Abstract
Background
Idiopathic pulmonary fibrosis is the most frequent interstitial disease with the worst prognosis. It is characterized by an uncontrolled fibrosis which is difficult to manage. The pathogenesis of this disease remains unclear with many theories resulting in multiple target therapies. The relation between fibrosis and vascular remodeling has been debated in the literature with different results that may seem contradictory.
Aim
We target to evaluate the connection between fibrosis and vascular remodeling in usual interstitial pneumonia.
Material and methods
26 cases of idiopathic pulmonary fibrosis were reviewed by 2 pathologists and the diagnosis of UIP was retained according to the American Thoracic Society's criteria. Fibrotic changes and vascular remodeling were evaluated blindly. The fibrotic changes were classified as severe, intermediate and mild. Vascular occlusion was graded in 4 grades extending from medial hypertrophy (grade 1) to plexiform lesions of the vascular wall (grade 4).
Results
We noticed that severe degrees of fibrosis were correlated with severe grades of vascular obstruction. In fact, our 26 cases were classified as severe fibrosis in 11 cases with grade IV vascular lesions in 6 cases, intermediate fibrosis in 12 cases with grade II vascular lesions in 8 cases and mild fibrosis in 3 cases with grade I vascular lesions in all cases.
Conclusion
Many theories have been reported concerning the UIP's pathogenesis. Recently, many authors reported that the primum movens of these lesions was an epithelial/endothelial injury which induces uncontrolled fibrosis and microvascular remodeling using different pathways. This puts emphasis on the necessity of multi-target therapies in order to improve the management of this fatal disease.
Keywords: Usual interstitial pneumonia, Fibrosis, Vascular remodeling
1. Introduction
Idiopathic pulmonary fibrosis (IPF) is the most frequent interstitial disease which is classified among idiopathic interstitial diseases (IIDs). This entity is a fibrosing disease characterized by the worse prognosis due to its difficult management. In fact, the only efficient treatment of this entity is the lung transplantation. Its better management depends on the knowledge of its complex pathogenesis. IPF is a clinical denomination and the histological correlation is usual interstitial pneumonia (UIP) which is characterized by a heterogeneous architecture determined by the succession of normal and fibrotic areas. Microvascular remodeling is a common phenomenon which is frequently reported in UIP. This phenomenon in association to other factors, deals with the development of a pulmonary hypertension (PH) which is associated with a poor survival [1], [2]. Many authors tried to assess the inter-connection between parenchymal fibrosis and vascular remodeling. We tried to explore this relationship among 26 cases of UIP diagnosed in our department of Pathology. We tried to emphasize on the fact that more effective therapeutics may be discovered if both phenomenon will be more integrated.
2. Materials
26 patients with the diagnosis of IPF/UIP were included in this study. All the cases were diagnosed on surgical lung sample according to the American Thoracic Society/European Respiratory Society International multidisciplinary consensus classification of the idiopathic interstitial pneumonias (IIP). All slides were reviewed by 2 pathologists. UIP was defined by alternating areas of normal parenchyma and injured parenchyma characterized by alveolar collapse, honeycombing and severe organizing fibrous airspace wall [3] (Fig. 1). Similarly to the article of Parra and coworkers, we evaluated the fibrosis using a semiquantitative manner [4]. Grade I was attributed to minimal fibrotic lesions defined as alveolar collapse with relatively normal lung parenchyma. Grade II lesions were characterized by moderate organizing fibrosis of the wall with fibroblast foci. Grades III and IV were defined by severe fibrosis of the wall with honeycombing and foci of actively proliferating fibroblasts and myofibroblasts (Fig. 2).
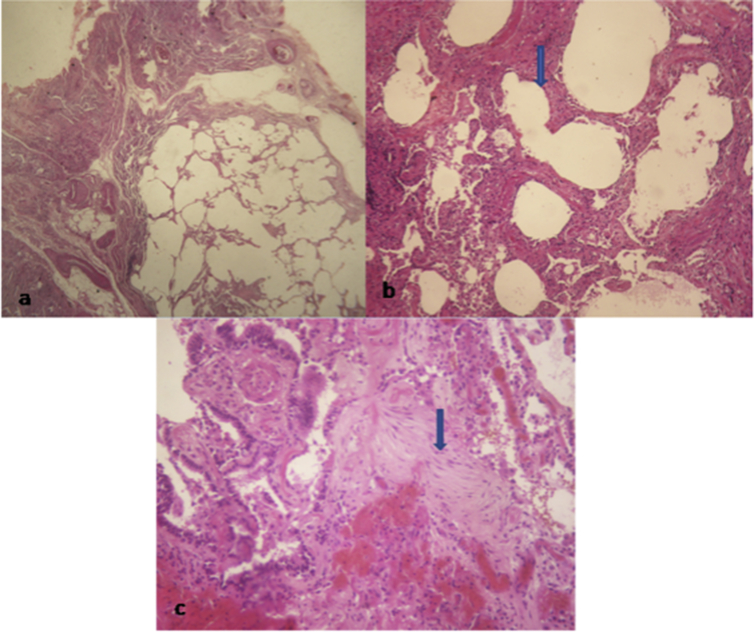
Fig. 1.
Diagnostic criteria of usual interstitial pneumonia: a/ alternating areas of relatively normal parenchyma and fibrosing areas with alveolar collapse. b/ honeycombing areas. c/ Fibroblast foci within alveolar wall.
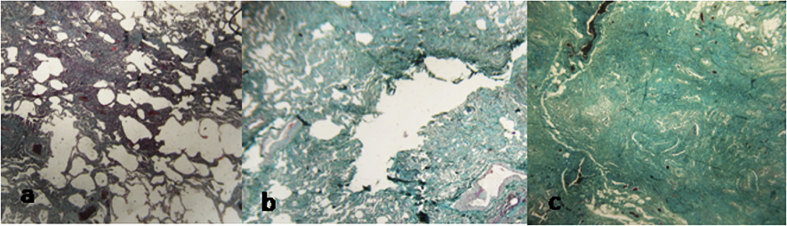
Fig. 2.
a/ UIP with minimal fibrosis characterized by alveolar collapse with mild interstitial thickening by fibrosis. c/ UIP with moderate fibrosis characterized by high degree of inflammation activity and moderate mural organizing fibrosis. c/ UIP with severe fibrosis characterized by severe mural organizing fibrosis.
In order to grade the vascular remodeling, we also used the criteria of Parra and colleagues. It was evaluated by a semiquantitative analysis for different level of vascular obstruction. Grade I defined isolated hypertrophy of the arterial media. Grade II corresponded to proliferative intimal lesions. Total occlusion of the arterial lumen defined grade III and grade IV was characterized by plexiform lesions (Fig. 3).
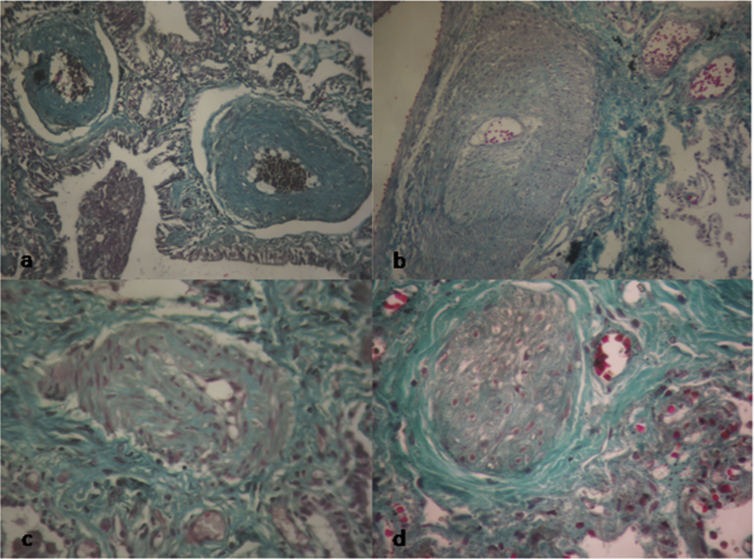
Fig. 3.
Distortion of the vascular wall architecture in fibrotic areas. a/ Grade I lesions characterized by isolated hypertrophy of the arterial media. b/ Grade II lesions characterized by proliferative intimal cells. c/ Grade III lesions characterized by the total occlusion of the arterial lumen by fibrosis tissue. d/ Grade IV lesions characterized by plexiform lesions and major proliferation of elastic fibres in the internal and external elastic lumina of the vascular wall.
3. Results
- Baseline characteristic: Between 2006 and 2010, 32 surgical biopsies were performed in order to explore ILDs. Among these cases, 80.6% (26 cases) were classified as UIP, 19.35% (6 cases) were classified as NSIP. We focused on the 26 cases of UIP. Our study was about 6 women and 20 men with a median age of 56.76 (range 37–75).
- Diagnostic criteria: To retain the diagnosis of UIP, all the items follow were present in all cases: a heterogenous architecture, honeycombing cysts, fibroblastic foci. Besides, the diagnosis of UIP was retained only after a correlation with clinical and radiological findings.
- Morphological results: The different grades of vascular obstruction were correlated with parenchymal changes. In fact, grades III and IV were observed in heavily fibrotic pulmonary parenchyma. Minimal fibrosis was noted in 3 cases with grade I. In all cases, vascular remodeling was of grade I. Intermediate fibrosis was noticed in 12 cases. 2 cases were classified as grade I, 8 cases were classified as grade II and 2 cases were classified as grade III. Severe fibrosis was noted in 11 cases. One case was classified as grade I, 1 case was classified as grade II, 3 cases were classified as grade III and 6 cases were classified as grade IV.
4. Discussion
Pulmonary fibrosis is refractory to treatment and induces a high mortality rate. It represents a heterogeneous group of lung disorders dealing with the progressive and irreversible destruction of lung architecture and death from respiratory failure [5]. IPF is of unknown etiology and is characterized by a heavy fibrosis dealing with a life expectancy of 2–6 years after diagnosis [6]. Many pathogenic theories have been reported within the last decade. Initial theories supposed that chronic inflammation played a key-role [7]. Then, many authors supposed that this theory wasn't convincing based on the finding that consisted in the presence of epithelial cell injury and abnormal wound repair in the absence of preceding inflammation [8]. Besides, intrinsic defects in the wound healing response involving lung epithelial cells and fibroblasts have supposed to play a major role in the pulmonary fibrosis's pathogenesis [9]. Finally, based on the multiplicity of the theories, many authors supposed that the pathogenic events must be more complex [10]. Vascular remodeling is a known phenomenon in IPF dealing with pulmonary hypertension whose prevalence in patients with IPF ranges from 32 to 85% [11]. In fact, using an experimental model, Farkas and colleagues have recently shown that the intimate interconnection between fibrosis and PH via effects of growth factors on the pulmonary microcirculation [12]. However, the accurate mechanisms haven't been assessed.
Vascular remodeling phenomenon seems to be the result of inflammatory changes instead of the modification of the vascular flow [4]. Plexiform lesions with abundant atypical endothelial cells are reported [13]. Regarding their origin, it has been postulated that these cells are derived from resident vascular smooth muscle cells and/or from adventitial fibroblasts via de-differentiation of the former or differentiation of the latter [14]. Numerous growth factors have been implicated in the development of the pulmonary vascular system. The most widely investigated is the Vascular Endothelial Growth Factor (VEGF) which is implicated in angiogenesis, vasculogenesis and alveologenesis. Epithelial cells are a predominant source of VEGF during lung development. In adult fibrotic lung, endothelial apoptosis is induced by injury and remodeling. This fact results in the decrease of the expression of VEGF in the damaged lung. This fact illustrates that epithelial injury results in both fibrosis and vascular remodeling. Besides, transforming growth factor TGF-B is important for coordinated vascular development.
It is documented that TGF B is a key factor in alveolar branching and increased TGF B activity is associated with progressive fibrosis in ILD and endothelial cell apoptosis [12].
Concerning the phenomenon of hypertrophy of the media, the pathways seem to be more complex. It is possible that the remodeled pulmonary microvasculature perpetuates the progression of the underlying ILD and pulmonary fibrosis via mediators secreted by injured endothelial cells, endothelial to mesenchymal transition and altered perivascular mesenchyma [15].
Recently, it has been reported that the pathobiology underlying the vascular remodeling and fibrosis of the parenchyma are closely intertwined but the results concerning vascular remodeling are quite discordant. In fact, Turner–Warwick has likely reported that fibrotic lungs have increased vessel density, on the other hand, Ebina and colleagues showed contradictory findings [15], [16], [17]. In our study, we tried to analyze only fibrotic regions and we noticed that the more severe the fibrosis is, the most important is the vasculature remodeling. These results may seem contradictory but may be explained by the heterogeneity of UIP and the difference in the areas focused on by the different authors. Published reports indicate that fibrotic areas have fewer blood vessels. This result was proved in our study. In the other hand, adjacent nonfibrotic tissue is highly vascularized [17], [18]. In fact, in fibrotic regions, the increase of angiostatic molecules and the decreased amount of VEGF because of the injury of the epithelial/endothelial cells is an attempt to decrease the progressive fibrogenesis [12]. On the other hand, in areas adjacent to fibrotic lung tissue, it is plausible that the increase of VEGF amount derived from apoptotic epithelial cells deals with a high vascular density [19]. The latter phenomenon may explain the authors of some authors who may focused on perifibrotic areas and who reported increased vascular remodeling with severe fibrosis. According to our results, pulmonary fibrosis and vascular remodeling result from the same primum movens which is the epithelial/endothelial cell injury of unknown origin, resulting in the activation of myofibroblasts in one side and the decrease of secretion of VEGF from apoptotic cells in fibrotic regions in the other side. The former phenomenon results in an uncontrolled fibrosis in predisposed persons and the latter results in the rarefaction of the vessels in fibrotic regions. The best knowledge of the pathogenesis of the IPF plays a key role in order to improve the management of this entity. In fact, till now, the lung transplant remains the only effective therapeutic modality in cases resistant to medical drugs [10]. Our results highlight the correlation between fibrosis and vascular remodeling and put emphasis on the necessity of multiple target therapies in order to control the fibrosis such as metalloproteases, micro RNA miR-21, epigenetic changes in fibroblasts but also vasculogenesis by targeting the most relevant vascular enhancers such as VEGF [10], [20], [21].
References
- 1.Lettieri C.J., Nathan S.D., Barnett S.D., Ahmad S., Shorr A.F. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006;129:746–752. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- 2.Glaser S., Noga O., Koch B. Impact of pulmonary hypertension on gas exchange and exercise capacity in patients with pulmonary fibrosis. Respir. Med. 2009;103:317–324. doi: 10.1016/j.rmed.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Demedts M., Costabel U. ATS/ERS international multidisciplinary consensus classification of the idiopathic interstitial pneumonias. Eur. Respir. J. 2002;19:794–796. doi: 10.1183/09031936.02.00492002. [DOI] [PubMed] [Google Scholar]
- 4.Parra E.R., Kairalla R.A., De Carvalho C.R.R., Capelozzi V.L. Abnormal deposition of collagen/elastic vascular fibres and prognostic significance in idiopathic interstitial pneumonias. Thorax. 2007;62(5):428–437. doi: 10.1136/thx.2006.062687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wynn T.A. Integrating mechanisms of pulmonary fibrosis. J. Exp. Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selman M., King T.E., Pardo A., American Thoracic Society: European Respiratory Society; American College of Chest physicians Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann. Intern Med. 2001;134:136–151. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 7.Ask K., Martin G.E.M., Kolb M., Gauldie J. Targeting genes for treatment in idiopathic pulmonary fibrosis. Proc. Am. Thorac. Soc. 2006;3:389–393. doi: 10.1513/pats.200602-021TK. [DOI] [PubMed] [Google Scholar]
- 8.Strieter R.M. Pathogenesis and natural history of usual interstitial pneumonia: the whole story or the last chapter of a long novel. Chest. 2005;128:526S–532S. doi: 10.1378/chest.128.5_suppl_1.526S. [DOI] [PubMed] [Google Scholar]
- 9.Lovgren A.K., Kovacs J.J., Xie T., Potts E.N., Foster W.M., Liang J., Meltzer E.B., Jiang D., Lefkowitz R.J., Noble P.W. Beta-arrestin deficiency protects against pulmonary fibrosis in mice and prevents fibroblast invasion of extracellular matrix. Sci. Transl. Med. 2011;3:74ra23. doi: 10.1126/scitranslmed.3001564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gharaee-Kermani M., Gyetko M.R., Hu B., Phan S.H. New insights into the pathogenesis and treatment of idiopathic pulmonary fibrosis: A potential role for stem cells in the lung parenchyma and implications for therapy. Pharm. Res. 2007;24:819–841. doi: 10.1007/s11095-006-9216-x. [DOI] [PubMed] [Google Scholar]
- 11.Lettieri C.J., Nathan S.D., Barnett S.D., Ahmad S., Shorr A.F. Prevalence and outcomes of pulmonary arterial hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2006;129:746–752. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- 12.Nathan S.D., Noble P.W., Tuder R.M. Idiopathic pulmonary fibrosis and pulmonary hypertension connecting the dots. Am. J. Respir. Crit. Care Med. 2007;175:875–880. doi: 10.1164/rccm.200608-1153CC. [DOI] [PubMed] [Google Scholar]
- 13.Farkas L., Farkas D., Ask K., Moller A., Gauldie J., Margetts P., Inman M., Kolb M. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J. Clin. Invest. 2009;119:1298–1311. doi: 10.1172/JCI36136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yeager M.E., Frid M.G., Stenmark K.R. Progenitor cells in pulmonary vascular remodeling. Pulm. Circ. 2011;1:4–16. doi: 10.4103/2045-8932.78095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farkas L., Kolb M. Pulmonary microcirculation in interstitial lung disease. Proc. Am. Thorac. Soc. 2011;8:516–521. doi: 10.1513/pats.201101-007MW. [DOI] [PubMed] [Google Scholar]
- 16.Turner-Warwick M. Precapillary systemic-pulmonary anastomoses. Thorax. 1963;18:225–237. doi: 10.1136/thx.18.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ebina M., Shimizukawa M., Shibata N., Kimura Y., Suzuki T., Endo M., Sasano H., Kondo T., Nukiwa T. heterogeneous increase in cd34-positive alveolar capillaries in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2004;169:1203–1208. doi: 10.1164/rccm.200308-1111OC. [DOI] [PubMed] [Google Scholar]
- 18.Simler N.R., Brenchley P.E., Horrocks A.W., Greaves S.M., Hasleton P.S., Egan J.J. Angiogenic cytokines in patients with idiopathic interstitial pneumonia. Thorax. 2004;59:581–585. doi: 10.1136/thx.2003.009860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakao S., Taraseviciene-Stewart L., Wood K., Cool C.D., Voelkel N.F. Apoptosis of pulmonary microvascular smooth muscle growth. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;291:L362–L368. doi: 10.1152/ajplung.00111.2005. [DOI] [PubMed] [Google Scholar]
- 20.Pandit K.V., Corcoran V.D., Yousef H., Yarlagadda M., Tzouvelekis A., Gibson K.F., Konishi K., Yousem A., Singh M., Handley D. Inhibition and role of let-7d in idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2010;182:220–229. doi: 10.1164/rccm.200911-1698OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bechtel W., McGoohan S., Zeisberg E.M., Muller G.A., Kalbacher H., Salant D.J., Muller C.A., Kalluri R., Zeisberg M. Methylation determines fibroblast activation and fibrogenesis in the kidney. Nat. Med. 2010;16 doi: 10.1038/nm.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]