Abstract
We describe the case of a 62-year-old man who presented with shortness of breath that had progressed over several years. He had a history of a paralyzed right hemidiaphragm for at least the previous 10 years. He also reported weakness in his proximal legs and daytime sleepiness. On examination, he was found to have thoracoabdominal paradox when in supine position. Pulmonary function testing revealed severe restriction; arterial blood gas showed chronic respiratory acidosis. Electromyography showed chronic phrenic neuropathy bilaterally, with mild proximal myopathy. Serum aldolase level was mildly elevated, but serologic tests for connective tissue disorders were within reference range. After extensive clinical investigations, the patient was found to have severely reduced acid α-glucosidase. Genetic analysis confirmed the diagnosis of adult-onset Pompe disease. The patient started treatment with bilevel positive airway pressure titrated during polysomnography, and acid α-glucosidase enzyme replacement was recommended.
Keywords: Acid α-glucosidase enzyme, Bilevel positive airway pressure, Enzyme replacement therapy, Forced vital capacity, Glycogen storage disease, Pompe disease
Abbreviations: FRC, functional residual capacity; GAA, acid α-glucosidase
1. Introduction
Pompe disease is a rare genetic, autosomal recessive glycogen storage disorder caused by an absence or a deficiency of the lysosomal enzyme acid α-glucosidase (GAA). Adult-onset Pompe disease is a rare cause of dyspnea, and its exact incidence in the United States is not known. Nevertheless, this condition is necessary for a clinician to keep in mind when considering the differential diagnosis of the causes of respiratory muscle weakness. Early recognition of Pompe disease can lead to the timely replacement of the deficient enzyme, which can be beneficial to some patients with this disease.
We report the case of an older man with severe restrictive lung disease, hypercapnic respiratory failure, chronic phrenic neuropathy bilaterally, and proximal muscle weakness who was found to have Pompe disease. Our case highlights the diagnostic challenges that clinicians may face while providing a work-up of this uncommon disease.
1.1. Patient information
A 62-year-old man with a long history of apparently idiopathic right hemidiaphragm paralysis presented with progressive shortness of breath, especially on exertion. He noted his symptoms to be more prominent over the past 6 months. He could walk only 1 city block at a slow pace before stopping because of breathlessness. Additional symptoms were hypersomnolence during the day and proximal muscle weakness with no sensory loss. His past medical history included chronic obstructive pulmonary disease, diabetes mellitus type 2, hypertension, hypercholesterolemia, and coronary artery disease.
About 12 years previously, the patient had elevated serum creatine kinase levels, after which statin therapy was discontinued. A rheumatologic consult was obtained at that time, and no evidence of connective tissue disease was found. At the same time, he also underwent muscle biopsy and electromyography, both of which showed nonspecific myopathic features associated with mild denervation. The patient was lost to follow-up after these investigations. He was an exsmoker with 40–80 pack-years of smoking. Medications at presentation to our facility included aspirin, clopidogrel, simvastatin, diltiazem, sitagliptin, metformin, budesonide and formoterol inhaler, tiotropium bromide inhaler, and albuterol inhaler.
1.2. Clinical findings
On presentation, the patient's vital signs were a temperature of 36.5 °C (97.7 °F); heart rate, 94 beats/minute; blood pressure, 104/70 mm Hg; respiratory rate, 22 breaths/minute; oxygen saturation, 90% when receiving 2 L of supplemental oxygen per minute; and body mass index, 29.9 kg/m2. A general examination showed a man with greater-than-ideal body weight who appeared sleepy. Chest examination showed decreased air entry bilaterally. The rest of the systemic examination was normal except for paradoxical respirations on supine position: Respiratory distress immediately developed when he was lying flat.
1.3. Diagnostic assessment
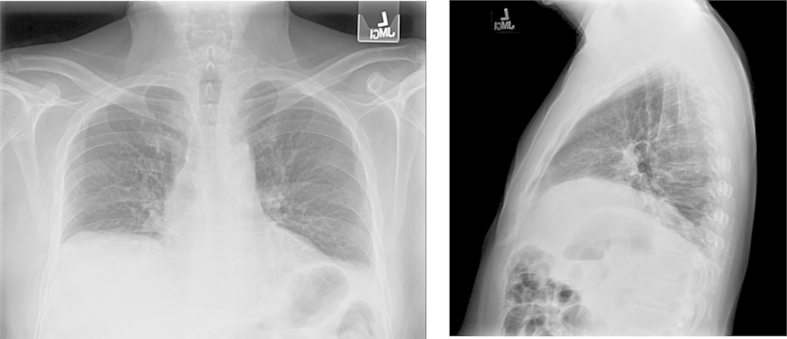
The patient's laboratory values were the following: hemoglobin, 120 g/L; platelets, 279 × 109/L; white blood cells, 7.7 × 109/L; sodium, 140 mmol/L; potassium, 5.1 mmol/L; bicarbonate, 40 mmol/L; serum urea nitrogen, 3.6 mmol/L; creatinine, 70.7 μmol/L; and calcium, 2.48 mmol/L Fig. 1 shows posteroanterior and lateral plain radiographs. Compared with prior images, computed tomography of the patient's chest showed a stable, mild elevation of the right hemidiaphragm, with bibasilar compressive atelectasis. Centrilobular emphysema with upper lung predominance and with fatty atrophy of paraspinal muscles and rotator cuff musculature was also found (Fig. 2). Tests for resting room-air arterial blood gases showed a pH of 7.29; PCO2, 89.4 mm Hg; PO2, 67.6 mm Hg; and serum bicarbonate, 43 mmol/L. Pulmonary function test results are shown in the Table 1.
Fig. 1.
Hemidiaphragm With Bibasilar Atelectasis. Posteroanterior and lateral chest radiographs show an elevated right hemidiaphragm and atelectasis.
Fig. 2.
Fatty Atrophy of Chest Musculature. Left panel, Fatty atrophy of right rotator cuff musculature (arrow). Right panel, Fatty atrophy of paraspinal muscles (arrows).
Table 1.
Pulmonary function tests.
| Function tested | Value |
|---|---|
| FVC, L sitting | 1.78 |
| % predicted | 39 |
| FVC, L lying supine | 1.36 |
| % predicted | 28 |
| FEV1, L | 1.20 |
| % predicted | 34 |
| FEV1/FVC | 69.6 |
| PI max, cm H2O | −27 |
| % predicted | 24 |
| PE max, cm H2O | 39 |
| % predicted | 19 |
| MVV, L | 38 |
| % predicted | 28 |
Abbreviations: FEV1, forced expiratory volume; FVC, forced vital capacity; max, maximum; MVV, maximum voluntary ventilation; PE, expiratory pressure; PI, inspiratory pressure.
Fluoroscopy showed right diaphragm paralysis and weakness in left hemidiaphragm. These findings prompted a consultation with neurology services. Electromyography showed complete absence of phrenic compound muscle action potentials bilaterally. No activation of motor unit potentials was seen within the diaphragm, either with respiration or with volitional effort. Ultrasonography showed that the left hemidiaphragm was hyperechoic, 0.14 cm thick at functional residual capacity (FRC), with a thickening ratio of 1.1 (the thickening ratio is thickness at total lung capacity/thickness at FRC). The right hemidiaphragm was hyperechoic, 0.15 cm thick at FRC, with thickening ratio of 0.9. (Normal thickness is >0.15 cm, normal thickening ratio is >1.2.) The aldolase concentration was slightly increased at 9 U/L (reference level, <7.7 U/L). The following tests were within reference range: creatine kinase, thyroid function tests, erythrocyte sedimentation rate, serum and protein electrophoresis, antinuclear antibody profile, and anti-Jo antibodies. To complete the evaluation for rarer causes of diaphragmatic dysfunction, neurology services requested testing for inherited neuropathies. Three weeks later, the patient's serum GAA level was 1.0 nmol/mL/hour (reference level, >7.4 nmol/mL/hour). This test was followed by genetic testing, and the patient was found to have c.525delT and c.-32-13T > G alterations, consistent with a diagnosis of Pompe disease.
Therapeutic intervention replacement treatment with recombinant acid α-glucosidase (GAA) was started. Consultation with sleep medicine services, followed by polysomnography, was obtained, and the patient received a prescription for bilevel positive airway pressure in the spontaneous, timed mode for respiratory failure from restrictive lung disease. Results of follow-up blood gases testing were pH of 7.40; PCO2, 46 mm Hg; PO2, 64 mm Hg; and bicarbonate, 29 mmol/L.
2. Discussion
Pompe disease, also known as glycogen storage disease type II, was first discovered by the Dutch pathologist Johann C. Pompe in 1932 when he carried out a postmortem examination of a 9-month-old girl who died of pneumonia [1]. In the autopsy, Pompe described accumulation of glycogen in muscle tissue. This rare genetic (autosomal recessive) lysosomal storage disorder is caused by the absence or deficiency of the lysosomal enzyme GAA. Glycogen degradation requires this enzyme; when a person has GAA deficiency, glycogen accumulates in tissues, although it mainly affects cardiac, skeletal, and smooth muscles. In the United States, the disease incidence of Pompe disease is not known. Three common mutations in the Dutch population have carrier frequencies that implicate an estimated frequency of late-onset Pompe disease as 1 in 57,000 persons [2].
Pompe disease is mainly divided into 2 types: infantile and late-onset (presenting after 1 year of age). The infantile type presents with cardiomegaly, generalized muscle weakness, hypotension, enlarged tongue, and hepatomegaly [3], [4]. This form results in severe Pompe disease and poor prognosis. In the late-onset or adult form, the heart and the liver are not involved. The late-onset form can present at any age, with the major characteristics of proximal muscle weakness and diaphragmatic involvement that leads to respiratory failure [5], [6], [7].
In contrast to the other myopathies, the respiratory neuromuscular unit is particularly susceptible to involvement by Pompe disease [8], [9]. Nighttime respiratory difficulty usually precedes its daytime symptoms [10]. Impaired cough and retained respiratory secretions lead to frequent pneumonia bouts and finally to acute respiratory failure. Approximately 60% of patients with late-onset Pompe disease have mild reductions in vital capacity and, according to 1 study, their vital capacity shows a variable, but in most cases progressively, deteriorating course, with a mean rate of decline of 1.6% per year [11]. Severe respiratory failure can occur independent of limb muscle weakness and is reported to be the most common cause of death [12].
Our patient had substantial phrenic nerve neuropathy. Newer studies support the finding that lysosomal dysfunction can lead to neuronal cell death. DeRuisseau et al. [13] provided a histologic description of phrenic motoneurons in the C4 spinal cord that showed swollen cell bodies, and biochemical survey indicated accumulation of glycogen. This phrenic motoneuron pathologic change contributes substantially to diaphragm motor deficits seen in Pompe disease [14], [15].
The diagnosis of late-onset Pompe disease is usually delayed because of the heterogeneity of the disease. GAA activity ranges from about 1% to 40% and hence presents with variable manifestations and disease severity [16], [17]. The symptoms can mimic other neuromuscular disease, and the tests usually performed for these patients, such as electromyography and muscle biopsy, may not provide the answer. Creatine kinase levels are usually elevated but can be at reference level, as in our patient [18]. Thus, the clinician must maintain a high index of suspicion in order to reach the diagnosis.
We did not perform muscle biopsy in our patient. However, when muscle biopsy shows abnormal glycogen accumulation and a vacuolar neuropathy, it is diagnostic for Pompe disease [19]. The diagnostic challenge is that vacuoles may not always contain glycogen, and electron microscopy may be needed for detection. The extent to which vacuolization occurs varies from patient to patient; even within the same patient, differences in vacuolization can occur among different muscles and fiber types [20].
The gold standard for diagnostic testing in Pompe disease is the finding of reduced or absent GAA activity either in blood, a cultured fibroblast, or muscle. Since the testing level of this enzyme can be falsely low, the diagnosis is usually made by either combining the enzyme activity test with DNA mutation analysis for GAA gene sequencing or with repeating the measurement of GAA level in a second sample [21].
In 2006, enzyme replacement therapy with alglucosidase-α for Pompe disease was approved in the United States and the European Union. In a randomized, double-blind placebo-controlled study of 90 patients with late-onset Pompe disease, van der Ploeg et al. [22] found a significant improvement in the 6-min walk test, as well as forced vital capacity [FVC], at 78 weeks of enzyme replacement therapy. This effect was observed in all the study's participants but was more pronounced in patients with better clinical status at the baseline. These findings reinforce that early diagnosis and the institution of treatment are essential to prevent progressive loss in function.
Conflict of interest
None.
Funding
None.
References
- 1.Hoyer S., Samuelson G. The man behind the syndrome: Joannes Cassianus Pompe. The first person to determine glycogen storage in heart enlargement: he died violently before an execution squad. Lakartidningen. 1986 Apr 16;83(16):1477–1479. (Swedish) [PubMed] [Google Scholar]
- 2.Ausems M.G., Verbiest J., Hermans M.P., Kroos M.A., Beemer F.A., Wokke J.H. Frequency of glycogen storage disease type II in The Netherlands: implications for diagnosis and genetic counselling. Eur. J. Hum. Genet. 1999 Sep;7(6):713–716. doi: 10.1038/sj.ejhg.5200367. [DOI] [PubMed] [Google Scholar]
- 3.Kishnani P.S., Hwu W.L., Mandel H., Nicolino M., Yong F., Corzo D. Infantile-Onset Pompe Disease Natural History Study Group. A retrospective, multinational, multicenter study on the natural history of infantile-onset Pompe disease. J. Pediatr. 2006 May;148(5):671–676. doi: 10.1016/j.jpeds.2005.11.033. [DOI] [PubMed] [Google Scholar]
- 4.Howell R.R., Byrne B., Darras B.T., Kishnani P., Nicolino M., van der Ploeg A. Diagnostic challenges for Pompe disease: an under-recognized cause of floppy baby syndrome. Genet. Med. 2006 May;8(5):289–296. doi: 10.1097/01.gim.0000204462.42910.b8. [DOI] [PubMed] [Google Scholar]
- 5.Hagemans M.L., Winkel L.P., Van Doorn P.A., Hop W.J., Loonen M.C., Reuser A.J. Clinical manifestation and natural course of late-onset Pompe's disease in 54 Dutch patients. Brain. 2005 Mar;128(Pt 3):671–677. doi: 10.1093/brain/awh384. Epub 2005 Jan 19. [DOI] [PubMed] [Google Scholar]
- 6.Hagemans M.L., Winkel L.P., Hop W.C., Reuser A.J., Van Doorn P.A., Van der Ploeg A.T. Disease severity in children and adults with Pompe disease related to age and disease duration. Neurology. 2005 Jun 28;64(12):2139–2141. doi: 10.1212/01.WNL.0000165979.46537.56. [DOI] [PubMed] [Google Scholar]
- 7.Engel A.G. Acid maltase deficiency in adults: studies in four cases of a syndrome which may mimic muscular dystrophy or other myopathies. Brain. 1970;93(3):599–616. doi: 10.1093/brain/93.3.599. [DOI] [PubMed] [Google Scholar]
- 8.Haley S.M., Fragala M.A., Skrinar A.M. Pompe disease and physical disability. Dev. Med. Child. Neurol. 2003 Sep;45(9):618–623. doi: 10.1017/s0012162203001129. [DOI] [PubMed] [Google Scholar]
- 9.Mellies U., Lofaso F. Pompe disease: a neuromuscular disease with respiratory muscle involvement. Respir. Med. 2009 Apr;103(4):477–484. doi: 10.1016/j.rmed.2008.12.009. Epub 2009 Jan 7. [DOI] [PubMed] [Google Scholar]
- 10.Mellies U., Stehling F., Dohna-Schwake C., Ragette R., Teschler H., Voit T. Respiratory failure in Pompe disease: treatment with noninvasive ventilation. Neurology. 2005 Apr 26;64(8):1465–1467. doi: 10.1212/01.WNL.0000158682.85052.C0. [DOI] [PubMed] [Google Scholar]
- 11.Van der Beek N.A., Hagemans M.L., Reuser A.J., Hop W.C., Van der Ploeg A.T., Van Doorn P.A. Rate of disease progression during long-term follow-up of patients with late-onset Pompe disease. Neuromuscul. Disord. 2009 Feb;19(2):113–117. doi: 10.1016/j.nmd.2008.11.007. Epub 2008 Dec 11. [DOI] [PubMed] [Google Scholar]
- 12.Bailey E.F., Fregosi R.F. Coordination of intrinsic and extrinsic tongue muscles during spontaneous breathing in the rat. J. Appl. Physiol. 1985;96(2):440–449. doi: 10.1152/japplphysiol.00733.2003. 2004 Feb. Epub 2003 Oct 3. [DOI] [PubMed] [Google Scholar]
- 13.DeRuisseau L.R., Fuller D.D., Qiu K., DeRuisseau K.C., Donnelly W.H., Jr., Mah C. Neural deficits contribute to respiratory insufficiency in Pompe disease. Proc. Natl. Acad. Sci. U. S. A. 2009 Jun 9;106(23):9419–9424. doi: 10.1073/pnas.0902534106. Epub 2009 May 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mah C.S., Falk D.J., Germain S.A., Kelley J.S., Lewis M.A., Cloutier D.A. Gel-mediated delivery of AAV1 vectors corrects ventilatory function in Pompe mice with established disease. Mol. Ther. 2010 Mar;18(3):502–510. doi: 10.1038/mt.2009.305. Epub 2010 Jan 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrne B.J., Falk D.J., Pacak C.A., Nayak S., Herzog R.W., Elder M.E. Pompe disease gene therapy. Hum. Mol. Genet. 2011 Apr 15;20(R1):R61–R68. doi: 10.1093/hmg/ddr174. Epub 2011 Apr 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kishnani P.S., Steiner R.D., Bali D., Berger K., Byrne B.J., Case L.E. Pompe disease diagnosis and management guideline. Genet. Med. 2006 May;8(5):267–288. doi: 10.1097/01.gim.0000218152.87434.f3. Erratum in: Genet Med. 2006 Jun;8(6):382. ACMG Work Group on Management of Pompe Disease [removed]; Case, Laura [corrected to Case, Laura E] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kishnani P.S., Howell R.R. Pompe disease in infants and children. J. Pediatr. 2004 May;144(5 Suppl):S35–S43. doi: 10.1016/j.jpeds.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 18.Winkel L.P., Hagemans M.L., van Doorn P.A., Loonen M.C., Hop W.J., Reuser A.J. The natural course of non-classic Pompe's disease: a review of 225 published cases. J. Neurol. 2005 Aug;252(8):875–884. doi: 10.1007/s00415-005-0922-9. [DOI] [PubMed] [Google Scholar]
- 19.Kretzschmar H.A., Wagner H., Hubner G., Danek A., Witt T.N., Mehraein P. Aneurysms and vacuolar degeneration of cerebral arteries in late-onset acid maltase deficiency. J. Neurol. Sci. 1990 Sep;98(2–3):169–183. doi: 10.1016/0022-510x(90)90258-o. [DOI] [PubMed] [Google Scholar]
- 20.Amato A.A. Acid maltase deficiency and related myopathies. Neurol. Clin. 2000 Feb;18(1):151–165. doi: 10.1016/s0733-8619(05)70182-1. [DOI] [PubMed] [Google Scholar]
- 21.American Association of Neuromuscular & Electrodiagnostic Medicine Diagnostic criteria for late-onset (childhood and adult) Pompe disease. Muscle Nerve. 2009 Jul;40(1):149–160. doi: 10.1002/mus.21393. [DOI] [PubMed] [Google Scholar]
- 22.van der Ploeg A.T., Clemens P.R., Corzo D., Escolar D.M., Florence J., Groeneveld G.J. A randomized study of alglucosidase alfa in late-onset Pompe's disease. N. Engl. J. Med. 2010 Apr 15;362(15):1396–1406. doi: 10.1056/NEJMoa0909859. [DOI] [PubMed] [Google Scholar]