Abstract
Minimal change disease (MCD) is an important cause of nephrotic syndrome and is characterized by massive proteinuria and hypoalbuminemia, resulting in edema and hypercholesterolemia. The podocyte plays a key role in filtration and its disruption results in a dramatic loss of function leading to proteinuria. Immunologic disturbance has been suggested in the pathogenesis of MCD. Because of its clinical features, such as recurrent relapse/remission course, steroid response in most patients, and rare familial cases, a genetic defect has been thought to be less likely in MCD. Recent progress in whole-exome sequencing reveals pathogenic mutations in familial cases in steroid-sensitive nephrotic syndrome (SSNS) and sheds light on possible mechanisms and key molecules in podocytes in MCD. On the other hand, in the majority of cases, the existence of circulating permeability factors has been implicated along with T lymphocyte dysfunction. Observations of benefit with rituximab added B cell involvement to the disease. Animal models are unsatisfactory, and the humanized mouse may be a good model that well reflects MCD pathophysiology to investigate suggested “T cell dysfunction” directly related to podocytes in vivo. Several candidate circulating factors and their effects on podocytes have been proposed but are still not sufficient to explain whole mechanisms and clinical features in MCD. Another circulating factor disease is focal segmental glomerulosclerosis (FSGS), and it is not clear if this is a distinct entity, or on the same spectrum, implicating the same circulating factor(s). These patients are mostly steroid resistant and often have a rapid relapse after transplantation. In clinical practice, predicting relapse or disease activity and response to steroids is important and is an area where novel biomarkers can be developed based on our growing knowledge of podocyte signaling pathways. In this review, we discuss recent findings in genetics and podocyte biology in MCD.
Keywords: Minimal change disease, steroid-sensitive nephrotic syndrome, focal segmental glomerulosclerosis, steroid-resistant nephrotic syndrome, circulating factor, permeability, podocyte
Introduction
Minimal change disease (MCD) is characterized by massive proteinuria without histological evidence of immune-mediated damage in the glomeruli. The glomerular podocyte plays a key role in filtration and its loss of function results in loss of protein, mainly albumin or smaller proteins, into the urine with high selectivity 1. Proteinuria in MCD is typically reversible with corticosteroid therapy 2. T cell dysfunction and circulating factors have long been implicated as a cause of the podocyte dysfunction in MCD 3, but their nature still remains to be elucidated.
Recent progress in genetics and cell biology has revealed the molecular mechanisms of dysfunction in podocytes 4. These findings give us clues to focus on target molecules on the podocyte to deduce what those circulating factors may be. At the same time, we can utilize those molecules as biomarkers not only as a diagnostic tool but also in predicting the disease activity or prognosis. This allows us to administer more accurate and precise treatment to patients with MCD while minimizing side effects caused by drugs.
Alongside MCD as one circulating factor disease is a subset of patients with the histological finding of focal segmental glomerulosclerosis (FSGS). These patients are mostly steroid resistant, and therefore the term steroid-resistant nephrotic syndrome (SRNS) is also used here. These patients often have a rapid relapse after transplantation, indicating another circulating factor disease. It is likely that at least a subset of patients with MCD progress to FSGS/SRNS, with a consistent circulating factor in both. The most compelling evidence for this is the observation that patients with initial steroid sensitivity (assumed to be MCD at that stage) who over subsequent years develop steroid resistance/FSGS, and renal failure, have a 90% chance of post-transplant disease recurrence – the archetypal manifestation of circulating factor disease 5. In this article, known pathogenesis and mechanisms underlying MCD are reviewed.
Clinical features of MCD
MCD is the most common cause of nephrotic syndrome in children 6 and around 15–20% of cases in adults 7, and is characterized by massive proteinuria and hypoalbuminemia, resulting in edema and hypercholesterolemia. Histological findings of the disease in glomeruli are typically normal by light microscopy and only electron microscopy shows effacement of podocyte foot processes without electron-dense immune deposits 8. These manifestations are typically reversible with the use of corticosteroid therapy in steroid-sensitive nephrotic syndrome (SSNS), so that progressive loss of renal function is rare.
The incidence of MCD in childhood is twofold higher in boys, with a prevalence that is inversely proportional to age. Relapse occurs in 50–80% of patients, and recurrent relapse tends to lessen after adolescence 9. A genetic defect cannot explain these phenomena in MCD.
Genetics in MCD
Pathogenic mutations in MCD
Familial cases are rather rare in MCD, therefore the genetic background of SSNS is largely unknown, while 23.6% of SRNS cases 10 and 29.5% of familial SRNS cases 11 are caused by gene mutation. More than 24 genes are currently known to be pathogenic in SRNS 12 and have already been clinically utilized in practice in SRNS cases.
Recently, using whole-exome sequencing, several mutations were found in pedigrees with SSNS, which shed light on new mechanisms of podocyte disruption in MCD. Epithelial membrane protein 2 (EMP2) is known to regulate the amount of caveolin-1 13, which contributes to endocytosis and the transcytosis of cholesterol and albumin 14. Lipopolysaccharide (LPS)-induced caveolin-1 phosphorylation was reported to lead to the increase of transcellular permeability 15.
More recently, recessive mutations in the KANK gene were identified in familial SSNS and in sporadic SRNS cases 16. Kidney ankyrin repeat-containing protein (KANK) family proteins have essential roles in podocyte/nephrocyte function and regulate Rho GTPase activity. KANK2 interacted with Rho GDP dissociation inhibitor alpha (ARHGDIA), a known regulator of Rho GTPases in podocytes found to be dysfunctional in SRNS 17. Knockdown of KANK2 in cultured podocytes increased active GTP-bound RHOA and decreased migration.
In these cases, we might have evidence of overlap of SSNS and SRNS. Also, it is important to know the mechanisms of how corticosteroid and immunosuppressants have their effect on nephrotic syndrome caused by single gene mutation.
T cell dysfunction in MCD
T cell dysfunction has long been postulated and many types of cytokines have been investigated. One of the difficulties in examining a hypothesis that immunological disruption underlies MCD in the laboratory is the lack of an animal model that reflects the pathophysiological mechanism. Haddad et al. employed unique methods and established a nephrotic syndrome model by injecting CD34+ peripheral stem cells obtained from FSGS and MCD patients 18 rather than injecting the supernatant of T cells or peripheral blood mononuclear cells (PBMCs) obtained from the patients 19. The injected cells successfully induced the engraftment of human CD45 leukocytes in the thymus, and only the injection of CD34+ stem cells from patients induced albuminuria. Interestingly, stem-cell-injected mice did not have CD3+ mature T cells, suggesting that the cells responsible for the pathogenesis of idiopathic nephrotic syndrome are more likely to be immature differentiating cells rather than mature peripheral T cells. Naïve T cells (Th0s) have been focused on to investigate the difference in DNA methylation in MCD patients 20. The change in DNA methylation patterns from remission to relapse occurs predominantly in Th0s. Epigenetic involvement in the pathogenesis of minimal change nephrotic syndrome in T cells has also been suggested in a report showing that nuclear factor related to kappaB binding protein (NFRKB) was highly expressed in the nuclear compartment in T lymphocytes of MCD patients during relapse and that NFRKB promotes hypomethylation of genomic DNA in HEK cells transfected with NFRKB expression plasmid 21.
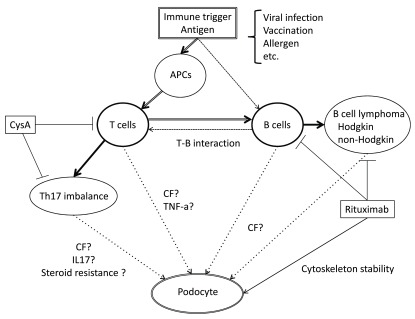
Another T cell dysfunction is a Th17 skew in MCD 22, 23 ( Figure 1). Patients with SSNS demonstrated after corticosteroid treatment that the Th17/regulatory T cell (Treg) balance returned to normal 24. More recently, it was reported that Th17 cells are strong candidate drivers for steroid resistance in immune diseases and have selective attenuation by cyclosporine A 25. This could be utilized to predict steroid response in early stages of nephrotic syndrome onset by testing peripheral Th17 levels.
Figure 1. Scheme of lymphocyte dysfunction and circulating factors in minimal change disease (MCD).
By immune trigger such as viral infection, vaccination, and exposure to allergen, antigen-presenting cells and memory B cells present antigen to T lymphocyte. These cells are stimulated to secrete circulating factors in MCD. Rituximab depletes B cells and induces remission; on the other hand, rituximab has an effect on cytoskeleton stability of podocytes and blocks albumin permeability. Th17 skew in MCD may cause steroid resistance and cyclosporine A selectively attenuates Th17. Abbreviations: APCs, antigen presenting cells; CysA, cyclosporine A; CF, circulating factor; IL17, interleukin 17; Th17, helper T subset 17; TNF-a, tumor necrosis factor alpha.
Rituximab and B cell dysfunction
A potential close pathophysiological relationship between MCD and chronic lymphoid neoplasms such as Hodgkin and non-Hodgkin lymphoma has been known since the 1950s, supporting a potential role for B cells in the pathogenesis of MCD ( Figure 1). A significant association of HLA-DQA1 (a major histocompatibility complex [MHC] class II) missense coding variants with SSNS recently suggested the possible role of an immune response and the implication of B cells in the pathogenesis of MCD 26.
Though the accurate mechanism by which rituximab, a monoclonal antibody against CD20, induces remission in MCD patients remains uncertain, recent observations of the effect of rituximab on complicated refractory SSNS 27– 29 suggests a pathophysiological role for B cells in MCD 30, 31 ( Figure 1). B cell depletion by rituximab resets and suppresses B cell and T cell interactions and keeps the Th17/Treg balance normal, which may lead to sustainable remission 32, 33. On the other hand, a direct role for rituximab on podocyte cytoskeleton stabilization was suggested: rituximab prevents disruption of the actin cytoskeleton in cultured normal human podocytes that have been exposed to FSGS patient sera in a sphingomyelin phosphodiesterase acid-like 3b-dependent manner 34.
Circulating factors and podocyte cell biology in MCD
A direct test of “circulating factor” activity is to expose human podocytes in culture to active human disease plasma and examine the direct cellular effects on this target cell. It has been shown using this method that nephrotic plasma alters slit diaphragm-dependent signaling and translocates nephrin, podocin, and CD2-associated protein in cultured human podocytes 35. This indicated that there is a certain factor increasing or missing in MCD disease plasma.
Hemopexin
Hemopexin (Hpx) is a circulating plasma protease that is synthesized in the liver. The active isoform of Hpx is increased in children with MCD 36. In vitro, podocytes showed dramatic reorganization of actin with loss of stress fibers after Hpx treatment 37. The Hpx effect on actin is dependent on nephrin followed by RhoA activation and protein kinase B phosphorylation in the downstream intracellular signaling pathway. The effects were reversible and were inhibited by pre-incubation with healthy human plasma or serine protease inhibitors. Though the mechanisms of Hpx activation in the disease are unclear, LPS and tumor necrosis factor (TNF)-α are indicated as possible triggers to activate Hpx in MCD 38.
PAR1 signaling axis and VASPp, or suPAR
Because it has serine protease activity 39, Hpx may act via the family of protease-activated receptors. There are also matrix metalloproteinases among those proteins that have Hpx homology domains. Recent studies investigated the possibility of a matrix metalloproteinase–protease-activated receptor 1 (PAR1) signaling axis 40. It was recently reported that proteases present in nephrotic plasma obtained from patients with FSGS can activate PAR1, leading to the podocin-dependent phosphorylation of the actin-associated protein vasodilator-stimulated phosphoprotein (VASP) in human podocytes and increased cell migration, suggesting a novel role for proteases and PARs in the pathogenesis of FSGS 41, 42. Although the exact component(s) of FSGS plasma that causes this response remains unknown, the soluble urokinase plasminogen activator receptor (suPAR) has been identified as a potential circulating factor in FSGS via activation of β3 integrin in podocytes and promotes cell motility 43– 45. However, correlation of disease activity with suPAR levels has been inconsistent in subsequent reports 46, 47. Urinary suPAR was increased in MCD relapse, but it is thought it may simply be a surrogate for proteinuria 48. These factors are found in FSGS but are potentially also relevant to MCD; this needs experimental verification.
CD80
CD80 (B7-1) is a T cell co-stimulatory molecule involved in antigen processing that is also unexpectedly expressed on podocytes in certain experimental and clinical disease states. Podocyte CD80 activation through Toll-like receptor (TLR) 3 and 4 by LPS, independent of T cells, causes proteinuria and foot process effacement 49.
Urinary CD80 levels are increased in MCD during relapse but are not increased in FSGS patients or MCD patients in remission 50. Sera from MCD patients in relapse, but not in remission, stimulated CD80 expression in cultured podocytes 51. The factor(s) in patients’ serum that stimulates podocytes is unknown. Most recently, it was reported that no significant up-regulation of podocyte CD80 was detected in MCD and FSGS patients' biopsies compared with controls using different primary antibodies and immunohistochemical assays, suggesting further confirmation is needed with CD80 in MCD 52.
TNF-α
TNF-α is suggested to be one of the circulating factors that exists in patient plasma of post-transplant recurrent FSGS 53, 54. The effect on the podocyte was actin cytoskeleton disruption and activation of β3 integrin. In MCD, it has been suggested that TNF-α synthesis in peripheral mononuclear cells from relapse is increased 55. Genome-wide DNA methylation analysis was performed in naïve T helper cells both in relapse and in remission of MCD 20 and it was found that the promoter region of TNF-α from relapse has a significant reduction in DNA methylation compared to that from remission in the same individuals, indicating predisposition of TNF-α synthesis in relapse in MCD [personal communication, Dr Yasuko Kobayashi].
Summarizing the data, an excess factor or missing/imbalance of factors in relapse plasma could be the primary cause of MCD, and interesting candidates with biological plausibility are Hpx, suPAR, and TNF-α. PAR1 or uPAR and β3 integrin are therefore potentially activated by circulating factors, and VASP-p is in the pathway downstream of PAR1 or integrins. CD80 is a product of podocyte stimulation by circulating factors. Reorganization of actin by Hpx is dependent on nephrin. The structural changes in actin result in foot process effacement and increase of permeability, which is the core feature in the disease.
The circulating factors might be secreted by peripheral blood cells such as T or B cells by mesangial or endothelial cells in a paracrine manner or by the podocyte itself in an autocrine manner.
Conclusion
Pathogenic gene mutation analysis in familial MCD has started to reveal insights into underlying mechanisms of pathophysiology in the podocyte, such as endocytosis or Rho GTPase, related to permeability.
We have less evidence of circulating factor activity or from genetic disease in MCD compared to FSGS, perhaps because of less disease severity and lower availability of patient samples in MCD. In terms of circulating factor diseases, findings in FSGS can be examined in relation to MCD. A humanized mouse model might give us a good tool to investigate T cell dysfunction directly related to podocytes.
There are several candidates for biomarkers to predict disease activity or steroid response that allow us to choose precise and acceptable treatment for each individual patient while reducing the side effects of long-term treatment.
New components might be inducible targeting of a specific molecule that is involved in the pathogenesis of MCD both for screening and for treatment.
Abbreviations
MCD, minimal change disease; SSNS, steroid-sensitive nephrotic syndrome; FSGS, focal segmental glomerulosclerosis; SRNS, steroid-resistant nephrotic syndrome; EMP2, epithelial membrane protein 2; LPS, lipopolysaccharide; KANK, kidney ankyrin repeat-containing protein; ARHGDIA, Rho GDP dissociation inhibitor (GDI) alpha; Th0s, naïve T cells; NFRKB, nuclear factor related to kappaB binding protein; Th17, helper T subset 17; Treg, regulatory T cell; PBMC, peripheral mononuclear cell; PAR1, protease activated receptor 1; VASP, vasodilator stimulated phosphoprotein; suPAR, soluble urokinase plasminogen activator receptor; TLR, Toll-like receptor; TNF-α, tumor necrosis factor alpha.
Editorial Note on the Review Process
F1000 Faculty Reviews are commissioned from members of the prestigious F1000 Faculty and are edited as a service to readers. In order to make these reviews as comprehensive and accessible as possible, the referees provide input before publication and only the final, revised version is published. The referees who approved the final version are listed with their names and affiliations but without their reports on earlier versions (any comments will already have been addressed in the published version).
The referees who approved this article are:
Vincent Audard, Centre de Référence Syndrome Néphrotique Idiopathique INSERM U955, Service de Néphrologie et Transplantation, CHU Henri Mondor, Université Paris Est Créteil, Créteil, Paris, France
Annette Bruchfeld, Department of Renal Medicine, Karolinska University Hospital, Karolinska Institutet, Stockholm, Sweden
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; referees: 2 approved]
References
- 1. Saleem MA: One hundred ways to kill a podocyte. Nephrol Dial Transplant. 2015;30(8):1266–71. 10.1093/ndt/gfu363 [DOI] [PubMed] [Google Scholar]
- 2. KDIGO: Chapter 3: Steroid-sensitive nephrotic syndrome in children. Kidney Int Suppl (2011). 2012;2(2):163–71. 10.1038/kisup.2012.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shalhoub RJ: Pathogenesis of lipoid nephrosis: a disorder of T-cell function. Lancet. 1974;2(7880):556–60. 10.1016/S0140-6736(74)91880-7 [DOI] [PubMed] [Google Scholar]
- 4. Saleem MA: New developments in steroid-resistant nephrotic syndrome. Pediatr Nephrol. 2013;28(5):699–709. 10.1007/s00467-012-2239-0 [DOI] [PubMed] [Google Scholar]
- 5. Ding WY, Koziell A, McCarthy HJ, et al. : Initial steroid sensitivity in children with steroid-resistant nephrotic syndrome predicts post-transplant recurrence. J Am Soc Nephrol. 2014;25(6):1342–8. 10.1681/ASN.2013080852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr. 1981;98(4):561–4. 10.1016/S0022-3476(81)80760-3 [DOI] [PubMed] [Google Scholar]
- 7. Mathieson PW: Minimal change nephropathy and focal segmental glomerulosclerosis. Semin Immunopathol. 2007;29(4):415–26. 10.1007/s00281-007-0094-z [DOI] [PubMed] [Google Scholar]
- 8. International Study of Kidney Disease in Children: Nephrotic syndrome in children: prediction of histopathology from clinical and laboratory characteristics at time of diagnosis. A report of the International Study of Kidney Disease in Children. Kidney Int. 1978;13(2):159–65. 10.1038/ki.1978.23 [DOI] [PubMed] [Google Scholar]
- 9. Tarshish P, Tobin JN, Bernstein J, et al. : Prognostic significance of the early course of minimal change nephrotic syndrome: report of the International Study of Kidney Disease in Children. J Am Soc Nephrol. 1997;8(5):769–76. [DOI] [PubMed] [Google Scholar]
- 10. Trautmann A, Bodria M, Ozaltin F, et al. : Spectrum of steroid-resistant and congenital nephrotic syndrome in children: the PodoNet registry cohort. Clin J Am Soc Nephrol. 2015;10(4):592–600. 10.2215/CJN.06260614 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 11. Sadowski CE, Lovric S, Ashraf S, et al. : A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2015;26(6):1279–89. 10.1681/ASN.2014050489 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 12. McCarthy HJ, Bierzynska A, Wherlock M, et al. : Simultaneous sequencing of 24 genes associated with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2013;8(4):637–48. 10.2215/CJN.07200712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gee HY, Ashraf S, Wan X, et al. : Mutations in EMP2 cause childhood-onset nephrotic syndrome. Am J Hum Genet. 2014;94(6):884–90. 10.1016/j.ajhg.2014.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 14. Parton RG, del Pozo MA: Caveolae as plasma membrane sensors, protectors and organizers. Nat Rev Mol Cell Biol. 2013;14(2):98–112. 10.1038/nrm3512 [DOI] [PubMed] [Google Scholar]
- 15. Wang N, Zhang D, Sun G, et al. : Lipopolysaccharide-induced caveolin-1 phosphorylation-dependent increase in transcellular permeability precedes the increase in paracellular permeability. Drug Des Devel Ther. 2015;9:4965–77. 10.2147/DDDT.S77646 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 16. Gee HY, Zhang F, Ashraf S, et al. : KANK deficiency leads to podocyte dysfunction and nephrotic syndrome. J Clin Invest. 2015;125(6):2375–84. 10.1172/JCI79504 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 17. Gee HY, Saisawat P, Ashraf S, et al. : ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J Clin Invest. 2013;123(8):3243–53. 10.1172/JCI69134 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 18. Sellier-Leclerc AL, Duval A, Riveron S, et al. : A humanized mouse model of idiopathic nephrotic syndrome suggests a pathogenic role for immature cells. J Am Soc Nephrol. 2007;18(10):2732–9. 10.1681/ASN.2006121346 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 19. Maruyama K, Tomizawa S, Shimabukuro N, et al. : Effect of supernatants derived from T lymphocyte culture in minimal change nephrotic syndrome on rat kidney capillaries. Nephron. 1989;51(1):73–6. 10.1159/000185246 [DOI] [PubMed] [Google Scholar]
- 20. Kobayashi Y, Aizawa A, Takizawa T, et al. : DNA methylation changes between relapse and remission of minimal change nephrotic syndrome. Pediatr Nephrol. 2012;27(12 ):2233–41. 10.1007/s00467-012-2248-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Audard V, Pawlak A, Candelier M, et al. : Upregulation of nuclear factor-related kappa B suggests a disorder of transcriptional regulation in minimal change nephrotic syndrome. PLoS One. 2012;7(1):e30523. 10.1371/journal.pone.0030523 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 22. Araya C, Diaz L, Wasserfall C, et al. : T regulatory cell function in idiopathic minimal lesion nephrotic syndrome. Pediatr Nephrol. 2009;24(9):1691–8. 10.1007/s00467-009-1214-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang L, Li Q, Wang L, et al. : The role of Th17/IL-17 in the pathogenesis of primary nephrotic syndrome in children. Kidney Blood Press Res. 2013;37(4–5):332–45. 10.1159/000350161 [DOI] [PubMed] [Google Scholar]
- 24. Liu LL, Qin Y, Cai JF, et al. : Th17/Treg imbalance in adult patients with minimal change nephrotic syndrome. Clin Immunol. 2011;139(3):314–20. 10.1016/j.clim.2011.02.018 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 25. Schewitz-Bowers LP, Lait PJ, Copland DA, et al. : Glucocorticoid-resistant Th17 cells are selectively attenuated by cyclosporine A. Proc Natl Acad Sci U S A. 2015;112(13):4080–5. 10.1073/pnas.1418316112 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 26. Gbadegesin RA, Adeyemo A, Webb NJ, et al. : HLA-DQA1 and PLCG2 Are Candidate Risk Loci for Childhood-Onset Steroid-Sensitive Nephrotic Syndrome. J Am Soc Nephrol. 2015;26(7):1701–10. 10.1681/ASN.2014030247 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 27. Iijima K, Sako M, Nozu K, et al. : Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2014;384(9950):1273–81. 10.1016/S0140-6736(14)60541-9 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 28. Bruchfeld A, Benedek S, Hilderman M, et al. : Rituximab for minimal change disease in adults: long-term follow-up. Nephrol Dial Transplant. 2014;29(4):851–6. 10.1093/ndt/gft312 [DOI] [PubMed] [Google Scholar]
- 29. Kronbichler A, Bruchfeld A: Rituximab in adult minimal change disease and focal segmental glomerulosclerosis. Nephron Clin Pract. 2014;128(3–4):277–82. 10.1159/000368590 [DOI] [PubMed] [Google Scholar]
- 30. Liu K, Mohan C: Altered B-cell signaling in lupus. Autoimmun Rev. 2009;8(3):214–8. 10.1016/j.autrev.2008.07.048 [DOI] [PubMed] [Google Scholar]
- 31. Chan OT, Hannum LG, Haberman AM, et al. : A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med. 1999;189(10):1639–48. 10.1084/jem.189.10.1639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sfikakis PP, Boletis JN, Lionaki S, et al. : Remission of proliferative lupus nephritis following B cell depletion therapy is preceded by down-regulation of the T cell costimulatory molecule CD40 ligand: an open-label trial. Arthritis Rheum. 2005;52(2):501–13. 10.1002/art.20858 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 33. Stasi R, Cooper N, Del Poeta G, et al. : Analysis of regulatory T-cell changes in patients with idiopathic thrombocytopenic purpura receiving B cell-depleting therapy with rituximab. Blood. 2008;112(4):1147–50. 10.1182/blood-2007-12-129262 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 34. Fornoni A, Sageshima J, Wei C, et al. : Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3(85):85ra46. 10.1126/scitranslmed.3002231 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 35. Coward RJ, Foster RR, Patton D, et al. : Nephrotic plasma alters slit diaphragm-dependent signaling and translocates nephrin, Podocin, and CD2 associated protein in cultured human podocytes. J Am Soc Nephrol. 2005;16(3):629–37. 10.1681/ASN.2004030172 [DOI] [PubMed] [Google Scholar]
- 36. Bakker WW, van Dael CM, Pierik LJ, et al. : Altered activity of plasma hemopexin in patients with minimal change disease in relapse. Pediatr Nephrol. 2005;20(10):1410–5. 10.1007/s00467-005-1936-3 [DOI] [PubMed] [Google Scholar]
- 37. Lennon R, Singh A, Welsh GI, et al. : Hemopexin induces nephrin-dependent reorganization of the actin cytoskeleton in podocytes. J Am Soc Nephrol. 2008;19(11):2140–9. 10.1681/ASN.2007080940 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 38. Kapojos JJ, Poelstra K, Borghuis T, et al. : Regulation of plasma hemopexin activity by stimulated endothelial or mesangial cells. Nephron Physiol. 2004;96(1):P1–10. 10.1159/000075574 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 39. Bakker WW, Borghuis T, Harmsen MC, et al. : Protease activity of plasma hemopexin. Kidney Int. 2005;68(2):603–10. 10.1111/j.1523-1755.2005.00438.x [DOI] [PubMed] [Google Scholar]
- 40. Goerge T, Barg A, Schnaeker EM, et al. : Tumor-derived matrix metalloproteinase-1 targets endothelial proteinase-activated receptor 1 promoting endothelial cell activation. Cancer Res. 2006;66(15):7766–74. 10.1158/0008-5472.CAN-05-3897 [DOI] [PubMed] [Google Scholar]
- 41. Harris JJ, McCarthy HJ, Ni L, et al. : Active proteases in nephrotic plasma lead to a podocin-dependent phosphorylation of VASP in podocytes via protease activated receptor-1. J Pathol. 2013;229(5):660–71. 10.1002/path.4149 [DOI] [PubMed] [Google Scholar]
- 42. Piccard H, Van den Steen PE, Opdenakker G: Hemopexin domains as multifunctional liganding modules in matrix metalloproteinases and other proteins. J Leukoc Biol. 2007;81(4):870–92. 10.1189/jlb.1006629 [DOI] [PubMed] [Google Scholar]
- 43. Alfano M, Cinque P, Giusti G, et al. : Full-length soluble urokinase plasminogen activator receptor down-modulates nephrin expression in podocytes. Sci Rep. 2015;5: 13647. 10.1038/srep13647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wei C, Möller CC, Altintas MM, et al. : Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14(1):55–63. 10.1038/nm1696 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 45. Wei C, El Hindi S, Li J, et al. : Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17(8):952–60. 10.1038/nm.2411 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 46. Sinha A, Bajpai J, Saini S, et al. : Serum-soluble urokinase receptor levels do not distinguish focal segmental glomerulosclerosis from other causes of nephrotic syndrome in children. Kidney Int. 2014;85(3):649–58. 10.1038/ki.2013.546 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 47. Wada T, Nangaku M, Maruyama S, et al. : A multicenter cross-sectional study of circulating soluble urokinase receptor in Japanese patients with glomerular disease. Kidney Int. 2014;85(3):641–8. 10.1038/ki.2013.544 [DOI] [PubMed] [Google Scholar]
- 48. Cara-Fuentes G, Wei C, Segarra A, et al. : CD80 and suPAR in patients with minimal change disease and focal segmental glomerulosclerosis: diagnostic and pathogenic significance. Pediatr Nephrol. 2014;29(8):1363–71. 10.1007/s00467-013-2679-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Reiser J, von Gersdorff G, Loos M, et al. : Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest. 2004;113(10):1390–7. 10.1172/JCI20402 [DOI] [PMC free article] [PubMed] [Google Scholar]; F1000 Recommendation
- 50. Garin EH, Mu W, Arthur JM, et al. : Urinary CD80 is elevated in minimal change disease but not in focal segmental glomerulosclerosis. Kidney Int. 2010;78(3):296–302. 10.1038/ki.2010.143 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 51. Ishimoto T, Cara-Fuentes G, Wang H, et al. : Serum from minimal change patients in relapse increases CD80 expression in cultured podocytes. Pediatr Nephrol. 2013;28(9):1803–12. 10.1007/s00467-013-2498-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Novelli R, Gagliardini E, Ruggiero B, et al. : Any value of podocyte B7-1 as a biomarker in human MCD and FSGS? Am J Physiol Renal Physiol. 2016;310(5):F335–41. 10.1152/ajprenal.00510.2015 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation
- 53. Saleem MA: The phenomenon of focal segmental glomerulosclerosis post-transplantation--a one-hit wonder? Pediatr Nephrol. 2012;27(12):2163–6. 10.1007/s00467-012-2218-5 [DOI] [PubMed] [Google Scholar]
- 54. Bitzan M, Babayeva S, Vasudevan A, et al. : TNFα pathway blockade ameliorates toxic effects of FSGS plasma on podocyte cytoskeleton and β3 integrin activation. Pediatr Nephrol. 2012;27(12):2217–26. 10.1007/s00467-012-2163-3 [DOI] [PubMed] [Google Scholar]
- 55. Bakr A, Shokeir M, El-Chenawi F, et al. : Tumor necrosis factor-alpha production from mononuclear cells in nephrotic syndrome. Pediatr Nephrol. 2003;18(6):516–20. 10.1007/s00467-003-1122-4 [DOI] [PubMed] [Google Scholar]; F1000 Recommendation