R7–R8. doi: 10.1016/s0014-2999(98)00285-4. [DOI] [PubMed] [Google Scholar]
- Faustman WO, Bardgett M, Faull KF, Pfefferbaum A, Csernansky JG. Cerebrospinal fluid glutamate inversely correlates with positive symptom severity in unmedicated male schizophrenic/schizoaffective patients. Biol Psychiatry. 1999;45:68–75. doi: 10.1016/s0006-3223(98)00207-8. [DOI] [PubMed] [Google Scholar]
- Federspiel A, Begré S, Kiefer C, Schroth G, Strik WK, Dierks T. Alterations of white matter connectivity in first episode schizophrenia. Neurobiol Dis. 2006;22:702–709. doi: 10.1016/j.nbd.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Flood JF, Morley JE, Roberts E. Memory-enhancing effects in male mice of pregnenolone and steroids metabolically derived from it. Proc Natl Acad Sci U S A. 1992;89:1567–1571. doi: 10.1073/pnas.89.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood JF, Morley JE, Roberts E. Pregnenolone sulfate enhances post-training memory processes when injected in very low doses into limbic system structures: the amygdala is by far the most sensitive. Proc Natl Acad Sci U S A. 1995;92:10806–10810. doi: 10.1073/pnas.92.23.10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Krystal JH, Mathalon DH. Neural synchrony in schizophrenia: from networks to new treatments. Schizophr Bull. 2007;33:848–852. doi: 10.1093/schbul/sbm062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey JM, Ticku MK, Huffman RD. GABAergic supersensitivity within the pars reticulata of the rat substantia nigra following chronic haloperidol administration. Brain Res. 1987;425:73–84. doi: 10.1016/0006-8993(87)90485-9. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Superoxide dismutases. Adv Enzymol Relat Areas Mol Biol. 1986;58:61–97. doi: 10.1002/9780470123041.ch2. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Adler DN, Davis KL. The role of norepinephrine in the pathophysiology of cognitive disorders: potential applications to the treatment of cognitive dysfunction in schizophrenia and Alzheimer’s disease. Biol Psychiatry. 1999;46:1243–1252. doi: 10.1016/s0006-3223(99)00232-2. [DOI] [PubMed] [Google Scholar]
- Fujimura M, Hashimoto K, Yamagami K. Effects of antipsychotic drugs on neurotoxicity, expression of fos-like protein and c-fos mRNA in the retrosplenial cortex after administration of dizocilpine. Eur J Pharmacol. 2000;398:1–10. doi: 10.1016/s0014-2999(00)00235-1. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Bedogni F, Maragnoli ME, Gennarelli M, Perez J, Racagni G, Riva MA. Dopaminergic D2 receptor activation modulates FGF-2 gene expression in rat prefrontal cortex and hippocampus. J Neurosci Res. 2003a;74:74–80. doi: 10.1002/jnr.10733. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Frasca A, Racagni G, Riva MA. Dynamic regulation of glutamatergic post-synaptic activity in rat prefrontal cortex by repeated administration antipsychotic drugs. Mol Pharmacol. 2008;73:1484–1490. doi: 10.1124/mol.107.043786. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Molteni R, Bedogni F, Gennarelli M, Perez J, Racagni G, Riva MA. Quetiapine regulates FGF-2 and BDNF expression in the hippocampus of animals treated with MK-801. Neuroreport. 2004;15:2109–2112. doi: 10.1097/00001756-200409150-00022. [DOI] [PubMed] [Google Scholar]
- Fumagalli F, Molteni R, Roceri M, Bedogni F, Santero R, Fossati C, Gennarelli M, Racagni G, Riva MA. Effect of antipsychotic drugs on brain-derived neurotrophic factor expression under reduced N-methyl-D-aspartate receptor activity. J Neurosci Res. 2003b;72:622–628. doi: 10.1002/jnr.10609. [DOI] [PubMed] [Google Scholar]
- Fuxe K, Ferré S, Genedani S, Franco R, Agnati LF. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol Behav. 2007;92:210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- Gale K. Chronic blockade of dopamine receptors by antischizophrenic drugs enhances GABA binding in substantia nigra. Nature. 1980;283:569–570. doi: 10.1038/283569a0. [DOI] [PubMed] [Google Scholar]
- Gao XM, Sakai K, Tamminga CA. Chronic olanzapine or sertindole treatment results in reduced oral chewing movements in rats compared to haloperidol. Neuropsychopharmacology. 1998;19:428–433. doi: 10.1016/S0893-133X(98)00039-6. [DOI] [PubMed] [Google Scholar]
- Garey LJ, Ong WY, Patel TS, Kanani M, Davis A, Mortimer AM, Barnes TR, Hirsch SR. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J Neurol Neurosurg Psychiatry. 1998;65:446–453. doi: 10.1136/jnnp.65.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattaz WF, Gasser T, Beckmann H. Multidimensional analysis of the concentrations of 17 substances in the CSF of schizophrenics and controls. Biol Psychiatry. 1985;20:360–366. doi: 10.1016/0006-3223(85)90038-1. [DOI] [PubMed] [Google Scholar]
- George MS, Guidotti A, Rubinow D, Pan B, Mikalauskas K, Post RM. CSF neuroactive steroids in affective disorders: pregnenolone, progesterone, and DBI. Biol Psychiatry. 1994;35:775–780. doi: 10.1016/0006-3223(94)91139-8. [DOI] [PubMed] [Google Scholar]
- Gerber U, Gee CE, Benquet P. Metabotropic glutamate receptors: intracellular signaling pathways. Curr Opin Pharmacol. 2007;7:56–61. doi: 10.1016/j.coph.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Ellenbroek B. Animal behavior models of the mechanisms underlying antipsychotic atypicality. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1071–1079. doi: 10.1016/j.pnpbp.2003.09.003. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology (Berl) 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Ghoumari AM, Ibanez C, El-Etr M, Leclerc P, Eychenne B, O’Malley BW, Baulieu EE, Schumacher M. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J Neurochem. 2003;86:848–859. doi: 10.1046/j.1471-4159.2003.01881.x. [DOI] [PubMed] [Google Scholar]
- Gibson CL, Constantin D, Prior MJ, Bath PM, Murphy SP. Progesterone suppresses the inflammatory response and nitric oxide synthase-2 expression following cerebral ischemia. Exp Neurol. 2005;193:522–530. doi: 10.1016/j.expneurol.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol. 2004;61:641–644. doi: 10.1001/archneur.61.5.641. [DOI] [PubMed] [Google Scholar]
- Girgis RR, Javitch JA, Lieberman JA. Antipsychotic drug mechanisms: links between therapeutic effects, metabolic side effects and the insulin signaling pathway. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.40. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glantz LA, Gilmore JH, Lieberman JA, Jarskog LF. Apoptotic mechanisms and the synaptic pathology of schizophrenia. Schizophr Res. 2006;81:47–63. doi: 10.1016/j.schres.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. doi: 10.1001/archpsyc.57.1.65. [DOI] [PubMed] [Google Scholar]
- Gleason SD, Shannon HE. Blockade of phencyclidine-induced hyperlocomotion by olanzapine, clozapine and serotonin receptor subtype selective antagonists in mice. Psychopharmacology (Berl) 1997;129:79–84. doi: 10.1007/s002130050165. [DOI] [PubMed] [Google Scholar]
- Goff DC, Hennen J, Lyoo IK, Tsai G, Wald LL, Evins AE, Yurgelun-Todd DA, Renshaw PF. Modulation of brain and serum glutamatergic concentrations following a switch from conventional neuroleptics to olanzapine. Biol Psychiatry. 2002;51:493–497. doi: 10.1016/s0006-3223(01)01321-x. [DOI] [PubMed] [Google Scholar]
- Goff DC, Tsai G, Beal MF, Coyle JT. Tardive dyskinesia and substrates of energy metabolism in CSF. Am J Psychiatry. 1995;152:1730–1736. doi: 10.1176/ajp.152.12.1730. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl) 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Litwin LC, Sutton EB, Malick JB. Seroquel: electrophysiological profile of a potential atypical antipsychotic. Psychopharmacology (Berl) 1993;112:293–298. doi: 10.1007/BF02244924. [DOI] [PubMed] [Google Scholar]
- Goossens V, Grooten J, Fiers W. The oxidative metabolism of glutamine. A modulator of reactive oxygen intermediate-mediated cytotoxicity of tumor necrosis factor in L929 fibrosarcoma cells. J Biol Chem. 1996;271:192–196. doi: 10.1074/jbc.271.1.192. [DOI] [PubMed] [Google Scholar]
- Goudie A, Taylor A. Comparative characterisation of the discriminative stimulus properties of clozapine and other antipsychotics in rats. Psychopharmacology. 1998;135:392–400. doi: 10.1007/s002130050527. [DOI] [PubMed] [Google Scholar]
- Goudie AJ, Smith JA. Discriminative stimulus properties of antipsychotics. Pharmacol Biochem Behav. 1999;64:193–201. doi: 10.1016/s0091-3057(99)00079-9. [DOI] [PubMed] [Google Scholar]
- Goudie AJ, Smith JA, Taylor A, Taylor MA, Tricklebank MD. Discriminative stimulus properties of the atypical neuroleptic clozapine in rats: tests with subtype selective receptor ligands. Behav Pharmacol. 1998;9:699–710. doi: 10.1097/00008877-199812000-00006. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2:260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould TD, Manji HK. Glycogen synthase kinase-3: a putative molecular target for lithium mimetic drugs. Neuropsychopharmacology. 2005;30:1223–1237. doi: 10.1038/sj.npp.1300731. [DOI] [PubMed] [Google Scholar]
- Green W, Patil P, Marsden CA, Bennett GW, Wigmore PM. Treatment with olanzapine increases cell proliferation in the subventricular zone and prefrontal cortex. Brain Res. 2006;1070:242–245. doi: 10.1016/j.brainres.2005.11.047. [DOI] [PubMed] [Google Scholar]
- Greene JG, Greenamyre JT. Characterization of the excitotoxic potential of the reversible succinate dehydrogenase inhibitor malonate. J Neurochem. 1995;64:430–436. doi: 10.1046/j.1471-4159.1995.64010430.x. [DOI] [PubMed] [Google Scholar]
- Greene JG, Greenamyre JT. Bioenergetics and glutamate excitotoxicity. Prog Neurobiol. 1996;48:613–634. doi: 10.1016/0301-0082(96)00006-8. [DOI] [PubMed] [Google Scholar]
- Greene R. Circuit analysis of NMDAR hypofunction in the hippocampus, in vitro, and psychosis of schizophrenia. Hippocampus. 2001;11:569–577. doi: 10.1002/hipo.1072. [DOI] [PubMed] [Google Scholar]
- Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10:704–711. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- Griffiths MR, Cooper AJ, Barber DJ, Mitchell IJ. Pharmacological mechanisms mediating phencyclidine-induced apoptosis of striatopallidal neurons: the roles of glutamate, dopamine, acetylcholine and corticosteroids. Brain Res. 2000;855:1–10. doi: 10.1016/s0006-8993(99)01917-4. [DOI] [PubMed] [Google Scholar]
- Grillo RW, Ottoni GL, Leke R, Souza DO, Portela LV, Lara DR. Reduced serum BDNF levels in schizophrenic patients on clozapine or typical antipsychotics. J Psychiatr Res. 2007;41:31–35. doi: 10.1016/j.jpsychires.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Grimwood S, Slater P, Deakin JF, Hutson PH. NR2-B-containing NMDA receptors are up-regulated in temporal cortex in schizophrenia. Neuroreport. 1999;10:461–465. doi: 10.1097/00001756-199902250-00004. [DOI] [PubMed] [Google Scholar]
- Gross A, Jockel J, Wei MC, Korsmeyer SJ. Enforced dimerization of BAX results in its translocation, mitochondrial dysfunction and apoptosis. EMBO J. 1998;17:3878–3885. doi: 10.1093/emboj/17.14.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti A, Auta J, Davis JM, Dong E, Grayson DR, Veldic M, Zhang X, Costa E. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology (Berl) 2005;180:191–205. doi: 10.1007/s00213-005-2212-8. [DOI] [PubMed] [Google Scholar]
- Guillin O, Abi-Dargham A, Laruelle M. Neurobiology of dopamine in schizophrenia. Int Rev Neurobiol. 2007;78:1–39. doi: 10.1016/S0074-7742(06)78001-1. [DOI] [PubMed] [Google Scholar]
- Guo H, Tang Z, Yu Y, Xu L, Jin G, Zhou J. Apomorphine induces trophic factors that support fetal rat mesencephalic dopaminergic neurons in cultures. Eur J Neurosci. 2002;16:1861–1870. doi: 10.1046/j.1460-9568.2002.02256.x. [DOI] [PubMed] [Google Scholar]
- Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry. 1998;155:1711–1717. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- Gur RE, Resnick SM, Alavi A, Gur RC, Caroff S, Dann R, Silver FL, Saykin AJ, Chawluk JB, Kushner M. Regional brain function in schizophrenia. I. A positron emission tomography study. Arch Gen Psychiatry. 1987;44:119–125. doi: 10.1001/archpsyc.1987.01800140021003. [DOI] [PubMed] [Google Scholar]
- Hajduch E, Litherland GJ, Hundal HS. Protein kinase B (PKB/Akt)—a key regulator of glucose transport? FEBS Lett. 2001;492:199–203. doi: 10.1016/s0014-5793(01)02242-6. [DOI] [PubMed] [Google Scholar]
- Hajós M. Targeting information-processing deficit in schizophrenia: a novel approach to psychotherapeutic drug discovery. Trends Pharmacol Sci. 2006;27:391–398. doi: 10.1016/j.tips.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Hakak Y, Walker JR, Li C, Wong WH, Davis KL, Buxbaum JD, Haroutunian V, Fienberg AA. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci U S A. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halim ND, Weickert CS, McClintock BW, Weinberger DR, Lipska BK. Effects of chronic haloperidol and clozapine treatment on neurogenesis in the adult rat hippocampus. Neuropsychopharmacology. 2004;29:1063–1069. doi: 10.1038/sj.npp.1300422. [DOI] [PubMed] [Google Scholar]
- Hanada S, Mita T, Nishino N, Tanaka C. [3H]Muscimol binding sites increased in autopsied brains of chronic schizophrenics. Life Sci. 1987;40:259–266. doi: 10.1016/0024-3205(87)90341-9. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122:593–624. doi: 10.1093/brain/122.4.593. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Engberg G, Shimizu E, Nordin C, Lindström LH, Iyo M. Reduced D-serine to total serine ratio in the cerebrospinal fluid of drug naïve schizophrenic patients. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:767–769. doi: 10.1016/j.pnpbp.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Fukushima T, Shimizu E, Komatsu N, Watanabe H, Shinoda N, Nakazato M, Kumakiri C, Okada S, Hasegawa H, et al. Decreased serum levels of D-serine in patients with schizophrenia: evidence in support of the N-methyl-D-aspartate receptor hypofunction hypothesis of schizophrenia. Arch Gen Psychiatry. 2003;60:572–576. doi: 10.1001/archpsyc.60.6.572. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Xu H, Yang Y, Zhang X, Li XM. Neuroprotective effects of olanzapine on methamphetamine-induced neurotoxicity are associated with an inhibition of hyperthermia and prevention of Bcl-2 decrease in rats. Brain Res. 2004;1018:186–192. doi: 10.1016/j.brainres.2004.05.060. [DOI] [PubMed] [Google Scholar]
- He J, Xu H, Yang Y, Zhang X, Li XM. Chronic administration of quetiapine alleviates the anxiety-like behavioral changes induced by a neurotoxic regimen of dl-amphetamine in rats. Behav Brain Res. 2005a;160:178–187. doi: 10.1016/j.bbr.2004.11.028. [DOI] [PubMed] [Google Scholar]
- He J, Yamada K, Zou LB, Nabeshima T. Spatial memory deficit and neurodegeneration induced by the direct injection of okadaic acid into the hippocampus in rats. J Neural Transm. 2001;108:1435–1443. doi: 10.1007/s007020100018. [DOI] [PubMed] [Google Scholar]
- He J, Yang Y, Xu H, Zhang X, Li XM. Olanzapine attenuates the okadaic acid-induced spatial memory impairment and hippocampal cell death in rats. Neuropsychopharmacology. 2005b;30:1511–1520. doi: 10.1038/sj.npp.1300757. [DOI] [PubMed] [Google Scholar]
- Healy DJ, Haroutunian V, Powchik P, Davidson M, Davis KL, Watson SJ, Meador-Woodruff JH. AMPA receptor binding and subunit mRNA expression in prefrontal cortex and striatum of elderly schizophrenics. Neuropsychopharmacology. 1998;19:278–286. doi: 10.1016/S0893-133X(98)00014-1. [DOI] [PubMed] [Google Scholar]
- Heckers S, Heinsen H, Heinsen Y, Beckmann H. Cortex, white matter, and basal ganglia in schizophrenia: a volumetric postmortem study. Biol Psychiatry. 1991;29:556–566. doi: 10.1016/0006-3223(91)90091-y. [DOI] [PubMed] [Google Scholar]
- Hertel P, Fagerquist MV, Svensson TH. Enhanced cortical dopamine output and antipsychotic-like effects of raclopride by alpha2 adrenoceptor blockade. Science. 1999;286:105–107. doi: 10.1126/science.286.5437.105. [DOI] [PubMed] [Google Scholar]
- Hertel P, Nomikos GG, Iurlo M, Svensson TH. Risperidone: regional effects in vivo on release and metabolism of dopamine and serotonin in the rat brain. Psychopharmacology (Berl) 1996;124:74–86. doi: 10.1007/BF02245607. [DOI] [PubMed] [Google Scholar]
- Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60:585–594. doi: 10.1001/archpsyc.60.6.585. [DOI] [PubMed] [Google Scholar]
- Hokama H, Shenton ME, Nestor PG, Kikinis R, Levitt JJ, Metcalf D, Wible CG, O’Donnell BF, Jolesz FA, McCarley RW. Caudate, putamen, and globus pallidus volume in schizophrenia: a quantitative MRI study. Psychiatry Res. 1995;61:209–229. doi: 10.1016/0925-4927(95)02729-h. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Hubl D, Koenig T, Strik W, Federspiel A, Kreis R, Boesch C, Maier SE, Schroth G, Lovblad K, Dierks T. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61:658–668. doi: 10.1001/archpsyc.61.7.658. [DOI] [PubMed] [Google Scholar]
- Huerta I, McCullumsmith RE, Haroutunian V, Giménez-Amaya JM, Meador-Woodruff JH. Expression of excitatory amino acid transporter interacting protein transcripts in the thalamus in schizophrenia. Synapse. 2006;59:394–402. doi: 10.1002/syn.20250. [DOI] [PubMed] [Google Scholar]
- Huffman RD, Ticku MK. The effects of chronic haloperidol administration on GABA receptor binding. Pharmacol Biochem Behav. 1983;19:199–204. doi: 10.1016/0091-3057(83)90039-4. [DOI] [PubMed] [Google Scholar]
- Humphrey WM, Dong H, Csernansky CA, Csernansky JG. Immediate and delayed hippocampal neuronal loss induced by kainic acid during early postnatal development in the rat. Brain Res Dev Brain Res. 2002;137:1–12. doi: 10.1016/s0165-3806(02)00344-9. [DOI] [PubMed] [Google Scholar]
- Hutchinson MA, Smith PF, Darlington CL. The behavioural and neuronal effects of the chronic administration of benzodiazepine anxiolytic and hypnotic drugs. Prog Neurobiol. 1996;49:73–97. doi: 10.1016/0301-0082(96)00011-1. [DOI] [PubMed] [Google Scholar]
- Ibanez C, Shields SA, El-Etr M, Leonelli E, Magnaghi V, Li WW, Sim FJ, Baulieu EE, Melcangi RC, Schumacher M, et al. Steroids and the reversal of age-associated changes in myelination and remyelination. Prog Neurobiol. 2003;71:49–56. doi: 10.1016/j.pneurobio.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Dai J, O’Laughlin IA, Fowler WL, Meltzer HY. Atypical, but not typical, antipsychotic drugs increase cortical acetylcholine release without an effect in the nucleus accumbens or striatum. Neuropsychopharmacology. 2002;26:325–339. doi: 10.1016/S0893-133X(01)00312-8. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Ishii H, Bonaccorso S, Fowler WL, O’Laughlin IA, Meltzer HY. 5-HT2A and D2 receptor blockade increases cortical DA release via 5-HT1A receptor activation: a possible mechanism of atypical antipsychotic-induced cortical dopamine release. J Neurochem. 2001;76:1521–1531. doi: 10.1046/j.1471-4159.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Kuroki T, Dai J, Meltzer HY. Effect of antipsychotic drugs on extracellular serotonin levels in rat medial prefrontal cortex and nucleus accumbens. Eur J Pharmacol. 1998;351:163–171. doi: 10.1016/s0014-2999(98)00308-2. [DOI] [PubMed] [Google Scholar]
- Imamura M, Prasad C. Modulation of GABA-gated chloride ion influx in the brain by dehydroepiandrosterone and its metabolites. Biochem Biophys Res Commun. 1998;243:771–775. doi: 10.1006/bbrc.1998.8177. [DOI] [PubMed] [Google Scholar]
- Ingvar DH, Franzén G. Abnormalities of cerebral blood flow distribution in patients with chronic schizophrenia. Acta Psychiatr Scand. 1974;50:425–462. doi: 10.1111/j.1600-0447.1974.tb09707.x. [DOI] [PubMed] [Google Scholar]
- Invernizzi R, Morali F, Pozzi L, Samanin R. Effects of acute and chronic clozapine on dopamine release and metabolism in the striatum and nucleus accumbens of conscious rats. Br J Pharmacol. 1990;100:774–778. doi: 10.1111/j.1476-5381.1990.tb14091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin RP, Lin SZ, Rogawski MA, Purdy RH, Paul SM. Steroid potentiation and inhibition of N-methyl-D-aspartate receptor-mediated intracellular Ca++ responses: structure-activity studies. J Pharmacol Exp Ther. 1994;271:677–682. [PubMed] [Google Scholar]
- Ishimaru M, Kurumaji A, Toru M. NMDA-associated glycine binding site increases in schizophrenic brains. Biol Psychiatry. 1992;32:379–381. doi: 10.1016/0006-3223(92)90043-y. [DOI] [PubMed] [Google Scholar]
- Iwamoto K, Bundo M, Kato T. Altered expression of mitochondria-related genes in postmortem brains of patients with bipolar disorder or schizophrenia, as revealed by large-scale DNA microarray analysis. Hum Mol Genet. 2005;14:241–253. doi: 10.1093/hmg/ddi022. [DOI] [PubMed] [Google Scholar]
- Jarskog LF, Gilmore JH, Selinger ES, Lieberman JA. Cortical bcl-2 protein expression and apoptotic regulation in schizophrenia. Biol Psychiatry. 2000;48:641–650. doi: 10.1016/s0006-3223(00)00988-4. [DOI] [PubMed] [Google Scholar]
- Jarskog LF, Glantz LA, Gilmore JH, Lieberman JA. Apoptic mechanisms in the pathophysiology of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:846–858. doi: 10.1016/j.pnpbp.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Jarskog LF, Selinger ES, Lieberman JA, Gilmore JH. Apoptotic proteins in the temporal cortex in schizophrenia: high Bax/Bcl-2 ratio without caspase-3 activation. Am J Psychiatry. 2004;161:109–115. doi: 10.1176/appi.ajp.161.1.109. [DOI] [PubMed] [Google Scholar]
- Javitt DC, Zukin SR. Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308. doi: 10.1176/ajp.148.10.1301. [DOI] [PubMed] [Google Scholar]
- Jentsch JD, Taylor JR, Roth RH. Subchronic phencyclidine administration increases mesolimbic dopaminergic system responsivity and augments stress-and psychostimulant-induced hyperlocomotion. Neuropsychopharmacology. 1998;19:105–113. doi: 10.1016/S0893-133X(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Jiang N, Chopp M, Stein D, Feit H. Progesterone is neuroprotective after transient middle cerebral artery occlusion in male rats. Brain Res. 1996;735:101–107. doi: 10.1016/0006-8993(96)00605-1. [DOI] [PubMed] [Google Scholar]
- Jockers-Scherübl MC, Rentzsch J, Danker-Hopfe H, Radzei N, Schürer F, Bahri S, Hellweg R. Adequate antipsychotic treatment normalizes serum nerve growth factor concentrations in schizophrenia with and without cannabis or additional substance abuse. Neurosci Lett. 2006;400:262–266. doi: 10.1016/j.neulet.2006.02.056. [DOI] [PubMed] [Google Scholar]
- Johnson EM, Jr, Greenlund LJ, Akins PT, Hsu CY. Neuronal apoptosis: current understanding of molecular mechanisms and potential role in ischemic brain injury. J Neurotrauma. 1995;12:843–852. doi: 10.1089/neu.1995.12.843. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325:529–531. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Johnson KM, Phillips M, Wang C, Kevetter GA. Chronic phencyclidine induces behavioral sensitization and apoptotic cell death in the olfactory and piriform cortex. J Neurosci Res. 1998;52:709–722. doi: 10.1002/(SICI)1097-4547(19980615)52:6<709::AID-JNR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Jones PB, Barnes TR, Davies L, Dunn G, Lloyd H, Hayhurst KP, Murray RM, Markwick A, Lewis SW. Randomized controlled trial of the effect on quality of life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1) Arch Gen Psychiatry. 2006;63:1079–1087. doi: 10.1001/archpsyc.63.10.1079. [DOI] [PubMed] [Google Scholar]
- Jordan S, Koprivica V, Dunn R, Tottori K, Kikuchi T, Altar CA. In vivo effects of aripiprazole on cortical and striatal dopaminergic and serotonergic function. Eur J Pharmacol. 2004;483:45–53. doi: 10.1016/j.ejphar.2003.10.025. [DOI] [PubMed] [Google Scholar]
- Kalkman HO. The role of the phosphatidylinositide 3-kinase-protein kinase B pathway in schizophrenia. Pharmacol Ther. 2006;110:117–134. doi: 10.1016/j.pharmthera.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Kalus P, Müller TJ, Zuschratter W, Senitz D. The dendritic architecture of prefrontal pyramidal neurons in schizophrenic patients. Neuroreport. 2000;11:3621–3625. doi: 10.1097/00001756-200011090-00044. [DOI] [PubMed] [Google Scholar]
- Kan O, Baldwin SA, Whetton AD. Apoptosis is regulated by the rate of glucose transport in an interleukin 3 dependent cell line. J Exp Med. 1994;180:917–923. doi: 10.1084/jem.180.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GB, Leite-Morris KA, Keith DJ. Differential effects of treatment with typical and atypical antipsychotic drugs on adenylyl cyclase and G proteins. Neurosci Lett. 1999;273:147–150. doi: 10.1016/s0304-3940(99)00610-2. [DOI] [PubMed] [Google Scholar]
- Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1081–1090. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Kapur S, Seeman P. Does fast dissociation from the dopamine D2 receptor explain the action of atypical antipsychotics? A new hypothesis. Am J Psychiatry. 2001;158:360–369. doi: 10.1176/appi.ajp.158.3.360. [DOI] [PubMed] [Google Scholar]
- Kargieman L, Santana N, Mengod G, Celada P, Artigas F. Antipsychotic drugs reverse the disruption in prefrontal cortex function produced by NMDA receptor blockade with phencyclidine. Proc Natl Acad Sci U S A. 2007;104:14843–14848. doi: 10.1073/pnas.0704848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karry R, Klein E, Ben Shachar D. Mitochondrial complex I subunits expression is altered in schizophrenia: a postmortem study. Biol Psychiatry. 2004;55:676–684. doi: 10.1016/j.biopsych.2003.12.012. [DOI] [PubMed] [Google Scholar]
- Kasai K, Shenton ME, Salisbury DF, Hirayasu Y, Lee CU, Ciszewski AA, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry. 2003;160:156–164. doi: 10.1176/appi.ajp.160.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith VA, Mansbach RS, Geyer MA. Failure of haloperidol to block the effects of phencyclidine and dizocilpine on prepulse inhibition of startle. Biol Psychiatry. 1991;30:557–566. doi: 10.1016/0006-3223(91)90025-h. [DOI] [PubMed] [Google Scholar]
- Kelley BM, Porter JH. The role of muscarinic cholinergic receptors in the discriminative stimulus properties of clozapine in rats. Pharmacol Biochem Behav. 1997;57:707–719. doi: 10.1016/s0091-3057(96)00342-5. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Bagwell WW, Haas GL, Sweeney JA, Schooler NR, Pettegrew JW. Changes in caudate volume with neuroleptic treatment. Lancet. 1994;344:1434. doi: 10.1016/s0140-6736(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Chopde CT. Serotonergic agents modulate antidepressant-like effect of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in mice. Brain Res. 2000;865:291–300. doi: 10.1016/s0006-8993(00)02373-8. [DOI] [PubMed] [Google Scholar]
- Khisti RT, Chopde CT, Jain SP. Antidepressant-like effect of the neurosteroid 3α-hydroxy-5α-pregnan-20-one in mice forced swim test. Pharmacol Biochem Behav. 2000;67:137–143. doi: 10.1016/s0091-3057(00)00300-2. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kornhuber HH, Schmid-Burgk W, Holzmüller B. Low cerebrospinal fluid glutamate in schizophrenic patients and a new hypothesis on schizophrenia. Neurosci Lett. 1980;20:379–382. doi: 10.1016/0304-3940(80)90178-0. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Kapur S, van der Kooy D. Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs. J Neurosci. 2005;25:5815–5823. doi: 10.1523/JNEUROSCI.1120-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama M, Fujioka T, Duman RS. Chronic olanzapine or fluoxetine administration increases cell proliferation in hippocampus and prefrontal cortex of adult rat. Biol Psychiatry. 2004;56:570–580. doi: 10.1016/j.biopsych.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Cohen AL, Karp E, Rogawski MA. Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology. 1996;35:1049–1056. doi: 10.1016/s0028-3908(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther. 1994;270:1223–1229. [PubMed] [Google Scholar]
- Kolluri N, Sun Z, Sampson AR, Lewis DA. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2005;162:1200–1202. doi: 10.1176/appi.ajp.162.6.1200. [DOI] [PubMed] [Google Scholar]
- Konradi C, Kobierski LA, Nguyen TV, Heckers S, Hyman SE. The cAMP-response-element-binding protein interacts, but Fos protein does not interact, with the proenkephalin enhancer in rat striatum. Proc Natl Acad Sci U S A. 1993;90:7005–7009. doi: 10.1073/pnas.90.15.7005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Kaufmann CA, Marnela KM, Weinberger DR. Cerebrospinal fluid amino acid concentrations in chronic schizophrenia. Psychiatry Res. 1987;20:337–345. doi: 10.1016/0165-1781(87)90095-3. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Amar S, Belmaker RH, Agam G. Psychotropic drugs affect Ser9-phosphorylated GSK-3β protein levels in rodent frontal cortex. Int J Neuropsychopharmacol. 2006;9:337–342. doi: 10.1017/S1461145705006097. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Belmaker RH, Agam G. Low GSK-3β immunoreactivity in postmortem frontal cortex of schizophrenic patients. Am J Psychiatry. 2000;157:831–833. doi: 10.1176/appi.ajp.157.5.831. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Belmaker RH, Agam G. Low GSK-3 activity in frontal cortex of schizophrenic patients. Schizophr Res. 2001;52:101–105. doi: 10.1016/s0920-9964(00)00174-2. [DOI] [PubMed] [Google Scholar]
- Kozlovsky N, Shanon-Weickert C, Tomaskovic-Crook E, Kleinman JE, Belmaker RH, Agam G. Reduced GSK-3β mRNA levels in postmortem dorsolateral prefrontal cortex of schizophrenic patients. J Neural Transm. 2004;111:1583–1592. doi: 10.1007/s00702-004-0166-3. [DOI] [PubMed] [Google Scholar]
- Krämer UM, Cunillera T, Càmara E, Marco-Pallarés J, Cucurell D, Nager W, Bauer P, Schüle R, Schöls L, Rodriguez-Fornells A, et al. The impact of catechol-O-methyltransferase and dopamine D4 receptor genotypes on neurophysiological markers of performance monitoring. J Neurosci. 2007;27:14190–14198. doi: 10.1523/JNEUROSCI.4229-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropp S, Kern V, Lange K, Degner D, Hajak G, Kornhuber J, Rüther E, Emrich HM, Schneider U, Bleich S. Oxidative stress during treatment with first- and second-generation antipsychotics. J Neuropsychiatry Clin Neurosci. 2005;17:227–231. doi: 10.1176/jnp.17.2.227. [DOI] [PubMed] [Google Scholar]
- Kung L, Roberts RC. Mitochondrial pathology in human schizophrenic striatum: a postmortem ultrastructural study. Synapse. 1999;31:67–75. doi: 10.1002/(SICI)1098-2396(199901)31:1<67::AID-SYN9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Küppers E, Beyer C. Dopamine regulates brain-derived neurotrophic factor (BDNF) expression in cultured embryonic mouse striatal cells. Neuroreport. 2001;12:1175–1179. doi: 10.1097/00001756-200105080-00025. [DOI] [PubMed] [Google Scholar]
- Kuroki T, Meltzer HY, Ichikawa J. Effects of antipsychotic drugs on extracellular dopamine levels in rat medial prefrontal cortex and nucleus accumbens. J Pharmacol Exp Ther. 1999;288:774–781. [PubMed] [Google Scholar]
- Kurumaji A, Ishimaru M, Toru M. α-[3H]amino-3-hydroxy-5-methylisoxazole-4-propionic acid binding to human cerebral cortical membranes: minimal changes in postmortem brains of chronic schizophrenics. J Neurochem. 1992;59:829–837. doi: 10.1111/j.1471-4159.1992.tb08320.x. [DOI] [PubMed] [Google Scholar]
- Kurumaji A, Nehls DG, Park CK, McCulloch J. Effects of NMDA antagonists, MK-801 and CPP, upon local cerebral glucose use. Brain Res. 1989;496:268–284. doi: 10.1016/0006-8993(89)91074-3. [DOI] [PubMed] [Google Scholar]
- Kyosseva SV. Mitogen-activated protein kinase signaling. Int Rev Neurobiol. 2004;59:201–220. doi: 10.1016/S0074-7742(04)59008-6. [DOI] [PubMed] [Google Scholar]
- Kyosseva SV, Elbein AD, Griffin WS, Mrak RE, Lyon M, Karson CN. Mitogen-activated protein kinases in schizophrenia. Biol Psychiatry. 1999;46:689–696. doi: 10.1016/s0006-3223(99)00104-3. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Koffel B, LaPorte D, Tamminga CA. Subanesthetic doses of ketamine stimulate psychosis in schizophrenia. Neuropsychopharmacology. 1995;13:9–19. doi: 10.1016/0893-133X(94)00131-I. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Tamara Michaelidis BA, Parwani A, Tamminga CA. Effects of ketamine in normal and schizophrenic volunteers. Neuropsychopharmacology. 2001;25:455–467. doi: 10.1016/S0893-133X(01)00243-3. [DOI] [PubMed] [Google Scholar]
- Lang UE, Puls I, Muller DJ, Strutz-Seebohm N, Gallinat J. Molecular mechanisms of schizophrenia. Cell Physiol Biochem. 2007;20:687–702. doi: 10.1159/000110430. [DOI] [PubMed] [Google Scholar]
- Langmead CJ, Watson J, Reavill C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther. 2008;117:232–243. doi: 10.1016/j.pharmthera.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Laruelle M. Imaging dopamine transmission in schizophrenia. A review and meta-analysis. Q J Nucl Med. 1998;42:211–221. [PubMed] [Google Scholar]
- Lawlor MA, Alessi DR. PKB/Akt: a key mediator of cell proliferation, survival and insulin responses? J Cell Sci. 2001;114:2903–2910. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- Lei G, Xia Y, Johnson KM. The role of Akt-GSK-3β signaling and synaptic strength in phencyclidine-induced neurodegeneration. Neuropsychopharmacology. 2008;33:1343–1353. doi: 10.1038/sj.npp.1301511. [DOI] [PubMed] [Google Scholar]
- Leveque JC, Macías W, Rajadhyaksha A, Carlson RR, Barczak A, Kang S, Li XM, Coyle JT, Huganir RL, Heckers S, et al. Intracellular modulation of NMDA receptor function by antipsychotic drugs. J Neurosci. 2000;20:4011–4020. doi: 10.1523/JNEUROSCI.20-11-04011.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–l162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Levin ED, Rezvani AH. Nicotinic interactions with antipsychotic drugs, models of schizophrenia and impacts on cognitive function. Biochem Pharmacol. 2007;74:1182–1191. doi: 10.1016/j.bcp.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewandowski KE. Relationship of catechol-O-methyltransferase to schizophrenia and its correlates: evidence for associations and complex interactions. Harv Rev Psychiatry. 2007;15:233–244. doi: 10.1080/10673220701650409. [DOI] [PubMed] [Google Scholar]
- Lewis DA. GABAergic local circuit neurons and prefrontal cortical dysfunction in schizophrenia. Brain Res Brain Res Rev. 2000;31:270–276. doi: 10.1016/s0165-0173(99)00042-9. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Volk DW, Hashimoto T. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology (Berl) 2004;174:143–150. doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- Lewis S, Lieberman J. CATIE and CUtLASS: can we handle the truth? Br J Psychiatry. 2008;192:161–163. doi: 10.1192/bjp.bp.107.037218. [DOI] [PubMed] [Google Scholar]
- Li Q, Clark S, Lewis DV, Wilson WA. NMDA receptor antagonists disinhibit rat posterior cingulated and retrosplenial cortices: a potential mechanism of neurotoxicity. J Neurosci. 2002;22:3070–3080. doi: 10.1523/JNEUROSCI.22-08-03070.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Rosborough KM, Friedman AB, Zhu W, Roth KA. Regulation of mouse brain glycogen synthase kinase-3 by atypical antipsychotics. Int J Neuropsychopharmacol. 2007;10:7–19. doi: 10.1017/S1461145706006547. [DOI] [PubMed] [Google Scholar]
- Li XM, Chlan-Fourney J, Juorio AV, Bennett VL, Shrikhande S, Keegan DL, Qi J, Boulton AA. Differential effects of olanzapine on the gene expression of superoxide dismutase and the low affinity nerve growth factor receptor. J Neurosci Res. 1999;56:72–75. doi: 10.1002/(SICI)1097-4547(19990401)56:1<72::AID-JNR9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Li XM, Perry KW, Wong DT, Bymaster FP. Olanzapine increases in vivo dopamine and norepinephrine release in rat prefrontal cortex, nucleus accumbens and striatum. Psychopharmacoloy (Berl) 1998;136:153–161. doi: 10.1007/s002130050551. [DOI] [PubMed] [Google Scholar]
- Li Z, Huang M, Ichikawa J, Dai J, Meltzer HY. N-Desmethylclozapine, a major metabolite of clozapine, increases cortical acetylcholine and dopamine release in vivo via stimulation of M1 muscarinic receptors. Neuropsychopharmacology. 2005;30:1986–1995. doi: 10.1038/sj.npp.1300768. [DOI] [PubMed] [Google Scholar]
- Li Z, Ichikawa J, Dai J, Meltzer HY. Aripiprazole, a novel antipsychotic drug, preferentially increases dopamine release in the prefrontal cortex and hippocampus in rat brain. Eur J Pharmacol. 2004;493:75–83. doi: 10.1016/j.ejphar.2004.04.028. [DOI] [PubMed] [Google Scholar]
- Lidow MS. Calcium signaling dysfunction in schizophrenia: a unifying approach. Brain Res Rev. 2003;43:70–84. doi: 10.1016/s0165-0173(03)00203-0. [DOI] [PubMed] [Google Scholar]
- Lieberman J, Chakos M, Wu H, Alvir J, Hoffman E, Robinson D, Bilder R. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry. 2001a;49:487–499. doi: 10.1016/s0006-3223(01)01067-8. [DOI] [PubMed] [Google Scholar]
- Lieberman J, Jody D, Geisler S, Alvir J, Loebel A, Szymanski S, Woerner M, Borenstein M. Time course and biologic correlates of treatment response in first-episode schizophrenia. Arch Gen Psychiatry. 1993;50:369–376. doi: 10.1001/archpsyc.1993.01820170047006. [DOI] [PubMed] [Google Scholar]
- Lieberman JA. Is schizophrenia a neurodegenerative disorder? A clinical and neurobiological perspective. Biol Psychiatry. 1999;46:729–739. doi: 10.1016/s0006-3223(99)00147-x. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Perkins D, Belger A, Chakos M, Jarskog F, Boteva K, Gilmore J. The early stages of schizophrenia: speculations on pathogenesis, pathophysiology and therapeutic approaches. Biol Psychiatry. 2001b;50:884–897. doi: 10.1016/s0006-3223(01)01303-8. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Malaspina D, Jarskog LF. Preventing clinical deterioration in the course of schizophrenia: the potential for neuroprotection. CNS Spectr. 2006;11(Suppl):1–13. [PubMed] [Google Scholar]
- Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005a;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Tollefson GD, Charles C, Zipursky R, Sharma T, Kahn RS, Keefe RS, Green AI, Gur RE, McEvoy J, et al. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005b;62:361–370. doi: 10.1001/archpsyc.62.4.361. [DOI] [PubMed] [Google Scholar]
- Liégeois JF, Ichikawa J, Meltzer HY. 5-HT2A receptor antagonism potentiates haloperidol-induced dopamine release in rat medial prefrontal cortex and inhibits that in the nucleus accumbens in a dose-dependent manner. Brain Res. 2002;947:157–165. doi: 10.1016/s0006-8993(02)02620-3. [DOI] [PubMed] [Google Scholar]
- Linn GS, Negi SS, Gerum SV, Javitt DC. Reversal of phencyclidine-induced prepulse inhibition deficits by clozapine in monkeys. Psychopharmacology (Berl) 2003;169:234–239. doi: 10.1007/s00213-003-1533-8. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Khaing ZZ, Weickert CS, Weinberger DR. BDNF mRNA expression in rat hippocampus and prefrontal cortex: effects of neonatal ventral hippocampal damage and antipsychotic drugs. Eur J Neurosci. 2001;14:135–144. doi: 10.1046/j.1460-9568.2001.01633.x. [DOI] [PubMed] [Google Scholar]
- Lockhart EM, Warner DS, Pearlstein RD, Penning DH, Mehrabani S, Boustany RM. Allopregnanolone attenuates N-methyl-D-aspartate-induced excitotoxicity and apoptosis in the human NT2 cell line in culture. Neurosci Lett. 2002;328:33–36. doi: 10.1016/s0304-3940(02)00448-2. [DOI] [PubMed] [Google Scholar]
- Löpez-Gil X, Babot Z, Amargós-Bosch M, Suñol C, Artigas F, Adell A. Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology. 2007;32:2087–2097. doi: 10.1038/sj.npp.1301356. [DOI] [PubMed] [Google Scholar]
- Lorrain DS, Baccei CS, Bristow LJ, Anderson JJ, Varney MA. Effects of ketamine and N-methyl-D-aspartate on glutamate and dopamine release in the rat prefrontal cortex: modulation by a group II selective metabotropic glutamate receptor agonist LY379268. Neuroscience. 2003;117:697–706. doi: 10.1016/s0306-4522(02)00652-8. [DOI] [PubMed] [Google Scholar]
- Lovestone S, Killick R, Di Forti M, Murray R. Schizophrenia as a GSK-3 dysregulation disorder. Trends Neurosci. 2007;30:142–149. doi: 10.1016/j.tins.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Lu XH, Bradley RJ, Dwyer DS. Olanzapine produces trophic effects in vitro and stimulates phosphorylation of Akt/PKB, ERK1/2 and the mitogen-activated protein kinase p38. Brain Res. 2004;1011:58–68. doi: 10.1016/j.brainres.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Lu XH, Dwyer DS. Second generation antipsychotic drugs, olanzapine, quetiapine, and clozapine enhance neurite outgrowth in PC12 cells via PI3K/AKT, ERK, and pertussis toxin-sensitive pathways. J Mol Neurosci. 2005;27:43–64. doi: 10.1385/jmn:27:1:043. [DOI] [PubMed] [Google Scholar]
- Luo C, Xu H, Li XM. Post-stress changes in BDNF and Bcl-2 immunoreactivities in hippocampal neurons: effect of chronic administration of olanzapine. Brain Res. 2004;1025:194–202. doi: 10.1016/j.brainres.2004.06.089. [DOI] [PubMed] [Google Scholar]
- Macciardi F, Lucca A, Catalano M, Marino C, Zanardi R, Smeraldi E. Amino acid patterns in schizophrenia: some new findings. Psychiatry Res. 1990;32:63–70. doi: 10.1016/0165-1781(90)90136-s. [DOI] [PubMed] [Google Scholar]
- Madeira C, Freitas ME, Vargas-Lopes C, Wolosker H, Panizzutti R. Increased brain D-amino acid oxidase (DAAO) activity in schizophrenia. Schizophr Res. 2008;101:76–83. doi: 10.1016/j.schres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingström A. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Demirgören S, Spivak CE, London ED. The neurosteroid dehydroepiandrosterone sulfate is an allosteric antagonist of the GABAA receptor. Brain Res. 1990;526:143–146. doi: 10.1016/0006-8993(90)90261-9. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Mienville JM, Vicini S. Neurosteroid pregnenolone sulfate antagonizes electrophysiological responses to GABA in neurons. Neurosci Lett. 1988;90:279–284. doi: 10.1016/0304-3940(88)90202-9. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, Breier A. NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology. 1996;14:301–307. doi: 10.1016/0893-133X(95)00137-3. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Carver J, Zorn SH. Blockade of drug-induced deficits in prepulse inhibition of acoustic startle by ziprasidone. Pharmacol Biochem Behav. 2001;69:535–542. doi: 10.1016/s0091-3057(01)00561-5. [DOI] [PubMed] [Google Scholar]
- Maragnoli ME, Fumagalli F, Gennarelli M, Racagni G, Riva MA. Fluoxetine and olanzapine have synergistic effects in the modulation of fibroblast growth factor 2 expression within the rat brain. Biol Psychiatry. 2004;55:1095–1102. doi: 10.1016/j.biopsych.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Marti HH. Erythropoietin and the hypoxic brain. J Exp Biol. 2004;207:3233–3242. doi: 10.1242/jeb.01049. [DOI] [PubMed] [Google Scholar]
- Martin P, Carlsson ML, Hjorth S. Systemic PCP treatment elevates brain extracellular 5-HT: a microdialysis study in awake rats. Neuroreport. 1998;9:2985–2988. doi: 10.1097/00001756-199809140-00012. [DOI] [PubMed] [Google Scholar]
- Marx C, Grobin A, Deutch A, Lieberman J. Atypical antipsychotics and stress. In: Steckler T, Kalin N, Reul J, editors. Handbook of Stress and the Brain. Elsevier Science; Amsterdam: 2005. pp. 301–313. [Google Scholar]
- Marx CE, Duncan GE, Gilmore JH, Lieberman JA, Morrow AL. Olanzapine increases allopregnanolone in the rat cerebral cortex. Biol Psychiatry. 2000;47:1000–1004. doi: 10.1016/s0006-3223(99)00305-4. [DOI] [PubMed] [Google Scholar]
- Marx CE, VanDoren MJ, Duncan GE, Lieberman JA, Morrow AL. Olanzapine and clozapine increase the GABAergic neuroactive steroid allopregnanolone in rodents. Neuropsychopharmacology. 2003;28:1–13. doi: 10.1038/sj.npp.1300015. [DOI] [PubMed] [Google Scholar]
- Marx CE, Shampine LJ, Khisti RT, Trost WT, Bradford DW, Grobin AC, Massing MW, Madison RD, Butterfield MI, Lieberman JA, et al. Olanzapine and fluoxetine administration and coadministration increase rat hippocampal pregnenolone, allopregnanolone and peripheral deoxycorticosterone: implications for therapeutic actions. Pharmacol Biochem Behav. 2006a;84:609–617. doi: 10.1016/j.pbb.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Marx CE, Stevens RD, Shampine LJ, Uzunova V, Trost WT, Butterfield MI, Massing MW, Hamer RM, Morrow AL, Lieberman JA. Neuroactive steroids are altered in schizophrenia and bipolar disorder: relevance to pathophysiology and therapeutics. Neuropsychopharmacology. 2006b;31:1249–1263. doi: 10.1038/sj.npp.1300952. [DOI] [PubMed] [Google Scholar]
- Massana G, Salgado-Pineda P, Junqué C, Pérez M, Baeza I, Pons A, Massana J, Navarro V, Blanch J, Morer A, et al. Volume changes in gray matter in first-episode neuroleptic-naive schizophrenic patients treated with risperidone. J Clin Psychopharmacol. 2005;25:111–117. doi: 10.1097/01.jcp.0000155818.29091.53. [DOI] [PubMed] [Google Scholar]
- Mateos JJ, Lomeña F, Parellada E, Mireia F, Fernandez-Egea E, Pavia J, Prats A, Pons F, Bernardo M. Lower striatal dopamine transporter binding in neuroleptic-naïve schizophrenic patients is not related to antipsychotic treatment but it suggests an illness trait. Psychopharmacology (Berl) 2007;191:805–811. doi: 10.1007/s00213-006-0570-5. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:148–157. doi: 10.1001/archpsyc.58.2.148. [DOI] [PubMed] [Google Scholar]
- Mathé JM, Nomikos GG, Blakeman KH, Svensson TH. Differential actions of dizocilpine (MK-801) on the mesolimbic and mesocortical dopamine systems: role of neuronal activity. Neuropharmacology. 1999;38:121–128. doi: 10.1016/s0028-3908(98)00163-4. [DOI] [PubMed] [Google Scholar]
- Mathew RJ, Duncan GC, Weinman ML, Barr DL. Regional cerebral blood flow in schizophrenia. Arch Gen Psychiatry. 1982;39:1121–1124. doi: 10.1001/archpsyc.1982.04290100001001. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Duan W. “Apoptotic” biochemical cascades in synaptic compartments: roles in adaptive plasticity and neurodegenerative disorders. J Neurosci Res. 1999;58:152–166. [PubMed] [Google Scholar]
- Matute C, Melone M, Vallejo-Illarramendi A, Conti F. Increased expression of the astrocytic glutamate transporter GLT-1 in the prefrontal cortex of schizophrenics. Glia. 2005;49:451–455. doi: 10.1002/glia.20119. [DOI] [PubMed] [Google Scholar]
- Maurer I, Möller HJ. Inhibition of complex I by neuroleptics in normal human brain cortex parallels the extrapyramidal toxicity of neuroleptics. Mol Cell Biochem. 1997;174:255–259. [PubMed] [Google Scholar]
- Maurer I, Zierz S, Möller H. Evidence for a mitochondrial oxidative phosphorylation defect in brains for patients with schizophrenia. Schizophr Res. 2001;48:125–136. doi: 10.1016/s0920-9964(00)00075-x. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Fisahn A. Interneurons unbound. Nat Rev Neurosci. 2001;2:11–23. doi: 10.1038/35049047. [DOI] [PubMed] [Google Scholar]
- McCullumsmith RE, Meador-Woodruff JH. Striatal excitatory amino acid transporter transcript expression in schizophrenia, bipolar disorder, and major depressive disorder. Neuropsychopharmacology. 2002;26:368–375. doi: 10.1016/S0893-133X(01)00370-0. [DOI] [PubMed] [Google Scholar]
- McDermott E, de Silva P. Impaired neuronal glucose uptake in pathogenesis of schizophrenia—can GLUT 1 and GLUT 3 deficits explain imaging, postmortem and pharmacological findings? Med Hypotheses. 2005;65:1076–1081. doi: 10.1016/j.mehy.2005.06.022. [DOI] [PubMed] [Google Scholar]
- McGuinness SM, Johansson R, Lundstrom J, Ross D. Induction of apoptosis by remoxipride metabolites in HL60 and CD34+/CD19− human bone marrow progenitor cells: potential relevance to remoxipride-induced aplastic anemia. Chem Biol Interact. 1999;121:253–265. doi: 10.1016/s0009-2797(99)00105-2. [DOI] [PubMed] [Google Scholar]
- McLeod MC, Sundram S, Dean B. Treatment with haloperidol and diazepam alters GABAA receptor density in the rat brain. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:560–567. doi: 10.1016/j.pnpbp.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Meisenzahl EM, Schmitt GJ, Scheuerecker J, Möller HJ. The role of dopamine for the pathophysiology of schizophrenia. Int Rev Psychiatry. 2007;19:337–345. doi: 10.1080/09540260701502468. [DOI] [PubMed] [Google Scholar]
- Melone M, Vitellaro-Zuccarello L, Vallejo-Illarramendi A, Pérez-Samartin A, Matute C, Cozzi A, Pellegrini-Giampietro DE, Rothstein JD, Conti F. The expression of glutamate transporter GLT-1 in the rat cerebral cortex is down-regulated by the antipsychotic drug clozapine. Mol Psychiatry. 2001;6:380–386. doi: 10.1038/sj.mp.4000880. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Matsubara S, Lee JC. Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J Pharmacol Exp Ther. 1989;251:238–246. [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull. 1999;25:233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Switzer RC, 3rd, Napier TC. Short-term, D2 receptor blockade induces synaptic degeneration, reduces levels of tyrosine hydroxylase and brain-derived neurotrophic factor, and enhances D2-mediated firing in the ventral pallidum. Brain Res. 2004;995:14–22. doi: 10.1016/j.brainres.2003.09.040. [DOI] [PubMed] [Google Scholar]
- Michel TM, Thome J, Martin D, Nara K, Zwerina S, Tatschner T, Weijers HG, Koutsilieri E. Cu, Zn-, and Mn-superoxide dismutase levels in brains of patients with schizophrenic psychosis. J Neural Transm. 2004;111:1191–1201. doi: 10.1007/s00702-004-0160-9. [DOI] [PubMed] [Google Scholar]
- Micheli F, Heidbreder C. Selective dopamine D3 receptor antagonists: a review 2001–2005. Recent Patents CNS Drug Discov. 2006;1:271–288. doi: 10.2174/157488906778773634. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Milev P, Ho BC, Arndt S, Nopoulos P, Andreasen NC. Initial magnetic resonance imaging volumetric brain measurements and outcome in schizophrenia: a prospective longitudinal study with 5-year follow-up. Biol Psychiatry. 2003;54:608–615. doi: 10.1016/s0006-3223(03)00293-2. [DOI] [PubMed] [Google Scholar]
- Millan MJ. N-Methyl-D-aspartate receptors as a target for improved antipsychotic agents: novel insights and clinical perspectives. Psychopharmacology (Berl) 2005;179:30–53. doi: 10.1007/s00213-005-2199-1. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Brocco M, Gobert A, Joly F, Bervoets K, Rivet J, Newman-Tancredi A, Audinot V, Maurel S. Contrasting mechanisms of action and sensitivity to antipsychotics of phencyclidine versus amphetamine: importance of nucleus accumbens 5-HT2A sites for PCP-induced locomotion in the rat. Eur J Neurosci. 1999a;11:4419–4432. doi: 10.1046/j.1460-9568.1999.00858.x. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Schreiber R, Monneyron S, Denorme B, Melon C, Queriaux S, Dekeyne A. S-16924, a novel, potential antipsychotic with marked serotonin1A agonist properties. IV. A drug discrimination comparison with clozapine. J Pharmacol Exp Ther. 1999b;289:427–436. [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Lewis DA, Levitt P. Analysis of complex brain disorders with gene expression microarrays: schizophrenia as a disease of the synapse. Trends Neurosci. 2001a;24:479–486. doi: 10.1016/s0166-2236(00)01862-2. [DOI] [PubMed] [Google Scholar]
- Mirnics K, Middleton FA, Stanwood GD, Lewis DA, Levitt P. Disease-specific changes in regulator of G-protein signaling 4 (RGS4) expression in schizophrenia. Mol Psychiatry. 2001b;6:293–301. doi: 10.1038/sj.mp.4000866. [DOI] [PubMed] [Google Scholar]
- Mitelman SA, Shihabuddin L, Brickman AM, Hazlett EA, Buchsbaum MS. MRI assessment of gray and white matter distribution in Brodmann’s areas of the cortex in patients with schizophrenia with good and poor outcomes. Am J Psychiatry. 2003;160:2154–2168. doi: 10.1176/appi.ajp.160.12.2154. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Duncan GE, Marx CE, Lieberman JA. Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry. 2005;10:79–104. doi: 10.1038/sj.mp.4001556. [DOI] [PubMed] [Google Scholar]
- Miyaoka T, Yasukawa R, Yasuda H, Shimizu M, Mizuno S, Sukegawa T, Inagaki T, Horiguchi J. Urinary excretion of biopyrrins, oxidative metabolites of bilirubin, increases in patients with psychiatric disorders. Eur Neuropsychopharmacol. 2005;15:249–252. doi: 10.1016/j.euroneuro.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Mizukami K, Ishikawa M, Hidaka S, Iwakiri M, Sasaki M, Iritani S. Immunohistochemical localization of GABAB receptor in the entorhinal cortex and inferior temporal cortex of schizophrenic brain. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:393–396. doi: 10.1016/s0278-5846(01)00247-0. [DOI] [PubMed] [Google Scholar]
- Modica-Napolitano JS, Lagace CJ, Brennan WA, Aprille JR. Differential effects of typical and atypical neuroleptics on mitochondrial function in vitro. Arch Pharm Res. 2003;26:951–959. doi: 10.1007/BF02980205. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Targeting metabotropic glutamate receptors for treatment of the cognitive symptoms of schizophrenia. Psychopharmacology (Berl) 2004;174:39–44. doi: 10.1007/s00213-004-1792-z. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Bunney BS. Acute effects of typical and atypical antipsychotic drugs on the release of dopamine from prefrontal cortex, nucleus accumbens, and striatum of the rat: an in vivo microdialysis study. J Neurochem. 1990;54:1755–1760. doi: 10.1111/j.1471-4159.1990.tb01230.x. [DOI] [PubMed] [Google Scholar]
- Mohler H, Knoflach F, Paysan J, Motejlek K, Benke D, Lüscher B, Fritschy JM. Heterogeneity of GABAA-receptors: cell-specific expression, pharmacology and regulation. Neurochem Res. 1995;20:631–636. doi: 10.1007/BF01694546. [DOI] [PubMed] [Google Scholar]
- Moley KH, Mueckler MM. Glucose transport and apoptosis. Apoptosis. 2000;5:99–105. doi: 10.1023/a:1009697908332. [DOI] [PubMed] [Google Scholar]
- Molina V, Reig S, Sanz J, Palomo T, Benito C, Sánchez J, Sarramea F, Pascau J, Desco M. Increase in gray matter and decrease in white matter volumes in the cortex during treatment with atypical neuroleptics in schizophrenia. Schizophr Res. 2005;80:61–71. doi: 10.1016/j.schres.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Moore NA, Calligaro DO, Wong DT, Bymaster FP, Tye NC. The pharmacology of olanzapine and other new antipsychotic agents. Curr Opin Investig Drugs. 1993;2:281–293. [Google Scholar]
- Moore NA, Tye NC, Axton MS, Risius FC. The behavioral pharmacology of olanzapine, a novel “atypical” antipsychotic agent. J Pharmacol Exp Ther. 1992;262:545–551. [PubMed] [Google Scholar]
- Moralí G, Letechipía-Vallejo G, López-Loeza E, Montes P, Hernández-Morales L, Cervantes M. Post-ischemic administration of progesterone in rats exerts neuroprotective effects on the hippocampus. Neurosci Lett. 2005;382:286–290. doi: 10.1016/j.neulet.2005.03.066. [DOI] [PubMed] [Google Scholar]
- Morris BJ, Cochran SM, Pratt JA. PCP: from pharmacology to modelling schizophrenia. Curr Opin Pharmacol. 2005;5:101–106. doi: 10.1016/j.coph.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow AL, Pace JR, Purdy RH, Paul SM. Characterization of steroid interactions with γ-aminobutyric acid receptor-gated chloride ion channels: evidence for multiple steroid recognition sites. Mol Pharmacol. 1990;37:263–270. [PubMed] [Google Scholar]
- Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987;142:483–485. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- Mouri A, Noda Y, Enomoto T, Nabeshima T. Phencyclidine animal models of schizophrenia: approaches from abnormality of glutamatergic neurotransmission and neurodevelopment. Neurochem Int. 2007;51:173–184. doi: 10.1016/j.neuint.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Moy SS, Fernandes A, Qian Y, Rotella DJ, Kostrewa RM, Breese GR. Effect of acute and chronic olanzapine treatment on phencyclidine-induced behavioral sensitization in rats with neonatal dopamine loss. Pharmacol Biochem Behav. 2004;78:47–56. doi: 10.1016/j.pbb.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Mueller HT, Haroutunian V, Davis KL, Meador-Woodruff JH. Expression of the ionotropic glutamate receptor subunits and NMDA receptor-associated intracellular proteins in the substantia nigra in schizophrenia. Mol Brain Res. 2004;121:60–69. doi: 10.1016/j.molbrainres.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Schnur DB, Reddy R. Family history of type 2 diabetes in schizophrenic patients. Lancet. 1989;1:495. doi: 10.1016/s0140-6736(89)91392-5. [DOI] [PubMed] [Google Scholar]
- Nair TR, Christensen JD, Kingsbury SJ, Kumar NG, Terry WM, Garver DL. Progression of cerebroventricular enlargement and the subtyping of schizophrenia. Psychiatry Res. 1997;74:141–150. doi: 10.1016/s0925-4927(97)00013-9. [DOI] [PubMed] [Google Scholar]
- Narr KL, Bilder RM, Toga AW, Woods RP, Rex DE, Szeszko PR, Robinson D, Sevy S, Gunduz-Bruce H, Wang YP, et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005;15:708–719. doi: 10.1093/cercor/bhh172. [DOI] [PubMed] [Google Scholar]
- Neeman G, Blanaru M, Bloch B, Kremer I, Ermilov M, Javitt DC, Heresco-Levy U. Relation of plasma glycine, serine, and homocysteine levels to schizophrenia symptoms and medication type. Am J Psychiatry. 2005;162:1738–1740. doi: 10.1176/appi.ajp.162.9.1738. [DOI] [PubMed] [Google Scholar]
- Nibuya M, Morinobu S, Duman RS. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci. 1995;15:7539–7547. doi: 10.1523/JNEUROSCI.15-11-07539.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Ninan I, Jardemark KE, Wang RY. Differential effects of atypical and typical antipsychotic drugs on N-methyl-D-aspartate- and electrically evoked responses in the pyramidal cells of the rat medial prefrontal cortex. Synapse. 2003a;48:66–79. doi: 10.1002/syn.10189. [DOI] [PubMed] [Google Scholar]
- Ninan I, Jardemark KE, Wang RY. Olanzapine and clozapine but not haloperidol reverse subchronic phencyclidine-induced functional hyperactivity of N-methyl-D-aspartate receptors in pyramidal cells of the rat medial prefrontal cortex. Neuropharmacology. 2003b;44:462–472. doi: 10.1016/s0028-3908(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Nishioka N, Arnold SE. Evidence for oxidative DNA damage in the hippocampus of elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry. 2004;12:167–175. [PubMed] [Google Scholar]
- Nishikawa T, Takashima M, Toru M. Increased [3H]kainic acid binding in the prefrontal cortex in schizophrenia. Neurosci Lett. 1983;40:245–250. doi: 10.1016/0304-3940(83)90046-0. [DOI] [PubMed] [Google Scholar]
- Noga JT, Hyde TM, Herman MM, Spurney CF, Bigelow LB, Weinberger DR, Kleinman JE. Glutamate receptor in the postmortem striatum of schizophrenic, suicide, and control brains. Synapse. 1997;27:168–176. doi: 10.1002/(SICI)1098-2396(199711)27:3<168::AID-SYN2>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- O’Connor JA, Hasenkamp W, Horman BM, Muly EC, Hemby SE. Region specific regulation of NR1 in rhesus monkeys following chronic antipsychotic drug administration. Biol Psychiatry. 2006;60:659–662. doi: 10.1016/j.biopsych.2006.03.044. [DOI] [PubMed] [Google Scholar]
- Ohta K, Kuno S, Mizuta I, Fujinami A, Matsui H, Ohta M. Effects of dopamine agonists bromocriptine, pergolide, cabergoline, and SKF-38393 on GDNF, NGF, and BDNF synthesis in cultured mouse astrocytes. Life Sci. 2003;73:617–626. doi: 10.1016/s0024-3205(03)00321-7. [DOI] [PubMed] [Google Scholar]
- Okazawa H, Murata M, Watanabe M, Kamei M, Kanazawa I. Dopaminergic stimulation up-regulates the in vivo expression of brain-derived neurotrophic factor (BDNF) in the striatum. FEBS Lett. 1992;313:138–142. doi: 10.1016/0014-5793(92)81430-t. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–637. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Sedvall GC, Agartz I. Reduced grey and white matter volumes in the temporal lobe of male patients with chronic schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2002;252:120–123. doi: 10.1007/s00406-002-0370-9. [DOI] [PubMed] [Google Scholar]
- Olianas MC, Maullu C, Onali P. Mixed agonist-antagonist properties of clozapine at different human cloned muscarinic receptor subtypes expressed in Chinese hamster ovary cells. Neuropsychopharmacology. 1999;20:263–270. doi: 10.1016/S0893-133X(98)00048-7. [DOI] [PubMed] [Google Scholar]
- Olney JW, Farber NB. Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- Palomino A, González-Pinto A, Aldama A, González-Gómez C, Mosquera F, González-García G, Matute C. Decreased levels of plasma glutamate in patients with first episode schizophrenia and bipolar disorder. Schizophr Res. 2007;95:174–178. doi: 10.1016/j.schres.2007.06.012. [DOI] [PubMed] [Google Scholar]
- Pandey GN, Conley RR, Pandey SC, Goel S, Roberts RC, Tamminga CA, Chute D, Smialek J. Benzodiazepine receptors in the post-mortem brain of suicide victims and schizophrenic subjects. Psychiatry Res. 1997;71:137–149. doi: 10.1016/s0165-1781(97)00060-7. [DOI] [PubMed] [Google Scholar]
- Parada MA, Hernandez L, Puig de Parada M, Rada P, Murzi E. Selective action of acute systemic clozapine on acetylcholine release in the rat prefrontal cortex by reference to the nucleus accumbens and striatum. J Pharmacol Exp Ther. 1997;281:582–588. [PubMed] [Google Scholar]
- Parikh V, Evans DR, Khan MM, Mahadik SP. Nerve growth factor in never-medicated first-episode psychotic and medicated chronic schizophrenic patients: possible implications for treatment outcome. Schizophr Res. 2003;60:117–123. doi: 10.1016/s0920-9964(02)00434-6. [DOI] [PubMed] [Google Scholar]
- Parikh V, Khan MM, Mahadik SP. Olanzapine counteracts reduction of brain-derived neurotrophic factor and TrkB receptors in rat hippocampus produced by haloperidol. Neurosci Lett. 2004a;356:135–139. doi: 10.1016/j.neulet.2003.10.079. [DOI] [PubMed] [Google Scholar]
- Parikh V, Khan MM, Terry A, Mahadik SP. Differential effects of typical and atypical antipsychotics on nerve growth factor and choline acetyltransferase expression in the cortex and nucleus basalis of rats. J Psychiatr Res. 2004b;38:521–529. doi: 10.1016/j.jpsychires.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Parikh V, Terry AV, Khan MM, Mahadik SP. Modulation of nerve growth factor and choline acetyltransferase expression in rat hippocampus after chronic exposure to haloperidol, risperidone, and olanzapine. Psychopharmacology (Berl) 2004c;172:365–374. doi: 10.1007/s00213-003-1669-6. [DOI] [PubMed] [Google Scholar]
- Park-Chung M, Malayev A, Purdy RH, Gibbs TT, Farb DH. Sulfated and unsulfated steroids modulate γ-aminobutyric acid A receptor function through distinct sites. Brain Res. 1999;830:72–87. doi: 10.1016/s0006-8993(99)01381-5. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. Neuroactive steroids. FASEB J. 1992;6:2311–2322. [PubMed] [Google Scholar]
- Pérez-Neri I, Ramírez-Bermúdez J, Montes S, Ríos C. Possible mechanisms of neurodegeneration in schizophrenia. Neurochem Res. 2006;31:1279–1294. doi: 10.1007/s11064-006-9162-3. [DOI] [PubMed] [Google Scholar]
- Perry TL. Normal cerebrospinal fluid and brain glutamate levels in schizophrenia do not support the hypothesis of glutamatergic neuronal dysfunction. Neurosci Lett. 1982;28:81–85. doi: 10.1016/0304-3940(82)90212-9. [DOI] [PubMed] [Google Scholar]
- Pietraszek M, Golembiowska K, Bijak M, Ossowska K, Wolfarth S. Differential effects of chronic haloperidol and clozapine administration on glutamatergic transmission in the fronto-parietal cortex in rats: microdialysis and electrophysiological studies. Naunyn Schmiedebergs Arch Pharmacol. 2002;366:417–424. doi: 10.1007/s00210-002-0619-x. [DOI] [PubMed] [Google Scholar]
- Pillai A, Mahadik SP. Differential effects of haloperidol and olanzapine on levels of vascular endothelial growth factor and angiogenesis in rat hippocampus. Schizophr Res. 2006;87:48–59. doi: 10.1016/j.schres.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Pillai A, Kale A, Joshi S, Napheda N, Sapkale S, Raju MSVK, Nasrallah HA, Buckley PF, Mahadik SP. Brain-derived neurotrophic factor levels are lower in plasma as well as cerebrospinal fluid from medication-naïve first episode schizophrenia patients. Biol Psychiatry. 2007;98:298–301. [Google Scholar]
- Pillai A, Terry AV, Jr, Mahadik SP. Differential effects of long-term treatment with typical and atypical antipsychotics on NGF and BDNF levels in rat striatum and hippocampus. Schizophr Res. 2006;82:95–106. doi: 10.1016/j.schres.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Pinna G, Dong E, Matsumoto K, Costa E, Guidotti A. In socially isolated mice, the reversal of brain allopregnanolone down-regulation mediates the anti-aggressive action of fluoxetine. Proc Natl Acad Sci U S A. 2003;100:2035–2040. doi: 10.1073/pnas.0337642100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirildar S, Gönül AS, Taneli F, Akdeniz F. Low serum levels of brain-derived neurotrophic factor in patients with schizophrenia do not elevate after antipsychotic treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:709–713. doi: 10.1016/j.pnpbp.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Porter JH, Varvel SA, Vann RE, Philibin SD, Wise LE. Clozapine discrimination with a low training dose distinguishes atypical from typical antipsychotic drugs in rats. Psychopharmacology (Berl) 2000;149:189–193. doi: 10.1007/s002139900366. [DOI] [PubMed] [Google Scholar]
- Pozzi L, Håkansson K, Usiello A, Borgkvist A, Lindskog M, Greengard P, Fisone G. Opposite regulation by typical and atypical anti-psychotics of ERK1/2, CREB and Elk-1 phosphorylation in mouse dorsal striatum. J Neurochem. 2003;86:451–459. doi: 10.1046/j.1471-4159.2003.01851.x. [DOI] [PubMed] [Google Scholar]
- Prabakaran S, Swatton JE, Ryan MM, Huffaker SJ, Huang JT, Griffin JL, Wayland M, Freeman T, Dudbridge F, Lilley KS, et al. Mitochondrial dysfunction in schizophrenia: evidence for compromised brain metabolism and oxidative stress. Mol Psychiatry. 2004;9:684–697. 643. doi: 10.1038/sj.mp.4001511. [DOI] [PubMed] [Google Scholar]
- Prince JA, Harro J, Blennow K, Gottfries CG, Oreland L. Putamen mitochondrial energy metabolism is highly correlated to emotional and intellectual impairment in schizophrenics. Neuropsychopharmacology. 2000;22:284–292. doi: 10.1016/S0893-133X(99)00111-6. [DOI] [PubMed] [Google Scholar]
- Prince JA, Yassin MS, Oreland L. Neuroleptic-induced mitochondrial enzyme alterations in the rat brain. J Pharmacol Exp Ther. 1997;280:261–267. [PubMed] [Google Scholar]
- Prince JA, Yassin MS, Oreland L. A histochemical demonstration of altered cytochrome oxidase activity in the rat brain by neuroleptics. Eur Neuropsychopharmacol. 1998;8:1–6. doi: 10.1016/s0924-977x(97)00036-9. [DOI] [PubMed] [Google Scholar]
- Pritchett DB, Lüddens H, Seeburg PH. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1989;245:1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- Prus AJ, Baker LE, Meltzer HY. Discriminative stimulus properties of 1.25 and 5.0 mg/kg doses of clozapine in rats: examination of the role of dopamine, serotonin, and muscarinic receptor mechanisms. Pharmacol Biochem Behav. 2004;77:199–208. doi: 10.1016/j.pbb.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Prus AJ, Philibin SD, Pehrson AL, Porter JH. Generalization to atypical antipsychotic drugs depends on training dose in rats trained to discriminate 1.25 mg/kg clozapine versus 5.0 mg/kg clozapine versus vehicle in a three-choice drug discrimination task. Behav Pharmacol. 2005;16:511–520. doi: 10.1097/01.fbp.0000172735.73876.06. [DOI] [PubMed] [Google Scholar]
- Qing H, Xu H, Wei Z, Gibson K, Li XM. The ability of atypical antipsychotic drugs vs. haloperidol to protect PC12 cells against MPP+-induced apoptosis. Eur J Neurosci. 2003;17:1563–1570. doi: 10.1046/j.1460-9568.2003.02590.x. [DOI] [PubMed] [Google Scholar]
- Radecki DT, Brown LM, Martinez J, Teyler TJ. BDNF protects against stress-induced impairments in spatial learning and memory and LTP. Hippocampus. 2005;15:246–253. doi: 10.1002/hipo.20048. [DOI] [PubMed] [Google Scholar]
- Raedler TJ, Bymaster FP, Tandon R, Copolov D, Dean B. Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry. 2007;12:232–246. doi: 10.1038/sj.mp.4001924. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther. 2007;113:523–536. doi: 10.1016/j.pharmthera.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjekar PK, Hinge A, Hegde MV, Ghate M, Kale A, Sitasawad S, Wagh UV, Debsikdar VB, Mahadik SP. Decreased antioxidant enzymes and membrane essential polyunsaturated fatty acids in schizophrenic and bipolar mood disorder patients. Psychiatry Res. 2003;121:109–122. doi: 10.1016/s0165-1781(03)00220-8. [DOI] [PubMed] [Google Scholar]
- Rapoport JL, Giedd J, Kumra S, Jacobsen L, Smith A, Lee P, Nelson J, Hamburger S. Childhood-onset schizophrenia. Progressive ventricular change during adolescence. Arch Gen Psychiatry. 1997;54:897–903. doi: 10.1001/archpsyc.1997.01830220013002. [DOI] [PubMed] [Google Scholar]
- Razoux F, Garcia R, Léna I. Ketamine, at a dose that disrupts motor behavior and latent inhibition, enhances prefrontal cortex synaptic efficacy and glutamate release in the nucleus accumbens. Neuropsychopharmacology. 2007;32:719–727. doi: 10.1038/sj.npp.1301057. [DOI] [PubMed] [Google Scholar]
- Reddy RD, Yao JK. Free radical pathology in schizophrenia: a review. Prostaglandins Leukot Essent Fatty Acids. 1996;55:33–43. doi: 10.1016/s0952-3278(96)90143-x. [DOI] [PubMed] [Google Scholar]
- Riley B, Kendler KS. Molecular genetic studies of schizophrenia. Eur J Hum Genet. 2006;14:669–680. doi: 10.1038/sj.ejhg.5201571. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Congy C, Santucci V, Simiand J, Gautret B, Neliat G, Labeeuw B, Le Fur G, Soubrie P, Breliere JC. Biochemical and pharmacological properties of SR 46349B, a new potent and selective 5-hydroxytryptamine2 receptor antagonist. J Pharmacol Exp Ther. 1992;262:759–768. [PubMed] [Google Scholar]
- Riva MA, Molteni R, Tascedda F, Massironi A, Racagni G. Selective modulation of fibroblast growth factor-2 expression in the rat brain by the atypical antipsychotic clozapine. Neuropharmacology. 1999;38:1075–1082. doi: 10.1016/s0028-3908(99)00031-3. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Fibiger HC. Effects of olanzapine on regional C-Fos expression in rat forebrain. Neuropsychopharmacology. 1996;14:105–110. doi: 10.1016/0893-133X(95)00196-K. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Matsumura H, Fibiger HC. Induction patterns of Fos-like immunoreactivity in the forebrain as predictors of atypical antipsychotic activity. J Pharmacol Exp Ther. 1994;271:1058–1066. [PubMed] [Google Scholar]
- Roh MS, Seo MS, Kim Y, Kim SH, Jeon WJ, Ahn YM, Kang UG, Juhnn YS, Kim YS. Haloperidol and clozapine differentially regulate signals upstream of glycogen synthase kinase 3 in the rat frontal cortex. Exp Mol Med. 2007;39:353–360. doi: 10.1038/emm.2007.39. [DOI] [PubMed] [Google Scholar]
- Roof RL, Duvdevani R, Braswell L, Stein DG. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp Neurol. 1994;129:64–69. doi: 10.1006/exnr.1994.1147. [DOI] [PubMed] [Google Scholar]
- Rosengarten H, Quartermain D. The effect of chronic treatment with typical and atypical antipsychotics on working memory and jaw movements in three- and eighteen-month-old rats. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:1047–1054. doi: 10.1016/s0278-5846(02)00221-x. [DOI] [PubMed] [Google Scholar]
- Rosenstein JM, Krum JM. New roles for VEGF in nervous tissue—beyond blood vessels. Exp Neurol. 2004;187:246–253. doi: 10.1016/j.expneurol.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Bristol LA, Hosler B, Brown RH, Jr, Kuncl RW. Chronic inhibition of superoxide dismutase produces apoptotic death of spinal neurons. Proc Natl Acad Sci U S A. 1994;91:4155–4159. doi: 10.1073/pnas.91.10.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roufogalis BD, Minocherhomjee AM, Al-Jobore A. Pharmacological antagonism of calmodulin. Can J Biochem Cell Biol. 1983;61:927–933. doi: 10.1139/o83-118. [DOI] [PubMed] [Google Scholar]
- Rowley HL, Kilpatrick IC, Needham PL, Heal DJ. Elevation of extracellular cortical noradrenaline may contribute to the antidepressant activity of zotepine: an in vivo microdialysis study in freely moving rats. Neuropharmacology. 1998;37:937–944. doi: 10.1016/s0028-3908(98)00094-x. [DOI] [PubMed] [Google Scholar]
- Rowley HL, Needham PL, Kilpatrick IC, Heal DJ. A comparison of the acute effects of zotepine and other antipsychotics on rat cortical dopamine release, in vivo. Naunyn Schmiedebergs Arch Pharmacol. 2000;361:187–192. doi: 10.1007/s002109900170. [DOI] [PubMed] [Google Scholar]
- Rujescu D, Bender A, Keck M, Hartmann AM, Ohl F, Raeder H, Giegling I, Genius J, McCarley RW, Möller HJ, et al. A pharmacological model for psychosis based on N-methyl-D-aspartate receptor hypofunction: molecular, cellular, functional and behavioral abnormalities. Biol Psychiatry. 2006;59:721–729. doi: 10.1016/j.biopsych.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Sakel M. The methodical use of hypoglycemia in the treatment of psychoses. Am J Psychiatry. 1994;151:240–247. doi: 10.1176/ajp.151.6.240. [DOI] [PubMed] [Google Scholar]
- Sanfilipo M, Lafargue T, Rusinek H, Arena L, Loneragan C, Lautin A, Feiner D, Rotrosen J, Wolkin A. Volumetric measure of the frontal and temporal lobe regions in schizophrenia: relationship to negative symptoms. Arch Gen Psychiatry. 2000;57:471–480. doi: 10.1001/archpsyc.57.5.471. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Sarter M, Nelson CL, Bruno JP. Cortical cholinergic transmission and cortical information processing in schizophrenia. Schizophr Bull. 2005;31:117–138. doi: 10.1093/schbul/sbi006. [DOI] [PubMed] [Google Scholar]
- Sastry PS, Rao KS. Apoptosis and the nervous system. J Neurochem. 2000;74:1–20. doi: 10.1046/j.1471-4159.2000.0740001.x. [DOI] [PubMed] [Google Scholar]
- Schaeffer HJ, Weber MJ. Mitogen-activated protein kinases: specific messages from ubiquitous messengers. Mol Cell Biol. 1999;19:2435–2444. doi: 10.1128/mcb.19.4.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheepers FE, de Wied CC, Hulshoff Pol HE, van de Flier W, van der Linden JA, Kahn RS. The effect of clozapine on caudate nucleus volume in schizophrenic patients previously treated with typical antipsychotics. Neuropsychopharmacology. 2001a;24:47–54. doi: 10.1016/S0893-133X(00)00172-X. [DOI] [PubMed] [Google Scholar]
- Scheepers FE, Gispen de Wied CC, Hulshoff Pol HE, Kahn RS. Effect of clozapine on caudate nucleus volume in relation to symptoms of schizophrenia. Am J Psychiatry. 2001b;158:644–646. doi: 10.1176/appi.ajp.158.4.644. [DOI] [PubMed] [Google Scholar]
- Schlesinger PH, Gross A, Yin XM, Yamamoto K, Saito M, Waksman G, Korsmeyer SJ. Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc Natl Acad Sci U S A. 1997;94:11357–11362. doi: 10.1073/pnas.94.21.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt CJ, Fadayel GM. Regional effects of MK-801 on dopamine release: effects of competitive NMDA or 5-HT2A receptor blockade. J Pharmacol Exp Ther. 1996;277:1541–1549. [PubMed] [Google Scholar]
- Schmitt A, Zink M, Petroianu G, May B, Braus DF, Henn FA. Decreased gene expression of glial and neuronal glutamate transporters after chronic anti-psychotic treatment in rat brain. Neurosci Lett. 2003;347:81–84. doi: 10.1016/s0304-3940(03)00653-0. [DOI] [PubMed] [Google Scholar]
- Schmitt GJ, Frodl T, Dresel S, la Fougère C, Bottlender R, Koutsouleris N, Hahn K, Möller HJ, Meisenzahl EM. Striatal dopamine transporter availability is associated with the productive psychotic state in first episode, drug-naïve schizophrenia patients. Eur Arch Psychiatry Clin Neurosci. 2006;256:115–121. doi: 10.1007/s00406-005-0618-2. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Wade T, Lidsky TI. Chronic neuroleptic treatment alters expression of glial glutamate transporter GLT-1 mRNA in the striatum. Neuroreport. 1998;9:133–136. doi: 10.1097/00001756-199801050-00026. [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, De Loore K, Leysen JE. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 1996;124:57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Akwa Y, Guennoun R, Robert F, Labombarda F, Desarnaud F, Robel P, De Nicola AF, Baulieu EE. Steroid synthesis and metabolism in the nervous system: trophic and protective effects. J Neurocytol. 2000;29:307–326. doi: 10.1023/a:1007152904926. [DOI] [PubMed] [Google Scholar]
- Schwarcz R, Rassoulpour A, Wu HQ, Medoff D, Tamminga CA, Roberts RC. Increased cortical kynurenate content in schizophrenia. Biol Psychiatry. 2001;50:521–530. doi: 10.1016/s0006-3223(01)01078-2. [DOI] [PubMed] [Google Scholar]
- See RE, Aravagiri M, Ellison GD. Chronic neuroleptic treatment in rats produces persisting changes in GABAA and dopamine D-2, but not dopamine D-1 receptors. Life Sci. 1989;44:229–236. doi: 10.1016/0024-3205(89)90600-0. [DOI] [PubMed] [Google Scholar]
- Seeger TF, Seymour PA, Schmidt AW, Zorn SH, Schulz DW, Lebel LA, McLean S, Guanowsky V, Howard HR, Lowe JA., 3rd Ziprasidone (CP-88,059): a new antipsychotic with combined dopamine and serotonin receptor antagonist activity. J Pharmacol Exp Ther. 1995;275:101–113. [PubMed] [Google Scholar]
- Seeman P, Ko F, Jack E, Greenstein R, Dean B. Consistent with dopamine supersensitivity, RGS9 expression is diminished in the amphetamine-treated animal model of schizophrenia and postmortem schizophrenia brain. Synapse. 2007;61:303–309. doi: 10.1002/syn.20368. [DOI] [PubMed] [Google Scholar]
- Seeman P, Lee T, Chau-Wong M, Wong K. Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature. 1976;261:717–719. doi: 10.1038/261717a0. [DOI] [PubMed] [Google Scholar]
- Seeman P, Schwarz J, Chen JF, Szechtman H, Perreault M, McKnight GS, Roder JC, Quirion R, Boksa P, Srivastava LK, et al. Psychosis pathways converge via D2High dopamine receptors. Synapse. 2006;60:319–346. doi: 10.1002/syn.20303. [DOI] [PubMed] [Google Scholar]
- Seeman P, Tallerico T. Rapid release of antipsychotic drugs from dopamine D2 receptors: An explanation for low receptor occupancy and early clinical relapse upon drug withdrawal of clozapine or quetiapine. Am J Psychiatry. 1999;156:876–884. doi: 10.1176/ajp.156.6.876. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Goldman-Rakic PS. The reduced neuropil hypothesis: a circuit based model of schizophrenia. Biol Psychiatry. 1999;45:17–25. doi: 10.1016/s0006-3223(98)00281-9. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28:1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]
- Shim SS, Hammonds MD, Kee BS. Potentiation of the NMDA receptor in the treatment of schizophrenia: focused on the glycine site. Eur Arch Psychiatry Clin Neurosci. 2008;258:16–27. doi: 10.1007/s00406-007-0757-8. [DOI] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Watanabe H, Komatsu N, Okamura N, Koike K, Shinoda N, Nakazato M, Kumakiri C, Okada S, et al. Serum brain-derived neurotrophic factor (BDNF) levels in schizophrenia are indistinguishable from controls. Neurosci Lett. 2003;351:111–114. doi: 10.1016/j.neulet.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Shirazi-Southall S, Rodriguez DE, Nomikos GG. Effects of typical and atypical antipsychotics and receptor selective compounds on acetylcholine efflux in the hippocampus of the rat. Neuropsychopharmacology. 2002;26:583–594. doi: 10.1016/S0893-133X(01)00400-6. [DOI] [PubMed] [Google Scholar]
- Shoval G, Weizman A. The possible role of neurotrophins in the pathogenesis and therapy of schizophrenia. Eur Neuropsychopharmacol. 2005;15:319–329. doi: 10.1016/j.euroneuro.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Shulman Y, Tibbo PG. Neuroactive steroids in schizophrenia. Can J Psychiatry. 2005;50:695–702. doi: 10.1177/070674370505001109. [DOI] [PubMed] [Google Scholar]
- Sigmundsson T, Suckling J, Maier M, Williams S, Bullmore E, Greenwood K, Fukuda R, Ron M, Toone B. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry. 2001;158:234–243. doi: 10.1176/appi.ajp.158.2.234. [DOI] [PubMed] [Google Scholar]
- Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27:1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JR, Isacson O. Mitochondrial impairment reduces the threshold for in vivo NMDA-mediated neuronal death in the striatum. Exp Neurol. 1993;121:57–64. doi: 10.1006/exnr.1993.1071. [DOI] [PubMed] [Google Scholar]
- Sirén AL, Knerlich F, Poser W, Gleiter CH, Brück W, Ehrenreich H. Erythropoietin and erythropoietin receptor in human ischemic/hypoxic brain. Acta Neuropathol (Berl) 2001;101:271–276. doi: 10.1007/s004010000297. [DOI] [PubMed] [Google Scholar]
- Skarsfeldt T. Differential effects of repeated administration of novel antipsychotic drugs on the activity of midbrain dopamine neurons in the rat. Eur J Pharmacol. 1995;281:289–294. doi: 10.1016/0014-2999(95)00260-r. [DOI] [PubMed] [Google Scholar]
- Skilbeck KJ, O’Reilly JN, Johnston GA, Hinton T. Antipsychotic drug administration differentially affects [3H]muscimol and [3H]flunitrazepam GABAA receptor binding sites. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:492–498. doi: 10.1016/j.pnpbp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;15:1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smythies J. Redox mechanisms at the glutamate synapse and their significance: a review. Eur J Pharmacol. 1999;370:1–7. doi: 10.1016/s0014-2999(99)00048-5. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–1281. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- Squires RF, Lajtha A, Saederup E, Palkovits M. Reduced [3H] flunitrazepam binding in cingulate cortex and hippocampus of postmortem schizophrenic brains: is selective loss of glutamatergic neurons associated with major psychoses? Neurochem Res. 1993;18:219–223. doi: 10.1007/BF01474687. [DOI] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- Steullet P, Neijt HC, Cuénod M, Do KQ. Synaptic plasticity impairment and hypofunction of NMDA receptors induced by glutathione deficit: relevance to schizophrenia. Neuroscience. 2006;137:807–819. doi: 10.1016/j.neuroscience.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Stockton ME, Rasmussen K. Electrophysiological effects of olanzapine, a novel atypical antipsychotic, on A9 and A10 dopamine neurons. Neuropsychopharmacology. 1996;14:97–105. doi: 10.1016/0893-133X(94)00130-R. [DOI] [PubMed] [Google Scholar]
- Stone JM, Davis JM, Leucht S, Pilowsky LS. Cortical dopamine D2/D3 receptors are a common site of action for antipsychotic drugs—an original patient data meta-analysis of the SPECT and PET in vivo receptor imaging literature. Schizophr Bull. 2008 doi: 10.1093/schbul/sbn009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss EB, Sands DE, Robinson AM, Tindall WJ, Stevenson WA. Use of dehydroisoandrosterone in psychiatric treatment; a preliminary survey. Br Med J. 1952;2:64–66. doi: 10.1136/bmj.2.4775.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous RD, Maayan R, Lapidus R, Stryjer R, Lustig M, Kotler M, Weizman A. Dehydroepiandrosterone augmentation in the management of negative, depressive, and anxiety symptoms in schizophrenia. Arch Gen Psychiatry. 2003;60:133–141. doi: 10.1001/archpsyc.60.2.133. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Anil AE, Jin D, Jayathilake K, Lee M, Meltzer HY. Plasma glycine and serine levels in schizophrenia compared to normal controls and major depression: relation to negative symptoms. Int J Neuropsychopharmacol. 2004;7:1–8. doi: 10.1017/S1461145703003900. [DOI] [PubMed] [Google Scholar]
- Sun Y, Jin K, Xie L, Childs J, Mao XO, Logvinova A, Greenberg DA. VEGF-induced neuroprotection, neurogenesis, and angiogenesis after focal cerebral ischemia. J Clin Invest. 2003;111:1843–1851. doi: 10.1172/JCI17977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Carruthers R, Rachleff I, Wattler S, Nehls M, Mc-Kinzie DL, Fienberg AA, Nomikos GG, Greengard P. Diverse psychotomimetics act through a common signaling pathway. Science. 2003;302:1412–1415. doi: 10.1126/science.1089681. [DOI] [PubMed] [Google Scholar]
- Swayze VW, 2nd, Andreasen NC, Alliger RJ, Yuh WT, Ehrhardt J. Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry. 1992;31:221–240. doi: 10.1016/0006-3223(92)90046-3. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Bakshi V, Geyer M. Seroquel restores sensorimotor gating in phencyclidine-treated rats. J Pharmacol Exp Ther. 1996;279:1290–1299. [PubMed] [Google Scholar]
- Tamminga CA. Schizophrenia and glutamatergic transmission. Crit Rev Neurobiol. 1998;12:21–36. doi: 10.1615/critrevneurobiol.v12.i1-2.20. [DOI] [PubMed] [Google Scholar]
- Tan YL, Zhou DF, Cao LY, Zou YZ, Zhang XY. Decreased BDNF in serum of patients with chronic schizophrenia on long-term treatment with antipsychotics. Neurosci Lett. 2005;382:27–32. doi: 10.1016/j.neulet.2005.02.054. [DOI] [PubMed] [Google Scholar]
- Tang AH, Franklin SR, Himes CS, Smith MW, Tenbrink RE. PNU-96415E, a potential antipsychotic agent with clozapine-like pharmacological properties. J Pharmacol Exp Ther. 1997;281:440–447. [PubMed] [Google Scholar]
- Taylor JL, Blanton RE, Levitt JG, Caplan R, Nobel D, Toga AW. Superior temporal gyrus differences in childhood-onset schizophrenia. Schizophr Res. 2005;73:235–241. doi: 10.1016/j.schres.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Parikh V, Gearhart DA, Pillai A, Hohnadel E, Warner S, Nasrallah HA, Mahadik SP. Time-dependent effects of haloperidol and ziprasidone on nerve growth factor, cholinergic neurons and spatial learning in rats. J Pharmacol Exp Ther. 2006;318:709–724. doi: 10.1124/jpet.105.099218. [DOI] [PubMed] [Google Scholar]
- Théberge J, Bartha R, Drost DJ, Menon RS, Malla A, Takhar J, Neufeld RW, Rogers J, Pavlosky W, Schaefer B, et al. Glutamate and glutamine measured with 4.0 T proton MRS in never-treated patients with schizophrenia and healthy volunteers. Am J Psychiatry. 2002;159:1944–1946. doi: 10.1176/appi.ajp.159.11.1944. [DOI] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- Tisch S, Silberstein P, Limousin-Dowsey P, Jahanshahi M. The basal ganglia: anatomy, physiology, and pharmacology. Psychiatr Clin North Am. 2004;27:757–799. doi: 10.1016/j.psc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Tkachev D, Mimmack ML, Ryan MM, Wayland M, Freeman T, Jones PB, Starkey M, Webster MJ, Yolken RH, Bahn S. Oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:798–805. doi: 10.1016/S0140-6736(03)14289-4. [DOI] [PubMed] [Google Scholar]
- Toki S, Saito T, Hatta S, Takahata N. Diazepam physical dependence and withdrawal in rats is associated with alteration in GABAA receptor function. Life Sci. 1996;59:1631–1641. doi: 10.1016/0024-3205(96)00494-8. [DOI] [PubMed] [Google Scholar]
- Tortorella A, Monteleone P, Fabrazzo M, Viggiano A, De Luca L, Maj M. Plasma concentrations of amino acids in chronic schizophrenics treated with clozapine. Neuropsychobiology. 2001;44:167–171. doi: 10.1159/000054937. [DOI] [PubMed] [Google Scholar]
- Toru M, Watanabe S, Shibuya H, Nishikawa T, Noda K, Mitsushio H, Ichikawa H, Kurumaji A, Takashima M, Mataga N. Neurotransmitters, receptors and neuropeptides in post-mortem brains of chronic schizophrenic patients. Acta Psychiatr Scand. 1988;78:121–137. doi: 10.1111/j.1600-0447.1988.tb06312.x. [DOI] [PubMed] [Google Scholar]
- Tosic M, Bovet P, Deppen P, Georghita F, Preisig M, Saraga M, Solida A, Cuenod M, Do KQ. Schizophrenia patients show reduced expression of the glutathione related genes. Soc Neurosci Abstr. 2004;30:909–1. [Google Scholar]
- Toyooka K, Asama K, Watanabe Y, Muratake T, Takahashi M, Someya T, Nawa H. Decreased levels of brain-derived neurotrophic factor in serum of chronic schizophrenic patients. Psychiatry Res. 2002;110:249–257. doi: 10.1016/s0165-1781(02)00127-0. [DOI] [PubMed] [Google Scholar]
- Tsai G, Passani LA, Slusher BS, Carter R, Baer L, Kleinman JE, Coyle JT. Abnormal excitatory neurotransmitter metabolism in schizophrenia brains. Arch Gen Psychiatry. 1995;52:829–836. doi: 10.1001/archpsyc.1995.03950220039008. [DOI] [PubMed] [Google Scholar]
- Turalba AV, Leite-Morris KA, Kaplan GB. Antipsychotics regulate cyclic AMP-dependent protein kinase and phosphorylated cyclic AMP response element-binding protein in striatal and cortical brain regions in mice. Neurosci Lett. 2004;357:53–57. doi: 10.1016/j.neulet.2003.11.059. [DOI] [PubMed] [Google Scholar]
- Ugale RR, Hirani K, Morelli M, Chopde CT. Role of neuroactive steroid allopregnanolone in antipsychotic-like action of olanzapine in rodents. Neuropsychopharmacology. 2004;29:1597–1609. doi: 10.1038/sj.npp.1300460. [DOI] [PubMed] [Google Scholar]
- Ukai W, Ozawa H, Tateno M, Hashimoto E, Saito T. Neurotoxic potential of haloperidol in comparison with risperidone: implication of Akt-mediated signal changes by haloperidol. J Neural Transm. 2004;111:667–681. doi: 10.1007/s00702-004-0109-z. [DOI] [PubMed] [Google Scholar]
- Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci U S A. 1998;95:3239–3244. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valjent E, Pagès C, Hervé D, Girault JA, Caboche J. Addictive and non-addictive drugs induce distinct and specific patterns of ERK activation in mouse brain. Eur J Neurosci. 2004;19:1826–1836. doi: 10.1111/j.1460-9568.2004.03278.x. [DOI] [PubMed] [Google Scholar]
- Vallée M, Mayo W, Darnaudéry M, Corpéchot C, Young J, Koehl M, Le Moal M, Baulieu EE, Robel P, Simon H. Neurosteroids: deficient cognitive performance in aged rats depends on low pregnenolone sulfate levels in the hippocampus. Proc Natl Acad Sci U S A. 1997;94:14865–14870. doi: 10.1073/pnas.94.26.14865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallée M, Purdy RH, Mayo W, Koob GF, Le Moal M. Neuroactive steroids: new biomarkers of cognitive aging. J Steroid Biochem Mol Biol. 2003;85:329–335. doi: 10.1016/s0960-0760(03)00227-9. [DOI] [PubMed] [Google Scholar]
- Vallejo-Illarramendi A, Torres-Ramos M, Melone M, Conti F, Matute C. Clozapine reduces GLT-1 expression and glutamate uptake in astrocyte cultures. Glia. 2005;50:276–279. doi: 10.1002/glia.20172. [DOI] [PubMed] [Google Scholar]
- van der Heijden FM, Tuinier S, Fekkes D, Sijben AE, Kahn RS, Verhoeven WM. Atypical antipsychotics and the relevance of glutamate and serotonin. Eur Neuropsychopharmacol. 2004;14:259–265. doi: 10.1016/j.euroneuro.2003.09.002. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Hulshoff Pol HE, Schnack HG, Cahn W, Mandl RC, Collins DL, Evans AC, Kahn RS. Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology. 2007;32:2057–2066. doi: 10.1038/sj.npp.1301347. [DOI] [PubMed] [Google Scholar]
- Veldic M, Caruncho HJ, Liu WS, Davis J, Satta R, Grayson DR, Guidotti A, Costa E. DNA methyltransferase-1 (DNMT1) is selectively overexpressed in telencephalic cortical GABAergic interneurons of schizophrenia brains. Proc Natl Acad Sci U S A. 2004;101:348–353. doi: 10.1073/pnas.2637013100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldic M, Guidotti A, Maloku E, Davis JM, Costa E. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc Natl Acad Sci U S A. 2005;102:2152–2157. doi: 10.1073/pnas.0409665102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrall L, Walker M, Rawlings N, Benzel I, Kew JN, Harrison PJ, Burnet PW. D-Amino acid oxidase and serine racemase in human brain: normal distribution and altered expression in schizophrenia. Eur J Neurosci. 2007;26:1657–1669. doi: 10.1111/j.1460-9568.2007.05769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volonté M, Monferini E, Cerutti M, Fodritto F, Borsini F. BIMG 80, a novel potential antipsychotic drug: evidence for multireceptor actions and preferential release of dopamine in prefrontal cortex. J Neurochem. 1997;69:182–190. doi: 10.1046/j.1471-4159.1997.69010182.x. [DOI] [PubMed] [Google Scholar]
- Wakade CG, Mahadik SP, Waller JL, Chiu FC. Atypical neuroleptics stimulate neurogenesis in adult rat brain. J Neurosci Res. 2002;69:72–79. doi: 10.1002/jnr.10281. [DOI] [PubMed] [Google Scholar]
- Walterfang M, Wood SJ, Velakoulis D, Pantelis C. Neuropathological, neurogenetic and neuroimaging evidence for white matter pathology in schizophrenia. Neurosci Biobehav Rev. 2006;30:918–948. doi: 10.1016/j.neubiorev.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Wang C, Anastasio N, Popov V, Leday A, Johnson KM. Blockade of N-methyl-D-aspartate receptors by phencyclidine causes the loss of corticostriatal neurons. Neuroscience. 2004a;125:473–483. doi: 10.1016/j.neuroscience.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Wang C, Fridley J, Johnson KM. The role of NMDA receptor upregulation in phencyclidine-induced cortical apoptosis in organotypic culture. Biochem Pharmacol. 2005a;69:1373–1383. doi: 10.1016/j.bcp.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Wang C, Kaufmann JA, Sanchez-Ross MG, Johnson KM. Mechanisms of N-methyl-D-aspartate-induced apoptosis in phencyclidine-treated cultured forebrain neurons. J Pharmacol Exp Ther. 2000;294:287–295. [PubMed] [Google Scholar]
- Wang C, McInnis J, Ross-Sanchez M, Shinnick-Gallagher P, Wiley JL, Johnson KM. Long-term behavioral and neurodegenerative effects of perinatal phencyclidine administration: implications for schizophrenia. Neuroscience. 2001;107:535–550. doi: 10.1016/s0306-4522(01)00384-0. [DOI] [PubMed] [Google Scholar]
- Wang C, McInnis J, West JB, Bao J, Anastasio N, Guidry JA, Ye Y, Salvemini D, Johnson KM. Blockade of phencyclidine-induced cortical apoptosis and deficits in prepulse inhibition by M40403, a superoxide dismutase mimetic. J Pharmacol Exp Ther. 2003;304:266–271. doi: 10.1124/jpet.102.041798. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Johnson KM. Differential effects of acute and subchronic administration on phencyclidine-induced neurodegeneration in the perinatal rat. J Neurosci Res. 2005;81:284–292. doi: 10.1002/jnr.20559. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Johnson KM. The role of caspase-3 activation in phencyclidine-induced neuronal death in postnatal rats. Neuropsychopharmacology. 2007;32:1178–1194. doi: 10.1038/sj.npp.1301202. [DOI] [PubMed] [Google Scholar]
- Wang CZ, Yang SF, Xia Y, Johnson KM. Postnatal phencyclidine administration selectively reduces adult cortical parvalbumin-containing interneurons. Neuropsychopharmacology. 2008;33:2442–2455. doi: 10.1038/sj.npp.1301647. [DOI] [PubMed] [Google Scholar]
- Wang HD, Deutch AY. Dopamine depletion of the prefrontal cortex induces dendritic spine loss: reversal by atypical antipsychotic drug treatment. Neuropsychopharmacology. 2008;33:1276–1286. doi: 10.1038/sj.npp.1301521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HD, Dunnavant FD, Jarman T, Deutch AY. Effect of antipsychotic drugs on neurogenesis in the forebrain of the adult rat. Neuropsychopharmacology. 2004b;29:1230–1238. doi: 10.1038/sj.npp.1300449. [DOI] [PubMed] [Google Scholar]
- Wang H, Xu H, Dyck LE, Li XM. Olanzapine and quetiapine protect PC12 cells from beta-amyloid peptide(25–35)-induced oxidative stress and the ensuing apoptosis. J Neurosci Res. 2005b;81:572–580. doi: 10.1002/jnr.20570. [DOI] [PubMed] [Google Scholar]
- Wang JM, Johnston PB, Ball BG, Brinton RD. The neurosteroid allopregnanolone promotes proliferation of rodent and human neural progenitor cells and regulates cell-cycle gene and protein expression. J Neurosci. 2005c;25:4706–4718. doi: 10.1523/JNEUROSCI.4520-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RY, Liang X. M100907 and clozapine, but not haloperidol or raclopride, prevent phencyclidine-induced blockade of NMDA responses in pyramidal neurons of the rat medial prefrontal cortical slice. Neuropsychopharmacology. 1998;19:74–85. doi: 10.1016/S0893-133X(98)00003-7. [DOI] [PubMed] [Google Scholar]
- Wassef A, Baker J, Kochan LD. GABA and schizophrenia: a review of basic science and clinical studies. J Clin Psychopharmacol. 2003;23:601–640. doi: 10.1097/01.jcp.0000095349.32154.a5. [DOI] [PubMed] [Google Scholar]
- Wei Z, Bai O, Richardson JS, Mousseau DD, Li XM. Olanzapine protects PC12 cells from oxidative stress induced by hydrogen peroxide. J Neurosci Res. 2003a;73:364–368. doi: 10.1002/jnr.10668. [DOI] [PubMed] [Google Scholar]
- Wei Z, Mousseau DD, Richardson JS, Dyck LE, Li XM. Atypical antipsychotics attenuate neurotoxicity of β-amyloid(25–35) by modulating Bax and Bcl-X(l/s) expression and localization. J Neurosci Res. 2003b;74:942–947. doi: 10.1002/jnr.10832. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Hyde TM, Lipska BK, Herman MM, Weinberger DR, Kleinman JE. Reduced brain-derived neurotrophic factor in prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2003;8:592–610. doi: 10.1038/sj.mp.4001308. [DOI] [PubMed] [Google Scholar]
- Weickert CS, Ligons DL, Romanczyk T, Ungaro G, Hyde TM, Herman MM, Weinberger DR, Kleinman JE. Reductions in neurotrophin receptor mRNAs in the prefrontal cortex of patients with schizophrenia. Mol Psychiatry. 2005;10:637–650. doi: 10.1038/sj.mp.4001678. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Illowsky BP. Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia. III. A new cohort and evidence for a monoaminergic mechanism. Arch Gen Psychiatry. 1988;45:609–615. doi: 10.1001/archpsyc.1988.01800310013001. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Burstein ES, Nash N, Croston GE, Currier EA, Vanover KE, Harvey SC, Donohue E, Hansen HC, Andersson CM, et al. 5-Hydroxytryptamine2A receptor inverse agonists as antipsychotics. J Pharmacol Exp Ther. 2001;299:268–276. [PubMed] [Google Scholar]
- Weiss B, Earl C, Prozialeck WC. Biochemical and possible neuropsychopharmacological implications of inhibiting calmodulin activity. Psychopharmacol Bull. 1983;19:378–386. [PubMed] [Google Scholar]
- Weller M, Paul SM. 3-Nitropropionic acid is an indirect excitotoxin to cultured cerebellar granule neurons. Eur J Pharmacol. 1993;248:223–228. doi: 10.1016/0926-6917(93)90048-u. [DOI] [PubMed] [Google Scholar]
- White FJ, Wang RY. Differential effects of classical and atypical antipsychotic drugs on A9 and A10 dopamine neurons. Science. 1983;221:1054–1057. doi: 10.1126/science.6136093. [DOI] [PubMed] [Google Scholar]
- Whiting PJ. GABAA receptor subtypes n the brain: a paradigm for CNS drug discovery? Drug Discov Today. 2003;8:445–450. doi: 10.1016/s1359-6446(03)02703-x. [DOI] [PubMed] [Google Scholar]
- Wieland S, Lan NC, Mirasedeghi S, Gee KW. Anxiolytic activity of the progesterone metabolite 5α-pregnan-3α-o1–20-one. Brain Res. 1991;565:263–268. doi: 10.1016/0006-8993(91)91658-n. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Porter JH. Serotonergic drugs do not substitute for clozapine in clozapine-trained rats in a two-lever drug discrimination procedure. Pharmacol Biochem Behav. 1992;43:961–965. doi: 10.1016/0091-3057(92)90433-g. [DOI] [PubMed] [Google Scholar]
- Wolkin A, Jaeger J, Brodie JD, Wolf AP, Fowler J, Rotrosen J, Gomez-Mont F, Cancro R. Persistence of cerebral metabolic abnormalities in chronic schizophrenia as determined by positron emission tomography. Am J Psychiatry. 1985;142:564–571. doi: 10.1176/ajp.142.5.564. [DOI] [PubMed] [Google Scholar]
- Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, Neiswender H, Dong XP, Wu J, Gassmann M, et al. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Woodruff-Pak DS, Gould TJ. Neuronal nicotinic acetylcholine receptors: involvement in Alzheimer’s disease and schizophrenia. Behav Cogn Neurosci Rev. 2002;1:5–20. doi: 10.1177/1534582302001001002. [DOI] [PubMed] [Google Scholar]
- Woods BT. Is schizophrenia a progressive neurodevelopmental disorder? Toward a unitary pathogenetic mechanism. Am J Psychiatry. 1998;155:1661–1670. doi: 10.1176/ajp.155.12.1661. [DOI] [PubMed] [Google Scholar]
- Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. doi: 10.1176/ajp.157.1.16. [DOI] [PubMed] [Google Scholar]
- Wroblewska B, Wroblewski JT, Pshenichkin S, Surin A, Sullivan SE, Neale JH. N-Aceylaspartylglutamate selectively activates mGluR3 receptors in transfected cells. J Neurochem. 1997;69:174–181. doi: 10.1046/j.1471-4159.1997.69010174.x. [DOI] [PubMed] [Google Scholar]
- Wu FS, Gibbs TT, Farb DH. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor. Mol Pharmacol. 1991;40:333–336. [PubMed] [Google Scholar]
- Wu Y, Rosenberg HC, Chiu TH, Zhao TJ. Subunit- and brain region-specific reduction of GABAA receptor subunit mRNAs during chronic treatment of rats with diazepam. J Mol Neurosci. 1994;5:105–120. doi: 10.1007/BF02736752. [DOI] [PubMed] [Google Scholar]
- Wyatt RJ. Neuroleptics and the natural course of schizophrenia. Schizophr Bull. 1991;17:325–351. doi: 10.1093/schbul/17.2.325. [DOI] [PubMed] [Google Scholar]
- Xu H, Qing H, Lu W, Keegan D, Richardson JS, Chlan-Fourney J, Li XM. Quetiapine attenuates the immobilization stress-induced decrease of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci Lett. 2002;321:65–68. doi: 10.1016/s0304-3940(02)00034-4. [DOI] [PubMed] [Google Scholar]
- Yamada K, Ohnishi T, Hashimoto K, Ohba H, Iwayama-Shigeno Y, Toyoshima M, Okuno A, Takao H, Toyota T, Minabe Y, et al. Identification of multiple serine racemase (SRR) mRNA isoforms and genetic analyses of SRR and DAO in schizophrenia and D-serine levels. Biol Psychiatry. 2005;57:1493–1503. doi: 10.1016/j.biopsych.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Yamakura T, Shimoji K. Subunit- and site-specific pharmacology of the NMDA receptor channel. Prog Neurobiol. 1999;59:279–298. doi: 10.1016/s0301-0082(99)00007-6. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Pehek EA, Meltzer HY. Brain region effects of clozapine on amino acid and monoamine transmission. J Clin Psychiatry. 1994;55(Suppl B):8–14. [PubMed] [Google Scholar]
- Yamamoto K, Hornykiewicz O. Proposal for a noradrenaline hypothesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:913–922. doi: 10.1016/j.pnpbp.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Yan XX, Najbauer J, Woo CC, Dashtipour K, Ribak CE, Leon M. Expression of active caspase-3 in mitotic and postmitotic cells of the rat forebrain. J Comp Neurol. 2001;433:4–22. doi: 10.1002/cne.1121. [DOI] [PubMed] [Google Scholar]
- Yang BH, Son H, Kim SH, Nam JH, Choi JH, Lee JS. Phosphorylation of ERK and CREB in cultured hippocampal neurons after haloperidol and risperidone administration. Psychiatry Clin Neurosci. 2004;58:262–267. doi: 10.1111/j.1440-1819.2004.01229.x. [DOI] [PubMed] [Google Scholar]
- Yang TT, Wang SJ. Effects of haloperidol and clozapine on glutamate release from nerve terminals isolated from rat prefrontal cortex. Synapse. 2005;56:12–20. doi: 10.1002/syn.20123. [DOI] [PubMed] [Google Scholar]
- Yao JK, Leonard S, Reddy RD. Increased nitric oxide radicals in postmortem brain from patients with schizophrenia. Schizophr Bull. 2004;30:923–934. doi: 10.1093/oxfordjournals.schbul.a007142. [DOI] [PubMed] [Google Scholar]
- Yao JK, Leonard S, Reddy R. Altered glutathione redox state in schizophrenia. Dis Markers. 2006;22:83–93. doi: 10.1155/2006/248387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Milatovic D, Schantz AM, Zhang J, Montine KS, Samii A, Deutch AY, Montine TJ. Dendritic degeneration in neostriatal medium spiny neurons in Parkinson disease. Neurology. 2005;64:545–547. doi: 10.1212/01.WNL.0000150591.33787.A4. [DOI] [PubMed] [Google Scholar]
- Zhang W, Perry KW, Wong DT, Potts BD, Bao J, Tollefson GD, Bymaster FP. Synergistic effects of olanzapine and other antipsychotic agents in combination with fluoxetine on norepinephrine and dopamine release in rat prefrontal cortex. Neuropsychopharmacology. 2000;23:250–262. doi: 10.1016/S0893-133X(00)00119-6. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Tan YL, Cao LY, Wu GY, Xu Q, Shen Y, Zhou DF. Antioxidant enzymes and lipid peroxidation in different forms of schizophrenia treated with typical and atypical antipsychotics. Schizophr Res. 2006;81:291–300. doi: 10.1016/j.schres.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Zhou DF, Cao LY, Zhang PY, Wu GY. Elevated blood superoxide dismutase in neuroleptic-free schizophrenia: association with positive symptoms. Psychiatry Res. 2003;117:85–88. doi: 10.1016/s0165-1781(02)00303-7. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Ksiezak-Reding H, Riggio S, Haroutunian V, Pasinetti GM. Insulin receptor deficits in schizophrenia and in cellular and animal models of insulin receptor dysfunction. Schizophr Res. 2006;84:1–14. doi: 10.1016/j.schres.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Zink M, Schmitt A, May B, Müller B, Braus DF, Henn FA. Differential effects of long-term treatment with clozapine or haloperidol on GABA transporter expression. Pharmacopsychiatry. 2004a;37:171–174. doi: 10.1055/s-2004-827173. [DOI] [PubMed] [Google Scholar]
- Zink M, Schmitt A, May B, Müller B, Demirakca T, Braus DF, Henn FA. Differential effects of long-term treatment with clozapine or haloperidol on GABAA receptor binding and GAD67 expression. Schizophr Res. 2004b;66:151–157. doi: 10.1016/S0920-9964(03)00088-4. [DOI] [PubMed] [Google Scholar]
- Zocchi A, Fabbri D, Heidbreder CA. Aripiprazole increases dopamine but not noradrenaline and serotonin levels in the mouse prefrontal cortex. Neurosci Lett. 2005;387:157–161. doi: 10.1016/j.neulet.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Zorn SH, Jones SB, Ward KM, Liston DR. Clozapine is a potent and selective muscarinic M4 receptor agonist. Eur J Pharmacol. 1994;269:R1–2. doi: 10.1016/0922-4106(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Zuo DY, Zhang YH, Cao Y, Wu CF, Tanaka M, Wu YL. Effect of acute and chronic MK-801 administration on extracellular glutamate and ascorbic acid release in the prefrontal cortex of freely moving mice on line with open-field behavior. Life Sci. 2006;78:2172–2178. doi: 10.1016/j.lfs.2005.09.022. [DOI] [PubMed] [Google Scholar]