Abstract
Objective
To determine whether microglial activity, measured using translocator-protein positron emission tomographic imaging (PET), is increased in unmedicated subjects presenting with sub-clinical symptoms indicating they are at ultra high risk of psychosis, and to determine if it is elevated in schizophrenia after controlling for a translocator specific genetic polymorphism.
Method
Here we use the second generation radioligand [11C]PBR28 and PET to image microglial activity in the brains of subjects at ultra high risk for psychosis. Subjects were recruited from early intervention centres. We also imaged a cohort of patients with schizophrenia and healthy controls for comparison, in total 56 subjects completed the study. At screening, subjects were genotyped to account for the rs6971 polymorphism in the gene encoding the 18Kd Translocator Protein. The main outcome measure was total grey matter [11C]PBR28 binding ratio, representing microglial activity.
Results
[11C]PBR28 binding ratio in grey matter was elevated in ultra high risk subjects, compared to matched controls, (p=0.004, F= 10.3, Cohen’s d >1.2), and was positively correlated with symptom severity (r= 0.730, p<0.01). Patients with schizophrenia also demonstrated elevated microglial activity with respect to matched controls (p<0.001, F= 20.8, Cohen’s d >1.7).
Conclusion
Microglial activity is elevated in schizophrenia and in subjects with sub-clinical symptoms who are at ultra high risk of psychosis, and is related to at risk symptom severity. This indicates that neuroinflammation is linked to the risk of psychosis and related disorders, and the expression of sub-clinical symptoms. Follow up of ultra high risk subjects will determine whether this is specific to the later development of schizophrenia or risk factors in general.
Introduction
Schizophrenia is a severe psychiatric disorder characterised by psychotic and cognitive symptoms, and is a leading cause of global disease burden (1). It is generally preceded by a prodromal phase of attenuated psychotic symptoms and functional impairment (2). Subjects meeting standardised criteria for this phase have an ultra high risk for developing a psychotic disorder, in most cases schizophrenia (3). Approximately ~35% of high risk subjects will develop a psychotic disorder within 24 months (4).
Whilst the pathoaetiology of schizophrenia is not fully understood, there is increasing evidence for the involvement of neuroinflammatory processes. Microglia are the resident immune cells of the central nervous system and several lines of evidence indicate microglial involvement in the pathology of psychosis (5-7). In ultra high risk subjects, there are elevations in the levels of pro-inflammatory cytokines (8) which are also elevated in patients with schizophrenia (9). The levels of such peripheral markers have also been associated with the reductions in grey matter volume in both ultra high risk subjects (10) and patients with schizophrenia (11). Post-mortem investigation of brain tissue has found elevated microglial cell density (with a hypertrophic morphology) in schizophrenia compared with controls (5), particularly in the frontal and temporal lobes (12), although some studies have found no differences (13). However, as microglial activity is dynamic, post-mortem studies may miss alterations early in the development of the disease.
Elevations in microglial activity can be measured in vivo with positron emission tomography (PET) using radioligands specific for the 18kD translocator-protein (TSPO), which is expressed on microglia (14). Investigations using the first generation radiotracer (R)-[11C]PK11195 have revealed an increase in TSPO binding in medicated patients with schizophrenia when compared to healthy controls (6, 7). The first investigation of microglia using PET in schizophrenia, in a cohort of 10 patients, revealed a total grey matter elevation of microglial activity in the five years following diagnosis (6). The most recent investigation in seven chronically medicated patients with schizophrenia using (R)-[11C]PK11195 demonstrated an elevation in hippocampal binding potential and a non-significant 30% increase in total grey matter binding potential (7).
Whilst these studies indicate elevated microglial activity in schizophrenia, they included patients in whom the disorder was already established. It is therefore unknown whether this elevation predates the onset of, or becomes evident after, the first episode of frank psychosis.
Therefore in the present investigation we seek to determine whether microglial activity is elevated in ultra high risk subjects using the novel TSPO radioligand [11C]PBR28. Our a priori hypothesis was that microglial activity would be elevated in the total grey matter in ultra high risk individuals compared to matched controls. An additional prediction was that this elevation would be evident in frontal and temporal cortical regions, brain areas that have been particularly implicated in ultra high risk pathophysiology (15). [11C]PBR28 is a second generation TSPO tracer with a higher affinity for TSPO than (R)-[11C]PK11195 (16). Recent in situ binding evidence shows that a genetic polymorphism (a C/T substitution at rs6971) influences the binding of TSPO tracers, including [11C]PBR28. This results in three TSPO binding profiles.
High affinity binders (HABs, C/C) have the greatest tracer affinity, low affinity binders (LABs, T/T) have a 50 fold reduction in affinity, and mixed affinity binders (MABs, C/T) express both HAB and LAB TSPO in approximately equal proportion (17). In view of this we included a cohort of patients to test the hypothesis that TSPO binding is elevated in schizophrenia after adjusting for this polymorphism, as this has not been taken into account previously. We also tested the secondary hypothesis that there would be a positive relationship between total grey matter microglial activity and symptom severity.
Methods
The study was approved by the local research ethics committee and was conducted in accordance with the Declaration of Helsinki. After complete description of the study to the subjects, written informed consent was obtained.
Subjects
A total of 56 subjects were recruited and completed the study; 14 subjects meeting ultra high risk criteria, as assessed on the comprehensive assessment of the at risk mental state (CAARMS) (2), were recruited from OASIS (Outreach and Support in South London) (18) (Mean age ± SD: 24.3 ± 5.40; (M:F=7:7)). Fourteen age (± 5 years) matched control subjects were recruited through newspaper and poster adverts. 14 subjects with schizophrenia (Mean age ± SD: 47.0 ± 9.31; (M:F=12:3)) were recruited from London mental health centres (South London and Maudsley NHS Foundation Trust). A further 14 age (± 5 years) matched healthy control subjects were recruited for comparison with this second cohort (Table 1.).
Table 1.
Demographic characteristics of experimental and control subjects
| Control | Stdev | Ultra high risk | Stdev | p-value | Control | Stdev | Schizophrenia | Stdev | p | |
|---|---|---|---|---|---|---|---|---|---|---|
| N = 14 | N = 14 | - | N=14 | N = 14 | - | |||||
| Age in years | 28.14 | 7.99 | 24.29 | 5.40 | 0.133 a | 46.21 | 13.62 | 47.00 | 9.31 | 0.982 a |
| Years of education | 14.8 | 3.0 | 14.3 | 1.6 | 0.344 a | 12.3 | 3.0 | 12.2 | 2.0 | 0.374a |
| Gender (M:F) | 10:4 | 7:7 | 0.352 b | 12:3 | 12:3 | 1.000 b | ||||
| TSPO genotype (HAB) | 10 | 7 | 0.352 b | 14 | 13 | 0.541 b | ||||
| CAARMS/PANSS | ||||||||||
| Positive | - | - | 11.2 | 4.5 | - | - | 17.0 | 6.1 | - | |
| Negative | - | - | 6.1 | 4.3 | - | - | 14.1 | 4.0 | - | |
| General | - | - | 19.1 | 12.3 | - | - | 32.6 | 8.7 | - | |
| Total | - | - | 49.5 | 21.6 | - | - | 63.7 | 18.1 | - |
independent samples t-test.
Mann-Whitney U test.
Symptom scales measured in high risk subjects on the CAARMS (comprehensive assessment of the ‘at risk mental state’) and in schizophrenia on the PANSS (positive and negative syndrome scale).
All subjects met the following inclusion criteria:
Aged 18+;
No significant physical or psychiatric health contraindications on assessment and medical examination by a trained physician.
Subjects were then screened based on the following exclusion criteria;
Exposure to any medications, including anti-inflammatory medications, in the last 1 month (see Supplemental Material for details).
History of substance abuse/dependence as determined by the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders IV (DSM-IV) (SCID) (19).
Benzodiazepine use, whether prescribed or not (20). One subject was excluded due to a positive result for benzodiazepines on the scan day.
History of head injury resulting in unconsciousness, or any physical medical condition associated with inflammation.
At the time of screening, subjects were tested for TSPO genotype to determine binding status (17). Subjects with a LAB genotype were excluded.
In ultra high risk and control subjects: antipsychotic medication exposure.
Significant prior exposure to radiation
Pregnancy or breast feeding.
Healthy control subjects with a personal history of a psychiatric disorder or a first degree relative with schizophrenia or a psychotic illness were excluded.
Clinical and neuropsychological measures
At screening all subjects were assessed using the SCID (19). Ultra high risk subjects were assessed on the CAARMS (2) by a trained investigator and patients with a diagnosis of schizophrenia were assessed on the positive and negative syndrome scale (PANSS) (21) by a clinician on the day of the PET scan. Depressive symptoms were assessed using the Beck Depression Inventory (BDI) (22).
PET imaging
An initial low dose transmission computer tomography (CT) scan (0.34 mSv) was acquired for attenuation and scatter correction using a Siemens Biograph™ TruePoint™ PET·CT scanner (Siemens Medical Systems, Germany). Subjects then received a bolus injection of [11C]PBR28 (mean Mbq activity ±SD: 325.31 ± 27.03) followed by a 90-minute emission scan. PET data were co-registered with whole brain structural images acquired with a 3T magnetic resonance imaging (MRI) scanner (Trio, Siemens Medical Systems, Germany). A 32 channel coil was used for all but one scan, where a 12 channel coil was used instead.
PET acquisition
PET data were acquired dynamically over a 90-minute time window and binned into 26 frames (durations: 8 × 15 s, 3 × 1 min, 5 × 2 min, 5 × 5 min, 5 × 10 min). Images were reconstructed using filtered back projection, which provides better data quality and signal-to-noise ratio over iterative methods (23), and corrected for attenuation and scatter. During the PET acquisition, arterial blood data were sampled via the radial artery using a combined automatic-manual approach. A continuous (one sample per second) sampling system (ABSS Allogg, Mariefred, Sweden) measured whole blood activity for the first 15 minutes of each scan (see on-line supplemental information for further details).
Structural MRI
Each subject underwent a T1 weighted MRI brain scan. MRI images were used for grey/white matter segmentation and region of interest (ROI) definition. A neuroanatomical atlas (24) was co-registered on each subject’s MRI scan and PET image using a combination of Statistical Parametric Mapping 8 (http://www.fil.ion.ucl.ac.uk/spm) and FSL (http://www.fsl.fmrib.ox.ac.uk/fsl) functions, implemented in MIAKAT™ (http://www.imanova.co.uk). The primary region of interest was total grey matter. Secondary regions of interest were temporal and frontal lobe grey matter (12).
Image analysis
All PET images were corrected for head movement using nonattenuation-corrected images, as they include greater scalp signal, which improves re-alignment compared to attenuation-corrected images (25). Frames were realigned to a single ‘reference’ space identified by the individual T1 MRI scan. The transformation parameters were then applied to the corresponding attenuation-corrected PET frames, creating a movement-corrected dynamic image for analysis. Regional time-activity curves (TACs) were obtained by sampling the image with the coregistered atlas. Hence quantification of [11C]PBR28 tissue distribution was performed using the two tissue compartmental model accounting for endothelial vascular TSPO binding (2TCM-1K) (26), as this has been shown to have improved performance compared with the two tissue compartmental model not accounting for endothelial binding (2TCM) (26).
Nevertheless, for completeness, we analysed the data using the 2TCM as well (see on-line supplemental information and Supplemental Table 1). Even after accounting for genotype, high inter-subject variability is seen in imaging with TSPO tracers. With PK11195 plasma protein binding is evident and may account for some levels of variability with TSPO imaging (27). Indeed TSPO ligand quantification approaches mostly use tissue reference methodologies (28). Analysis of PK11195 is conducted using simplified reference tissue models (SRTM) and supervised cluster analysis (29). This method is not applicable to second generation TSPO tracers, including PBR28, as the higher ligand affinity leads to appreciable endothelial binding in the blood brain barrier (BBB) (26). As a result, it is not possible to identify a supervised cluster for reference. Our outcome measure therefore was the distribution volume ratio (DVR, the ratio of the VT in the ROI to VT in the whole brain) as this accounts for inter-subject variability in the input function. In secondary analyses, we tested the regional specificity of group changes by comparing DVR between groups in regions (the cerebellum and brainstem) where we did not expect marked inflammatory changes based on the post-mortem studies and grey matter changes seen in people at risk of psychosis (30). To ensure non-specific effects of normalization, our parameters used to normalize the PET signal were tested for group differences. There are no differences in blood or normalization regions used (see Supplemental Table 2).
Statistical analysis
Data, other than for gender and genotype, were shown to have a normal distribution following a Shapiro-Wilk test (31). Hence parametric tests were implemented for all but gender and affinity analyses, where a Mann-Whitney U test was used. Demographic data and tracer activity data were analysed using independent-samples t-tests. Multiple analysis of covariance (ANCOVA) with Bonferroni correction (32) was used to determine whether there was an effect of group on [11C]PBR28 binding associated microglial activity in the total grey matter, frontal lobe, and temporal lobe. There is data to suggest that cortical microglial density, hence TSPO binding, is elevated with aging (33), which is also evident in our data (see Supplemental Table 3). For this reason, we performed group level analysis using age as a covariate. There is a significantly higher binding of tracer in HABs than MABs and the variation in signal from HAB and MAB subjects is high, with a large degree of overlap across the binding statuses (Supplemental Figure 3). Hence we pooled the binding statuses in our groups and co-varied for genotype in our analysis. (17). For all statistical comparisons alpha was set at a 0.05 threshold (two-tailed) for significance. Statistical analysis was performed using SPSS 21 (IBM, USA). Partial correlation analysis was used to test the association of microglial activity with symptom severity and total grey matter volumes, with age and affinity as covariates of no interest.
Results
Demographic Comparisons and Tracer Dosing
No significant demographic differences between the two groups of controls and respective patient groups were observed (Table 1). There were no differences in the injected dose, injected mass, specific activity, parent plasma fraction or plasma over blood ratio between ultra high risk subjects or patients with schizophrenia and their respective controls (Supplemental Table 4).
[11C]PBR28 distribution in total grey matter regions
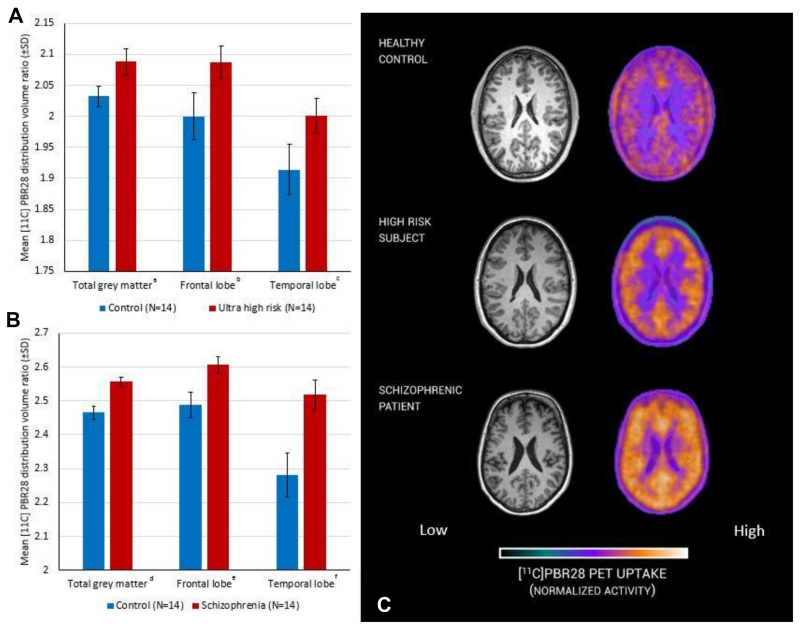
The [11C]PBR28 distribution volume ratios in total grey matter, frontal lobe and temporal lobe grey matter were significantly increased in ultra high risk when compared with matched control subjects (Figure 1 A and Table 2). Similarly, patients with a diagnosis of schizophrenia had elevated [11C]PBR28 DVRs in total, frontal lobe and temporal lobe grey matter with respect to matched control subjects (Figure 1 B and Table 2). Secondary analysis to investigate regional specificity revealed no difference between ultra high risk or schizophrenia and respective controls in cerebellar or brainstem DVR (Table 2). Representative PET images of control, ultra high risk and patients with schizophrenia are presented in Figure 1 C. When comparing regions using VT, with either 2TCM or 2TCM-1K, no significant group difference was observed (supplemental Table 5).
Figure 1. Microglial activity measured with PET in ultra high risk subjects, patients with schizophrenia and matched controls.
Significant difference between experimental (red) and control (blue) groups, ANCOVA (covarying for age and genotype). A: a (df=21 p=0.004), b (df=21 p=0.030), c (df=21 p=0.047); B: d (df=21 p<0.001), e (df=21 p=0.005), f (df=21 p=0.001); C representative [11C]PBR28 PET image from a subject from each group.
Table 2.
Microglial activity, as measured by PBR28 distribution volume ratio, is elevated in subjects at ultra high risk of psychosis (df=21 p=0.004) and patients with schizophrenia (df=21 p<0.001) in the total grey matter, frontal and temporal cortical regions of interest but not in control regions (the cerebellum and brainstem). The mean regional distribution volume ratios are shown for each group together with those for matched controls. The results of the ANCOVA covarying for age and translocator-protein genotype are shown for each case-matched control comparison
| Mean Regional DVR of [11C]PBR28 | Control | Stdev | Ultra high risk | Stdev | F | P | Cohen’s d | Control | Stdev | Schizophrenia | SD | F | p | Cohen’s d |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total grey Matter | 2.032 | 0.017 | 2.088 | 0.021 | 10.332 | 0.004 | 1.244 | 2.465 | 0.020 | 2.557 | 0.014 | 20.802 | <0.001 | 1.769 |
| Frontal lobe | 2.000 | 0.038 | 2.087 | 0.026 | 5.339 | 0.030 | 0.894 | 2.489 | 0.037 | 2.606 | 0.025 | 9.883 | 0.005 | 1.245 |
| Temporal lobe | 1.914 | 0.041 | 2.001 | 0.028 | 4.417 | 0.047 | 0.829 | 2.282 | 0.065 | 2.518 | 0.044 | 13.089 | 0.001 | 1.430 |
| Cerebellum | 2.307 | 0.055 | 2.287 | 0.081 | 0.062 | 0.805 | - | 2.863 | 0.060 | 2.873 | 0.063 | 0.015 | 0.905 | - |
| Brain stem | 2.291 | 0.191 | 2.489 | 0.28 | 0.500 | 0.487 | - | 2.514 | 0.154 | 2.097 | 0.234 | 3.194 | 0.088 | - |
Two subjects in the ultra high risk group had taken citalopram in the past. However only one was using the medication at the time of scan, and no other UHR subjects had taken psychotropic drugs. Re-analysis excluding the two subjects who had taken citalopram did not alter the significant elevation in [11C]PBR28 DVR in the high risk group in the total (F=6.601, p=0.018) and frontal lobe (F=5.392, p=0.030) grey matter but the finding in the temporal cortex was no longer significant (p=0.149).
Genotype specific analysis
In further sensitivity analyses, we repeated the analyses separately for HABs and MABs in the UHR. This showed that the elevation in the ultra high subject was present irrespective of whether the analysis was restricted to HABs or MABs (Supplemental Table 6 and Supplemental Figure 3).
Relationship between [11C]PBR28 distribution and symptom severity
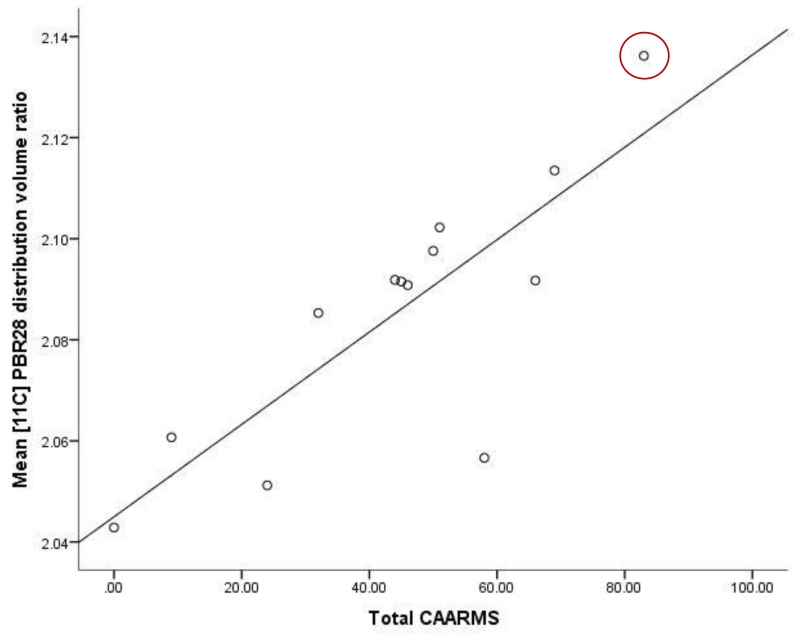
In ultra high risk subjects, there was a positive correlation between the total CAARMS symptom severity score and [11C]PBR28 DVR in total grey matter (r = 0.730, p = 0.011, Figure 2). No correlation was observed between [11C]PBR28 DVR in total grey matter and duration of ultra high risk symptoms (r= −0.086, p= 0.802). In patients with schizophrenia, there was no significant correlation between total grey matter DVR and total PANSS score (Supplemental Figure 2). There was no relationship between depressive symptom severity (Beck Depression Inventory score) and total grey matter DVR in either patients with schizophrenia (r= 0.478 p= 0.162) or ultra high risk subjects (r=−0.339 p= 0.506).
Figure 2. Microglial activity and symptom scores in ultra high risk subjects.
Significant correlation between measures. Partial correlation including age and genotype as covariates (N=13, data were missing for 1 subject r= 0.730, p= 0.011). Highlighted subject, transition case.
Exploratory analysis of DVR normalization
To evaluate whether our findings were influenced by the signal used for normalization, we conducted exploratory analyses using the cerebellum and white matter as alternative normalization regions. Cerebellar normalization did not alter the major regional findings (frontal lobe p=0.001; temporal lobe p=0.006). White matter normalization performed similarly to the cerebellum (see supplemental table 7).
Discussion
Our main finding is that [11C]PBR28 binding ratio, a marker of microglial activity, is elevated in people at ultra high risk of psychosis, with a large effect size (Cohen’s d >1.2). Furthermore [11C]PBR28 binding ratio was associated with the severity of symptoms in ultra high risk subjects, linking elevated microglial activity to the expression of sub-clinical psychotic symptoms. Importantly we found no relationship with depressive symptoms, suggesting elevated microglial activity is specific to the development of psychotic-like symptoms, rather than psychiatric symptoms in general. It would be valuable to examine change in [11C]PBR28 signal during the course of the prodromal period to determine if there is a change during the prodromal phase. As the ultra high risk subjects, who had recently presented to psychiatric services, were medication naïve and had no history of psychotic disorder, these findings cannot be attributed to effects of previous illness or its treatment. Interestingly, at the time of writing, one ultra high risk subject has transitioned to first episode psychosis. This subject had the highest total grey matter [11C]PBR28 signal in the cohort (DVR=2.14). Follow up of the remaining subjects is required to determine the role of elevated TSPO availability in the onset of psychosis.
The present findings are consistent with recent evidence of elevated peripheral inflammatory markers in people at high risk of psychosis (8, 10), and suggest that elevated microglial activity predates the onset of frank psychosis. We also found evidence of elevated microglia activity in people with schizophrenia relative to controls with a large effect size (Cohen’s d >1.7). This extends previous PET studies which have not controlled for TSPO genotype (34), a potential confound as genotype influences binding, by showing that TSPO binding is elevated after controlling for TSPO genotype. Our findings are also consistent with the findings of a post-mortem study in schizophrenia, which also used PBR28. However, because it was in vitro, was able to use a two-point assay to quantify specific PBR28 binding to show elevated PBR28 binding in schizophrenia (35). We did not find the same symptom correlation in schizophrenia as we did in ultra high risk subjects. This may be due to the fact that these patients were not acutely unwell.
Limitations
Antipsychotic treatment is a potential confound in the schizophrenia group (see Supplemental Table 6) but not the ultra high risk group. There is growing evidence to suggest an influence of antipsychotic medication on microglial cell dynamics, including evidence that antipsychotics may reduce microglial activity (36-38). Hence in future studies it would be preferable to investigate patients with schizophrenia who were medication naïve.
In this investigation, we have used an approach to analysis (accounting for endothelial and vascular binding), which has been shown to be more reliable than alternative approaches (26). This was applied in a standardized automated manner across groups, and also applied to control regions (brain stem and cerebellum) to examine the specificity for our findings. A limitation of all current approaches to imaging microglia, including with [11C]PBR28, is that the outcome measure is VT. Thus the elevation in grey matter could reflect increased non-specific tracer binding as well as biological signal. However, blocking studies have shown that a substantial proportion of VT for [11C]PBR28 is specific binding to the TSPO (39), although the proportion in schizophrenia remains to be determined.
In our sample, plasma input analysis results in a ~30% level of VT variability using the 2TCM approach. This variability is due in part to a small free fraction (fp), which has been reported to introduce 29% variability (see Supplemental Table 8 and (26)). This being the case, the noise and measurement error from fp appears to be greater than the component it represents (40) and obscures the signal difference in our cohorts. Indeed, the variability of fp in our cohorts reaches 35% (9.9± 3.5), resulting in a large VT variability.
We used the distribution volume ratio (DVR), in this case with whole brain signal as our normalization region, as our outcome measure. We also showed that the main findings remained significant when other regions were used, suggesting that the findings are robust to the method of normalization. The use of DVR analysis is a standard approach in PET imaging that has recently been applied to second generation TSPO tracers (41, 42), including using whole brain normalization (43), as well as to the first generation TSPO tracer PK11195 (44, 45). Preclinical studies have demonstrated that the DVR approach is able to detect microglial changes due to inflammatory stimuli and confirmed that elevated DVR signal corresponds to elevated levels of TSPO and other markers of microglia measured ex vivo using immunohistochemistry and/or autoradiography (46-49). These preclinical studies thus indicate the functional significance of elevated [11C]PBR28 DVR and support further in vivo investigation in patients.
Normalisation by the whole brain VT raises a conceptual issue as the whole brain VT includes the grey matter VT as one constituent. The whole brain VT also includes the VT in other tissue compartments including white matter and sub-cortical structures. Thus the elevation we see in the ultra high risk and schizophrenia groups could indicate a reduced VT in one or more of these other compartments. Further work to investigate changes in these compartments, for example using selective TSPO blockers, is required to test this interpretation.
Preclinical studies have demonstrated that the DVR approach is able to detect microglial changes due to inflammatory stimuli and confirmed that elevated DVR signal corresponds to elevated levels of TSPO and other markers of microglia measured ex vivo using immunohistochemistry and/or autoradiography (46-49). These preclinical studies thus indicate the functional significance of elevated [11C]PBR28 DVR but further in vivo investigation in patients will be required to confirm the functional significance.
The normalization approach would likely account for global group differences in non-specific binding but we cannot exclude a grey matter selective increase in non-specific binding contributing to the elevations seen. Whilst the signal-to-noise ratio of [11C]PBR28 PET imaging is better relative to first generation tracers, it remains relatively low. However this noise would obscure a difference between groups, so is unlikely to account for our findings. In this study we did not correct for possible partial volume effects. Given that brain volume is generally reduced in schizophrenia, these would tend to underestimate the elevations observed here and not account for our group differences. There is a relatively higher binding in control subjects matched to patients with schizophrenia over those matched to the ultra high risk group. This can be explained in part by age associated increases in TSPO but also by an increased number of MABs in the ultra high risk matched controls. Finally, it is important to note that not all the ultra high risk subjects will go on to develop a psychotic disorder and we will conduct clinical follow-up to determine whether the elevated microglial activity is specific to the development of the disorder or risk factors for psychosis.
Implications
Whilst TSPO may be expressed on astrocytes (50) and some neuronal sub-types (51), it is predominantly expressed on microglia (52). The direct biological relationship between microglia and TSPO binding in vivo is not fully understood. However, in non-human primates inflammation induced increases in microglial activity cause marked increases in [11C]PBR28 signal, confirmed post mortem to be largely due to microglial binding (53). Microglia perform immune surveillance roles, mount inflammatory response to injury (54) and are involved in synaptic modulation in experience dependent plasticity (55). Interpretation of elevated activity is therefore complex and not defined by ‘activated’ or ‘resting’. The elevations presented here might reflect a protective response triggered by associated pathology, such as glutamatergic excitotoxicity (56) or indicate a primary neuroinflammatory process linked to risk factors for psychosis and the development of sub-clinical symptoms. When our findings are interpreted with evidence that anti-inflammatory drugs are effective in schizophrenia (57), particularly in addressing early negative symptoms (58), they suggest a neuroinflammatory process is involved in the development of psychotic disorders. Whilst this indicates that anti-inflammatory treatment may be effective in preventing the onset of the disorder, further studies are required to determine the clinical significance of elevated microglial activity.
Conclusions
Here we provide, to our knowledge, the first evidence of elevated brain microglial activity in people at ultra high risk of psychosis, and show that greater microglial activity is associated with greater symptom severity. We also demonstrate the first in vivo elevations of TSPO binding in schizophrenia with a second generation tracer after adjusting for TSPO genotyping. These findings are consistent with increasing evidence that that there is a neuroinflammatory component in the development of psychotic disorders, raising the possibility that it plays a role in its pathogenesis.
Supplementary Material
Patient perspectives.
Patient 1 – Clinical vignette
‘Well I like the idea of taking part in research. It’s nice to try to help people find out what’s wrong.’
Patient 1 was the first patient I’d met while conducting clinical academic research. I had spoken to her care coordinator with the local mental health trust in South London and after talking on the phone, she visited the Hammersmith Hospital. We had a long chat about the research and I, with support from Dr Selvaraj, screened her for the study. Patient 1 had lived in London for her adult life and relied greatly on support from her family and the local mental health trust, she had been diagnosed with schizophrenia 13 years ago, at which point she was placed under section (involuntary admission). Patient 1 spoke of her experience of schizophrenia and her struggles to find a suitable medication, she was experiencing extrapyramidal side effects from her current regime of paliperidone, however was ‘sticking with it for now’. Following the MRI scan, she seemed glad to be out of such a confined space and responded in a more positive way to the open space of the PET scanner. I phoned patient 1 after 24 hours to check on the arterial site condition which was, ‘a little bruised, but getting better.’ We stayed in touch until honorarium reached her account, at which point she asked to be told of more research opportunities.
Acknowledgements
The study was awarded funding by King’s College London and the Medical Research Council. The authors would like to thank all the clinical imaging staff at Imanova for their help with this study. Particular thanks go to Qi Guo for her initial input and help with PET methodology.
References
- 1.Howes OD, Murray RM. Schizophrenia: an integrated sociodevelopmental-cognitive model. The Lancet. 2014;383:1677–1687. doi: 10.1016/S0140-6736(13)62036-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yung AR, Pan Yuen H, McGorry PD, Phillips LJ, Kelly D, Dell’olio M, Francey SM, Cosgrave EM, Killackey E, Stanford C, Godfrey K, Buckby J. Mapping the Onset of Psychosis: The Comprehensive Assessment of At-Risk Mental States. Australian and New Zealand Journal of Psychiatry. 2005;39:964–971. doi: 10.1080/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 3.Fusar-Poli P, Bechdolf A, Taylor MJ, Bonoldi I, Carpenter WT, Yung AR, McGuire P. At Risk for Schizophrenic or Affective Psychoses? A Meta-Analysis of DSM/ICD Diagnostic Outcomes in Individuals at High Clinical Risk. Schizophrenia Bulletin. 2013;39:923–932. doi: 10.1093/schbul/sbs060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: Meta-analysis of transition outcomes in individuals at high clinical risk. Archives of General Psychiatry. 2012;69:220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- 5.Bayer TA, Buslei R, Havas L, Falkai P. Evidence for activation of microglia in patients with psychiatric illnesses. Neuroscience Letters. 1999;271:126–128. doi: 10.1016/s0304-3940(99)00545-5. [DOI] [PubMed] [Google Scholar]
- 6.van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, Luurtsema G, Windhorst AD, Cahn W, Lammertsma AA, Kahn RS. Microglia Activation in Recent-Onset Schizophrenia: A Quantitative (R)-[11C]PK11195 Positron Emission Tomography Study. Biological Psychiatry. 2008;64:820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 7.Doorduin J, de Vries EFJ, Willemsen ATM, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in Schizophrenia-Related Psychosis: A PET Study. Journal of Nuclear Medicine. 2009;50:1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- 8.Perkins DO, Jeffries CD, Addington J, Bearden CE, Cadenhead KS, Cannon TD, Cornblatt BA, Mathalon DH, McGlashan TH, Seidman LJ, Tsuang MT, Walker EF, Woods SW, Heinssen R. Towards a Psychosis Risk Blood Diagnostic for Persons Experiencing High-Risk Symptoms: Preliminary Results From the NAPLS Project. Schizophrenia Bulletin. 2014 doi: 10.1093/schbul/sbu099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-Analysis of Cytokine Alterations in Schizophrenia: Clinical Status and Antipsychotic Effects. Biological Psychiatry. 2011;70:663–671. doi: 10.1016/j.biopsych.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cannon TD, Chung Y, He G, Sun D, Jacobson A, van Erp TGM, McEwen S, Addington J, Bearden CE, Cadenhead K, Cornblatt B, Mathalon DH, McGlashan T, Perkins D, Jeffries C, Seidman LJ, Tsuang M, Walker E, Woods SW, Heinssen R. Progressive Reduction in Cortical Thickness as Psychosis Develops: A Multisite Longitudinal Neuroimaging Study of Youth at Elevated Clinical Risk. Biological Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meisenzahl EMRD, Kirner A. Association of an interleukin-1β genetic polymorphism with altered brain structure in patients with schizophrenia. Am J Psychiatry. 2001;158:1316–1319. doi: 10.1176/appi.ajp.158.8.1316. [DOI] [PubMed] [Google Scholar]
- 12.Radewicz K, Garey LJ, Gentleman SM, Reynolds R. Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J Neuropathol Exp Neurol. 2000;59:137–150. doi: 10.1093/jnen/59.2.137. [DOI] [PubMed] [Google Scholar]
- 13.Steiner J, Mawrin C, Ziegeler A, Bielau H, Ullrich O, Bernstein H-G, Bogerts B. Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathologica. 2006;112:305–316. doi: 10.1007/s00401-006-0090-8. [DOI] [PubMed] [Google Scholar]
- 14.Karlstetter M, Nothdurfter C, Aslanidis A, Moeller K, Horn F, Scholz R, Neumann H, Weber BH, Rupprecht R, Langmann T. Translocator protein (18kDa) (TSPO) is expressed in reactive retinal microglia and modulates microglial inflammation and phagocytosis. Journal of Neuroinflammation. 2014;11:3. doi: 10.1186/1742-2094-11-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bose SK, Turkheimer FE, Howes OD, Mehta MA, Cunliffe R, Stokes PR, Grasby PM. Classification of schizophrenic patients and healthy controls using [18F] fluorodopa PET imaging. Schizophrenia Research. 2008;106:148–155. doi: 10.1016/j.schres.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Kreisl WC, Fujita M, Fujimura Y, Kimura N, Jenko KJ, Kannan P, Hong J, Morse CL, Zoghbi SS, Gladding RL, Jacobson S, Oh U, Pike VW, Innis RB. Comparison of [11C]-(R)-PK 11195 and [11C]PBR28, two radioligands for translocator protein (18 kDa) in human and monkey: Implications for positron emission tomographic imaging of this inflammation biomarker. NeuroImage. 2010;49:2924–2932. doi: 10.1016/j.neuroimage.2009.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, Rhodes C, Pulford DJ, Bennacef I, Parker CA, StJean PL, Cardon LR, Mooser VE, Matthews PM, Rabiner EA, Rubio JP. An 18-kDa Translocator Protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2011;32:1–5. doi: 10.1038/jcbfm.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fusar-Poli P, Byrne M, Badger S, Valmaggia LR, McGuire PK. Outreach and support in south London (OASIS), 2001-2011: ten years of early diagnosis and treatment for young individuals at high clinical risk for psychosis. European psychiatry: the journal of the Association of European Psychiatrists. 2013;28:315–326. doi: 10.1016/j.eurpsy.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Spitzer RL, Williams JW, Gibbon M, First MB. The structured clinical interview for dsm-iii-r (scid): I: history, rationale, and description. Archives of General Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- 20.Rao VLR, Butterworth RF. Characterization of binding sites for the ω3 receptor ligands [3H]PK11195 and [3H]RO5-4864 in human brain. European Journal of Pharmacology. 1997;340:89–99. doi: 10.1016/s0014-2999(97)01395-2. [DOI] [PubMed] [Google Scholar]
- 21.Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for Schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, Ward CH, Mendelson MM, Mock JJ, Erbaugh JJ. AN inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 23.Reilhac A, Tomeï S, Buvat I, Michel C, Keheren F, Costes N. Simulation-based evaluation of OSEM iterative reconstruction methods in dynamic brain PET studies. NeuroImage. 2008;39:359–368. doi: 10.1016/j.neuroimage.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 24.Tziortzi AC, Searle GE, Tzimopoulou S, Salinas C, Beaver JD, Jenkinson M, Laruelle M, Rabiner EA, Gunn RN. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: Dissection of D3 signal and anatomy. NeuroImage. 2011;54:264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 25.Montgomery AJ, Thielemans K, Mehta MA, Turkheimer F, Mustafovic S, Grasby PM. Correction of Head Movement on PET Studies: Comparison of Methods. Journal of Nuclear Medicine. 2006;47:1936–1944. [PubMed] [Google Scholar]
- 26.Rizzo G, Veronese M, Tonietto M, Zanotti-Fregonara P, Turkheimer FE, Bertoldo A. Kinetic modeling without accounting for the vascular component impairs the quantification of [lsqb]11C[rsqb]PBR28 brain PET data. J Cereb Blood Flow Metab. 2014 doi: 10.1038/jcbfm.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lockhart A, Davis B, Matthews JC, Rahmoune H, Hong G, Gee A, Earnshaw D, Brown J. The peripheral benzodiazepine receptor ligand PK11195 binds with high affinity to the acute phase reactant alpha1-acid glycoprotein: implications for the use of the ligand as a CNS inflammatory marker. Nucl Med Biol. 2003;30:199–206. doi: 10.1016/s0969-8051(02)00410-9. [DOI] [PubMed] [Google Scholar]
- 28.Turkheimer FE, Edison P, Pavese N, Roncaroli F, Anderson AN, Hammers A, Gerhard A, Hinz R, Tai YF, Brooks DJ. Reference and target region modeling of [11C]-(R)-PK11195 brain studies. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2007;48:158–167. [PubMed] [Google Scholar]
- 29.Yaqub M, van Berckel BN, Schuitemaker A, Hinz R, Turkheimer FE, Tomasi G, Lammertsma AA, Boellaard R. Optimization of supervised cluster analysis for extracting reference tissue input curves in (R)-[(11)C]PK11195 brain PET studies. J Cereb Blood Flow Metab. 2012;32:1600–1608. doi: 10.1038/jcbfm.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood SJ, Pantelis C, Velakoulis D, Yücel M, Fornito A, McGorry PD. Progressive Changes in the Development Toward Schizophrenia: Studies in Subjects at Increased Symptomatic Risk. Schizophrenia bulletin. 2008;34:322–329. doi: 10.1093/schbul/sbm149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shapiro S, Wilk MB. An analysis of variance test for normality (complete samples) Biometrika. 1965;52:591–611. [Google Scholar]
- 32.Dunn OJ. Multiple Comparisons among Means. Journal of the American Statistical Association. 1961;56:52–64. [Google Scholar]
- 33.Schuitemaker A, van der Doef TF, Boellaard R, van der Flier WM, Yaqub M, Windhorst AD, Barkhof F, Jonker C, Kloet RW, Lammertsma AA, Scheltens P, van Berckel BNM. Microglial activation in healthy aging. Neurobiology of Aging. 2012;33:1067–1072. doi: 10.1016/j.neurobiolaging.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Takano A, Arakawa R, Ito H, Tateno A, Takahashi H, Matsumoto R, Okubo Y, Suhara T. Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. The International Journal of Neuropsychopharmacology. 2010;13:943–950. doi: 10.1017/S1461145710000313. [DOI] [PubMed] [Google Scholar]
- 35.Kreisl WC, Jenko KJ, Hines CS, Hyoung Lyoo C, Corona W, Morse CL, Zoghbi SS, Hyde T, Kleinman JE, Pike VW, McMahon FJ, Innis RB. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J cereb blood flow metab. 2013 doi: 10.1038/jcbfm.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu FZY, Ding Y-q, Liu Y, Zhang X, et al. Minocycline and Risperidone Prevent Microglia Activation and Rescue Behavioral Deficits Induced by Neonatal Intrahippocampal Injection of Lipopolysaccharide in Rats. PLoS ONE. 2014;9(4):e93966. doi: 10.1371/journal.pone.0093966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bian Q, Kato T, Monji A, Hashioka S, Mizoguchi Y, Horikawa H, Kanba S. The effect of atypical antipsychotics, perospirone, ziprasidone and quetiapine on microglial activation induced by interferon-Î3. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2008;32:42–48. doi: 10.1016/j.pnpbp.2007.06.031. [DOI] [PubMed] [Google Scholar]
- 38.Kato T, Mizoguchi Y, Monji A, Horikawa H, Suzuki SO, Seki Y, Iwaki T, Hashioka S, Kanba S. Inhibitory effects of aripiprazole on interferon-γ-induced microglial activation via intracellular Ca2+ regulation in vitro. Journal of Neurochemistry. 2008;106:815–825. doi: 10.1111/j.1471-4159.2008.05435.x. [DOI] [PubMed] [Google Scholar]
- 39.Owen DR, Guo Q, Kalk NJ, Colasanti A, Kalogiannopoulou D, Dimber R, Lewis YL, Libri V, Barletta J, Ramada-Magalhaes J, Kamalakaran A, Nutt DJ, Passchier J, Matthews PM, Gunn RN, Rabiner EA. Determination of [C]PBR28 binding potential in vivo: a first human TSPO blocking study. J Cereb Blood Flow Metab. 2014 doi: 10.1038/jcbfm.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hines CS, Fujita M, Zoghbi SS, Kim JS, Quezado Z, Herscovitch P, Miao N, Ferraris Araneta MD, Morse C, Pike VW, Labovsky J, Innis RB. Propofol Decreases In Vivo Binding of 11C-PBR28 to Translocator Protein (18 kDa) in the Human Brain. Journal of Nuclear Medicine. 2013;54:64–69. doi: 10.2967/jnumed.112.106872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coughlin JM, Wang Y, Ma S, Yue C, Kim PK, Adams AV, Roosa HV, Gage KL, Stathis M, Rais R, Rojas C, McGlothan JL, Watkins CC, Sacktor N, Guilarte TR, Zhou Y, Sawa A, Slusher BS, Caffo B, Kassiou M, Endres CJ, Pomper MG. Regional brain distribution of translocator protein using [(11)C]DPA-713 PET in individuals infected with HIV. Journal of neurovirology. 2014;20:219–232. doi: 10.1007/s13365-014-0239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimber R, Vera Rojas J, Guo Q, Adonis A, Bishop C, Newbould R, Winston A, Gunn R, Taylor G, Rabiner E. Imaging brain TSPO availability with [11C]PBR28 PET in patients with retroviral (HTLV1 and HIV-1) infection. J NUCL MED MEETING ABSTRACTS. 2014;55:376. [Google Scholar]
- 43.Loggia ML, Chonde DB, Akeju O, Arabasz G, Catana C, Edwards RR, Hill E, Hsu S, Izquierdo-Garcia D, Ji R-R, Riley M, Wasan AD, Zürcher NR, Albrecht DS, Vangel MG, Rosen BR, Napadow V, Hooker JM. Evidence for brain glial activation in chronic pain patients. 2015 doi: 10.1093/brain/awu377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arias R, Tuisku Dickens, Anthony Rinne. In Vivo PET Imaging of Activated Microglial Cells Can Be Used to Differentiate Between Inactive and Active Chronic Lesions in Progressive Multiple Sclerosis. Neurology. 2014;82 no. 10 Supplement S44.003. [Google Scholar]
- 45.Rissanen E, Tuisku J, Rokka J, Paavilainen T, Parkkola R, Rinne JO, Airas L. In Vivo Detection of Diffuse Inflammation in Secondary Progressive Multiple Sclerosis Using PET Imaging and the Radioligand 11C-PK11195. Journal of Nuclear Medicine. 2014;55:939–944. doi: 10.2967/jnumed.113.131698. [DOI] [PubMed] [Google Scholar]
- 46.Maeda J, Zhang M-R, Okauchi T, Ji B, Ono M, Hattori S, Kumata K, Iwata N, Saido TC, Trojanowski JQ, Lee VMY, Staufenbiel M, Tomiyama T, Mori H, Fukumura T, Suhara T, Higuchi M. In vivo positron emission tomographic imaging of glial responses to amyloid-β and tau pathologies in mouse models of Alzheimer’s disease and related disorders. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2011;31:4720–4730. doi: 10.1523/JNEUROSCI.3076-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Imaizumi M, Kim H-J, Zoghbi SS, Briard E, Hong J, Musachio JL, Ruetzler C, Chuang D-M, Pike VW, Innis RB, Fujita M. PET imaging with [11C]PBR28 can localize and quantify upregulated peripheral benzodiazepine receptors associated with cerebral ischemia in rat. Neuroscience Letters. 2007;411:200–205. doi: 10.1016/j.neulet.2006.09.093. [DOI] [PubMed] [Google Scholar]
- 48.Converse AK, Larsen EC, Engle JW, Barnhart TE, Nickles RJ, Duncan ID. 11C-(R)-PK11195 PET Imaging of Microglial Activation and Response to Minocycline in Zymosan-Treated Rats. Journal of Nuclear Medicine. 2011;52:257–262. doi: 10.2967/jnumed.110.082743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martín A, Boisgard R, Thézé B, Van Camp N, Kuhnast B, Damont A, Kassiou M, Dollé F, Tavitian B. Evaluation of the PBR/TSPO radioligand [(18)F]DPA-714 in a rat model of focal cerebral ischemia. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:230–241. doi: 10.1038/jcbfm.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Martin A, Boisgard R, Theze B, Van Camp N, Kuhnast B, Damont A, Kassiou M, Dolle F, Tavitian B. Evaluation of the PBR/TSPO radioligand [lsqb]18F[rsqb]DPA-714 in a rat model of focal cerebral ischemia. J Cereb Blood Flow Metab. 2009;30:230–241. doi: 10.1038/jcbfm.2009.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Varga B, Markó K, Hádinger N, Jelitai M, Demeter K, Tihanyi K, Vas Á , Madarász E. Translocator protein (TSPO 18 kDa) is expressed by neural stem and neuronal precursor cells. Neuroscience Letters. 2009;462:257–262. doi: 10.1016/j.neulet.2009.06.051. [DOI] [PubMed] [Google Scholar]
- 52.Taylor RA, Sansing LH. Microglial Responses after Ischemic Stroke and Intracerebral Hemorrhage. Clinical and Developmental Immunology. 2013;2013:10. doi: 10.1155/2013/746068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hannestad J, Gallezot J-D, Schafbauer T, Lim K, Kloczynski T, Morris ED, Carson RE, Ding Y-S, Cosgrove KP. Endotoxin-induced systemic inflammation activates microglia: [11C]PBR28 positron emission tomography in nonhuman primates. NeuroImage. 2012;63:232–239. doi: 10.1016/j.neuroimage.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kettenmann H, Hanisch U-K, Noda M, Verkhratsky A. Physiology of Microglia. Physiological Reviews. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 55.Tremblay M-Ãv, Lowery RL, Majewska AK. Microglial Interactions with Synapses Are Modulated by Visual Experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: An update for the 21st century. Journal of Psychopharmacology. 2015;29:97–115. doi: 10.1177/0269881114563634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Müller N, Riedel M, Scheppach C, Brandstätter B, Sokullu S, Krampe K, Ulmschneider M, Engel RR, Möller HJ, Schwarz MJ. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry. 2002;159:1029–1034. doi: 10.1176/appi.ajp.159.6.1029. [DOI] [PubMed] [Google Scholar]
- 58.Chaudhry IB, Hallak J, Husain N, Minhas F, Stirling J, Richardson P, Dursun S, Dunn G, Deakin B. Minocycline benefits negative symptoms in early schizophrenia: a randomised double-blind placebo-controlled clinical trial in patients on standard treatment. Journal of psychopharmacology. 2012;26:1185–1193. doi: 10.1177/0269881112444941. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.