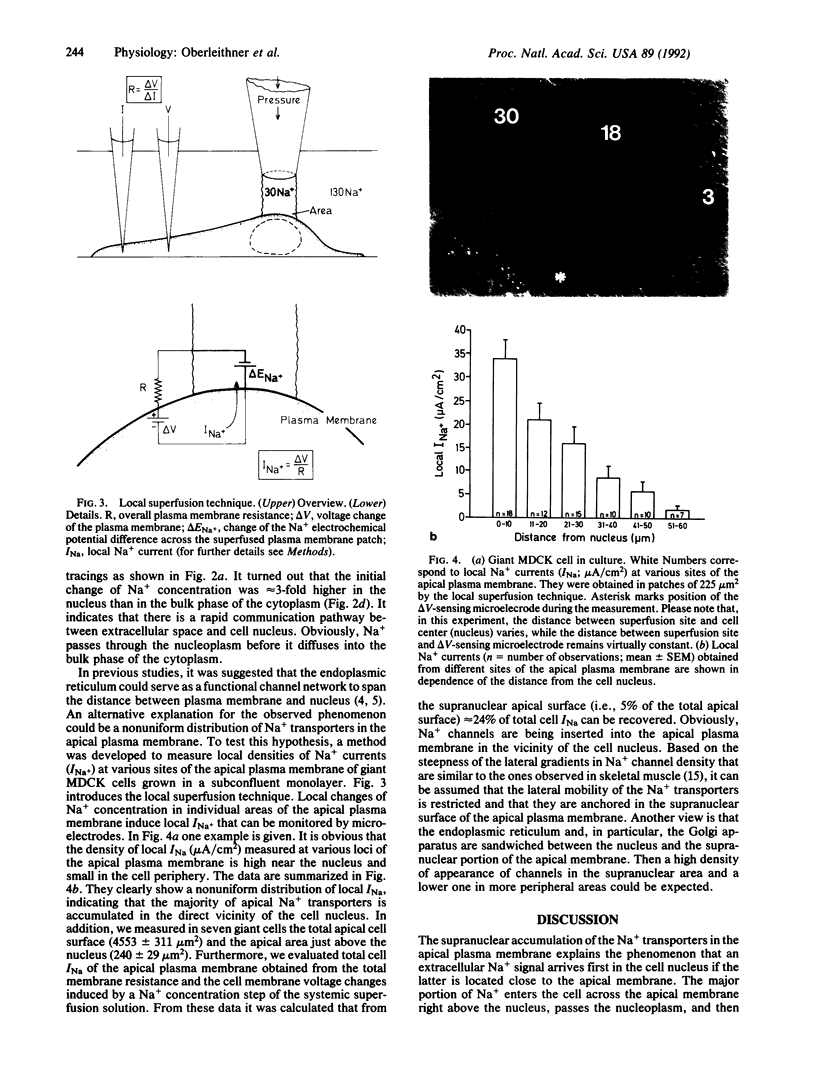
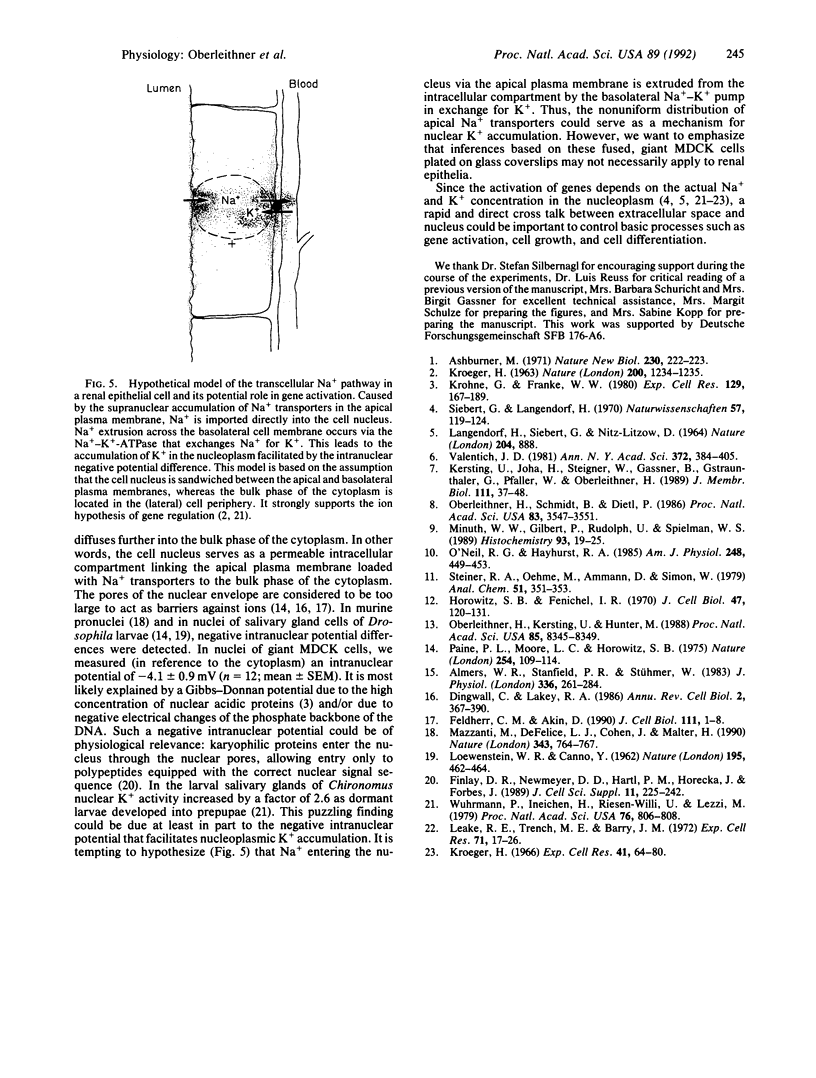
Abstract
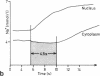
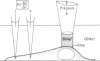
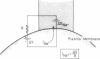
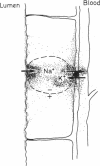
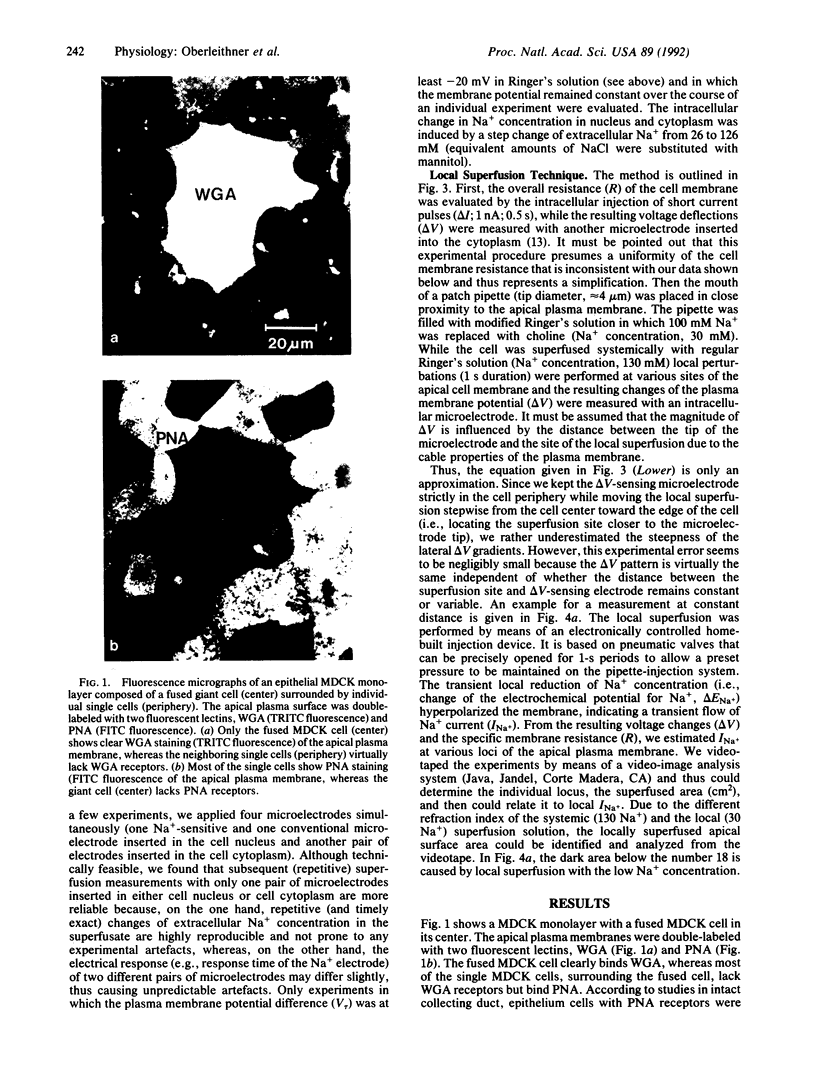
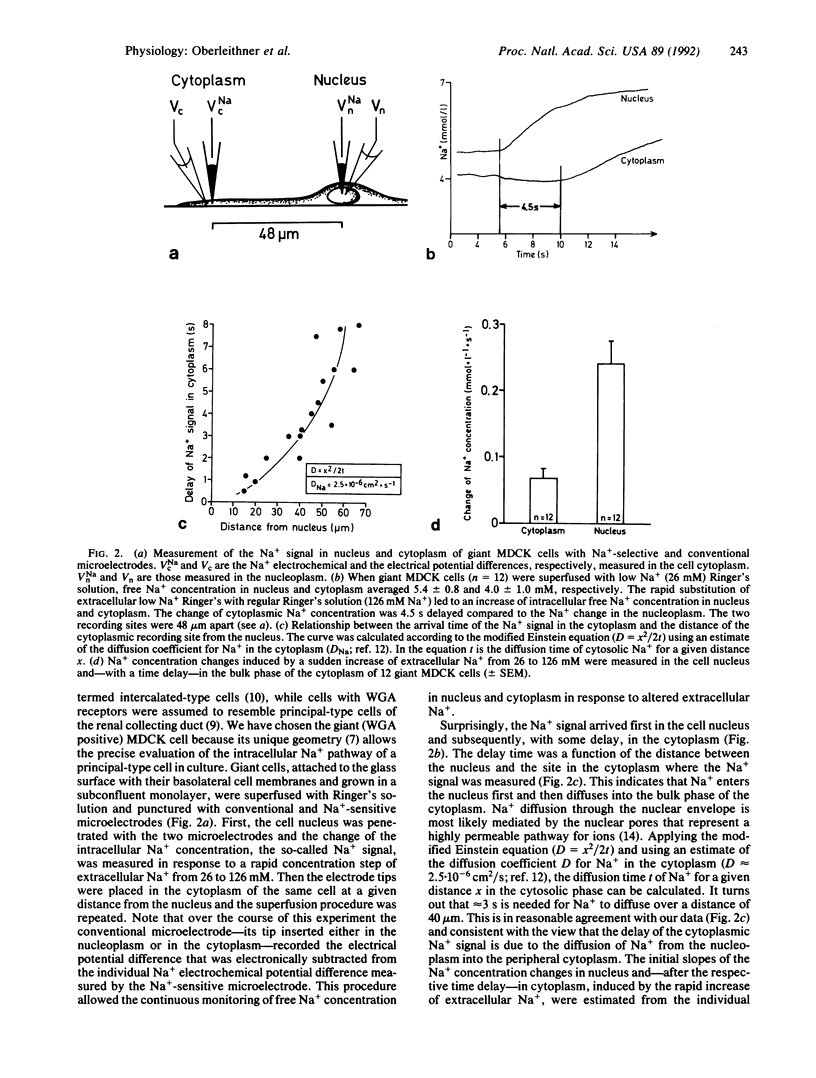
Intracellular Na+ activities and local current densities were measured in fused Madin-Darby canine kidney cells using Na+ and voltage-sensing microelectrodes. Na+ that enters the cell across the apical plasma membrane accumulates initially in the nucleoplasm, several seconds ahead of its appearance in the cell cytoplasm. The spatial distribution of Na+ currents, produced by a local superfusion of the cell surface, indicates a nonuniform, patchy accumulation of apical Na+ transporters in the vicinity of the nucleus. Such pathways for direct Na+ flux between extracellular space and cell nucleus could be potentially important for gene activation.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Stanfield P. R., Stühmer W. Lateral distribution of sodium and potassium channels in frog skeletal muscle: measurements with a patch-clamp technique. J Physiol. 1983 Mar;336:261–284. doi: 10.1113/jphysiol.1983.sp014580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner M. Induction of puffs in polytene chromosomes of in vitro cultured salivary glands of Drosophila melanogaster by ecdysone and echysone analogues. Nat New Biol. 1971 Apr 14;230(15):222–224. doi: 10.1038/newbio230222a0. [DOI] [PubMed] [Google Scholar]
- Dingwall C., Laskey R. A. Protein import into the cell nucleus. Annu Rev Cell Biol. 1986;2:367–390. doi: 10.1146/annurev.cb.02.110186.002055. [DOI] [PubMed] [Google Scholar]
- Feldherr C. M., Akin D. The permeability of the nuclear envelope in dividing and nondividing cell cultures. J Cell Biol. 1990 Jul;111(1):1–8. doi: 10.1083/jcb.111.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay D. R., Newmeyer D. D., Hartl P. M., Horecka J., Forbes D. J. Nuclear transport in vitro. J Cell Sci Suppl. 1989;11:225–242. doi: 10.1242/jcs.1989.supplement_11.17. [DOI] [PubMed] [Google Scholar]
- Horowitz S. B., Fenichel I. R. Analysis of sodium transport in the amphibian oocyte by extractive and radioautographic techniques. J Cell Biol. 1970 Oct;47(1):120–131. doi: 10.1083/jcb.47.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROEGER H. CHEMICAL NATURE OF THE SYSTEM CONTROLLING GENE ACTIVITIES IN INSECT CELLS. Nature. 1963 Dec 21;200:1234–1235. doi: 10.1038/2001234a0. [DOI] [PubMed] [Google Scholar]
- Kersting U., Joha H., Steigner W., Gassner B., Gstraunthaler G., Pfaller W., Oberleithner H. Fusion of cultured dog kidney (MDCK) cells: I. Technique, fate of plasma membranes and of cell nuclei. J Membr Biol. 1989 Oct;111(1):37–48. doi: 10.1007/BF01869207. [DOI] [PubMed] [Google Scholar]
- Kroeger H. Potentialdifferenz und Puff-Muster. Elektrophysiolgische und cytologische Untersuchungen an den Speicheldrüsen von Chironomus thummi. Exp Cell Res. 1966 Jan;41(1):64–80. doi: 10.1016/0014-4827(66)90547-7. [DOI] [PubMed] [Google Scholar]
- Krohne G., Franke W. W. A major soluble acidic protein located in nuclei of diverse vertebrate species. Exp Cell Res. 1980 Sep;129(1):167–189. doi: 10.1016/0014-4827(80)90341-9. [DOI] [PubMed] [Google Scholar]
- LANGENDORF H., SIEBERT G., NITZ-LITZOW D. PARTICIPATION OF RAT LIVER NUCLEI IN MOVEMENTS OF SODIUM. Nature. 1964 Nov 28;204:888–888. doi: 10.1038/204888a0. [DOI] [PubMed] [Google Scholar]
- LOEWENSTEIN W. R., KANNO Y. Some electrical properties of the membrane of a cell nucleus. Nature. 1962 Aug 4;195:462–464. doi: 10.1038/195462a0. [DOI] [PubMed] [Google Scholar]
- Leake R. E., Trench M. E., Barry J. M. Effect of cations on the consideration of hen erythrocyte nuclei and its relation to gene activation. Exp Cell Res. 1972 Mar;71(1):17–26. doi: 10.1016/0014-4827(72)90257-1. [DOI] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J., Cohn J., Malter H. Ion channels in the nuclear envelope. Nature. 1990 Feb 22;343(6260):764–767. doi: 10.1038/343764a0. [DOI] [PubMed] [Google Scholar]
- Minuth W. W., Gilbert P., Rudolph U., Spielman W. S. Successive histochemical differentiation steps during postnatal development of the collecting duct in rabbit kidney. Histochemistry. 1989;93(1):19–25. doi: 10.1007/BF00266842. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Kersting U., Hunter M. Cytoplasmic pH determines K+ conductance in fused renal epithelial cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8345–8349. doi: 10.1073/pnas.85.21.8345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberleithner H., Schmidt B., Dietl P. Fusion of renal epithelial cells: a model for studying cellular mechanisms of ion transport. Proc Natl Acad Sci U S A. 1986 May;83(10):3547–3551. doi: 10.1073/pnas.83.10.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine P. L., Moore L. C., Horowitz S. B. Nuclear envelope permeability. Nature. 1975 Mar 13;254(5496):109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- Siebert G., Langendorf H. Ionenhaushalt im Zellkern. Naturwissenschaften. 1970 Mar;57(3):119–124. doi: 10.1007/BF00600046. [DOI] [PubMed] [Google Scholar]
- Valentich J. D. Morphological similarities between the dog kidney cell line MDCK and the mammalian cortical collecting tubule. Ann N Y Acad Sci. 1981;372:384–405. doi: 10.1111/j.1749-6632.1981.tb15490.x. [DOI] [PubMed] [Google Scholar]
- Wuhrmann P., Ineichen H., Riesen-Willi U., Lezzi M. Change in nuclear potassium electrochemical activity and puffing of potassium-sensitive salivary chromosome regions during Chironomus development. Proc Natl Acad Sci U S A. 1979 Feb;76(2):806–808. doi: 10.1073/pnas.76.2.806. [DOI] [PMC free article] [PubMed] [Google Scholar]