Abstract
A 28-year-old male was referred to our hospital with dyspnea. He was diagnosed as having chronic thromboembolic pulmonary hypertension, and a pulmonary endarterectomy (PEA) was performed. However, exertional dyspnea remained because of residual pulmonary hypertension; therefore, the patient was re-admitted to our hospital 1 year after PEA. We performed computed tomography and pulmonary angiography and found web and band lesions in the distal pulmonary artery with a high pulmonary artery pressure. Although further management was complicated because the patient had an anaphylactic shock to iodine-based contrast media, we eventually completed five sessions of balloon pulmonary angioplasty (BPA) using gadolinium contrast medium. His symptoms and hemodynamics dramatically improved after a series of BPA. After 15 months, mean pulmonary arterial pressure reduced from 67 mmHg to 20 mmHg, and subjective symptoms improved from stage Ⅳ to I as per the WHO classification system. BPA is a potential procedure for residual pulmonary hypertension after PEA and could be safely performed using gadolinium contrast medium for patients with iodine allergy.
Keywords: Chronic thromboembolic pulmonary hypertension, Balloon pulmonary angioplasty, Iodine Allergy, Gadolinium contrast medium
1. Background
The effectiveness of balloon pulmonary angioplasty (BPA) for chronic thromboembolic pulmonary hypertension (CTEPH) is known [1], [2]; however, the management of anaphylaxis to contrast media is rarely reported in this context. Here we report a 28-year-old man with no history of iodine allergy who presented with refractory CTEPH and developed anaphylaxis to iodine contrast media during BPA. We describe the case history and use of gadolinium contrast media for BPA.
2. Case presentation
A 28-year-old male had never been diagnosed with an iodine allergy. At 27 years of age, the patient underwent pulmonary endarterectomy (PEA) for central CTEPH caused by antiphospholipid antibody syndrome. At that time, his mean pulmonary arterial pressure (mPAP) was reduced from 56 mmHg to 20 mmHg, and his subjective symptoms improved from stage III to I as per the World Health Organization (WHO) classification system. Warfarin anticoagulation was initiated before performing PEA, and good control was achieved with a target of a PT-INR of approximately 2.0–2.5. However, 1 year later, the patient's condition worsened because of an acute embolism. At this time, he was classified as WHO stage II and was subsequently hospitalized for additional treatment. Blood analysis indicated elevated lupus anticoagulant and D-dimer levels, along with findings indicative of right ventricular failure, including elevated B-type natriuretic peptide, uric acid, GOT, and GPT levels (Table 1). Chest X-ray revealed an enlarged pulmonary artery shadow with a cardiothoracic ratio of 55% and protrusion of the left second and fourth aortic arches (Fig. 1). Electrocardiogram revealed right axis deviation; a pulmonary P wave in leads II, III, and aVF; and a negative T wave in leads III, aVF, and V1–4 (Fig. 2). Echocardiogram revealed enlargement of the right atrium and right ventricle with left-ventricular displacement (Fig. 3). Marked elevation of 95 mmHg was also noted in the tricuspid regurgitation pressure gradient (TRPG). We confirmed an exacerbation of pulmonary hypertension by finding mPAP of 57 mmHg and a pulmonary vascular resistance of 12.3 Wood units on cardiac catheterization. Pulmonary capillary wedge pressure was 6 mmHg, right atrial pressure was 2 mmHg, and the cardiac index was 2.4 L/min/m2 (Table 2). Pulmonary arteriography revealed webbing, bands, abrupt narrowing, and disruption (Fig. 4), and pulmonary ventilation/perfusion scintigraphy revealed a mismatch (Fig. 5). The findings were consistent with CTEPH.
Table 1.
Blood sample analysis.
| WBC (μl) | 7600 | GOT (IU/l) | 40 | BUN (mg/dl) | 15 |
| RBC (μl) | 510 × 104 | GPT (IU/l) | 75 | PT-INR | 2.05 |
| Hb (g/dl) | 17 | LDH (IU/l) | 308 | D-dimer (μg/ml) | 3.4 |
| Plt (μl) | 24 × 104 | T-bil (mg/dl) | 1.9 | FDP (μg/ml) | 13 |
| Na (mEq/l) | 143 | UA (mg/dl) | 7.6 | BNP (pg/ml) | 100 |
| K (mEq/l) | 3.9 | Cre (mg/dl) | 1.01 | Lupus AC | 1.39 |
WBC: White Blood cell, RBC: Red Blood Cell, Hb: Hemoglobin, Plt: Platelet, Na: Natrium, K: Kalium, GOT: Glutamic Oxaloacetic Transaminase, GPT: Glutamic Pyruvic Transaminase, LDH: Lactate Dehydrogenase, T-bil: Total Bilirubin, UA: Uric Acid, Cre: Creatinine, BUN: Blood Urea Nitrogen, PT-INR: Prothrombin Time International Normalized Ratio, FDP: Fibrin Degradation Products, BNP: Brain Natriuretic Peptide, LA: Lupus Anticoagulant.
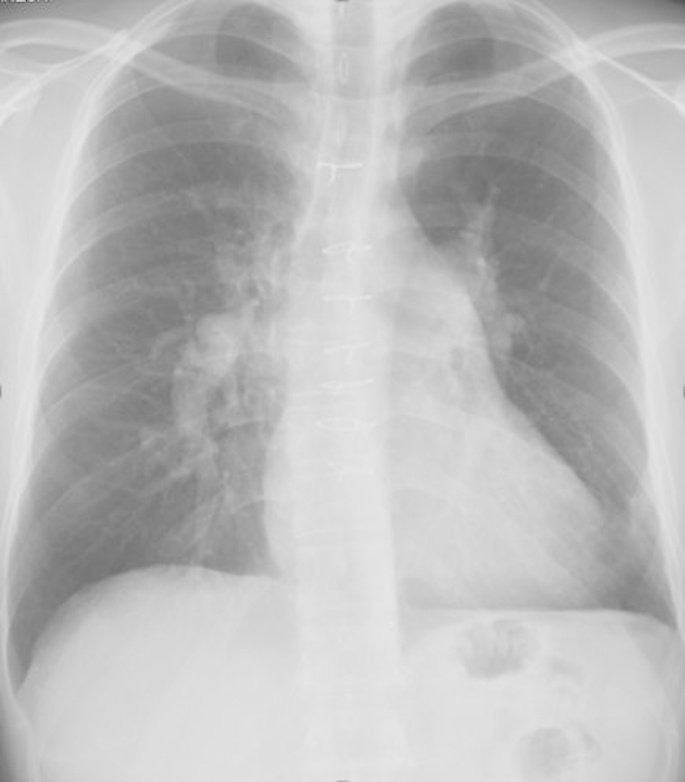
Fig. 1.
Chest X-ray Film. Chest X-ray images showing cardiac dilatation with a cardiothoracic ratio of 55%, enlarged pulmonary shadow and enlargement of the left second and fourth aortic arches.
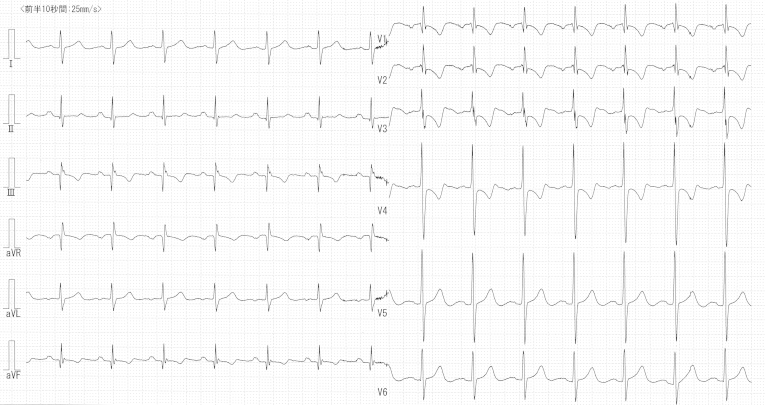
Fig. 2.
12-Lead Electrocardiogram. Electrocardiogram showing right axis deviation, a pulmonary P wave in leads II, III, and aVF, and a negative T wave in leads III, aVF, and V1–4.
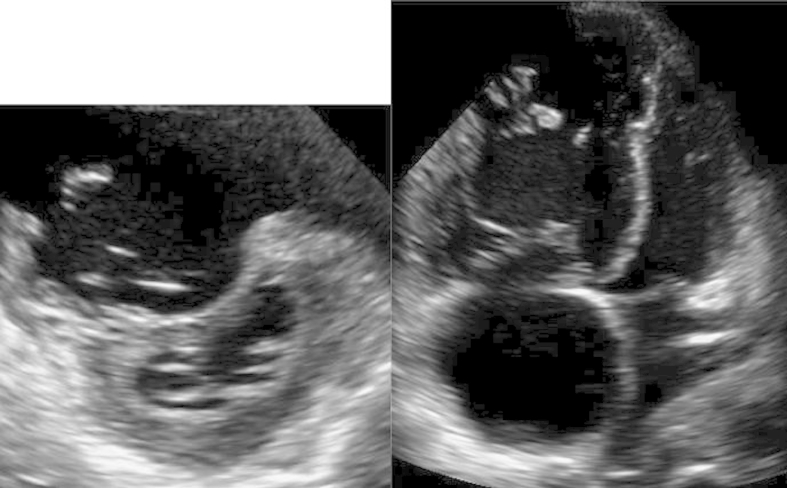
Fig. 3.
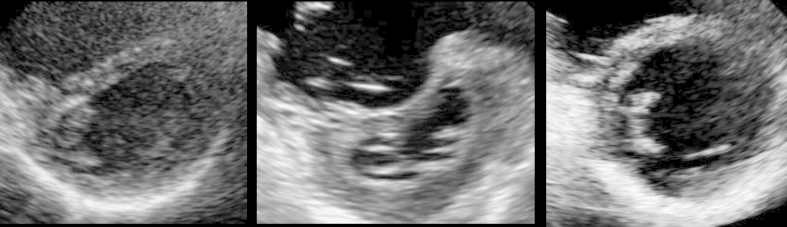
Echocardiography. The left and right images show the short-axis and four-chamber views, respectively. Enlargement of the right atrium and ventricle and marked left-ventricular displacement, are observed.
Table 2.
Clinical coarse.
| pre PEA | post PEA | Hospitalization | Exacerbation | BPA 1st | BPA 2nd | BPA 3rd | BPA 4th | BPA 5th | 4 month | 15 month | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| WHO-FC | Ⅲ | Ⅰ | Ⅲ | Ⅳ | Ⅲ | Ⅲ | Ⅲ | Ⅲ | Ⅱ | Ⅰ | Ⅰ |
| O2 (L)/DOB(γ) | room air/0 | room air/0 | room air/0 | 5.0/2.0 | 5.0/2.0 | 5.0/2.0 | 4.0/0 | 4.0/0 | 4.0/0 | room air/0 | room air/0 |
| BP (mmHg) | 109/73 | 127/81 | 118/75 | 105/72 | 110/68 | 120/68 | 120/68 | 122/68 | 127/69 | 124/76 | 105/70 |
| HR (bpm) | 86 | 101 | 88 | 106 | 120 | 112 | 102 | 95 | 98 | 98 | 88 |
| BNP (pg/ml) | 67 | 18 | 100 | 196 | 96 | 92 | 87 | 18 | 28 | 6 | 14 |
| TRPG (mmHg) | 58 | 24 | 95 | 120 | 100 | 97 | 60 | 42 | 47 | 30 | 22 |
| RAP (mmHg) | 0 | 0 | 2 | 9 | 6 | 3 | 3 | 7 | 1 | 1 | 1 |
| PA (mPAP) (mmHg) | 96/40 (56) | 30/14 (20) | 96/37 (57) | 110/37 (67) | 108/40 (65) | 104/38 (63) | 79/32 (49) | 82/35 (48) | 56/24 (35) | 45/20 (28) | 34/13 (20) |
| PCW (mmHg) | 10 | 2 | 6 | 6 | 12 | 6 | 6 | 12 | 4 | 12 | 6 |
| PVR (wood.units) | 11.3 | 3.8 | 12.3 | 11.5 | 9 | 11 | 7.8 | 5.9 | 5.3 | 2.5 | 2.5 |
| SvO2 (%) | 69 | 70 | 67 | 58 | 76 | 74 | 78 | 79 | 83 | 72 | 75 |
| CO/CI (L/m, L/m/m2) | 4.1/2.3 | 4.8/2.9 | 4.2/2.4 | 5.3/3.1 | 5.8/3.4 | 5.6/3.4 | 6.7/4.0 | 6.6/4.0 | 7.9/4.8 | 6.5/3.9 | 5.9/3.5 |
| MRI: RVEF (%) | 11 | 23 | 31 | ||||||||
| MRI: LVEF (%) | 40 | 46 | 50 | ||||||||
| pO2 (torr) | 58 | 80 | 80 | 66 | 88 | 88 | 124 | 85 | 117 | 110 | 85 |
| pCO2 (torr) | 30 | 37 | 37 | 36 | 37 | 40 | 33 | 39 | 37 | 40 | 39 |
| DLCO (%) | 69 | 45 | 64 | 56 | 57 | 57 | 60 | 55 | 60 | 64 | |
| VC (%) | 104 | 77 | 100 | 82 | 86 | 90 | 93 | 96 | 98 | 100 | |
| FEV1.0 (%) | 78 | 99 | 82 | 82 | 83 | 80 | 84 | 82 | 83 | 87 | |
| peak VO2 (ml/min/kg) | 26.4 | ||||||||||
| AT (ml/min/kg) | 7.8 | ||||||||||
| 6MWD (m) | 260 | 370 | 560 | 570 | |||||||
| Amount of Gd (ml) | 90 | 50 | 59 | 40 | 45 |
WHO FC: World Health Organization Function Class, O2: Oxygen, DOB: Dobutamine Hydrochloride, BP: Blood Pressure, HR: Heart Rate, TRPG: Tricuspid Regurgitation Pressure Gradient, mPAP: mean Pulomonary Artery Pressure, PCW: Pulmonary Capillary Wedge Pressure, PVR: Pulmonary Vascular Resistance, SvO2: Venous Oxygen Saturation, CO: Cardiac Output, CI: Cardiac Index, MRI: Magnetic Resonance Imaging, RVEF: Right Ventricular Ejection Fraction, LVEF: Left Ventricular Ejection Fraction, pO2: Partial Pressure of Oxygen, pCO2: Partial Pressure of Carbon Dioxide, DLCO: Diffuse Capacity of Carbon Monoxide, FEV1.0: Forced Expiratory Volume % one second, Peak VO2: Peak Oxygen Uptake, AT: Anaerobic Threshold, 6MWD: Six Minutes Walk Distance, Gd: Gadolinium contrast medium.
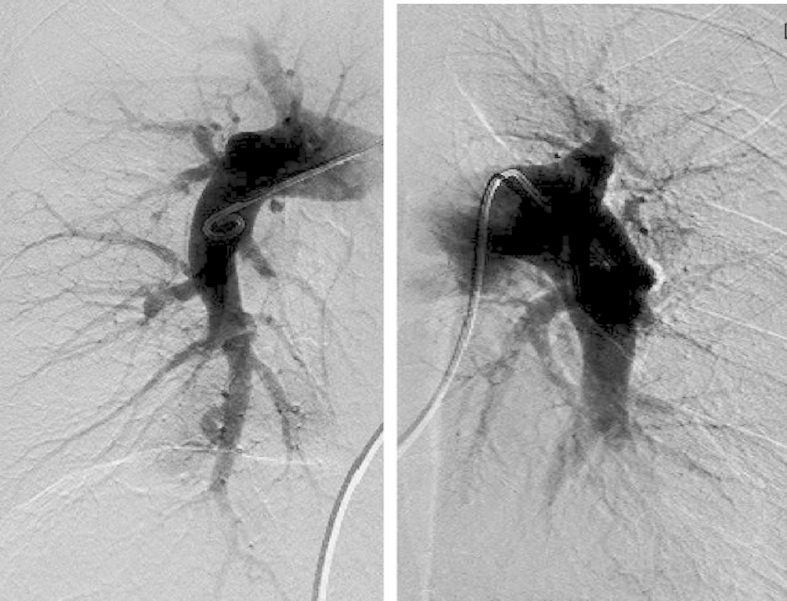
Fig. 4.
Pulmonary Angiography. The left and right images show the right and left pulmonary arteries, respectively. Pulmonary arteriography shows webbing, bands, abrupt narrowing, and disruption but no centrally operable lesion.
Fig. 5.
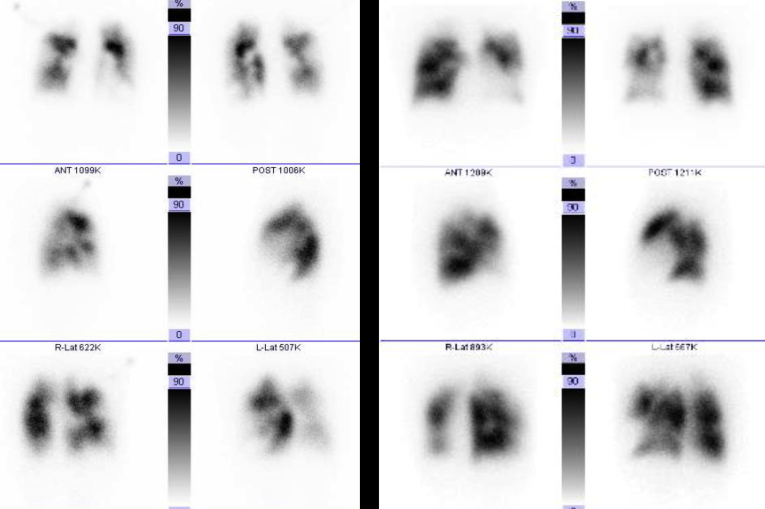
Lung Scintigraphy. The left and right images are of ventilation and perfusion scans, respectively. The perfusion imaging shows multiple segmental blood flow decreases; the ventilation imaging shows a ventilation–perfusion mismatch, with no filling defect.
We suspected that a new pulmonary thromboembolism had developed and, although we performed thrombolytic therapy with urokinase, the effects were limited. His PT-INR was controlled at 2.5–3.0with warfarin and aspirin administration. However, one month later, his mPAP reached 67 mmHg, and he developed hemoptysis. His condition worsened to WHO stage IV, and he lapsed into a catecholamine-dependent state. Sildenafil (60 mg), 5 mg ambrisentan, and 180 μg beraprost were used as specific therapies to treat pulmonary hypertension, but they had little effect. When iodine contrast medium was used in an investigation after hospitalization, the patient had developed a rash. Therefore, 30 mg prednisolone, 20 mg famotidine, and 2 mg d-chlorpheniramine maleate were administered as premedications before contrast-enhanced computed tomography with iopamidol (Iopamiron 370, Bayer, Land Nordrhein-Westfalen, Germany). Despite our efforts, the patient suffered an anaphylactic shock after the test. The CT images we obtained suggested progression of the pulmonary arterial lesion. However, the anaphylaxis meant that it was difficult to perform tests and treatments that relied on iodine-based contrast agents. Moreover, we had to consider the fact that within a very short period, the patient's condition had drastically worsened and he was in a life-threatening state.
At this point, we examined a report describing percutaneous transluminal coronary angioplasty using gadolinium and digital subtraction angiography for a patient with unstable angina who was allergic to iodine and another report that described the use of percutaneous transluminal angioplasty for a pediatric patient with pulmonary stenosis [3], [4]. It was determined that there was a high risk that repeat surgery would worsen the patient's general condition; therefore, we decided to perform BPA with digital subtraction angiography using meglumine gadopentetate (Magnevist; Schering, Berlin, Germany).
The procedure involved placing a sheath (Arrow-Flex; Teleflex, Durham, NC) in the vein and using a 0.035-inch guidewire (Radifocus Guide Wire M; Terumo, Tokyo, Japan) to insert a 6-french-gauge (Fr) wedge-pressure catheter to the bifurcation of the pulmonary trunk. Then, this was replaced with a 6-Fr long sheath (Bright Tip sheath introducer; Cordis/Johnson & Johnson, New Brunswick, NJ) before placing the catheter in the target pulmonary artery. A 6-Fr multipurpose catheter (Mach 1 peripheral MP; Boston Scientific, Natick, MA) was inserted through this and placed in the pulmonary artery trunk. A 0.014-inch wire (Cruise; Asahi Intecc, Tokyo, Japan) was then used to cross the lesion site and was inflated to 6–12 atm using a balloon catheter (2–4 mm, IKAZUCHI PAD, Kaneka, Osaka, Japan). Digital subtraction angiography images were acquired at a rate of 7.5 frames/s with settings of 78 Kv and 41 mAs.
The vessel branches that were dilated in each session were as follows: in session 1, the posterior basal segmental artery of the right lung (Rt.A10a, 10c); in session 2, the lateral basal segmental artery of the left lung (Lt.A9b) and the posterior basal segmental artery of the right lung (Lt.A10b); in session 3, the lateral basal segmental artery of the right lung (Rt.A9b); in session 4, the anterior basal segmental artery of the right lung (Rt.A8); and in session 5, the anterior basal segmental artery of the left lung (Lt.A8). Fig. 6 shows contrast-enhanced images from before and after additional treatment on the right lower lobe branch. The five sessions were concluded without any allergic reactions or complications.
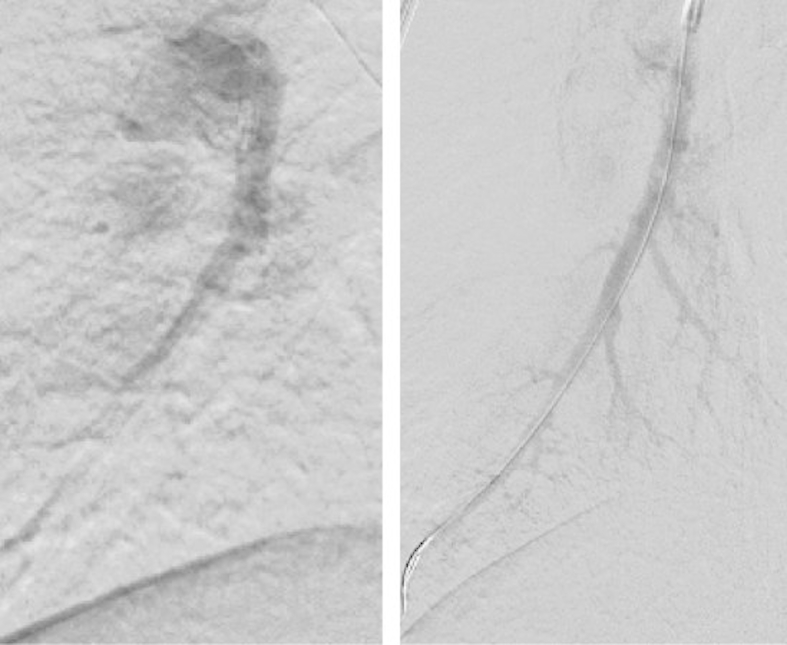
Fig. 6.
Balloon Pulmonary Angioplasty. The right and left images are pre-BPA and post-BPA, respectively. Contrast-enhanced images from before and after additional treatment showing the lateral basal segmental artery of right lung (Rt.A9b). On DSA images, many peripheral vessels are seen post-BPA. Abbreviations: BPA, balloon pulmonary angioplasty; DSA, digital subtraction angiography.
The follow-up right heart catheterization at 4 months indicated improvement to mild pulmonary hypertension with an mPAP of 28 mmHg and a pulmonary vascular resistance of 2.5 Wood units. The pulmonary capillary wedge pressure was 4 mmHg, right atrial pressure 1 mmHg, and cardiac index 3.9 L/min/m2, indicating increased cardiac output. Improved right cardiac function was shown by the right ventricular ejection fraction increasing from 11% to 23% on magnetic resonance imaging. Using 5 L/min of oxygen, the partial pressure of oxygen (pO2) was 66 Torr; in room air, the pO2 was 110 Torr. Furthermore, exercise tolerance markedly improved with the improved oxygenation and cardiac index: the patient could walk 560 m in 6 min, and his maximal oxygen consumption was 26.4 mL/min/kg during cardiopulmonary exercise testing, despite previously being bedridden. His subjective symptoms also improved from WHO stage IV to I and his body weight decreased to 53 kg from 60.5 kg in the most exacerbated stage.
At the 15-month follow-up examination, the patient had no further exacerbations and remained at WHO stage I. Moreover, his mPAP had improved to 20 mmHg, the pulmonary vascular resistance had remained at 2.5 Wood units, and his right ventricular ejection fraction had improved to 31%. Table 2 and Fig. 7 show the changes in clinical data. The changes observed in chest X-ray images, 12-lead electrocardiograms, echocardiograms, and ventilation scintigraphy in Fig. 8, Fig. 9, Fig. 10, Fig. 11 are consistent with pulmonary hypertension pressure dynamics. The patient is being followed-up on an outpatient basis and has reported no problems in his daily life.
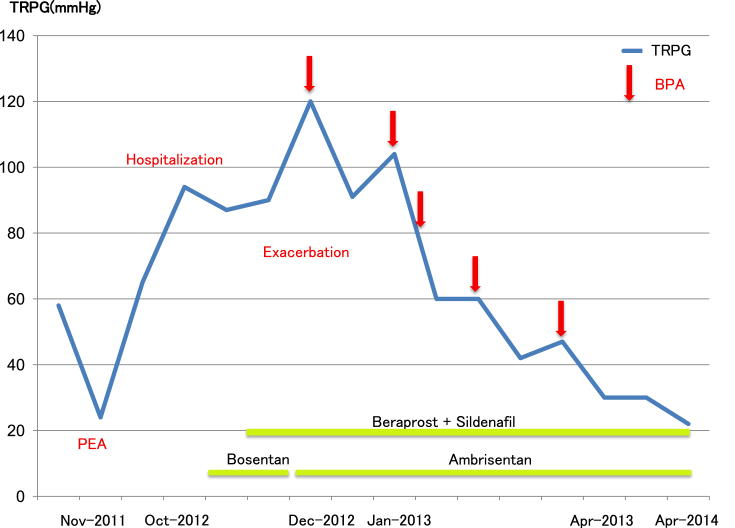
Fig. 7.
Clinical Course. This figure shows changes in the pulmonary arterial pressure with TRPG measured on cardiac echocardiography as an index. After PEA, the TRPG dropped to 24 mmHg but it later reached 95 mmHg during an exacerbation. After hospitalization, gradual exacerbation was observed despite the use of targeted medical therapy, and TRPG eventually reached 120 mmHg. After that, TRPG decreased with each BPA procedure. After five BPA procedures, TRPG decreased to 30 mmHg at 4 months and 22 mmHg at 15 months. Abbreviations: Apr, April; BPA, balloon pulmonary angioplasty; Dec, December; Jan, January; PEA, pulmonary endarterectomy; TRPG, tricuspid regurgitation pressure gradient; Nov, November; Oct, October.
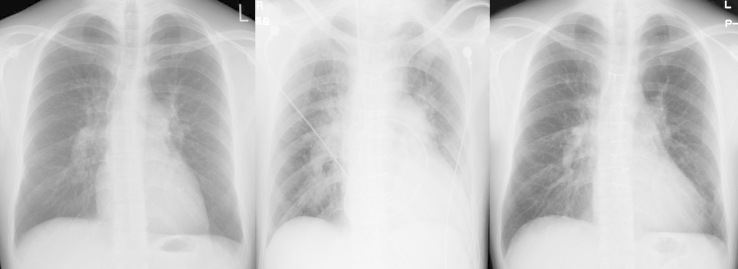
Fig. 8.
Changes over Time in Chest X-ray Film. Left image is post-PEA, center image is during an exacerbation after hospitalization, and right image is 15 months after BPA. During exacerbation, enlargement of the second and fourth aortic arches is seen together with blood congestion, but these improved after BPA. Abbreviations: BPA, balloon pulmonary angioplasty; PEA, pulmonary endarterectomy.
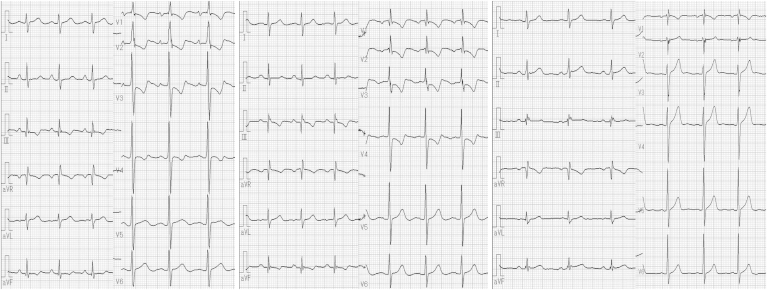
Fig. 9.
Changes over Time in 12-Lead Electrocardiograms. Left image is post-PEA, center image is during an exacerbation after hospitalization, and right image is 15 months after BPA. During exacerbation, right axis deviation can be seen with descending type decreased ST and a negative T waves in the precordial leads. However, these improved after BPA. Abbreviations: BPA, balloon pulmonary angioplasty; PEA, pulmonary endarterectomy.
Fig. 10.
Changes over Time in Echocardiography. Left image is post-PEA, center image is during an exacerbation after hospitalization, and right image is 15 months after BPA. The D-shape showing left-ventricular displacement disappeared after BPA. Abbreviations: BPA, balloon pulmonary angioplasty; PEA, pulmonary endarterectomy.
Fig. 11.
Changes over Time in Lung Scintigraphy Perfusion Imaging. Left image is pre-BPA and right image is 4 months after BPA. This figure shows changes in lung scintigraphy from after PEA to during exacerbation, and at 4 months after BPA. The blood flow in treated regions improved. Abbreviations: BPA, balloon pulmonary angioplasty; PEA, pulmonary endarterectomy.
3. Discussion
PEA for central CTEPH is a safe and effective procedure with a reported five-year survival rate of 82%, 10-year survival rate of 75%, and perioperative mortality rate of 4.4% [5]. However, pulmonary hypertension remains postoperatively in 10%–15% of patients, and prognosis is generally poor in such cases [6], [7]. In the present case, although pulmonary hypertension improved after PEA, the lesion peripherally persisted. We believe that the thromboembolism then progressed because of the underlying antiphospholipid antibody syndrome, resulting in the later acute exacerbation. Furthermore, because his symptoms rapidly worsened soon after hospitalization and because therapy was ineffective for the pulmonary arterial hypertension, there was a high risk of early mortality without treatment.
Panitan et al. reported that allergies to iodine contrast media occurred in 579 (1.05%) of 55,286 patients receiving the media. Of these, 16 patients (0.03%) experienced serious symptoms and one (0.002%) died. Thus, while the incidence is low, serious symptoms can sometimes occur. In this case, the patient exhibited serious symptoms due to anaphylactic shock despite changing the type of iodine contrast medium and despite the use of premedication [8].
Recently, it has been reported that BPA is effective for inoperable cases of peripheral CTEPH. In addition, gadolinium can be used for patients who are allergic to iodine contrast media; however, there have been no previous reports of cases in which BPA was performed using gadolinium in a patient with CTEPH. We report our experiences of performing five sessions of BPA on a young patient who we considered would have had a poor prognosis without treatment. The surgery was successfully concluded without complication, and the patient's condition sufficiently improved for him to return to his normal activities of daily living.
Meglumine gadopentetate is a gadolinium formulation to which meglumine and diethylenetriaminepentaacetic acid (DTPA) are added as a pH adjuster and stabilizer, respectively. For each contrast, 10 mL of meglumine gadopentetate at a dilution of 0.5 mmol/mL was used.
The benefit of using gadolinium contrast medium is that it can also be used on patients with severe iodine allergies. Problems associated with gadolinium are its low viscosity, the fact that the high speed of pulmonary arterial blood flow makes it difficult to view the blood flow with fluoroscopy, and that the technique is more difficult than when performed with iodine contrast. The effect of gadolinium contrast has also been reported to be approximately one fifth that of an iodine contrast [9]; however, when an increased amount of gadolinium contrast medium is used, it can cause renal dysfunction. However, according to the guidelines for the use of gadolinium contrast for patients with renal failure, as recommended by the Japan Radiological Society and the Japan Society of Nephrology, there is insufficient evidence to support the claim that renal function of GFR ≥60 mL/min/1.73 m2 increases the risk of nephrogenic systemic fibrosis (NSF). In the present case, GFR was 73.7 mL/min/1.73 m2. Therefore, during treatment, symptoms such as itching sensation in skin, swelling, hardening and joint stiffness were frequently checked and renal function was periodically monitored through blood tests to confirm that renal function had not deteriorated. Importantly, gadolinium contrast medium is also expensive, and because it is usually exclusively used with magnetic resonance imaging, it is not covered by health insurance when used for BPA. However, in this case, as the surgeon became familiar with the procedure over repeated sessions and as the vessels were more accurately targeted by advance examination and detailed planning, the amount of gadolinium contrast medium used was reduced from 90 mL to 40 mL, as shown in Table 2. We believe that this amount could be further reduced with the use of optical coherence tomography [10].
We conclude that BPA using gadolinium may become an option for severe CTEPH in patients who are allergic to iodine.
References
- 1.Feistein J.A., Goldhaber S.Z., Landzberg M.J. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation. 2001;103:10–13. doi: 10.1161/01.cir.103.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Hoeper M.M., Michael M.M., Norifumi N. Chronic thromboembolic pulmonary hypertension. Lancet Respir. Med. 2014;2:573–582. doi: 10.1016/S2213-2600(14)70089-X. [DOI] [PubMed] [Google Scholar]
- 3.Furuichi S., Yasuda S., Arita Y. Gadopentetate dimeglumine as a potential alternative contrast medium during percutaneous coronary intervention: a case report. Circ. J. 2004;68:972–973. doi: 10.1253/circj.68.972. [DOI] [PubMed] [Google Scholar]
- 4.Sugiyama H., Kadono T., Hoshiai M. Gadolinium-based balloon angioplasty for pulmonary artery stenosis in an infant with a right isomerism. Catheter. Cardiovasc. Interv. 2004;63:346–350. doi: 10.1002/ccd.20186. [DOI] [PubMed] [Google Scholar]
- 5.Madani M.M., Auger W.R., Pretorius V., Sakakibara N. Pulmonary endarterectomy: recent changes in a single institution's experience of more than 2700 patients. Ann. Thorac. Surge. 2012;94:97–103. doi: 10.1016/j.athoracsur.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Jamieson S.W., Kapelanski D.P., Sakakibara N. Pulmonary endarterectomy: experience and lessons learned in 1500 cases. Ann. Thorac. Surge. 2003;76:1457–1464. doi: 10.1016/s0003-4975(03)00828-2. [DOI] [PubMed] [Google Scholar]
- 7.Fedullo P.F., Auger W.R., Channick R.N. Chronic thromboembolic pulmonary hypertension. Clin. Chest Med. 2001;22:561–581. doi: 10.1016/s0272-5231(05)70292-6. [DOI] [PubMed] [Google Scholar]
- 8.Panitan P., Dhana N., Jongjarearnprasert K. Adverse reactions to iodinated contrast media: prevalence, risk factors and outcome-the results of a 3-year period. Asian Pac. J. Allergy Immunol. 2013;31:299–306. doi: 10.12932/AP0297.31.4.2013. [DOI] [PubMed] [Google Scholar]
- 9.Nagashima H., Sakamoto H., Sano Y. Fundamental study of DSA images using Gadolinium contrast agent. Nihon Hoshasen Gijutsu Zasshi. 2002;58:1369–1376. doi: 10.6009/jjrt.kj00003111387. [DOI] [PubMed] [Google Scholar]
- 10.Tatebe S., Fukumoto Y., Sugimura K. Optical coherence tomography as a novel diagnostic tool for distal type chronic thromboembolic pulmonary hypertension. Circ. J. 2010;74:1742–1744. doi: 10.1253/circj.cj-10-0160. [DOI] [PubMed] [Google Scholar]