Abstract
Background and Purpose
The presence of white-matter hyperintensities (WMHs) has been linked to intracerebral hemorrhage (ICH). We sought to determine whether the severity of WMHs influences hematoma growth and ICH volume.
Methods
We retrospectively reviewed prospectively collected clinical, laboratory, and radiologic data from 79 consecutive ICH patients who had brain magnetic resonance imaging performed within 72 hours of ICH symptom onset. We assessed the severity of WMHs on magnetic resonance imaging on the modified Scheltens scale and performed logistic-regression analysis to examine the association between WMHs and ICH volume. We also examined the association between WMH score and hematoma growth in a subset of 34 patients who had a baseline computed tomography scan within 12 hours of ICH onset and a follow-up scan within 72 hours.
Results
The ICH volume at 37.6±22.3 hours from ICH onset was 2-fold higher in patients with a high WMH score (≥14) than in those with a lower score. A high WMH score was independently associated with a larger ICH volume (odds ratio=1.152; 95% CI, 1.035 to 1.282; P=0.01). There was a trend for an association between WMH score and ICH volume growth (odds ratio=1.286; 95% CI, 0.978 to 1.692; P=0.062).
Conclusion
Severe WMHs are associated with larger ICH volumes and, to a lesser extent, with hematoma growth. Our findings suggest that WMHs may provide important prognostic information on patients with ICH and may have implications for treatment stratification. These findings require prospective validation, and the links between WMHs and ICH growth require further investigations.
Keywords: white matter hyperintensities, hematoma, leukoaraiosis, intracerebral hemorrhage
White-matter hyperintensities (WMHs), also referred to as leukoaraiosis, are often seen on brain magnetic resonance imaging (MRI) scans of older patients and appear as increased signal intensity on T2-weighted and fluid-attenuated inversion recovery (FLAIR) sequences (the Figure). Several studies have linked WMHs to intracerebral hemorrhage (ICH).1,2 The presence of WMHs is a risk factor for ICH during oral anticoagulation and after treatment with tissue plasminogen activator in ischemic stroke patients.3–5 WMHs are also correlated with the risk of recurrent lobar ICHs.6 WMHs are thought to represent areas of white-matter ischemic damage attributed to degenerative changes of small vessels, including intimal hyperplasia, atherosclerosis, lipohyalinosis, and amyloidosis.7 Studies examining the histopathologic correlates of WMHs have revealed diffuse vacuolization and spongiform changes, arteriosclerosis, tissue rarefaction associated with loss of myelin and axons, widening of perivascular spaces, and a reduction in the densities of glial cells.8,9 Emerging literature suggests that the development of WMHs is related to diminished regional cerebral blood flow, disruption of capillary permeability, and a damaged blood-brain barrier (BBB).7,10–13 Therefore, we hypothesized that the severity of WMHs, as an indicator of their impact on underlying brain tissue density and on vascular and BBB damage, would be correlated with hematoma volume, hematoma expansion, and perihematoma edema (PHE), and we undertook the present study to examine these associations.
Figure.
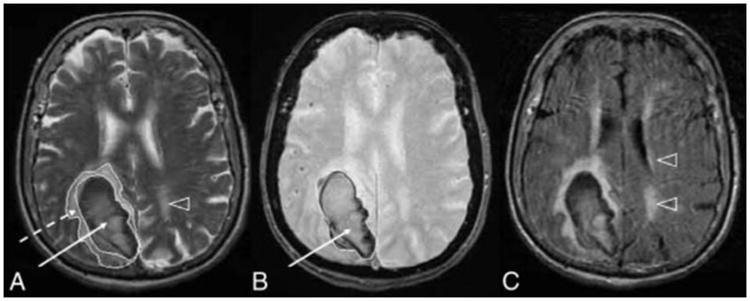
Examples of acute ICH and deep WMHs on MRI T2-weighted (A), GRE (B), and FLAIR (C) images in a patient with lobar ICH. Arrowheads point to WMHs; dashed arrow, to PHE; and solid arrow, to the hematoma.
Methods
Patient Selection and Data Collection
We retrospectively reviewed our prospectively collected database for consecutive patients with ICH admitted during 2006 to 2008. We identified patients with ICH age ≥45 years who had brain MRI performed within 72 hours of ICH symptom onset, when known, or from the last time the patient was known to be symptom-free. Patients with secondary causes of ICH, including underlying aneurysm, vascular malformation or tumor, head trauma, venous infarction, or hemorrhagic transformation of ischemic infarction, and patients who underwent surgical evacuation or craniectomy before MRI were excluded.
We retrieved baseline clinical and demographic information, including age; sex; comorbid conditions, including history of hypertension, atrial fibrillation, diabetes mellitus, coronary artery disease, dementia, hyperlipidemia, ischemic stroke or transient ischemic attack, or prior ICHs; medications used on admission, such as antiplatelet agents, anticoagulants, and statins; systolic and diastolic blood pressures on initial evaluation; and laboratory results, including serum glucose level, platelet count, international normalized ratio, and partial thromboplastin time.
Radiologic Data
MRI Acquisition and Analysis
All MRI studies were performed according to a standardized protocol including T2-weighted, T2* gradient-echo (GRE), and FLAIR imaging. The severity of WMHs was rated visually on axial FLAIR images on the modified Scheltens scale, in which scores ranging from 0 to 2 are given for 3 periventricular regions (frontal horn, occipital horn, and lateral bands) and scores from 0 to 6 are given for frontal, parietal, and occipital subcortical white-matter regions, depending on the size and number of the lesions (supplemental Table I, available online at http://stroke.ahajournals.org).14 The ratings for the aforementioned regions were summed to obtain a total score, where a higher score indicates a more severe WMH. Because some hemorrhages obscured the underlying brain parenchyma, we analyzed WMHs in the hemisphere without ICH (the Figure). The images were reviewed independently by 2 raters on 20 randomly selected scans. The weighted kappa scores for interrater and intrarater agreements were 0.84 and 0.83, respectively. Therefore, only 1 operator analyzed the remaining MRIs.
Axial T2-weighted images were used to measure the hematoma and PHE volumes on MRI with the use of National Institutes of Health (NIH) image-processing software. Experienced operators manually drew regions of interest by tracing the perimeters of the hematoma and PHE in each slice, throughout the hemorrhagic lesion, as described previously.15 The traced regions of interest ROIs in the contiguous voxels were then summed after adjusting for slice thickness to yield a hematoma volume and an absolute edema volume (the volume of the surrounding PHE). We then calculated the relative PHE volume according to the following equation: relative PHE volume=absolute edema volume/hematoma volume, to express the PHE volume as a ratio of the associated hematoma volume. The intraclass correlation coefficients for intra- and inter-rater agreements for ICH volumetric measurements were 0.99 and 0.98 and for PHE measurements, were 0.95 and 0.96, respectively. Hematoma location and the presence of intraventricular hemorrhage were noted; ICH was defined as lobar when a cortical region was involved.
We used axial GRE images to detect the presence of microbleeds in both hemispheres. Microbleeds were defined as focal areas of very low signal intensity on GRE scans, smaller than 10 mm. Signal voids caused by sulcal vessels, calcifications in the basal ganglia, choroid plexus, pineal calcifications, and low-signal averaging from adjacent bone were excluded.16
CT Acquisition and Analysis
To determine hematoma growth, we identified patients who had both baseline computed tomography (CT) scans within 12 hours and repeated CT scans within 72 hours of ICH onset. We limited the baseline scan to 12 hours because most of the hematoma expansion occurs during the first 24 hours.17 Hematoma volumes were measured with NIH imaging software, as previously described. CT scans were reviewed independently by 2 investigators. The test-retest intraclass correlation coefficients for inter- and intra-observer agreements were both 0.99.
Statistical Analysis
Patients were dichotomized according to ICH volume as measured on MRI into those with ICH volume ≥30 mL (large) versus those with ICHs <30 mL (small), on the basis of the prognostic utility of this cutoff value in previous studies.18 ICH volume growth was calculated by subtracting the baseline ICH volume from the follow-up volume on CT scans and dividing the product by the baseline volume to express the change in volume as a ratio of the initial ICH volume; ICH expansion was defined as an increase in ICH volume ≥30%. We used Fisher's exact test to compare dichotomous variables between groups and the Wilcoxon rank-sum test for continuous and ordinal variables. Variables with a P<0.1 in univariate regression analyses were included in the multivariate logistic-regression models. The Spearman correlation coefficient was used to determine the correlation between WMHs, ICH volume, number of microbleeds, ICH volume growth, and PHE. Statistical significance was set at P≤0.05.
Results
We identified 243 consecutive ICH patients who presented during the study period. Of these, 129 patients did not have an MRI, 18 had a secondary cause for ICH, and 6 underwent surgical evacuation before MRI. A total of 90 subjects met our inclusion criteria and none of our exclusion criteria. Of these, 11 with a prior history of stroke or ICH were excluded to avoid the confounding effect of prior lesions on radiologic assessments. The remaining 79 were included in this analysis. Their ages ranged from 45 to 96 years (mean±SD, 73±14). Forty-seven patients had lobar and 32 had nonlobar hemorrhages; intraventricular hemorrhage was present in 10 patients and was seen in 6% of patients with lobar hemorrhages compared with 22% of nonlobar ICH cases (P=0.047). The mean time from ICH onset to MRI was 37.6±22.3 hours. Twenty-six patients (33%) were discharged to home and 63% to extended-care facilities and rehabilitation; 4% died during hospitalization. There were no significant differences in baseline characteristics and ICH location between the patients who were included in our analysis and those who were not. As expected, in-hospital mortality was higher among patients who did not undergo MRI (16%; P=0.006).
The total WMH scores ranged from 1 to 23, with a median of 14 (mean±SD, 12.7±6.4). Table 1 shows the baseline characteristics of the cohort classified according to WMH score dichotomized at the median. Patients with a higher WMH score were older and had a higher prevalence of microbleeds. As expected, the total WMH score was correlated with age (r=0.361; P=0.001). It was also correlated with the total number of microbleeds in both hemispheres (r=0.272; P=0.015) and in the hemisphere ipsilateral to the ICH (r=0.263; P=0.019). More patients with a lower WMH score were discharged to home compared with those having a higher score (44% vs 23%; P=0.058). All of the patients who died during hospitalization had higher WMH scores.
Table 1. Baseline Characteristics Classified According to WMH Score Dichotomized at the Median.
| WMH Score | P Value | ||
|---|---|---|---|
|
| |||
| 0–13 (n=39) | 14–24 (n=40) | ||
| Age, y, mean±SD | 68±13 | 77±13 | 0.003 |
| Female | 13 (33%) | 18 (45%) | 0.359 |
| Past medical history, n | |||
| Hypertension | 28 (72%) | 25 (63%) | 0.474 |
| Atrial fibrillation | 4 (10%) | 9 (23%) | 0.225 |
| Coronary artery disease | 5 (13%) | 11 (28%) | 0.161 |
| Diabetes mellitus | 10 (26%) | 6 (15%) | 0.274 |
| Hyperlipidemia | 11 (28%) | 8 (20%) | 0.439 |
| Dementia | 0 (0%) | 3 (8%) | 0.241 |
| Clinical variables | |||
| Glucose, mg/dL | 140.8±56.8 | 130.9±34.0 | 0.806 |
| Systolic blood pressure, mm Hg | 159.0±28.1 | 156.3±27.6 | 0.617 |
| Diastolic blood pressure, mm Hg | 82.3±21.5 | 83.1 ±20.8 | 0.830 |
| Platelet count, 1000/μL | 250.6±78.0 | 243.4±95.2 | 0.593 |
| Partial thromboplastin time, sec | 26.1 ±3.2 | 27.3±4.2 | 0.405 |
| International Normalized Ratio | 1.2±0.5 | 1.4±0.6 | 0.051 |
| Medications, n | |||
| Use of antiplatelet agents on admission | 9 (23%) | 12 (30%) | 0.612 |
| Use of warfarin on admission | 4 (10%) | 8 (20%) | 0.348 |
| Use of statin | 11 (28%) | 7 (18%) | 0.293 |
| Radiologic data | |||
| Time to MRI, h | 39.2±24.8 | 36.0±20.0 | 0.967 |
| Lobar ICH, n | 20 (51%) | 27 (68%) | 0.173 |
| ICH volume, mL | 17.4±20.8 | 36.0±45.2 | 0.022 |
| PHE volume, mL | 19.6±17.9 | 26.2±18.7 | 0.114 |
| Relative PHE | 1.60±0.86 | 1.25±0.80 | 0.05 |
| Presence of intraventricular hemorrhage, n | 6 (15%) | 4 (10%) | 0.518 |
| Presence of microbleed, n | 3 (8%) | 11 (28%) | 0.037 |
| No. of microbleeds | |||
| Total (both hemispheres) | 2.1 ±7.5 | 5.2±10.8 | 0.042 |
| Ipsilateral to ICH hemisphere | 1.3±4.3 | 2.7±5.2 | 0.024 |
| Discharge destination | |||
| Home | 17 (44%) | 9 (23%) | 0.058 |
| Extended-care facilities | 22 (56%) | 28 (70%) | 0.248 |
| Died during hospitalization | 0 | 3 (8%) | 0.241 |
The mean ICH volume was twice as high in patients with a high WMH score than in those with a low WMH score, and there was a significant correlation between WMH score and ICH volume on MRI (r=0.30; P=0.007). We did not find a significant difference in the absolute edema volumes between patients with high and low WMH scores (26.2±18.7 vs 19.6±17.9 mL; P=0.114). The relative PHE volume was lower in patients with high versus low WMH scores (1.25±0.80 vs 1.60±0.86; P=0.05). There was no correlation between the total number of microbleeds and ICH volume (P=0.433).
Tables 2 and 3 list the results of univariate and multivariate logistic-regression analyses of the risk factors for ICH volume ≥30 mL. Only a high WMH score was independently associated with large hemorrhage ≥30 mL on multivariate regression.
Table 2. Univariate Risk Factors for Large Hemorrhage (ICH Volume ≥30 mL).
| ICH Volume | P Value | ||
|---|---|---|---|
|
| |||
| Large (n=22) | Small (n=57) | ||
| Age, y, mean±SD | 76±12 | 71±14 | 0.125 |
| Female | 9 (41%) | 22 (39%) | 1.0 |
| Past medical history, n | |||
| Hypertension | 12 (55%) | 41 (72%) | 0.183 |
| Atrial fibrillation | 4 (18%) | 9 (16%) | 0.748 |
| Coronary artery disease | 4 (18%) | 12 (21%) | 1.0 |
| Diabetes mellitus | 3 (14%) | 13 (23%) | 0.535 |
| Hyperlipidemia | 3 (14%) | 16 (28%) | 0.245 |
| Dementia | 3 (14%) | 0 (0%) | 0.019 |
| Clinical variables | |||
| Glucose, mg/dL | 141.9±45.4 | 133.4±47.3 | 0.373 |
| Systolic blood pressure, mm Hg | 154.6±33.2 | 158.8±25.4 | 0.337 |
| Diastolic blood pressure, mm Hg | 83.4±18.1 | 82.4±22.1 | 0.672 |
| Platelet count, 1000/μL | 238.3±54.3 | 250.3±96.5 | 0.831 |
| Partial thromboplastin time, sec | 26.2±4.0 | 26.9±3.7 | 0.566 |
| International Normalized Ratio | 1.4±0.7 | 1.3±0.5 | 0.910 |
| Medications | |||
| Use of antiplatelet agents on admission | 10 (45%) | 18 (32%) | 0.261 |
| Use of warfarin on admission | 4 (18%) | 10 (18%) | 0.729 |
| Use of statin on admission | 4 (18%) | 19 (33%) | 0.370 |
| Radiologic data | |||
| Time to MRI, h | 30.1 ±17.1 | 40.6±23.5 | 0.150 |
| WMH score | 16.8±5.1 | 11.1 ±6.1 | 0.001 |
| Presence of intraventricular hemorrhage | 1 (5%) | 8 (14%) | 0.717 |
| Lobar ICH | 18 (82%) | 29 (51%) | 0.020 |
| No. of microbleeds (total) | 6.5±11.4 | 2.6±8.4 | 0.110 |
| No. of microbleeds (ipsilateral) | 3.4±5.6 | 1.5±4.4 | 0.067 |
Table 3. Multivariate Logistic Regression Analysis of Risk Factors for Large ICHs.
| Odds Ratio | 95% CI | P Value | |
|---|---|---|---|
| WMH score | 1.152 | 1.035–1.282 | 0.012 |
| Lobar ICH | 2.725 | 0.757–9.805 | 0.139 |
| History of dementia | 3.247 | 0.290–36.411 | 0.340 |
| No. of ipsilateral microbleeds | 1.021 | 0.916–1.139 | 0.703 |
Thirty-four of the 79 patients had both a baseline CT scan within 12 hours of ICH onset and another CT scan during the following 72 hours. In this subset of patients, the mean ICH onset-to-imaging time was 6.1 hours (range, 40 minutes to 12 hours) for the baseline CT scan and 29.8 hours (range, 10 to 72 hours) for the follow-up scan. The baseline characteristics of subjects who had a follow-up CT scan and those who did not were largely comparable, except for a higher prevalence of dementia in the first group.
The mean ICH volume was 22.3±20.3 mL on the initial CT scan and 24.4±21.8 mL on the repeat CT scan. The WMH score was significantly correlated with ICH growth on direct testing (r=0.369; P=0.035). However, we only observed a trend for an association between WMH score and hematoma growth after adjusting for other confounding variables that could influence ICH growth (odds ratio=1.29; P=0.062). Tables 4 and 5 list the results of univariate and multivariate logistic-regression analyses of the risk factors for ICH growth. Only a history of hyperlipidemia emerged as a significant predictor of hematoma expansion (odds ratio=43.48; P=0.014).
Table 4. Univariate Analysis of Hematoma Expansion.
| All Patients (n=34) | P Value | ||
|---|---|---|---|
|
| |||
| With Expansion (n=5) | No Expansion (n=29) | ||
| Age, y, mean±SD | 79±9 | 73±11 | 0.420 |
| Female | 2 (40%) | 14 (48%) | 1.0 |
| Past medical history, n | |||
| Hypertension | 5 (100%) | 21 (72%) | 0.309 |
| Atrial fibrillation | 1 (20%) | 5 (17%) | 1.0 |
| Coronary artery disease | 2 (40%) | 4 (14%) | 0.205 |
| Diabetes mellitus | 1 (20%) | 7 (24%) | 1.0 |
| Hyperlipidemia | 4 (80%) | 5 (17%) | 0.012 |
| Dementia | 1 (20%) | 3 (10%) | 0.488 |
| Clinical variables | |||
| Glucose, mg/dL | 161.2±32.6 | 142.3±49.3 | 0.295 |
| Systolic blood pressure, mm Hg | 162.2±13.7 | 152.6±34.2 | 0.252 |
| Diastolic blood pressure, mm Hg | 81.0±6.6 | 81.0±22.2 | 0.827 |
| Platelet count, 1000/μL | 241.6±64.6 | 231.8±71.2 | 0.706 |
| Partial thromboplastin time | 28.1 ±3.8 | 26.9±4.6 | 0.603 |
| International Normalized Ratio | 1.8±0.8 | 1.4±0.6 | 0.089 |
| Medications | |||
| Use of antiplatelet agents on admission | 1 (20%) | 10 (34%) | 1.0 |
| Use of warfarin on admission | 1 (20%) | 5 (17%) | 1.0 |
| Use of statin on admission | 1 (20%) | 10 (34%) | 1.0 |
| Radiologic data | |||
| Time from onset to first CT, h | 6.8±5.3 | 6.0±3.4 | 0.888 |
| Time from onset to second CT, h | 20.8±10.3 | 31.4±15.6 | 0.149 |
| WMH score | 18.2±4.1 | 12.5±7.0 | 0.073 |
| Presence of intraventricular hemorrhage | 0 | 7 (24%) | 0.559 |
| Lobar ICH | 5 (100%) | 17 (59%) | 0.137 |
| No. of microbleeds | 4.8±7.2 | 7.6±14.1 | 0.880 |
Table 5. Multivariate Logistic Regression Analysis of Hematoma Expansion.
| Odds Ratio | 95% CI | P Value | |
|---|---|---|---|
| WMH score | 1.286 | 0.978–1.692 | 0.062 |
| History of hyperlipidemia | 43.482 | 2.165–873.207 | 0.014 |
| International Normalized Ratio | 1.853 | 0.404–8.509 | 0.428 |
Discussion
We found an independent association between high WMH score and large ICHs (those exceeding 30 mL) in this cohort of patients. WMH score was also correlated with ICH volume growth, suggesting that white-matter damage may predict ICH volume and growth.
Histopathologic studies have shown that areas of WMHs are correlated with spongiform changes and tissue rarefaction, widening of perivascular spaces, and reduced density of brain tissue.8,9 Recent observations have also suggested regional BBB disruption in patients with WMHs.19 For example, the MR contrast agent leaks into the brain more intensively in those with WMHs than in controls11; plasma proteins leak around perforating arteries in patients with WMHs12; and haplotypes of the genes encoding matrix metalloproteinases, which can influence capillary permeability by degrading components of the extracellular matrix, are associated with WMH volume.13 An underlying vascular etiology for WMHs was strengthened by the study of Young et al,14 who showed a significant decrease in vascular integrity in areas of WMHs, and of Black et al,20 who reported that periventricular intraparenchymal venular disease was correlated with the severity of WMHs. It is well established that vasculopathic changes, including microaneurysmal dilatation, fibrinoid necrosis, and microhemorrhages, are implicated in the mechanism of ICH in both hypertension and amyloid angiopathy (AA)21 and that hematoma growth is attributed, at least partly, to BBB disruption after ICH.22 These common pathophysiologic features between WMHs and ICH, and the impact of WMHs on brain density, provide mechanistic links accounting for our findings, whereby weakening of vascular integrity, rarefaction of brain tissue, widening of perivascular spaces, and BBB disruption in severe WMHs can result in larger ICH volumes and a greater tendency for hematoma growth.
A lobar location and history of dementia were associated with a larger ICH on univariate analysis. The pathophysiologic basis for our findings and their potential relation to WMHs that frequently accompany AA require further investigations. They may solely reflect the fact that deep cerebral hemorrhages are more likely to rupture into the ventricular system than do their lobar counterparts, thus partially preventing further extension of the hematoma. It is also possible that they may be an artifact of selection bias, whereby more patients with lobar ICH underwent GRE MRI and as a result, were included in our analysis, to further evaluate the possibility of AA than those with deep ICH.
Previous studies reported an association between WMHs and the number of microbleeds identified on GRE MRI6,23 and found that the number of microbleeds predicts the risk of future symptomatic ICH in patients with probable AA and lobar hemorrhage.24,25 These findings indicate that WMHs and microbleeds may share common mechanisms and imply that WMHs and microbleeds should have similar effects on ICH. Concordant with previous studies, the total number of microbleeds in the brain and in the hemisphere ipsilateral to the ICH was greater among patients with high WMH scores in our study. Although the number of microbleeds in the ipsilateral hemisphere was greater among patients with large ICHs on univariate analysis, we did not observe an association between the number of microbleeds and hematoma growth. Our results may be attributable to the relatively small sample size or an indication that microbleeds do not mediate the effects of WMHs on ICHs, perhaps because their presence reflects a more severe and downstream vasculopathy.
Contrary to our expectations, we found no correlation between WMHs and absolute edema volume. There are 2 possible explanations for this finding: (1) PHE formation is not directly related to the volume of the underlying hematoma26,27 and (2) other factors, unrelated to the pathogenesis of WMHs, have been implicated in the development of PHE, including inflammatory mediators (eg, cytokines and chemokines), thrombin, hemoglobin breakdown products, and oxidative stress.26,28
The ability to predict hematoma growth after ICH has important prognostic and therapeutic implications. Hematoma growth is an independent predictor of mortality and diminished functional outcomes29 and is a target for therapeutic intervention with hemostatic therapy. We found a trend for an association between WMHs and ICH volume growth and a significant association between history of hyperlipidemia and ICH expansion. These findings are intriguing. However, we only examined hematoma growth in a small subset of 34 patients, of whom only 5 showed evidence of hematoma expansion. These results, therefore, should only be considered preliminary and hypothesis-generating. They require prospective investigations in larger cohorts. The association between history of hyperlipidemia and ICH growth does not seem to be related to statin use because a smaller percentage of patients with ICH expansion, compared with those without expansion, were on a statin before their ICH. It is possible that this finding may be related to differences in total and LDL cholesterol levels between the groups. However, this seems unlikely and cannot be ascertained because we did not routinely check the lipid profiles of our patients. It is also possible that this finding is simply due to a chance association as an artifact of small sample size.
Our study has other limitations, largely imposed by its retrospective nature. We found that patients with low WMH scores tended to have better outcomes on discharge compared with patients with high scores. However, we did not have long-term follow-up data for most patients to assess the effect of WMHs on long-term functional outcomes and mortality. In addition, we only analyzed a single MRI, obtained at ≈38 hours after ICH onset, and graded WMHs in the hemisphere contralateral to the ICH to minimize the obscuring effects of the ICH on visible WMHs in the ipsilateral hemisphere. Therefore, one cannot entirely rule out that the presence of acute ICH did not affect WMHs. This seems unlikely, however, as Smith et al3 have recently shown the lack of influence of acute ICH on WMH grade. We measured ICH volumes on MRIs obtained at ≈38 hours after ICH instead of admission CT scans. Although this approach facilitated simultaneous assessment of the relation between ICH and WMHs at the same time point, the ICH volume, measured on MRIs at this later time point, may not truly represent the initial ICH volume and likely included some growth that occurred after presentation. Similarly, we only measured PHE at 1 time point and as a result, do not have serial measurements of PHE volumes to evaluate the temporal relation between ICH and PHE volumes and their impact on morbidity and mortality. Finally, we used a qualitative method to assess WMHs. Our results may be partly related to the use of a qualitative rather than a fully quantitative volumetric method to assess WMH burden. Future prospective studies should address these limitations.
In conclusion, we found that the severity of WMHs in patients with ICH is correlated with ICH volume. We also found a trend for an association between WMHs and hematoma growth. Our findings suggest that WMHs may provide important prognostic information in patients with ICH and may have implications for treatment stratification. Further studies are needed to elucidate the pathophysiologic link between WMHs and ICH and to confirm our findings.
Acknowledgments
Sources of Funding: Dr Lou is supported by a Zhejiang University Academic Research Fellowship, the National Natural Science Foundation of China (30500175), and the Science and Technology Department of Zhejiang Province (2008C14078). Dr Novak is supported by NIH/NINDS (1R01-NS045745–01A2). Dr Selim is supported in part by NIH/NINDS (1R01-NS 057127–01A1).
Footnotes
Disclosures: None.
References
- 1.Inzitari D, Giordano GP, Ancona AL, Pracucci G, Mascalchi M, Amaducci L. Leukoaraiosis, intracerebral hemorrhage, and arterial hypertension. Stroke. 1990;21:1419–1423. doi: 10.1161/01.str.21.10.1419. [DOI] [PubMed] [Google Scholar]
- 2.Selekler K, Erzen C. Leukoaraiosis and intracerebral hematoma. Stroke. 1989;20:1016–1020. doi: 10.1161/01.str.20.8.1016. [DOI] [PubMed] [Google Scholar]
- 3.Smith EE, Rosand J, Knudsen KA, Hylek EM, Greenberg SM. Leukoaraiosis is associated with warfarin-related hemorrhage following ischemic stroke. Neurology. 2002;59:193–197. doi: 10.1212/wnl.59.2.193. [DOI] [PubMed] [Google Scholar]
- 4.Gorter JW. Major bleeding during anticoagulation after cerebral ischemia: patterns and risk factors; Stroke Prevention In Reversible Ischemia Trial (SPIRIT) European Atrial Fibrillation Trial (EAFT) study groups. Neurology. 1999;53:1319–1327. doi: 10.1212/wnl.53.6.1319. [DOI] [PubMed] [Google Scholar]
- 5.Palumbo V, Boulanger JM, Hill MD, Inzitari D, Buchan AM CASES Investigators. Leukoaraiosis and intracerebral hemorrhage after thrombolysis in acute stroke. Neurology. 2007;68:1020–1024. doi: 10.1212/01.wnl.0000257817.29883.48. [DOI] [PubMed] [Google Scholar]
- 6.Smith EE, Gurol ME, Eng JA, Engel CR, Nguyen TN, Rosand J, Greenberg SM. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004;63:1606–1612. doi: 10.1212/01.wnl.0000142966.22886.20. [DOI] [PubMed] [Google Scholar]
- 7.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 8.Erkinjuntti T, Benavente O, Eliasziw M, Munoz DG, Sulkava R, Haltia M, Hachinski V. Diffuse vacuolization (spongiosis) and arteriolosclerosis in the frontal white matter occurs in vascular dementia. Arch Neurol. 1996;53:325–332. doi: 10.1001/archneur.1996.00550040053014. [DOI] [PubMed] [Google Scholar]
- 9.Matsusue E, Sugihara S, Fujii S, Ohama E, Kinoshita T, Ogawa T. White matter changes in elderly people: MR-pathologic correlations. Magn Reson Med Sci. 2006;5:99–104. doi: 10.2463/mrms.5.99. [DOI] [PubMed] [Google Scholar]
- 10.Brickman AM, Zahra A, Muraskin J, Steffener J, Holland CM, Habeck C, Borogovac A, Ramos MA, Brown TR, Asllani I, Stern Y. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry Res. 2009;172:117–120. doi: 10.1016/j.pscychresns.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starr JM, Wardlaw J, Ferguson K, MacLullich A, Deary IJ, Marshall I. Increased blood-brain barrier permeability in type II diabetes demonstrated by gadolinium magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2003;74:70–76. doi: 10.1136/jnnp.74.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akiguchi I, Tomimoto H, Suenaga T, Wakita H, Budka H. Blood-brain barrier dysfunction in Binswanger's disease; an immunohistochemical study. Acta Neuropathol. 1998;95:78–84. doi: 10.1007/s004010050768. [DOI] [PubMed] [Google Scholar]
- 13.Fornage M, Mosley TH, Jack CR, de Andrade M, Kardia SL, Boerwinkle E, Turner ST. Family-based association study of matrix metalloproteinase-3 and -9 haplotypes with susceptibility to ischemic white matter injury. Hum Genet. 2007;120:671–680. doi: 10.1007/s00439-006-0236-8. [DOI] [PubMed] [Google Scholar]
- 14.Young VG, Halliday GM, Kril JJ. Neuropathologic correlates of white matter hyperintensities. Neurology. 2008;71:804–811. doi: 10.1212/01.wnl.0000319691.50117.54. [DOI] [PubMed] [Google Scholar]
- 15.Lou M, Lieb K, Selim M. The relationship between hematoma iron content and perihematoma edema: an MRI study. Cerebrovasc Dis. 2009;27:266–271. doi: 10.1159/000199464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vernooij MW, van der Lugt A, Ikram MA, Wielopolski PA, Niessen WJ, Hofman A, Krestin GP, Breteler MM. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70:1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 17.Brott T, Broderick J, Kothari R, Barsan W, Tomsick T, Sauerbeck L, Spilker J, Duldner J, Khoury J. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Hemphill JC, III, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 19.Wardlaw JM, Sandercock PA, Dennis MS, Starr J. Is breakdown of the blood-brain barrier responsible for lacunar stroke, leukoaraiosis, and dementia? Stroke. 2003;34:806–812. doi: 10.1161/01.STR.0000058480.77236.B3. [DOI] [PubMed] [Google Scholar]
- 20.Black S, Gao F, Bilbao J. Understanding white matter disease: imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009;40(suppl):S48–S52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- 21.McCarron MO, Nicoll JA. Cerebral amyloid angiopathy and thrombolysis-related intracerebral haemorrhage. Lancet Neurol. 2004;3:484–492. doi: 10.1016/S1474-4422(04)00825-7. [DOI] [PubMed] [Google Scholar]
- 22.Wada R, Aviv RI, Fox AJ, Sahlas DJ, Gladstone DJ, Tomlinson G, Symons SP. CT angiography ‘spot sign’ predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38:1257–1262. doi: 10.1161/01.STR.0000259633.59404.f3. [DOI] [PubMed] [Google Scholar]
- 23.Chen YW, Gurol ME, Rosand J, Viswanathan A, Rakich SM, Groover TR, Greenberg SM, Smith EE. Progression of white matter lesions and hemorrhages in cerebral amyloid angiopathy. Neurology. 2006;67:83–87. doi: 10.1212/01.wnl.0000223613.57229.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenberg SM, Eng JA, Ning M, Smith EE, Rosand J. Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke. 2004;35:1415–1420. doi: 10.1161/01.STR.0000126807.69758.0e. [DOI] [PubMed] [Google Scholar]
- 25.Greenberg SM, O'Donnell HC, Schaefer PW, Kraft E. MRI detection of new hemorrhages: potential marker of progression in cerebral amyloid angiopathy. Neurology. 1999;53:1135–1138. doi: 10.1212/wnl.53.5.1135. [DOI] [PubMed] [Google Scholar]
- 26.Castillo J, Dávalos A, Alvarez-Sabín J, Pumar JM, Leira R, Silva Y, Montaner J, Kase CS. Molecular signatures of brain injury after intracerebral hemorrhage. Neurology. 2002;58:624–629. doi: 10.1212/wnl.58.4.624. [DOI] [PubMed] [Google Scholar]
- 27.Mehdiratta M, Kumar S, Hackney D, Schlaug G, Selim M. Association between serum ferritin and perihematoma edema volume in patients with spontaneous intracerebral hemorrhage. Stroke. 2008;39:1165–1170. doi: 10.1161/STROKEAHA.107.501213. [DOI] [PubMed] [Google Scholar]
- 28.Xi G, Keep RF, Hoff JT. Pathophysiology of brain edema formation. Neurosurg Clin North Am. 2002;13:371–383. doi: 10.1016/s1042-3680(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 29.Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, Begtrup K, Steiner T Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–1181. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]


