Abstract
This study aimed to validate the physiological importance of Arabidopsis thaliana alternative oxidase 1a (AtAOX1a) in alleviating oxidative stress using Saccharomyces cerevisiae as a model organism. The AOX1a transformant (pYES2AtAOX1a) showed cyanide resistant and salicylhydroxamic acid (SHAM)‐sensitive respiration, indicating functional expression of AtAOX1a in S. cerevisiae. After exposure to oxidative stress, pYES2AtAOX1a showed better survival and a decrease in reactive oxygen species (ROS) when compared to S. cerevisiae with empty vector (pYES2). Furthermore, pYES2AtAOX1a sustained growth by regulating GPX2 and/or TSA2, and cellular NAD +/NADH ratio. Thus, the expression of AtAOX1a in S. cerevisiae enhances its respiratory tolerance which, in turn, maintains cellular redox homeostasis and protects from oxidative damage.
Keywords: alternative oxidase 1a, oxidative stress, reactive oxygen species, redox homeostasis, respiration, Saccharomyces cerevisiae
Abbreviations
- ADH
alcohol dehydrogenase
- AOX
alternative oxidase
- AtAOX1a
Arabidopsis thaliana alternative oxidase 1a
- COX
cytochrome c oxidase
- DCPIP
dichlorophenolindophenol
- IPTG
isopropyl‐β‐d‐thiogalactopyranoside
- PCD
programmed cell death
- PG
propyl gallate
- PMS
phenazine methosulfate
- PVDF
polyvinylidene difluoride
- ROS
reactive oxygen species
- SHAM
salicylhydroxamic acid
- SOD
superoxide dismutase
- t‐BOOH
tert‐butyl hydroperoxide
- TCA
tricarboxylic acid
- UQ
ubiquinone
Alternative oxidase (AOX) is a nonproton pumping ubiquinol oxidase localized in the inner mitochondrial membrane of higher plants, fungi, some protists and was recently identified in 28 animal species 1. In contrast to cytochrome c oxidase (COX), it is cyanide resistant and branches from the ‘standard’ mitochondrial respiratory chain at the level of ubiquinone (UQ). It is considered as a sink for excess electrons as it reduces the molecular oxygen to water, bypassing the oxidative phosphorylation at both complex III and IV. Thus, AOX plays an important role in maintaining the cellular energy balance 2, 3, 4. A crystal structure of AOX from Trypanosome brucei revealed that it is a homodimer, which exists as an integral interfacial membrane protein with a nonhaem diiron carboxylate active site buried within a four helix bundle. The active site is ligated by four glutamate residues and a highly conserved Tyr220, which mediates its catalytic activity. Furthermore, the two hydrophobic cavities occur per monomer which bind to ubiquinol and Tyr220 for catalytic cycle and O2 reduction 5, 6, 7.
AOX was first identified in thermogenic plants to provide favorable temperature during floral development to attract pollinators 8, 9, 10, 11. In nonthermogenic plants, AOX is known to prevent over‐reduction of UQ and generation of reactive oxygen species (ROS) while allowing continued operation of the tricarboxylic acid (TCA) cycle 12, 13, 14. On exposure to abiotic stress, AOX‐deficient plants showed an increase in intracellular ROS and a decrease in photosynthetic performance as compared to wild‐type plants 15, 16, 17, 18, 19. On the other hand, AOX overexpression lines showed an enhanced photosynthetic efficiency with lower levels of cellular ROS when compared with wild‐type plants during abiotic stress conditions 20, 21, 22. In Arabidopsis, overexpression of AOX1a alleviated the Al‐induced programmed cell death (PCD) by decreasing the ROS production due to efficient mitochondrial electron flux and caspase‐3‐like activation 23. Also, the role of AOX has been studied extensively in lower organisms since last two decades. Kumar and Söll 24 reported for the first time heterologous expression of Arabidopsis thaliana AOX into hemA‐deficient strains of Escherichia coli, which acquired resistance to cyanide and exhibited aerobic respiration. Later, several other studies also demonstrated the expression of AOX in many yeast, fungal, and bacterial species, which resulted in the successful operation of cyanide‐insensitive respiration 25, 26, 27, 28, 29, 30. Recently, Honda et al. 29 demonstrated the visual expression of AOX in Aspergillus niger transformants (harboring fusion gene aox1‐egfp) upon exposure to heat shock, oxidative, and osmotic stress. Furthermore, expression of AOX from Hansenula anomala in Saccharomyces cerevisiae resulted in up‐regulation of several proteins related to major metabolic pathways such as Krebs cycle and amino acid biosynthesis suggesting the physiological role of AOX in mitoproteome plasticity 31. The role of AOX is also revealed in the survival of pathogenic fungi such as Aspergillus fumigatus and Histoplasma capsulatum inside the host under stress conditions 32, 33. Similar to plants, the AOX mutant of pathogenic yeast Cryptococcus neoformans showed susceptibility to oxidative stress 34.
Yeast cells have become one of the most preferred experimental models to study the PCD and aging under oxidative stress, owing to special characteristics such as short life cycle and ease for genetic manipulation along with presence of core cellular processes similar to eukaryotes 35. In most of the aerobic cells, respiration is the major source for generation of superoxide radical (O2 −) as electrons leak out from the mitochondrial electron transport chain at Complex I and Complex III. Furthermore, dismutation of O2 − by superoxide dismutase (SOD) generates H2O2, a quite stable toxic product which creates oxidative environment inside the cell 36. To detoxify the cellular H2O2, mitochondria have evolved an efficient antioxidant defense system such as catalase and peroxiredoxins, which include glutathione peroxidase/glutathione reductase and thioredoxin peroxidase/thioredoxin reductase 37. In spite of the existence of such a strong antioxidant defense system, several pet mutants (impaired in mitochondrial electron transport chain) of S. cerevisiae showed accumulation of H2O2. However, the addition of exogenous cytochrome c to isolated mitoplasts significantly decreased the H2O2 levels 38. In Candida albicans and Aspergillus niger, AOX was also induced along with cytochrome c under oxidizing conditions 30, 39. Thus, AOX pathway is known to play an important role in the alleviation of ROS and thereby oxidative stress, either independently or in association with the COX pathway and/or antioxidant defense system. Furthermore, a direct or an indirect role of AOX has also been demonstrated in maintaining redox homeostasis in higher plants in response to several abiotic stresses 18, 19, 40, 41, 42. However, such type of significance for AOX is yet to be elucidated in lower organisms.
In Arabidopsis, AOX1a is known to be induced under various oxidative stresses (imposed by biotic and abiotic stresses) and developmental stages 15, 16, 18, 43, 44, 45, which indicate that genetic engineering of AOX1a might be a promising tool to combat oxidative stress in AOX deficient strains or organisms. In the present study, AtAOX1a was heterologously expressed in S. cerevisiae (an eukaryotic organism devoid of AOX) to characterize its role in response to oxidative stress. To create an oxidative environment inside the cells, S. cerevisiae were incubated with H2O2 and tertiary‐butyl hydroperoxide (t‐BOOH). The functional expression of AtAOX1a and its characterization have been studied by monitoring the changes in respiration, growth, viability, ROS, antioxidant system, and redox state of S. cerevisiae under oxidizing conditions.
Materials and methods
Strains and culture conditions
Escherichia coli (E. coli) DH5α or BL21(DE3)pLysS (Invitrogen™, Waltham, MA, USA) were grown at 37 °C in Luria–Bertani medium. Saccharomyces cerevisiae strain INVSc1 (Invitrogen™) was grown at 30 °C either in YPD medium (1% w/v yeast extract, 2% w/v peptone and 2% w/v dextrose) or SC‐URA¯ minimal medium (0.67% w/v yeast nitrogen base without amino acids, 2% w/v glucose as carbon source) and amino acids.
Cloning of AtAOX1a and plasmid construction
Total RNA was isolated from A. thaliana wild‐type leaves using TRI reagent (Sigma‐Aldrich, St. Louis, MO, USA). One microgram of total RNA was used for the first‐strand cDNA synthesis using iScript™ cDNA synthesis kit (Bio‐Rad, Hercules, CA, USA). AtAOX1a encoding a mature protein was amplified by Phusion DNA polymerase (Clontech, CA, USA) using the following primers: F‐GAGAATTCGCTAGCACGATCACTCTGG and R‐GGCTCGAGTCAATGATACCCAATTGGAG, and cloned into a pET28a(+)™ expression vector. In contrast, AtAOX1a encoding a mature protein along with its leader sequence was amplified by using the primers: F‐GGGAATTCTGATGATGATAACTCGCGGTGG and R‐GGCTCGAGTCAATGATACCCAATTGGAG, and cloned into a pYES2/NT expression vector. Clones were confirmed by DNA sequencing. The recombinant plasmids were transformed into their respective host strains, i.e., BL21(DE3)pLysS and INVSc1.
Protein expression, purification, and antibody generation
The expression of AtAOX1a in E. coli BL21(DE3)pLysS was induced by 0.1 mm isopropyl‐β‐d‐thiogalactopyranoside (IPTG) at 28 °C for 4 h. The recombinant protein was purified under denaturing conditions with Ni‐NTA agarose column using standard protocols and the purified protein from the gel slice was subjected to a matrix‐assisted laser desorption ionization time‐of‐flight mass spectrometry (MALDI‐TOF/TOF) analysis as described in ref. 46 for confirmation as AtAOX1a. The purified protein was used to generate a polyclonal antibody in rabbit using standard protocols (Animal ethics approval number is UH/IAEC/KPMS/2014‐1/24).
AtAOX1a protein expression in Saccharomyces cerevisiae
For heterologous protein expression, S. cerevisiae with empty vector (pYES2) or transformed with AtAOX1a (pYES2AtAOX1a) were grown overnight in SC‐URA¯ minimal media containing 2% galactose as a carbon source. Protein was extracted using trichloroacetic acid (TCA) method 47 and separated on a 12.5% SDS/PAGE. For immunodetection, protein gel was electroblotted onto polyvinylidene difluoride (PVDF) membrane and treated with a polyclonal AOX1a antibody (generated as mentioned in section ‘Protein expression, purification, and antibody generation’) at 1 : 1000 dilutions followed by a goat anti‐rabbit IgG‐alkaline phosphate conjugate (Sigma, USA) at 1 : 5000 dilutions. The blot was developed using 5‐bromo‐4‐chloro‐3‐indolyl phosphate/nitro blue tetrazolium (BCIP/NBT) system.
Oxidative stress
Oxidative stress analyses were performed as described earlier 48. Treatment duration was different for each set of experiments depending on their feasibility. The duration of oxidative stress treatment was fixed at 10 min for ROS estimation, 4 h for survival rate and growth recovery assay, and 75 min for pyridine nucleotides and transcript level analyses.
Measurement of O2 uptake and cell survival rate
The respiratory O2 uptake measurements (10 min) were performed using Clark‐type O2 electrode 49, 50. The viability of cells was examined with fluctuation assay as reported by Dalal et al. 50.
Measurement of ROS
The intracellular ROS level was measured following Jang et al. 51. The cells were incubated with 100 μm 2′,7′‐ dichlorodihydrofluorescein diacetate (H2DCF‐DA; Sigma) for 5 min in the dark at 25 °C, and the change in DCF fluorescence was imaged under a laser‐scanning confocal fluorescence microscope (LSM 710 NLO ConfoCor 3; Carl Zeiss, Jena, Germany).
Measurement of pyridine nucleotide content
The extraction and estimation of NAD+ and NADH were done as per Queval and Noctor 52. The assay involves phenazine methosulfate (PMS) catalyzed reduction of dichlorophenolindophenol (DCPIP) in the presence of alcohol dehydrogenase (ADH) and ethanol. The NAD+ and NADH content were calculated using the relevant standard (0–40 pmole).
RNA isolation and expression analysis
Total RNA was isolated using the acid‐phenol method 53. First strand cDNA was synthesized with 2 μg of total RNA using SuperScript® III (Invitrogen) according to manufacturer's instructions. Primers used for real‐time PCR analysis are listed in Table 1 42. Comparative C T method was used to analyze the relative gene expression levels 54.
Table 1.
List of primers used in real‐time PCR study. ACT1 was used as housekeeping gene
| Gene | Accession no. | Primer sequence (5′ to 3′) | Amplicon length (bp) |
|---|---|---|---|
| SOD1 | YJR104C | R‐TAACGACGCTTCTGCCTACA | 191 |
| SOD2 | YHR008C | 191 | |
| GPX2 | YBR244W | 172 | |
| TSA2 | YDR453C | 159 | |
| ACT1 | YFL039C | 184 |
Statistical analysis
All values are presented as means ± standard errors of the means (SEM). The statistical evaluation of the data was performed with one‐way analysis of variance (ANOVA), Tukey test of multiple comparison analysis using sigma plot 11.0 software (Systat, San Jose, CA, USA). P values of < 0.05 were considered as statistically significant.
Results
Expression of AtAOX1a in Escherichia coli and mass analysis
The expression of AtAOX1a protein induced in the presence of 0.1 mm IPTG in E. coli was visualized on SDS/PAGE as a ~ 36 kDa band as it includes AtAOX1a sequence encoding a mature protein (32.34 kDa) and pET28a(+) vector sequence (3.83 kDa) (Fig. 1A). Four major peptide fragments obtained during MALDI‐TOF‐TOF analysis of a trypsin‐digested protein showed the following sequences in Biotools: WPTDLFFQR (1209.81 Da), DVNHFASDIHYQGR (1658.04 Da), GNIENVPAPAIAIDYWR (1898.27 Da), and ELDKGNIENVPAPAIAIDYWR (2383.58 Da). As the sequences from these peptides showed 100% matching with Arabidopsis AOX1a (Fig. 1B, and Figs S1, S2A–D), the purified protein was injected into a rabbit and the polyclonal antibody was obtained.
Figure 1.
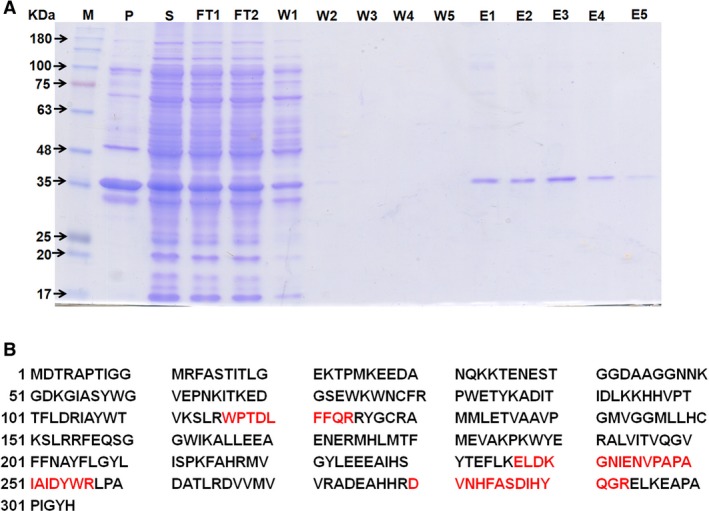
Purification profile and molecular mass analysis of a polyhistidine tagged pET28a‐AtAOX1a recombinant protein: (A) 12.5% SDS/PAGE depicting a ~ 36 kDa AtAOX1a protein in different fractions of the purification protocol. Abbreviations used are as follows: M‐marker, P‐pellet (insoluble protein), S‐supernatant (soluble protein), FT‐flow through (supernatant passed through Ni‐NTA column), W‐washing fractions, E‐elute (purified protein). (B) The sequences corresponding to peptide fragments with molecular masses of 1209.819, 1659.057, 1899.286, and 2384.592 Da, respectively, obtained from MALDI‐TOF‐TOF analysis of trypsin digested purified protein showed 100% matching to internal sequences (indicated in red font) of Arabidopsis thaliana AOX1a (AT3G22370), retrieved from NCBI database.
Functional characterization of AtAOX1a in Saccharomyces cerevisiae
The protein expression of AtAOX1a in S. cerevisiae was confirmed through western blot analysis (Fig. 2A). To ascertain the function of AtAOX1a, cyanide‐sensitive respiration was monitored using 1 mm KCN, an inhibitor of complex IV in COX pathway, while cyanide‐insensitive respiration was monitored in the presence of 2 mm salicylhydroxamic acid (SHAM) or 100 μm propyl gallate (PG), inhibitors of AOX in the alternative pathway. In the absence of metabolic inhibitors, the respiratory rates of pYES2AtAOX1a (8.6 ± 0.11 nmol O2 s−1) were similar to pYES2 (8.45 ± 0.09 nmol O2 s−1). But, in the presence of KCN, pYES2 showed a pronounced decrease in respiratory rates when compared with pYES2AtAOX1a. In contrast, addition of SHAM or PG significantly decreased the respiratory rates of pYES2AtAOX1a but not of pYES2 (Fig. 2B).
Figure 2.
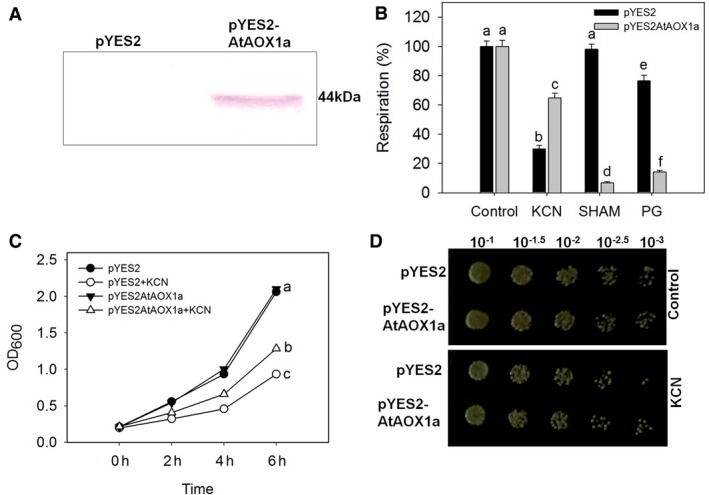
Functional expression of AtAOX1a in Saccharomyces cerevisiae. (A) Western blot showing the AtAOX1a protein (44 kDa) expression in pYES2AtAOX1a (right side) but not in PYES2 (left side); (B) Rates of oxygen uptake by pYES2 and pYES2AtAOX1a in the absence or presence of KCN (1 mm), SHAM (2 mm), and PG (100 μm); (C) Time‐dependent growth curve of pYES2 and pYES2AtAOX1a in the absence or presence of KCN (1 mm) and (D) Growth recovery in pYES2 and pYES2AtAOX1a after KCN (1 mm) treatment for 4 h. Different lowercase alphabetical letters indicate statistically significant difference (P < 0.05).
The exponential growth pattern of both pYES2 and pYES2AtAOX1a were found to be similar (OD600 = 2.1) up to 6 h. But, treatment with KCN remarkably decreased the exponential growth in yeast cells (Fig. 2C). However, the decrease in exponential growth of pYES2 was more significant when compared with pYES2AtAOX1a. Furthermore, in the presence of KCN, growth recovery was found to be higher in pYES2AtAOX1a than pYES2 (Fig. 2D). Taken together, these results indicate that AtAOX1a was successfully expressed and functional in S. cerevisiae.
Changes in cellular ROS during oxidative stress
Under control conditions, the cellular ROS was minimal in both pYES2 and pYES2AtAOX1a as indicated by DCF fluorescence. However, upon treatment with KCN, H2O2, or t‐BOOH, the fluorescence increased significantly in pYES2. In contrast, pYES2AtAOX1a restricted the increase in fluorescence during oxidative stress indicating the importance of AOX1a in preventing and/or regulating the ROS generation (Fig. 3).
Figure 3.
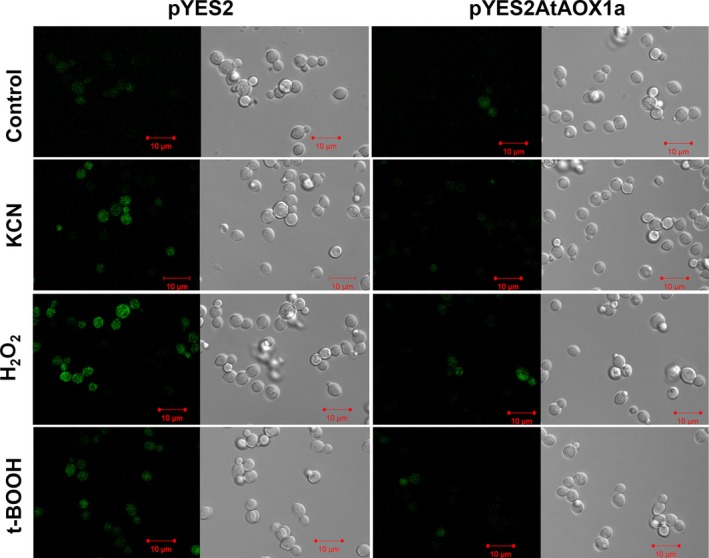
Effect of H2O2 (2 mm) or t‐BOOH (0.25 mm) on the intracellular ROS generation. ROS were monitored in pYES2 and pYES2AtAOX1a at 488 nm (excitation) and 525 nm (emission) wavelengths under a confocal fluorescence microscope as DCF fluorescence produced by the action of esterases on H2 DCFDA. Sample treated with KCN (1 mm) was used as a positive control.
Changes in cell survival rate and growth recovery during oxidative stress
Among the two oxidants, H2O2 was found to be more lethal than t‐BOOH. Upon treatment with these oxidants, the survival rate of pYES2 decreased drastically as compared to pYES2AtAOX1a (Fig. 4A). Also, recovery assays clearly indicated an enhanced colony number in pYES2AtAOX1a than in pYES2 under oxidizing conditions with a clear visible difference at 1 × 10−2.5 and 1 × 10−3 dilutions (Fig. 4B). It appears that AOX1a plays a critical role in decreasing the rates of cell death and improving their growth recovery under oxidizing conditions.
Figure 4.
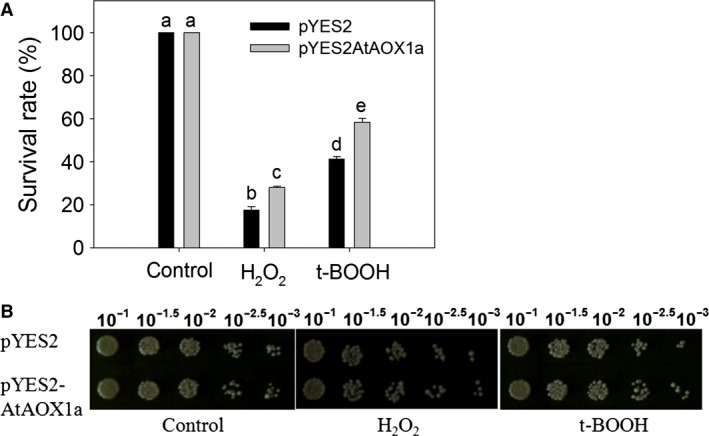
Effect of H2O2 (2 mm) or t‐BOOH (0.25 mm) on (A) the cell survival rate and (B) return to growth assay in pYES2 and pYES2AtAOX1a. Different lowercase alphabetical letters indicate statistically significant difference (P < 0.05).
Differential antioxidant gene expression profile during oxidative stress
The ROS scavenging efficiency of pYES2 and pYES2AtAOX1a was measured by monitoring the changes in transcript levels of antioxidant genes viz., Superoxide dismutase 1 (SOD1), Superoxide dismutase 2 (SOD2), Glutathione peroxidase 2 (GPX2), and Thioredoxin peroxidase 2 (TSA2) during oxidative stress (Fig. 5A–D). Under control conditions, the expression of these antioxidant genes was approximately similar in both pYES2 and pYES2AtAOX1a. Upon treatment with H2O2 or t‐BOOH, the expression of SOD1 (> 8‐fold), SOD2 (> 6‐fold), GPX2 (> 52‐fold), and TSA2 (> 157‐fold) increased significantly by several fold in both pYES2 and pYES2AtAOX1a (Fig. 5A–D). But, the expression of GPX2 was down‐regulated significantly in pYES2AtAOX1a when compared with pYES2 in the presence of both H2O2 and t‐BOOH (Fig. 5C). In contrast, the expression of TSA2 was down‐regulated significantly in pYES2AtAOX1a when treated with t‐BOOH, while remained unchanged in the presence of H2O2 (Fig. 5D).
Figure 5.
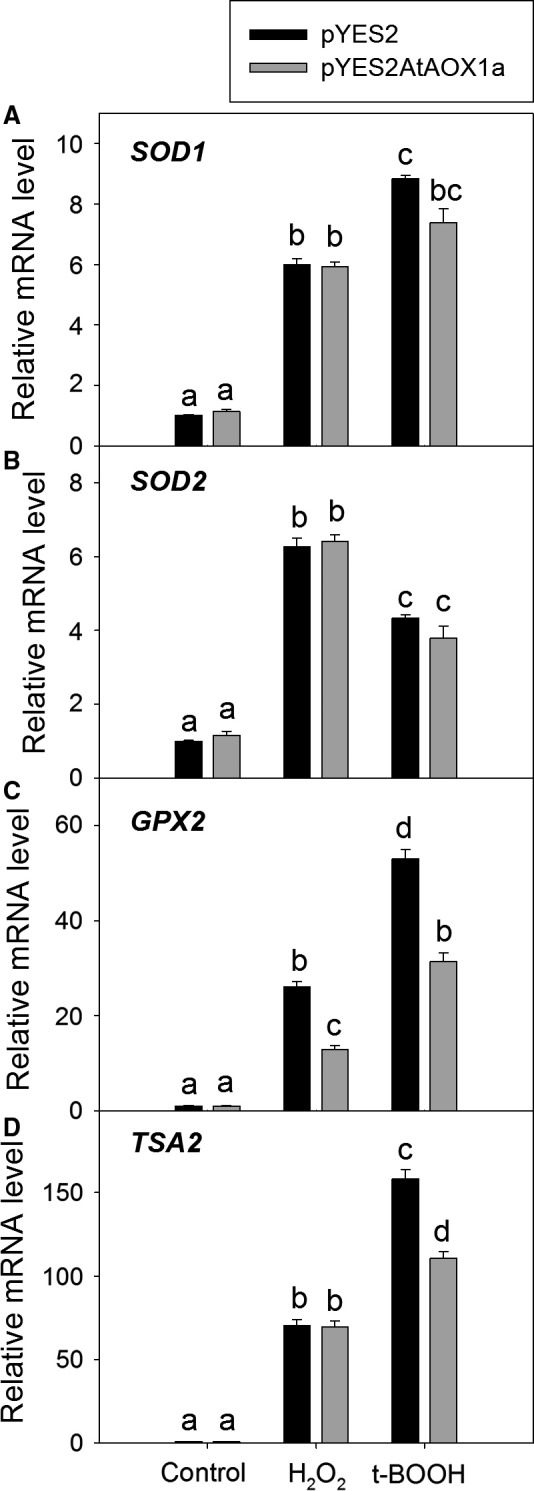
Relative mRNA profile of the antioxidant genes (A) SOD1, (B) SOD2, (C) GPX2, and (D) TSA2 in pYES2 and pYES2AtAOX1a after exposure to H2O2 (2 mm) or t‐BOOH (0.25 mm). ACT1 was used as housekeeping gene. Different lowercase alphabetical letters indicate statistically significant difference (P < 0.05).
Changes in cellular redox during oxidative stress
The role of AtAOX1a in maintaining the cellular redox balance during oxidative stress was revealed by monitoring the changes in pyridine nucleotide (NAD+ and NADH) redox couple. In control, the cellular levels of NAD+, NADH, and the redox ratio of NAD+/NADH were similar in both pYES2 and pYES2AtAOX1a. Upon treatment with H2O2, the cellular NAD+ levels decreased significantly in both pYES2 and pYES2AtAOX1a (Fig. 6A). In contrast, the decrease in cellular NADH levels was significant in pYES2AtAOX1a alone (Fig. 6B). Consequently, the cellular redox ratio of NAD+/NADH was maintained at much higher levels in pYES2AtAOX1a when compared with pYES2 in the presence of H2O2 (Fig. 6C).
Figure 6.
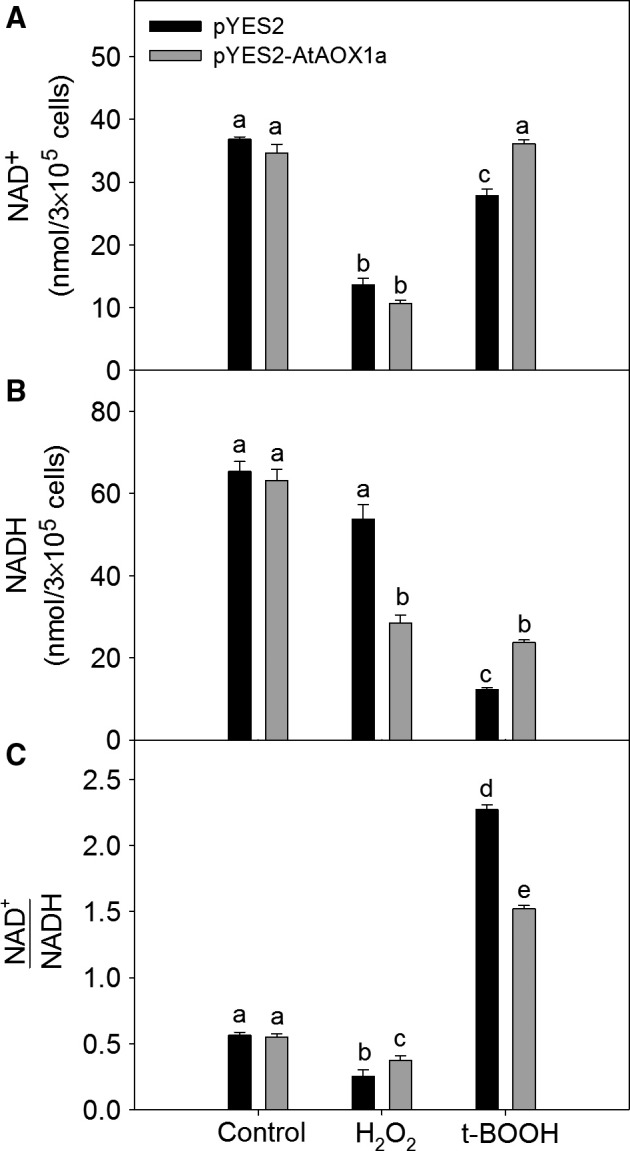
Changes in the total cellular pyridine nucleotides (A) NAD +; (B) NADH; and (C) ratio of NAD + to NADH in pYES2 and pYES2AtAOX1a upon treatment with H2O2 (2 mm) or t‐BOOH (0.25 mm). Different lowercase alphabetical letters indicate statistically significant difference (P < 0.05).
The responses of NAD+, NADH, and consequently NAD+/NADH were quite different in t‐BOOH‐treated samples as compared to H2O2 treatment. In the presence of t‐BOOH, both NAD+ and NADH levels increased significantly, while the redox ratio of NAD+/NADH decreased drastically in pYES2AtAOX1a when compared with pYES2 (Fig. 6A–C).
Discussion
In higher plants, AOX is known to perform several mitochondrial and extramitochondrial functions, viz: (a) alleviation of reactive oxygen and nitrogen species, and cell death 16, 55, 56, 57, (b) preventing over‐reduction of chloroplastic/mitochondrial electron transport carriers, particularly plastoquinone or UQ 13, (c) maintenance of cellular redox and carbon balance 18, 19, 58, (d) modulation of cellular energy level 59, and (e) optimization of photosynthesis during a wide range of biotic and abiotic stresses 18, 19, 60, 61. The role of AOX in alleviating ROS levels and oxidative stress is not only confined to plants but was also revealed in several nonphotosynthetic organisms including fungi, protists, bacteria, and human cells 29, 39, 62, 63. These observations suggest that engineering of AOX into such species which are deficient in AOX may help them to cope up against various biotic and abiotic stresses.
Saccharomyces cerevisiae lacks an AOX homolog 64. Therefore, AtAOX1a was expressed in S. cerevisiae to validate its physiological function during oxidative stress (Fig. 2A). It is well known that any restriction of electron flow through the COX pathway or exposure to oxidative stress leads to an induction of AOX in plants and fungi 21, 27, 60, 65. Corroborating with these studies, restriction of electron transport through the COX pathway by KCN caused a significant reduction in the total respiratory rates of pYES2 and pYES2AtAOX1a. However, due to an AOX catalyzed respiration, pYES2AtAOX1a showed higher respiratory rates compared to pYES2. While the SHAM‐insensitive respiration in pYES2 indicates the absence of AOX‐catalyzed respiration, SHAM or PG‐sensitive respiration in pYES2AtAOX1a confirms the functional expression of AtAOX1a in yeast (Fig. 2B) 29, 39. Any increase in the respiratory activity is known to increase the chronological and replicative lifespan of yeast 66. Also, the recovery in the growth curve assays and a rise in the total respiratory rates of pYES2AtAOX1a in the presence of KCN reveal the significance of AOX‐catalyzed respiration in the maintenance of yeast cell growth (Fig. 2B–D).
ROS production is a common phenomenon in cells, which occurs during aerobic respiration or in response to several biotic or abiotic stresses. But, excessive ROS production leads to oxidative stress 67, 68, 69. Yeast cells show a range of responses depending on the concentration of cellular ROS. At very low levels of ROS, the cells try to adapt themselves, while at higher levels of ROS, the cells activate their antioxidant defense system mediated by Yap1p and Msn2,4p transcription factors 70. Beyond this, ROS might arrest the cell cycle leading to apoptosis 71, 72. In the present study, the higher levels of cellular ROS induced by KCN, H2O2, or t‐BOOH in pYES2 were positively correlated with cell death and negatively correlated with growth recovery. In contrast, the lower levels of ROS, better survival rate, and growth recovery recorded under oxidizing environment in pYES2AtAOX1a indicate the importance of AOX catalyzed respiration in mitigating the cellular ROS production (Figs 3 and 4A,B).
Redox homeostasis is a basic requirement to maintain the cellular metabolism and ROS, particularly during aging 72, 73. Accumulation of NADH decreases the Sir2 activity, which is essential for chromatin silencing and extension of life span. Thus, any increase in the redox ratio of NAD+/NADH extended the chronological as well as replicating life span of yeast cells 74, 75. The pYES2AtAOX1a showed an increase in the NAD+/NADH ratio when compared with pYES2 upon treatment with H2O2. In contrast, pYES2AtAOX1a maintained the cellular redox homeostasis by minimizing the redox ratio of NAD+/NADH raised by t‐BOOH. These results elucidate the importance of AtAOX1a in the maintenance of cellular redox homeostasis to increase the life span as evident by cell survival rate of yeast (Figs 4A,B and 6C).
Furthermore, the sulphydryl (‒SH) group plays a critical role in proper functioning of several of the enzymes, transcription factors, and membrane proteins, which in turn play a significant role in maintaining the cellular redox homeostasis 73. During oxidative stress, cysteine sulfhydryl residues are oxidized to disulfide bonds, thereby leading to a loss in protein activity. Small heat‐stable oxidoreductases, glutaredoxins, and thioredoxins catalyze the reduction of disulfides to thiols using thiolated cysteine residues present in the active sites 73, 76, 77. A few studies reported the role of glutaredoxins and thioredoxins in supplying reducing equivalents to the regulatory sulfhydryl/disulfide system of AOX to activate it, which in turn play a role in preventing the over‐reduction of mitochondrial electron transport carriers and thereby ROS generation 78, 79, 80. In the present study, a several fold increase in the transcript levels of GPX2 and TSA2 in pYES2 and their down‐regulation in pYES2AtAOX1a in the presence of t‐BOOH and/or H2O2 suggests the role of AOX1a in regulating the expression of these antioxidant enzymes, which play an important role in the detoxification of ROS and the maintenance of cellular redox balance (Figs 3, 5C,D and 6C).
The results from the present study suggest that transformation of AtAOX1a introduced AOX‐catalyzed respiration in S. cerevisiae, which in turn mitigated ROS generation by regulating GPX2 and TSA2 to maintain cellular redox homeostasis and better cell survival rate during oxidative stress.
Author contributions
KP conceived and supervised the study; KP, SDT and AV designed the experiments; AV and AD acquired the data; KP, SDT, PBK and AV analyzed and interpreted the data; AV wrote the paper; KP edited the paper; SDT and PBK contributed important intellectual content.
Supporting information
Fig. S1 MALDI‐TOF‐TOF mass spectrum of trypsin digested purified AtAOX1a protein between 500 and 5000 m/z.
Fig. S2 Lift spectrum and Biotools display of four major peaks from trypsin digested AtAOX1a protein: (A) m/z 1209.819, (B) 1659.057, (C) 1899.286, and (D) 2384.592.
Acknowledgements
This work was supported by grants from DST‐FIST and UGC‐SAP‐DRS, both from New Delhi, India to the DoBB and DoPS, SLS facilities of UoH. AV thanks CSIR for senior research fellowship. AD thanks UoH for doctoral research fellowship. We gratefully acknowledge Prof. Renate Scheibe, University of Osnabrueck, for a kind gift of Arabidopsis Columbia seeds; Prof. A. S. Raghavendra, UoH, for extending his lab facilities; Prof. Prakash Babu and Dr. Suraj, UoH, for their help in raising the antibody. We thank Ms. Nalini, CFN, UoH, for technical assistance in confocal microscope and Mrs. Monica, SLS, UoH, for MALDI‐TOF‐TOF analysis. No conflict of interest declared.
References
- 1. McDonald AE, Vanlerberghe GC and Staples JF (2009) Alternative oxidase in animals: unique characteristics and taxonomic distribution. J Exp Biol 212, 2627–2634. [DOI] [PubMed] [Google Scholar]
- 2. Moore AL and Siedow JN (1991) The regulation and nature of the cyanide‐resistant alternative oxidase of plant mitochondria. Biochim Biophys Acta 1059, 121–140. [DOI] [PubMed] [Google Scholar]
- 3. Vanlerberghe GC and McIntosh L (1997) Alternative oxidase: from gene to function. Annu Rev Plant Biol 48, 703–734. [DOI] [PubMed] [Google Scholar]
- 4. Millenaar F and Lambers H (2003) The alternative oxidase: in vivo regulation and function. Plant Biol 5, 2–15. [Google Scholar]
- 5. Moore AL, Shiba T, Young L, Harada S, Kita K and Ito K (2013) Unraveling the heater: new insights into the structure of the alternative oxidase. Annu Rev Plant Biol 64, 637–663. [DOI] [PubMed] [Google Scholar]
- 6. Shiba T, Kido Y, Sakamoto K, Inaoka DK, Tsuge C, Tatsumi R, Takahashi G, Balogun EO, Nara T, Aoki T et al (2013) Structure of the trypanosome cyanide‐insensitive alternative oxidase. Proc Natl Acad Sci USA 110, 4580–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Young L, Shiba T, Harada S, Kita K, Albury MS and Moore AL (2013) The alternative oxidases: simple oxidoreductase proteins with complex functions. Biochem Soc Trans 41, 1305–1311. [DOI] [PubMed] [Google Scholar]
- 8. Meeuse BJ (1975) Thermogenic respiration in aroids. Annu Rev Plant Physiol 26, 117–126. [Google Scholar]
- 9. Watling JR, Robinson SA and Seymour RS (2006) Contribution of the alternative pathway to respiration during thermogenesis in flowers of the sacred lotus. Plant Physiol 140, 1367–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wagner AM, Krab K, Wagner MJ and Moore AL (2008) Regulation of thermogenesis in flowering Araceae: the role of the alternative oxidase. Biochim Biophys Acta 1777, 993–1000. [DOI] [PubMed] [Google Scholar]
- 11. Miller RE, Grant NM, Giles L, Ribas‐Carbo M, Berry JA, Watling JR and Robinson SA (2011) In the heat of the night–alternative pathway respiration drives thermogenesis in Philodendron bipinnatifidum . New Phytol 189, 1013–1026. [DOI] [PubMed] [Google Scholar]
- 12. Maxwell DP, Wang Y and McIntosh L (1999) The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA 96, 8271–8276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yoshida K, Watanabe CK, Hachiya T, Tholen D, Shibata M, Terashima I and Noguchi K (2011) Distinct responses of the mitochondrial respiratory chain to long‐ and short‐term high‐light environments in Arabidopsis thaliana . Plant, Cell Environ 34, 618–628. [DOI] [PubMed] [Google Scholar]
- 14. Araújo WL, Nunes‐Nesi A and Fernie AR (2014) On the role of plant mitochondrial metabolism and its impact on photosynthesis in both optimal and sub‐optimal growth conditions. Photosynth Res 119, 141–156. [DOI] [PubMed] [Google Scholar]
- 15. Giraud E, Ho LH, Clifton R, Carroll A, Estavillo G, Tan YF, Howell KA, Ivanova A, Pogson BJ, Millar AH et al (2008) The absence of ALTERNATIVE OXIDASE1a in Arabidopsis results in acute sensitivity to combined light and drought stress. Plant Physiol 147, 595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Strodtkötter I, Padmasree K, Dinakar C, Speth B, Niazi PS, Wojtera J, Voss I, Do PT, Nunes‐Nesi A, Fernie AR et al (2009) Induction of the AOX1D isoform of alternative oxidase in A. thaliana T‐DNA insertion lines lacking isoform AOX1A is insufficient to optimize photosynthesis when treated with antimycin A. Mol Plant 2, 284–297. [DOI] [PubMed] [Google Scholar]
- 17. Watanabe CK, Hachiya T, Takahara K, Kawai M, Uchimiya H, Uesono Y, Terashima I and Noguchi K (2010) Effects of AOX1a deficiency on plant growth, gene expression of respiratory components, and metabolic profile under low‐nitrogen stress in Arabidopsis thaliana plants. Plant Cell Physiol 51, 810–822. [DOI] [PubMed] [Google Scholar]
- 18. Vishwakarma A, Bashyam L, Senthilkumaran B, Scheibe R and Padmasree K (2014) Physiological role of AOX1a in photosynthesis and maintenance of cellular redox homeostasis under high light in Arabidopsis thaliana . Plant Physiol Biochem 81, 44–53. [DOI] [PubMed] [Google Scholar]
- 19. Vishwakarma A, Tetali SD, Selinski J, Scheibe R and Padmasree K (2015) Importance of alternative oxidase pathway in regulating cellular redox and ROS homeostasis to optimize photosynthesis during restriction of cytochrome oxidase pathway in Arabidopsis thaliana . Ann Bot 116, 555–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Umbach AL, Fiorani F and Siedow JN (2005) Characterization of transformed Arabidopsis with altered alternative oxidase levels and analysis of effects on reactive oxygen species in tissue. Plant Physiol 139, 1806–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abu‐Romman S, Shatnawi M, Hasan M, Qrunfleh I, Omar S and Salem N (2012) cDNA cloning and expression analysis of a putative alternative oxidase HsAOX1 from wild barley (Hordeum spontaneum). Gene Genomic 34, 59–66. [Google Scholar]
- 22. Mhadhbi H, Fotopoulos V, Mylona PV, Jebara M, Aouani ME and Polidoros AN (2013) Alternative oxidase 1 (Aox1) gene expression in roots of Medicago truncatula is a genotype‐specific component of salt stress tolerance. J Plant Physiol 170, 111–114. [DOI] [PubMed] [Google Scholar]
- 23. Liu J, Li Z, Wang Y and Xing D (2014) Overexpression of ALTERNATIVE OXIDASE1a alleviates mitochondria‐dependent programmed cell death induced by aluminium phytotoxicity in Arabidopsis . J Exp Bot 65, 4465–4478. [DOI] [PubMed] [Google Scholar]
- 24. Kumar AM and Söll D (1992) Arabidopsis alternative oxidase sustains Escherichia coli respiration. Proc Natl Acad Sci USA 89, 10842–10846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Albury MS, Dudley P, Watts FZ and Moore AL (1996) Targeting the plant alternative oxidase protein to Schizosaccharomyces pombe mitochondria confers cyanide‐insensitive respiration. J Biol Chem 271, 17062–17066. [DOI] [PubMed] [Google Scholar]
- 26. Affourtit C, Albury MS, Krab K and Moore AL (1999) Functional expression of the plant alternative oxidase affects growth of the yeast Schizosaccharomyces pombe . J Biol Chem 274, 6212–6218. [DOI] [PubMed] [Google Scholar]
- 27. Magnani T, Soriani FM, Martins VP, Nascimento AM, Tudella VG, Curti C and Uyemura SA (2007) Cloning and functional expression of the mitochondrial alternative oxidase of Aspergillus fumigatus and its induction by oxidative stress. FEMS Microbiol Lett 271, 230–238. [DOI] [PubMed] [Google Scholar]
- 28. Martins VP, Dinamarco TM, Soriani FM, Tudella VG, Oliveira SC, Goldman GH, Curti C and Uyemura SA (2011) Involvement of an alternative oxidase in oxidative stress and mycelium‐to‐yeast differentiation in Paracoccidioides brasiliensis . Eukaryot Cell 10, 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Honda Y, Hattori T and Kirimura K (2012) Visual expression analysis of the responses of the alternative oxidase gene (aox1) to heat shock, oxidative, and osmotic stresses in conidia of citric acid‐producing Aspergillus niger . J Biosci Bioeng 113, 338–342. [DOI] [PubMed] [Google Scholar]
- 30. Papagianni M and Avramidis N (2012) Cloning and functional expression of the mitochondrial alternative oxidase gene (aox1) of Aspergillus niger in Lactococcus lactis and its induction by oxidizing conditions. Enzyme Microb Technol 50, 17–21. [DOI] [PubMed] [Google Scholar]
- 31. Mathy G, Navet R, Gerkens P, Leprince P, De Pauw E, Sluse‐Goffart CM, Sluse FE and Douette P (2006) Saccharomyces cerevisiae mitoproteome plasticity in response to recombinant alternative ubiquinol oxidase. J Proteome Res 5, 339–348. [DOI] [PubMed] [Google Scholar]
- 32. Tudella VG, Curti C, Soriani FM, Santos AC and Uyemura SA (2004) In situ evidence of an alternative oxidase and an uncoupling protein in the respiratory chain of Aspergillus fumigatus . Int J Biochem Cell Biol 36, 162–172. [DOI] [PubMed] [Google Scholar]
- 33. Johnson CH, Prigge JT, Warren AD and McEwen JE (2003) Characterization of an alternative oxidase activity of Histoplasma capsulatum . Yeast 20, 381–388. [DOI] [PubMed] [Google Scholar]
- 34. Akhter S, McDade HC, Gorlach JM, Heinrich G, Cox GM and Perfect JR (2003) Role of alternative oxidase gene in pathogenesis of Cryptococcus neoformans . Infect Immun 71, 5794–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Carmona‐Gutierrez D, Eisenberg T, Buttner S, Meisinger C, Kroemer G and Madeo F (2010) Apoptosis in yeast: triggers, pathways, subroutines. Cell Death Differ 17, 763–773. [DOI] [PubMed] [Google Scholar]
- 36. Turrens JF (1997) Superoxide production by the mitochondrial respiratory chain. Biosci Rep 17, 3–8. [DOI] [PubMed] [Google Scholar]
- 37. Kowaltowski AJ, de Souza‐Pinto NC, Castilho RF and Vercesi AE (2009) Mitochondria and reactive oxygen species. Free Radic Biol Med 47, 333–343. [DOI] [PubMed] [Google Scholar]
- 38. Barros MH, Netto LE and Kowaltowski AJ (2003) H2O2 generation in Saccharomyces cerevisiae respiratory pet mutants: effect of cytochrome c. Free Radical Biol Med 35, 179–188. [DOI] [PubMed] [Google Scholar]
- 39. Brown S and Tuffery R (2010) Induction of alternative oxidase activity in Candida albicans by oxidising conditions. Int J Biol Life Sci 6, 26–30. [Google Scholar]
- 40. Vanlerberghe GC, Cvetkovska M and Wang J (2009) Is the maintenance of homeostatic mitochondrial signaling during stress a physiological role for alternative oxidase? Physiol Plant 137, 392–406. [DOI] [PubMed] [Google Scholar]
- 41. Zhang DW, Xu F, Zhang ZW, Chen YE, Du JB, Jia SD, Yuan S and Lin HH (2010) Effects of light on cyanide‐resistant respiration and alternative oxidase function in Arabidopsis seedlings. Plant Cell Environ 33, 2121–2131. [DOI] [PubMed] [Google Scholar]
- 42. Zhang DW, Yuan S, Xu F, Zhu F, Yuan M, Ye HX, Guo HQ, Lv X, Yin Y and Lin HH (2016) Light intensity affects chlorophyll synthesis during greening process by metabolite signal from mitochondrial alternative oxidase in Arabidopsis . Plant Cell Environ 39, 12–25. [DOI] [PubMed] [Google Scholar]
- 43. Saisho D, Nambara E, Naito S, Tsutsumi N, Hirai A and Nakazono M (1997) Characterization of the gene family for alternative oxidase from Arabidopsis thaliana . Plant Mol Biol 35, 585–596. [DOI] [PubMed] [Google Scholar]
- 44. Saisho D, Nakazono M, Tsutsumi N and Hirai A (2001) ATP synthesis inhibitors as well as respiratory inhibitors increase steady‐state level of alternative oxidase mRNA in Arabidopsis thaliana . J Plant Physiol 158, 241–245. [Google Scholar]
- 45. Clifton R, Millar AH and Whelan J (2006) Alternative oxidases in Arabidopsis: a comparative analysis of differential expression in the gene family provides new insights into function of non‐phosphorylating bypasses. Biochim Biophys Acta 1757, 730–741. [DOI] [PubMed] [Google Scholar]
- 46. Swathi M, Lokya V, Swaroop V, Mallikarjuna N, Kannan M, Dutta‐Gupta A and Padmasree K (2014) Structural and functional characterization of proteinase inhibitors from seeds of Cajanus cajan (cv. ICP 7118). Plant Physiol Biochem 83, 77–87. [DOI] [PubMed] [Google Scholar]
- 47. Laskar S, Bhattacharyya MK, Shankar R and Bhattacharyya S (2011) HSP90 controls SIR2 mediated gene silencing. PLoS One 6, e23406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Luhua S, Ciftci‐Yilmaz S, Harper J, Cushman J and Mittler R (2008) Enhanced tolerance to oxidative stress in transgenic Arabidopsis plants expressing proteins of unknown function. Plant Physiol 148, 280–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Agrimi G, Brambilla L, Frascotti G, Pisano I, Porro D, Vai M and Palmieri L (2011) Deletion or overexpression of mitochondrial NAD+ carriers in Saccharomyces cerevisiae alters cellular NAD and ATP contents and affects mitochondrial metabolism and the rate of glycolysis. Appl Environ Microbiol 77, 2239–2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dalal A, Vishwakarma A, Singh NK, Gudla T, Bhattacharyya MK, Padmasree K, Viehhauser A, Dietz KJ and Kirti PB (2014) Attenuation of hydrogen peroxide‐mediated oxidative stress by Brassica juncea annexin‐3 counteracts thiol‐specific antioxidant (TSA1) deficiency in Saccharomyces cerevisiae . FEBS Lett 588, 584–593. [DOI] [PubMed] [Google Scholar]
- 51. Jang HH, Lee KO, Chi YH, Jung BG, Park SK, Park JH, Lee JR, Lee SS, Moon JC, Yun JW et al (2004) Two enzymes in one; two yeast peroxiredoxins display oxidative stress‐dependent switching from a peroxidase to a molecular chaperone function. Cell 117, 625–635. [DOI] [PubMed] [Google Scholar]
- 52. Queval G and Noctor G (2007) A plate reader method for the measurement of NAD, NADP, glutathione, and ascorbate in tissue extracts: Application to redox profiling during Arabidopsis rosette development. Anal Biochem 363, 58–69. [DOI] [PubMed] [Google Scholar]
- 53. Schmitt ME, Brown TA and Trumpower BL (1990) A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae . Nucleic Acids Res 18, 3091–3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Livak KJ and Schmittgen TD (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2‐ΔΔCT method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- 55. Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7, 405–410. [DOI] [PubMed] [Google Scholar]
- 56. Amirsadeghi S, Robson CA, McDonald AE and Vanlerberghe GC (2006) Changes in plant mitochondrial electron transport alter cellular levels of reactive oxygen species and susceptibility to cell death signaling molecules. Plant Cell Physiol 47, 1509–1519. [DOI] [PubMed] [Google Scholar]
- 57. Igamberdiev AU, Ratcliffe RG and Gupta KJ (2014) Plant mitochondria: source and target for nitric oxide. Mitochondrion 19, 329–333. [DOI] [PubMed] [Google Scholar]
- 58. Sieger SM, Kristensen BK, Robson CA, Amirsadeghi S, Eng EW, Abdel‐Mesih A, Møller IM and Vanlerberghe GC (2005) The role of alternative oxidase in modulating carbon use efficiency and growth during macronutrient stress in tobacco cells. J Exp Bot 56, 1499–1515. [DOI] [PubMed] [Google Scholar]
- 59. Padmasree K and Raghavendra AS (1999) Importance of oxidative electron transport over oxidative phosphorylation in optimizing photosynthesis in mesophyll protoplasts of pea (Pisum sativum L.). Physiol Plant 105, 546–553. [Google Scholar]
- 60. Dinakar C, Abhaypratap V, Yearla SR, Raghavendra AS and Padmasree K (2010) Importance of ROS and antioxidant system during the beneficial interactions of mitochondrial metabolism with photosynthetic carbon assimilation. Planta 231, 461–474. [DOI] [PubMed] [Google Scholar]
- 61. Vanlerberghe GC (2013) Alternative oxidase: a mitochondrial respiratory pathway to maintain metabolic and signaling homeostasis during abiotic and biotic stress in plants. Int J Mol Sci 14, 6805–6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Matsukawa K, Kamata T and Ito K (2009) Functional expression of plant alternative oxidase decreases antimycin A‐induced reactive oxygen species production in human cells. FEBS Lett 583, 148–152. [DOI] [PubMed] [Google Scholar]
- 63. Rogov A and Zvyagilskaya R (2015) Physiological role of alternative oxidase (from yeasts to plants). Biochemistry (Moscow) 80, 400–407. [DOI] [PubMed] [Google Scholar]
- 64. Minagawa N and Yoshimoto A (1987) The induction of cyanide‐resistant respiration in Hansenula anomala . J Biochem 101, 1141–1146. [DOI] [PubMed] [Google Scholar]
- 65. Vanlerberghe GC, Robson CA and Yip JY (2002) Induction of mitochondrial alternative oxidase in response to a cell signal pathway down‐regulating the cytochrome pathway prevents programmed cell death. Plant Physiol 129, 1829–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Barros MH, Bandy B, Tahara EB and Kowaltowski AJ (2004) Higher respiratory activity decreases mitochondrial reactive oxygen release and increases life span in Saccharomyces cerevisiae . J Biol Chem 279, 49883–49888. [DOI] [PubMed] [Google Scholar]
- 67. Finkel T (2003) Oxidant signals and oxidative stress. Curr Opin Cell Biol 15, 247–254. [DOI] [PubMed] [Google Scholar]
- 68. Apel K and Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55, 373–399. [DOI] [PubMed] [Google Scholar]
- 69. Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141, 391–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Perrone GG, Tan S‐X and Dawes IW (2008) Reactive oxygen species and yeast apoptosis. Biochim Biophys Acta (BBA)‐Mol. Cell Res 1783, 1354–1368. [DOI] [PubMed] [Google Scholar]
- 71. Farrugia G and Balzan R (2012) Oxidative stress and programmed cell death in yeast. Front Oncol 2, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ayer A, Gourlay CW and Dawes IW (2014) Cellular redox homeostasis, reactive oxygen species and replicative ageing in Saccharomyces cerevisiae . FEMS Yeast Res 14, 60–72. [DOI] [PubMed] [Google Scholar]
- 73. Wheeler GL and Grant CM (2004) Regulation of redox homeostasis in the yeast Saccharomyces cerevisiae . Physiol Plant 120, 12–20. [DOI] [PubMed] [Google Scholar]
- 74. Lin S‐J, Ford E, Haigis M, Liszt G and Guarente L (2004) Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev 18, 12–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lin S‐J, Kaeberlein M, Andalis AA, Sturtz LA, Defossez P‐A, Culotta VC, Fink GR and Guarente L (2002) Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature 418, 344–348. [DOI] [PubMed] [Google Scholar]
- 76. Rietsch A and Beckwith J (1998) The genetics of disulfide bond metabolism. Annu Rev Genet 32, 163–184. [DOI] [PubMed] [Google Scholar]
- 77. Herrero E, Ros J, Bellí G and Cabiscol E (2008) Redox control and oxidative stress in yeast cells. Biochim Biophys Acta 1780, 1217–1235. [DOI] [PubMed] [Google Scholar]
- 78. Purvis AC (1997) Role of the alternative oxidase in limiting superoxide production by plant mitochondria. Physiol Plant 100, 165–170. [Google Scholar]
- 79. Gelhaye E, Rouhier N, Gérard J, Jolivet Y, Gualberto J, Navrot N, Ohlsson P‐I, Wingsle G, Hirasawa M, Knaff DB et al (2004) A specific form of thioredoxin h occurs in plant mitochondria and regulates the alternative oxidase. Proc Natl Acad Sci USA 101, 14545–14550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Arnholdt‐Schmitt B, Costa JH and de Melo DF (2006) AOX – a functional marker for efficient cell reprogramming under stress? Trends Plant Sci 11, 281–287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1 MALDI‐TOF‐TOF mass spectrum of trypsin digested purified AtAOX1a protein between 500 and 5000 m/z.
Fig. S2 Lift spectrum and Biotools display of four major peaks from trypsin digested AtAOX1a protein: (A) m/z 1209.819, (B) 1659.057, (C) 1899.286, and (D) 2384.592.


