Abstract
Idiopathic pulmonary fibrosis (IPF) is a dreaded disease of uncertain etiology and no available cure. It is still unclear if a causal relationship exists between gastro-esophageal reflux (GER) and IPF, but studies have shown an increased prevalence of acid reflux in patients with IPF. We describe a patient with achalasia and GER who went on to develop IPF. She underwent a rapidly worsening course punctuated by acute exacerbations of IPF, despite best efforts to manage the acid GER. We also reviewed the literature on the role of GER in the etiology and progression of IPF and the impact of antireflux measures on its course.
Keywords: Gastroesophageal reflux, Idiopathic pulmonary fibrosis, Pulmonary fibrosis, Acid reflux, Proton pump inhibitor, Fundoplication
1. Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive disease with a steady downhill course, despite a variety of treatment strategies. Gastro-esophageal reflux (GER) is known to occur in a high proportion of patients with IPF. We describe a patient with IPF whose disease progression was drastically altered by GER.
2. Case
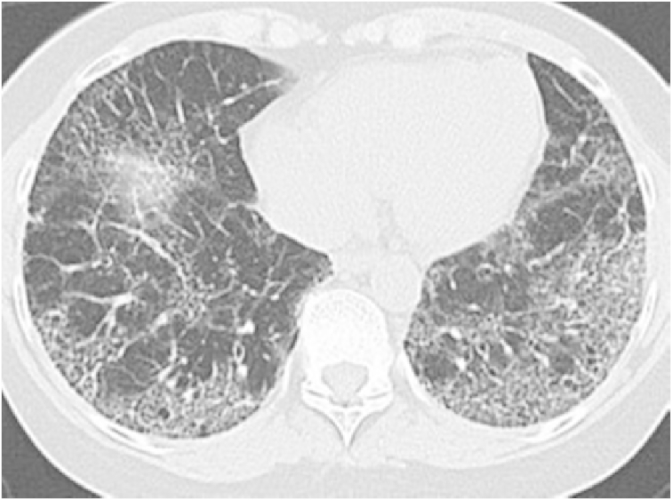
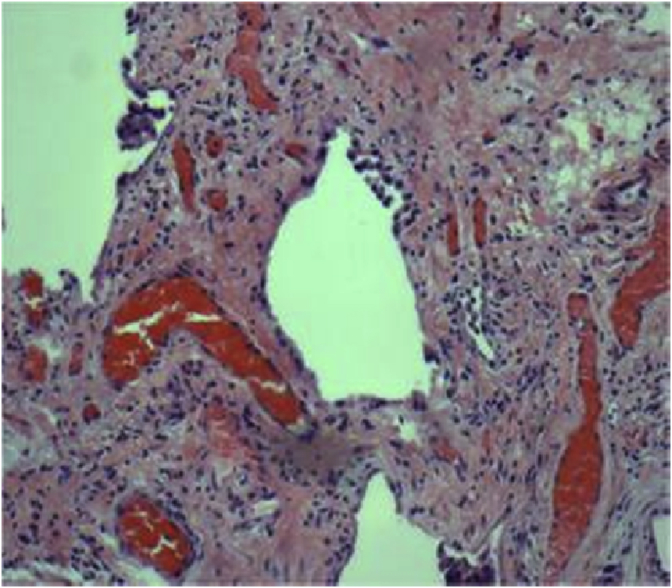
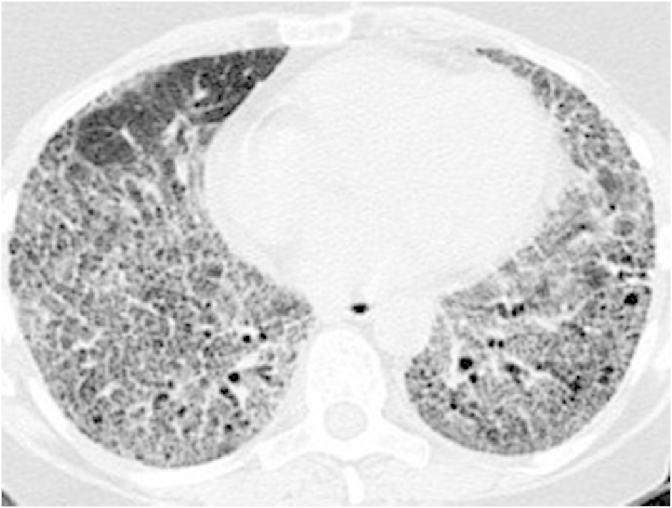
A 54 year old female with a history of celiac disease was on treatment with proton pump inhibitors (PPI) for her new onset of reflux symptoms. Upper gastrointestinal endoscopy was unremarkable. Esophageal manometry and motility studies revealed a hypertensive lower esophageal sphincter with mild esophageal dysmotility, suggestive of achalasia. PPIs were continued on a twice daily basis. She also received endoscopic injections of botulinum toxin in the lower esophageal sphincter (LES) with partial and transient relief of symptoms. Six months following the onset of her reflux symptoms, she developed slowly progressive shortness of breath and cough. A chest radiograph showed bilateral interstitial thickening. A high-resolution computed tomography (CT) of the chest revealed a bilateral prominent interstitial reticular pattern predominantly in the lower lobes and subpleural regions with mild honeycombing (Fig. 1), consistent with IPF. Pulmonary function tests (PFTs) showed an FVC of 61%, FEV1 of 64% and FEV1/FVC of 106% suggestive of a restrictive pattern. Total lung capacity and vital capacity were diminished, the residual volume to total lung capacity ratio was elevated at 122% and the diffusion capacity for carbon monoxide was diminished at 39%. Video assisted thoracoscopic lung biopsy showed chronic active interstitial pneumonitis and fibrosis with maximal changes beneath the pleura. Histopathology demonstrated a usual interstitial pneumonia (UIP) pattern, corresponding to the clinical diagnosis of IPF (Fig. 2). PPIs were continued and she also followed the lifestyle changes advised for GER. She was placed on a trial of N-acetyl cysteine without obvious benefit.
Fig. 1.
On initial evaluation, high resolution computed tomography of the chest shows bilateral interstitial reticular pattern on the lower lobes with mild honey combing.
Fig. 2.
Video assisted thoracoscopic lung biopsy showed chronic active interstitial pneumonitis and fibrosis representing usual interstitial pneumonia pattern.
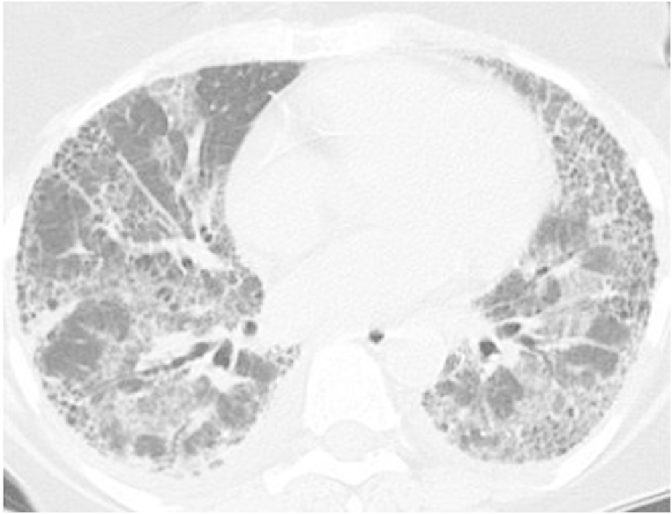
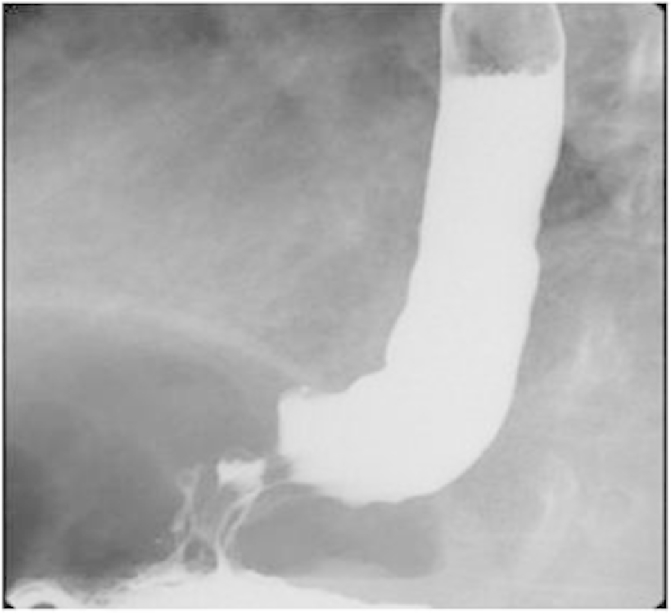
Over the next year, while stable symptomatically, PFTs showed a gradual decline in lung volumes, forced vital capacity and diffusion capacity. In the next few months, she developed progressive worsening shortness of breath. A repeat CT of the chest demonstrated bilaterally increasing interstitial fibrosis and traction bronchiectasis (Fig. 3). Since she also had progressive reflux symptoms despite medical treatment, she underwent a laparoscopic Heller's myotomy (LHM) with Dor fundoplication. In the next three months her reflux symptoms worsened, and she deteriorated with severe shortness of breath and hypoxia, requiring continuous nasal oxygen. A double contrast barium swallow revealed no evidence of a leak and persistent dilatation at the region of fundoplication (Fig. 4). Gastric emptying studies showed no delay. Repeat imaging revealed severe extensive honey combing and air trapping with superimposed ground glassing (Fig. 5). Her respiratory status continued to decline and she died of respiratory failure.
Fig. 3.
Repeat high resolution computed tomography of the chest done for worsening symptoms reveals increasing fibrosis and traction bronchiectasis.
Fig. 4.
Double contrast barium swallow done 3 months following laparoscopic Heller's myotomy with fundoplication shows persistent dilatation at the region of fundoplication.
Fig. 5.
Computed tomography of the chest done 3 months after the surgery reveals extensive honey combing and ground glassing indicating superimposed pneumonitis.
3. Discussion
IPF is a devastating disease with a progressive downhill course. It has a serious impact on the quality of life and carries a median survival rate of only 3–5 years [1]. Despite the burden imposed by IPF on health care and numerous clinical trials with several agents, with an absence to date of a clear etiopathogenesis there has been no improvement in mortality. Research recently focuses on alveolar epithelial injury resulting in an exaggerated tissue reaction and fibrosis [2]. There is growing consensus on the role of chronic asymptomatic microaspiration causing the initial insult, resulting in aberrant wound healing. Animal models have demonstrated that aspiration of gastric fluid can result in activation of the fibrosis cascade in the pulmonary parenchyma through increased concentration of profibrotic and inflammatory cytokines IL-1, IL-2, TNF-alpha and TGF-beta [3]. Though similar studies are not possible in humans, there has been much emphasis in the association between IPF and GER, which is the most important risk factor for microaspiration. Esophageal 24-h pH monitoring has shown abnormal esophageal acid exposure in 68–94% of patients with IPF [4], [5], [6], which was clearly much higher than the prevalence of GER in the controls and the general population. Many of these patients did not have the classical clinical symptoms of reflux, and standard dose PPI therapy may not suppress the acid GER in this group. The role of non-acid reflux and chronic micro-aspiration of gastric contents has also been studied in these patients. Bronchoalveolar lavage (BAL) pepsin, which has been proven to be a good biomarker for gastric aspiration in several population subsets, was found to be elevated in patients with IPF when compared to the general population. Also, BAL pepsin levels have been found to be significantly higher in patients with acute exacerbation when compared to the stable cohort of IPF patients, which suggests that aspiration plays an important part in the progression of the disease by causing acute exacerbations [7].
Though an association between GER and IPF is proven, it is difficult to establish a cause-effect relationship. Gastro-esophageal reflux disease (GERD) is very common in the general population, and it is unclear why only a very small proportion develops pulmonary fibrosis. One argument against the causal relationship is that increased reflux of gastric contents may be secondary to the mechanical effects of IPF including poor lung compliance, distortion of mediastinal anatomy and weakening of the lower esophageal sphincter [8]. In a sizeable proportion of patients, the course of IPF is characterised by periods of clinical stability interspersed with acute exacerbations, which result in a decline of respiratory status and worsen the clinical outcome of the disease [9]. GER and aspiration are proposed mechanisms for these exacerbations, though the exact cause remains unknown [10].
The case described above not only reinforces the postulated role of GER in the progression of IPF, but also underlines the fact that reflux can exacerbate IPF and contribute to accelerated deterioration of respiratory function. Our patient was on medical therapy for GERD. Since she had an anatomical abnormality in the form of achalasia that predisposed her to acid reflux, laparoscopic Dor fundoplication was done. However the surgery failed to correct the reflux in her case and she continued to decline. Very few studies on the effect of acid suppression therapy in IPF are available in the literature. A case series on four patients with IPF and GER showed that treatment of acid GER with PPI therapy can stabilize pulmonary functions and prevent exacerbations [11]. Another retrospective study concluded that the use of medications for GER was associated with reduced radiological fibrosis and increased survival in patients with IPF [12]. A recent study made an interesting observation that patients with IPF who were on anti-acid treatment had a smaller decline in FVC when compared to those who were not [13]. Peri-operative data from a lung transplant center showed that laparoscopic Nissen fundoplication and laparoscopic Dor fundoplication were associated with reduced episodes of acute rejection after transplant and improvement in lung functions [14]. Another case series on IPF patients awaiting lung transplantation demonstrated stabilization of oxygen requirements with laparoscopic Nissen fundoplication [15]. There are no studies directly comparing anti-acid pharmacotherapy and anti-reflux surgery in patients with IPF. Most of the support in favor of the use of PPIs in IPF patients is based on the fact that acid suppression can reduce the stimulus for further fibrosis by limiting lung damage and that these agents are much less expensive and toxic than the other agents available for the management of the disease. However given the chronicity of IPF, this also means that anti-acid treatment has to be used for a long duration, which is fraught with risks. Long term use of PPIs has been shown to increase the risk of enteric infections, especially Clostridium difficile and hospital-acquired pneumonia [16], [17], [18]. It is also questionable if they would address the influence of non-acid reflux reflux in the progression of the disease.
In conclusion, GER has a high prevalence in patients with IPF and may be associated with the pathogenesis and progression of the latter. Clinical symptoms of reflux are poor predictors of GER in this population and hence there should be a low threshold to investigate and treat them for reflux. The exact subset of patients who need to be treated and the optimal acid suppression therapy in patients with IPF that can prevent disease progression is not clear. Also, the data on the efficacy of anti-reflux surgery in patients with IPF is yet to be clarified.
Conflict of interest
The authors have no conflicts of interest to declare.
References
- 1.Nathan S.D., Shlobin O.A., Weir N., Ahmad S., Kaldjob J.M., Battle E. Long-term course and prognosis of idiopathic pulmonary fibrosis in the new millennium. Chest. 2011;140(1):221–229. doi: 10.1378/chest.10-2572. [DOI] [PubMed] [Google Scholar]
- 2.Strieter R.M., Mehrad B. New mechanisms of pulmonary fibrosis. Chest. 2009;136(5):1364–1370. doi: 10.1378/chest.09-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appel J.Z., 3rd, Lee S.M., Hartwig M.G., Li B., Hsieh C.C., Cantu E., 3rd Characterization of the innate immune response to chronic aspiration in a novel rodent model. Respir. Res. 2007;8:87. doi: 10.1186/1465-9921-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raghu G., Freudenberger T.D., Yang S., Curtis J.R., Spada C., Hayes J. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur. Respir. J. 2006;27(1):136–142. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- 5.Salvioli B., Belmonte G., Stanghellini V., Baldi E., Fasano L., Pacilli A.M. Gastro-oesophageal reflux and interstitial lung disease. Dig. Liver Dis. 2006;38(12):879–884. doi: 10.1016/j.dld.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Tobin R.W., Pope C.E., 2nd, Pellegrini C.A., Emond M.J., Sillery J., Raghu G. Increased prevalence of gastroesophageal reflux in patients with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 1998;158(6):1804–1808. doi: 10.1164/ajrccm.158.6.9804105. [DOI] [PubMed] [Google Scholar]
- 7.Lee J.S., Song J.W., Wolters P.J., Elicker D.M., King T.E., King D.S. Bronchoalveolar lavage pepsin in acute exacerbation of idiopathic pulmonary fibrosis. Eur. Respir. J. 2012;39(2):352–358. doi: 10.1183/09031936.00050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J.S., Collard H.R., Raghu G., Sweet M.P., Hays S.R., Campos G.M. Does chronic microaspiration cause idiopathic pulmonary fibrosis? Am. J. Med. 2010;123(4):304–311. doi: 10.1016/j.amjmed.2009.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collard H.R., Moore B.B., Flaherty K.R., Brown K.K., Kaner R.J., King T.E., Jr. Acute exacerbations of idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2007;176(7):636–643. doi: 10.1164/rccm.200703-463PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fahim A., Crooks M., Hart S.P. Gastroesophageal reflux and idiopathic pulmonary fibrosis: a review. Pulm. Med. 2011;2011:634613. doi: 10.1155/2011/634613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raghu G., Yang S.T., Spada C., Hayes J., Pellegrini C.A. Sole treatment of acid gastroesophageal reflux in idiopathic pulmonary fibrosis: a case series. Chest. 2006;129(3):794–800. doi: 10.1378/chest.129.3.794. [DOI] [PubMed] [Google Scholar]
- 12.Lee J.S., Ryu J.H., Elicker B.M., Lydell C.P., Jones K.D., Wolters P.J. Gastroesophageal reflux therapy is associated with longer survival in patients with idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2011;184(12):1390–1394. doi: 10.1164/rccm.201101-0138OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J.S., Collard H.R., Anstrom K.J., Martinez F.J., Noth I., Roberts R.S. Anti-acid treatment and disease progression in idiopathic pulmonary fibrosis: an analysis of data from three randomised controlled trials. Lancet Respir. Med. 2013;1(5):369–376. doi: 10.1016/S2213-2600(13)70105-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoppo T., Jarido V., Pennathur A., Morrell M., Crespo M., Shigemura N. Antireflux surgery preserves lung function in patients with gastroesophageal reflux disease and end-stage lung disease before and after lung transplantation. Arch. Surg. 2011;146(9):1041–1047. doi: 10.1001/archsurg.2011.216. [DOI] [PubMed] [Google Scholar]
- 15.Linden P.A., Gilbert R.J., Yeap B.Y., Boyle K., Deykin A., Jaklitsch M.T. Laparoscopic fundoplication in patients with end-stage lung disease awaiting transplantation. J. Thorac. Cardiovasc Surg. 2006;131(2):438–446. doi: 10.1016/j.jtcvs.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 16.Bavishi C., Dupont H.L. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment. Pharmacol. Ther. 2011;34(11–12):1269–1281. doi: 10.1111/j.1365-2036.2011.04874.x. [DOI] [PubMed] [Google Scholar]
- 17.Barletta J.F., Scar D.A. Proton pump inhibitors increase the risk for hospital-acquired clostridium difficile infection in critically ill patients. Crit. Care. 2014;18(6):714. doi: 10.1186/s13054-014-0714-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herzig S.J., Howell M.D., Ngo L.H., Marcantonio E.R. Acid-suppressive medication use and the risk for hospital-acquired pneumonia. JAMA. 2009;301(20):2120–2128. doi: 10.1001/jama.2009.722. [DOI] [PubMed] [Google Scholar]