Abstract
Chemokines, acting on their cognate receptors on infiltrating leukocytes, drive the inflammatory response. We have been interested in determining roles and potential mechanisms for the atypical chemokine-scavenging receptor D6 in the regulation of inflammation. Here we show that a ‘psoriasis-like’ pathology that arises in inflamed skins of D6 deficient mice, is characterised by a massive and aberrant localisation of neutrophils to the dermal/epidermal junction which is associated with development of the pathology. Such misplacement of neutrophils is also seen with D6 deficient mice in other inflammatory models suggesting a role for D6 in the spatial positioning of neutrophils within inflamed sites. We further show that D6 functions cell-autonomously in this context and that D6, expressed by neutrophils, limits their migrational responses to CCR1 ligands such as CCL3. Our data therefore indicate that D6 is able to play a cell-autonomous role as a migratory ‘rheostat’ restricting migration of D6-expressing cells such as neutrophils towards ligands for co-expressed inflammatory chemokine receptors. These data have important implications for our understanding of the roles for D6 in regulating inflammation and for our understanding of the control of spatial positioning of leukocytes at inflamed sites.
Introduction
The movement of inflammatory leukocytes is controlled by members of the chemokine family(1) which is defined on the basis of chemotactic activity and the presence of a conserved cysteine motif in all members of the family. The family can be divided into four subfamilies (CC, CXC, XC, CX3C) according to the specific nature of the cysteine motif. There are almost 50 mammalian chemokines identified and their biology is therefore complex. However, it is possible to impose a relatively simple two-fold functional subdivision and to define chemokines as being either homeostatic, or inflammatory, according to the contexts in which they function(2, 3). Thus homeostatic chemokines regulate basal leukocyte trafficking whilst inflammatory chemokines regulate migration of leukocytes to inflamed or damaged sites. Chemokines interact with their target cells through seven-transmembrane spanning receptors(4) which again can be defined as being either homeostatic or inflammatory. In addition to the classical signalling chemokine receptors, there exists a small subfamily of atypical receptors characterised by promiscuity of ligand binding and an apparent inability to signal following ligand binding(5-7). This family includes the Duffy Antigen Receptor for Chemokines (DARC), CCXCKR, CXCR7 and D6(8, 9), which is the focus of this study.
D6 is a promiscuous receptor for at least 12 CC-chemokines and has a binding preference for CC-chemokines that are involved in inflammatory responses(10-12). D6 does not bind CC-chemokines involved in homeostatic cellular migration or chemokines from the other subfamilies, and does not appear to signal following ligand binding. D6 is expressed predominantly in barrier tissues such as the skin, gut, lung and placenta and we, and others, have shown expression of D6 on lymphatic endothelial cells(13-15) and syncitiotrophoblasts(16, 17) at these sites. In addition we have detected expression of D6 on leukocytes(18, 19). Notably, although D6 does not signal following ligand binding, it is not inert and actively internalises ligands and targets them for intracellular degradation(11, 20). This has led us, and others, to propose a role for D6 as a ‘scavenging’ receptor for inflammatory CC-chemokines. Numerous in vivo studies using D6-deficient mice have confirmed this scavenging role and have demonstrated an essential role, in vivo, for D6 in the resolution phase of cutaneous(21, 22), intestinal(23) and pulmonary(24) inflammatory responses and for the effective maintenance of pregnancy under systemic inflammatory conditions(16). Importantly D6 has also been shown to play roles in a range of human inflammatory pathologies including those of cutaneous(25, 26), respiratory(27) and cardiovascular(28) origin. In addition, the enhanced inflammatory response in the D6-deficient mice is associated with an exaggerated tumorigenic programme in murine models of skin(29) and intestinal(23) carcinogenesis. Finally D6-deficient mice display an exaggerated systemic inflammatory response to infection by Mycobacterium tuberculosis(30).
Our initial in vivo study(21) demonstrated that D6-deficient mice, in contrast to wild type (WT) mice, were incapable of efficiently removing inflammatory CC-chemokines from TPA-treated skin and the ensuing exaggerated inflammation precipitated the development of a ‘psoriasis-like’ pathology. The purpose of the present study was to attempt to understand, in more detail, the involvement of D6 dysfunction in the emergence of this cutaneous pathology in the D6-deficient mice. Here we present evidence supporting a novel role for D6 in the cell-autonomous regulation of neutrophil migration during inflammatory responses. In the absence of D6, neutrophils displayed exaggerated migratory responses, in vitro and in vivo, to inflammatory CC-chemokines. This resulted in the inappropriate localization of neutrophils at the dermal/epidermal boundary and was associated with destruction of the dermal/epidermal barrier and epidermal shedding. Overall, our results suggest a novel role for D6 as a ‘migratory rheostat’ limiting inappropriate intra-tissue movement of neutrophils during inflammatory responses. These results therefore shed new light on the range of roles played by D6 in the regulation of the inflammatory response.
Materials and Methods
Mice and in vivo treatments
WT and D6-deficient mice were maintained as described(21) and all animal experimentation was approved by our local ethical review committee and licensed by the United Kingdom Government Home Office. Cutaneous inflammation was initiated by application of either a single application, or 3 applications at 24 hour intervals, of 150μl of a 50μM TPA (12-O-tetradecanoylphorbol-13-acetate: Sigma, Poole, UK) solution in acetone to shaved dorsal skin(21). The CCR1 blocker, BX471(31), was administered orally (200μl of a 25mg/ml solution in 0.5% hydroxyethyl cellulose) 3 times per day for the duration of the mouse treatment. Vehicle control animals received 200μl of 0.5% hydroxyethyl cellulose. For the anti-CXCL2 experiments, 250μg of antibody (R&D Systems Inc, Minneapolis, USA) or isotype control (rat IgG2B) in PBS was injected intra peritoneally (i.p.), daily, throughout the study. Neutrophil depletion was performed by daily i.p. administration of 100μg/mouse of the RB6-8C5 anti-Gr-1 or isotype control (rat IgG2b) antibody. Compound 48/80 (20μl of a 10mg/ml solution; Sigma, Poole, UK) was injected intradermally on 2 sites either side of the dorsal midline of the mice.
Tissue processing and imaging
Following killing, the skins were removed from the mice and processed for routine H&E, or myeloperoxidase, staining as described(21). Caspase-3 staining was performed using an anti-Caspase 3 antibody from Santa Cruz Biotechnology (Heidelberg, Germany). Images were captured on a Zeiss Axiostar Plus microscope with Axiovision software (Carl Zeiss Inc, Welwyn Garden City, UK). CCL3 in skin sections was visualised using anti-CCL3 antibodies from R&D Systems (Minneapolis, USA). Skin thicknesses were measured at multiple sites per section using an eyepiece graticule. Analysis of neutrophil numbers was carried out by assessing the number of neutrophils in 10 random 12.5μm2 field per section. The relative proximity of neutrophil deposits to the dermal/epidermal junction was assessed by measuring the average distance between the base of the epidermal layer and the centre of the neutrophil influx. Again this analysis was performed using an eyepiece graticule.
Neutrophil isolation and labelling and in vivo transfer
Neutrophils were obtained from murine bone marrow in a laminar flow hood and isolated (c. 96% purity) by positive selection using a Ly6G Isolation kit (Miltenyi Biotec, Surrey, UK). For CFSE and TAMRA labelling, neutrophils were resuspended in prewarmed PBS/0.1% BSA at a concentration of 1×106 cells/ml. CFSE (Invitrogen, Paisley, UK) was added to give a final concentration of 10μM, and incubated at 37°C for 10 mins and TAMRA (Invitrogen, Paisley, UK) was added to give a final concentration of 5μM, and incubated at 37°C for 20 mins. Staining was quenched by adding 5 volumes of ice cold PBS and incubating on ice for 5 mins. Cells were washed 3 times in ice cold culture media, before re-suspending at the appropriate concentration for adoptive transfer. Neutrophils were transferred to mice in which the skin had been inflamed with TPA 11 hours earlier. Tissue was harvested from these mice six hours later and processed for imaging of labelled neutrophils.
Confocal Analysis of labelled neutrophil entry into inflamed skin
5mm-punch biopsies were taken from treated mice and snap frozen in liquid nitrogen. 4μm sections were cut on a cryostat and mounted with Vectashield plus DAPI (Vector Labs, Peterborough, UK). Images were taken using the LSM510 META confocal laser scanning microscope (Carl Zeiss Inc, Welwyn Graden City, UK), at 20× magnification for the whole length of the skin section. The distance from the epidermis to the position of each labelled cell was measured in micrometres, using AxioVision Rel. 4.6 software (Carl Zeiss Inc, Welwyn G will raden City, UK).
Neutrophil extravasation induced by i.p. injection of CCL3
CCL3 (1μg/mouse and 5μg/mouse; R&D Systems Inc., Minneapolis, USA) was injected into the peritoneal cavities of D6-deficient and WT mice (n=3). PBS was used as a negative control. After 4 hours, mice were sacrificed, peritoneal cavities lavaged and the number of neutrophils determined from absolute and differential cell counts.
Boyden type chemotaxis assay
Blood was drawn from WT and D6-deficient mice into heparinized tubes and peripheral blood neutrophils isolated using Polymorphoprep (Axis-Shield PoC AS, Oslo, Norway) according to the manufacturer’s instructions. Migration of the neutrophils to CCL2, CCL3, CCL4, CCL5 and CXCL8 was measured after incubation for 30min at 37°C in a Boyden type chamber using a 5μm PVPF Filter.
Statistics
For 2-way comparisons, Student’s ‘t’ test was used and for more complex comparisons, one-way ANOVA with Tukey’s multiple comparison test were used. All statistical analyses were carried out using GraphPad Prism software.
Results
Dermal/Epidermal disruption is associated with exaggerated inflammation in D6-deficient mice
Whilst there are no differences in the resting skin of WT and D6-deficient mice(21), we have previously shown exaggerated cutaneous inflammation in D6-deficient mice(21) in response to 3 applications of TPA. This treatment lead to the development of a ‘psoriasis like’ pathology in the D6-deficient mice which was not seen in similarly treated WT mice (Figure 1A). To investigate the cellular basis for the early development of this pathology we examined the skins of WT and D6-deficient mice 24 hours after 1, 2 and 3 daily applications of TPA. As shown in Figure 1B, repeated application of TPA to WT skin induced a modest but progressive increase in dermal and epidermal thickness, and inflammation, within the skin. In contrast, even after a single application of TPA, a marked accumulation of leukocytes was seen at the dermal/epidermal junction in the D6-deficient mice. After the 2nd and 3rd TPA application to D6-deficient mice these leukocytes appeared to be associated with disruption of the dermal/epidermal junction and eventual shedding of the epidermis. Thus enhanced inflammation seen in D6 deficient mouse skin is associated with leukocyte-mediated destruction of the dermal/epidermal junction.
Figure 1.
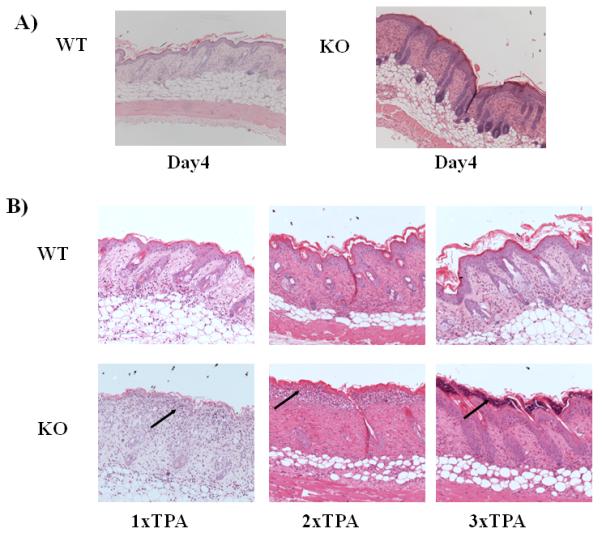
the Psoriasis-like phenotype in D6-deficient mouse skin is associated with leukocyte-mediated destruction of the dermal/epidermal junction
A) H&E staining of skin from WT and D6-deficient (KO) mice 4 days after they have received 3 daily applications of TPA. Note the markedly thickened and inflamed skin in the KO section.
B) H&E staining of skin sections from WT and D6-deficient (KO) mice at 24 hours after 1, 2 and 3 TPA applications (leukocyte accumulations are arrowed).
Misplaced neutrophils are associated with the dermal/epidermal disruption
The majority of the cells at the dermal/epidermal junction displayed the characteristic morphology of neutrophils (Figure 2A). Their nature was confirmed by positive staining for myeloperoxidase (MPO) which further demonstrated neutrophil accumulation to be restricted predominantly to positions lower in the dermal compartment in WT mice but to the dermal/epidemal junction in D6-deficient mice (Figure 2B). Notably, treatment of the D6-deficient mice with the neutrophil-depleting antibody Gr1 resulted in significant attenuation of the development of the ‘psoriasis-like’ pathology as assessed quantitatively by measurement of increased skin thickness (Figure 2Ci). In addition use of the anti-Gr1 antibody was associated with a marked decrease in neutrophil numbers in the blood (data not shown) as well as dramatically impaired neutrophil recruitment to the skin thus confirming the success of the neutrophil depletion (Figure 2Cii). Overall, these data demonstrate that the ‘psoriasis-like’ pathology that develops in D6-deficient mice following TPA application is associated with, and dependent on, an aberrant accumulation of neutrophils at the dermal/epidermal junction.
Figure 2.
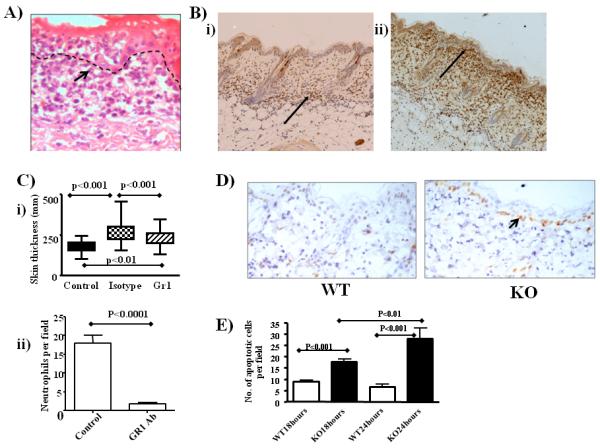
Neutrophils are the key cell type accumulating at the dermal/epidermal junction
A) High-magnification image showing the neutrophilic morphology of cells (arrowed) accumulating at the dermal/epidermal junction.
B) Myeloperoxidase staining revealing the neutrophilic nature (arrowed) of the misplaced leukocytes in D6-deficient (ii) compared to WT (i) mouse skin.
C) i) Extent of inflammation as reflected in skin thickness measurements in mice with, or without, Gr1-mediated in vivo neutrophil depletion. Control values refer to those obtained from untreated D6-deficient mouse skins, isotype values are from mice treated with the appropriate isotype control (rat IgG2b) and Gr1 values are from mice treated with the Gr1 antibody. Overall significance using ANOVA is p<0.0001 and the specific significance of each comparison is noted on the figure. ii) Quantitation of the number of neutrophils in isotype control and Gr1-treated D6 deficient mouse skins. Neutrophils were measured as numbers per 12.5μm2 field with 10 fields measured per mouse skin and 5 mice per group.
D) Skin sections were collected 24 hours after a single application of TPA and apoptosis was visualised by staining for Caspase 3. Large numbers of apoptotic basal-layer keratinocytes are seen in the inflamed D6-deficient (KO) mouse skin (arrowed).
E) Quantitation of the numbers of apoptotic (Caspase 3+ve) cells in WT and KO epidermis at 18 and 24 hours after TPA application. Counts are means +/− S.D. from 10 sections with 20 counts per random field per section. For this comparison, ANOVA demonstrated overall significance of p<0.0001 and the inter-group significance levels are noted on the figure.
Closer analysis of the dermal/epidermal junction at the site of neutrophil accumulation revealed a disruption of the basement membrane (not shown) in the TPA treated D6-deficient mice. This was associated with an early increase in keratinocyte apoptosis which was detectable, using ‘activated-caspase-3’ staining, as early as 18 hours after the first application of TPA (Figure 2D). Quantitation of caspase 3 staining showed that the extent of apoptosis in the basal keratinocyte layer increased between 18 and 24 hours, a time frame that is associated with the disruption of the dermal/epidermal junction (Figure 2E). Importantly, similar levels of apoptosis were not seen in WT mouse skin following TPA application (Figures 2D and E). The positioning of the caspase 3 positive cells specifically along the basal layer of keratinocytes, and not deeper within the dermis, strongly suggest that they are keratinocytes and not neutrophils. Thus the aberrant accumulation of neutrophils at the dermal/epidermal junction in D6-deficient mice is associated with induction of apoptosis amongst basal keratinocytes, destruction of the dermal epidermal boundary and ultimately epidermal shedding (Figure 1B).
The accumulation of neutrophils at the dermal/epidermal junction is dependent on CCR1
We next determined which chemokine receptors were responsible for this aberrant positioning of neutrophils in the inflamed skins from D6-deficient mice. Overall, as assessed by skin thickness measurements, the ‘psoriasis-like’ pathology is significantly dependent on the neutrophil attracting chemokine CXCL2 (Figure 3A) but CXCL2 is not a ligand for D6 suggesting that whilst CXCL2 may regulate neutrophil entry into inflamed skin, other chemokines and their receptors may separately regulate the spatial positioning of neutrophils within the skin. Therefore we used PCR to investigate expression of other chemokine receptors in WT murine neutrophils (Figure 3B). This analysis revealed expression of the major neutrophil chemokine receptor, CXCR2, in neutrophils and, in accord with other reports, also showed expression of CCR1(32, 33). Interestingly, and in agreement with previous findings(18), the WT mouse derived neutrophils were also positive for D6. Therefore, to investigate roles for CCR1 ligands in the aberrant positioning of the neutrophils in D6-deficient mice we examined the neutrophil positioning in inflamed skins of D6-deficient mice treated with a well-characterised antagonist of CCR1(31). As shown in Figure 3C, administration of this CCR1 antagonist effectively reversed the aberrant accumulation of neutrophils in D6-deficient mouse skins in response to TPA challenge. This was quantified by measuring the distance between the base of the epidermis and the centre of the neutrophil deposits. This analysis demonstrated that the antagonist was associated with a significant reduction in neutrophil accumulation at the dermal/epidermal border (Figure 3Di). In addition the reduction in neutrophil accumulation at this site was associated with a related decrease in skin thickness (a useful surrogate measure of inflammation) confirming a role for neutrophil positioning in the exaggerated skin inflammation in D6-deficient mice (Figure 3Dii). Importantly, there was no significant impact of the CCR1 antagonist on neutrophil accumulation (Figure 3Diii) suggesting that the antagonist alters neutrophil positioning but not recruitment to the inflamed skin. Next we investigated the reasons underlying the specific CCR1-dependent accumulation of the D6-deficient neutrophils at the dermal/epidermal boundary. Using immunohistochemistry, we showed that, following TPA application, the major site of production of CCR1-binding inflammatory CC-chemokines such as CCL3 (Figure 3E) is the epidermal compartment. Thus, these data suggest that, in D6-deficient mice, CCR1 is important for the directed, migration of neutrophils through the dermis towards inflammatory CC-chemokines produced within the epidermis.
Figure 3.
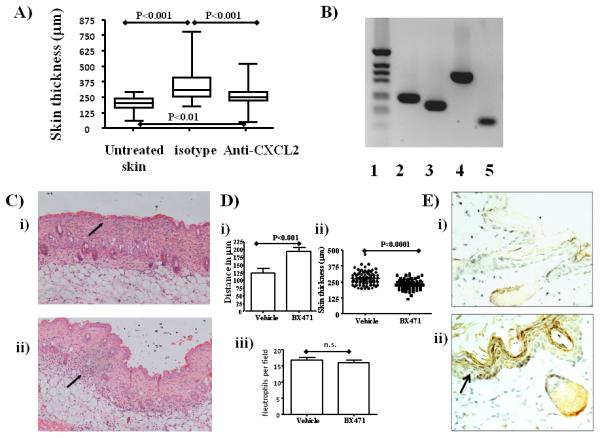
The aberrant neutrophil positioning is CCR1-dependent
A) D6-deficient mice were either left untreated or were treated 3 times with TPA and left for 4 days for the psoriatic phenotype to develop as described. TPA-treated D6-deficient mice were either administered antibodies to CXCL2 or isotype control antibodies. Skin thickness as a measure of inflammation was assessed in 10 sections per point with 20 measurements per section. ANOVA indicated p<0.0001 for the complete data set and inter-group significance levels are noted on the figure.
B) PCR analysis of expression of 2) CCR1 3) CXCR2 4) D6 and 5) actin in neutrophils isolated by positive selection. 1) is the molecular weight marker track.
C) The effects of the CCR1 blocker BX471 on the positioning of neutrophils in the inflamed D6-defient mouse skin. Mice were treated i.p. with either i) vehicle or ii) BX471 and then TPA was applied to the skin. Skins were collected 24 hours later and H&E stained (neutrophils are arrowed).
D) Quantitation of the effects of CCR1 blockade on i) neutrophil positioning. These results report the average distances from the bottom of the epidermis to the centre of the neutrophil deposits in the skins of the vehicle, or BX471, treated D6-deficient mice. ii) Skin thickness as an index of inflammation; iii) number of neutrophils per 12.5μm2 field with 10 fields measured per mouse skin and 5 mice per group.
E) Immunostaining for CCL3 expression reveals the epidermis to be the major site of expression following TPA-induced inflammation. D6-deficient mice were topically treated with acetone (i), or with TPA (ii), for 24 hours, then skins were collected and stained for CCL3 expression.
Aberrant neutrophil accumulation is also seen in TPA-independent models of inflammation in D6-deficient mice
To examine roles for D6 in regulating neutrophil positioning in other models of D6-deficient cutaneous inflammatory responses we intra-dermally injected, into resting WT and D6-deficient mice, compound 48/80 which induces mast cell degranulation. Strikingly, this compound was associated with severe tissue damage at the site of injection in D6-deficient mice but a lesser response was seen in WT mice (Figure 4A). Examination of the cells accumulating at the site of tissue damage again revealed them to be predominantly neutrophilic in nature (data not shown) suggesting that the inflammation associated with mast cell degranulation leads to recruitment of neutrophils which are again aberrantly positioned in D6-deficient mice leading to extensive damage at the dermal/epidermal junction of the treated skin. In contrast to the TPA induced inflammation model, the tissue damage associated with compound 48/80 was so severe that objective measurement of neutrophil numbers or position was not possible. Nevertheless such severe tissue damage was seen in all compound 48/80 treated D6-deficient, but not WT, mice. Thus, the contribution of aberrantly positioned neutrophils to tissue damage is not restricted to the TPA painting model of skin inflammation.
Figure 4.
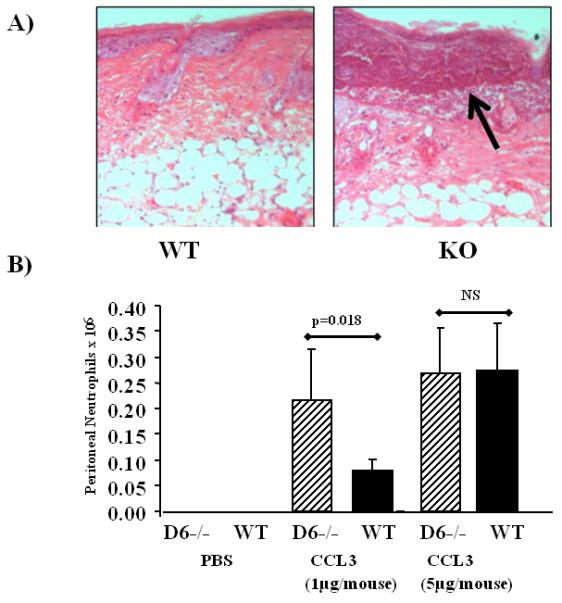
Aberrant neutrophil accumulation is also seen in other inflammatory models
A) Mice were administered compound 48/80 (20μl of 10mg/ml) intradermally and the mice killed and skins collected 24 hours later. Skin sections were stained with H&E and the neutrophil associated damage is arrowed.
B) Mice were injected i.p. with CCL3 at the indicated concentrations and recruited neutrophils counted 24 hours later.
Next we examined neutrophil accumulation following intraperitoneal injection of the D6 ligand CCL3 in WT and D6-deficient mice. As shown in Figure 4B, administration of 1μg of CCL3 induced a significantly higher accumulation of neutrophils in the peritoneal cavity of D6-deficient mice than was seen in WT mice. Interestingly, higher levels of CCL3 induced similar levels of neutrophil accumulation in WT and D6-deficient mouse peritoneum suggesting that the negative impact of D6 on neutrophil accumulation in WT mice can be overcome under conditions of high chemokine exposure. Thus, together, these data show that the limiting effects of D6 on neutrophil accumulation are not restricted to the TPA skin-painting model and can be overcome under conditions of exaggerated chemokine exposure.
D6 displays a cell-autonomous ability to regulate neutrophil migration and positioning in vivo
Based on the above observations there are two possible explanations for the aberrant positioning of neutrophils in D6-deficient mice. Firstly, the inability to effectively clear chemokines in D6-deficient mice(14, 15) may leave excess chemokines in the vicinity of the dermal/epidermal junction that may chemoattract neutrophils via CCR1. Alternatively, as we have shown D6 expression in neutrophils (Figure 3B), it is possible, as also suggested by the in vitro studies of Bonecchi et al(10), that D6 regulates leukocyte migration towards inflammatory CC-chemokines on a cell-autonomous basis by interfering with CCR1 function. To test whether the aberrant localization of neutrophils related to hemopoietic or non-hemopoietic D6 expression, we generated radiation chimeras. As shown in figure 5A, D6-deficient mice, in receipt of WT bone marrow (i), did not display accumulation of neutrophils at the dermal/epidermal junction. In contrast, WT mice receiving D6-deficient bone marrow did (ii). As a control we also generated chimeras in which D6-deficient mice received D6-deficient bone marrow and in these mice there was also mislocalization of neutrophils at the dermal/epidermal junction (iii). Neurophil mislocalisation was quantified by measuring the distance from the base of the epidermis to the centre of the neutrophil infiltrate. As shown in Figure 5B this analysis demonstrated a significantly enhanced ability of D6-deficient neutrophils to migrate to the dermal/epidermal boundary (reduced distance measure in Figure 5B) compared to WT neutrophils (increased distance measure in Figure 5B). These data therefore show that D6 regulation of neutrophil positioning in inflamed skin is dependent on hematopoietic expression of D6 and, along with the data in Figure 3B, suggests that D6 may regulate neutrophil migration in a cell-autonomous manner. To investigate this directly, we examined the response of WT and D6-deficient neutrophils to a range of chemokine ligands in Boyden chamber assays. As shown in Figure 6A, there were no differences in the migration of WT or D6-deficient neutrophils towards the CXCR2 ligand CXCL8 or to the CCR5-specific ligand CCL4. However there was a marked difference in the ability of WT and D6-deficient neutrophils to migrate towards the CCR1 and CCR5 interacting ligand CCL3 with D6-deficient neutrophils displaying a significantly more marked migration towards this chemokine. Unusually we only managed to demonstrate a weak migratory responses of the neutrophils to another CCR1 ligand, CCL5, and at present we have no explanation for this observation. As shown in the stained filter from a Boyden chamber assay (Figure 6B), the enhanced migration of D6 deficient neutrophils is seen over a range of concentrations of CCL3. These data suggest that D6 functions, in a cell-autonomous manner, to limit migration of neutrophils towards CCR1 ligands and that the absence of D6 in D6-deficient mice allows unrestricted movement of the neutrophils specifically towards sources of CCL3.
Figure 5.
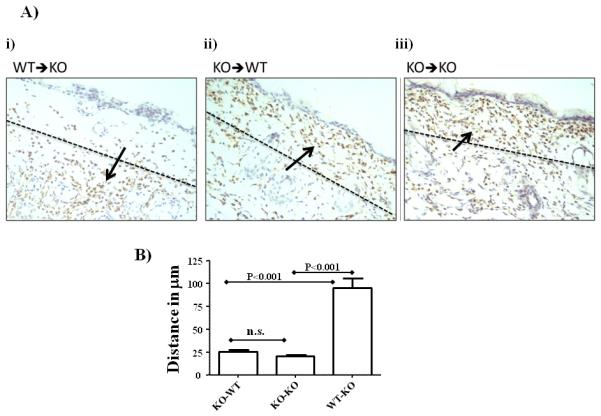
D6 exerts a cell-autonomous effect on neutrophil migration
A) Histological analysis of neutrophil migration in radiation chimeric mice. Neutrophil accumulation is detected by staining for myeloperoxidase and marked by the arrows. i) WT donor and D6-deficient (KO) recipient mice; ii) D6-deficient donors and WT recipient mice; iii) D6-deficient recipient and D6-deficient donor mice.
B) Quantitation of the distance from the base of the epidermal layer to the centre of the neutrophil influx. For each experimental group, 10 separate measurements for each skin sample were made from 5 separate mice.
Figure 6.
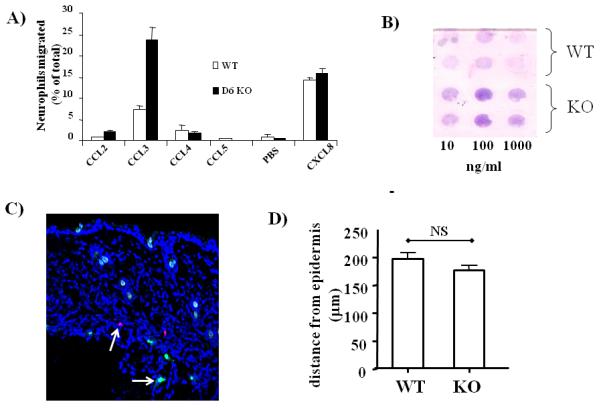
D6-deficient neutrophils display enhanced responses to CCL3
A) D6-deficient (KO) neutrophils display an exaggerated migration towards CCL3 in Boyden chamber assays.
B) Stained filter from a Boyden Chamber assay showing differential responses of D6-deficient and WT neutrophils to a range of CCL3 concentrations.
C) Confocal imaging of frozen skin sections from mice to which WT (CMTMR-labelled) and KO (CFSE-labelled) neutrophils (indicated by arrows) had been transferred and then skins inflamed by TPA treatment.
D) Quantitation of the distance of neutrophil entry from the epidermis assessed by measuring the distance from the base of the epidermal layer to the point of neutrophil entry.
To formally rule out the possibility that the accumulation of neutrophils in D6-deficient mice relates to entry of the D6-deficient neutrophils at higher positions within the dermis than WT neutrophils, we separately dye-labelled WT and D6-deficient neutrophils and adoptively transferred them into D6-deficient mice which had been treated with TPA. As shown in Figure 6C, confocal imaging revealed similar spatial positioning of the WT and D6-deficient neutrophils soon after transfer. Quantification revealed (Figure 6D) that there were no significant differences in the levels of entry (i.e. distance from the base of the epidermis) of WT and D6-deficient neutrophils in the dermis of the D6-deficient mice. It is important to note that whilst this will the preceding data would suggest that the D6-deficient neutrophils in this experiment might have been expected to have migrated higher within the dermis than their WT counterparts, these cells are most likely at the end of their lifespan given the time taken to harvest them and to perform this homing assay. We suggest, therefore, that by the time that the neutrophils enter the skin they are likely to be unresponsive to further chemokine stimulation. However, we cannot formally exclude the possibility that in this experimental context, a contribution of D6 expression on other haematopoietic cells may have had an unanticipated effect on the intra-cutaneous movement of the D6-deficient neutrophils.
Thus, together the majority of our data point to a cell-autonomous role for D6 in regulating neutrophil movement and in restricting the accumulation of neutrophils at the dermal/epidermal junction in WT mice. In contrast, the D6-deficient neutrophils do not have this restriction and so migrate more freely towards the epidermally-expressed chemokines.
Discussion
The chemokine scavenging receptor, D6, has been shown to be an important regulator of the in vivo inflammatory response(8). We have now analysed the early stages of the involvement of D6 in the generation of the ‘psoriasis-like’ pathology seen in the skins of D6-deficient mice following TPA treatment. Our data indicate that, in addition to scavenging chemokines within the inflamed skins, D6 is also involved in controlling the spatial positioning of neutrophils within inflamed sites. As we have previously shown mast cells to also be misplaced towards the dermal/epidermal junction in the D6-deficient mice(21), this role for D6 may not be restricted to neutrophils. The importance of regulating the spatial distribution of cells such as neutrophils and mast cells within tissues that represent barriers between the organism and the outside world is apparent from the extensive damage that such cells can cause. Indeed a number of pathologies result from tissue destruction arising as a consequence of aberrantly accumulating neutrophils at a range of distinct anatomical sites(34, 35).
The mechanism behind this aberrant accumulation of neutrophils depends on expression of both D6 and CCR1 by neutrophils. Numerous previous studies have reported expression of CCR1 by both murine(33, 36) and human(36-38) neutrophils and the present results would indicate that this is an important regulator of positioning within inflamed tissues. One question that emerges from this is, why neutrophils co-express CCR1 and D6 if D6 blocks CCR1 function. Why not simply transcriptionally silence CCR1? Interestingly, we have noticed that the restricted motility of neutrophils co-expressing D6 and CCR1 can be overcome by exposure to high levels of chemokines (e.g. Figure 4B). We therefore propose that D6 is not a full suppresser of CCR1 responses in neutrophils, but that it serves as a ‘rheostat’ controlling the movement of neutrophils towards inflammatory events at the dermal/epidermal interface. Under conditions of extreme tissue damage in which shedding of the damaged epidermis may be advantageous (e.g. in the context of significant epidermal infection), the rheostat function of D6 is overcome allowing neutrophils (and potentially mast cells) to move to the dermal/epidermal interface and to induce shedding of the damaged epidermis and subsequent tissue regeneration.
Our present data, and our proposal for a role for D6 as a ‘migratory rheostat’, are in keeping with the in vitro data reported by Bonnechi and colleagues(10) demonstrating the ability of D6 to dominantly suppress migration of cells to ligands for co-expressed CCR4. Thus the cell-autonomous effects of D6 are unlikely to be specific for CCR1 and are likely to be seen with any of the co-expressed receptors for inflammatory CC-chemokines.
Therefore, in summary, our data indicate a role for the atypical chemokine receptor, D6, in the regulation of cellular migration in vivo. Accordingly, these data broaden our understanding of the in vivo roles for D6 and suggest that, as well as being a scavenger of inflammatory CC-chemokines, D6 may also act as a cell-autonomous ‘migratory rheostat’ for leukocytes. The primary aim of this may be to protect crucial barrier tissues from the unnecessary toxic effects of neutrophil and mast cell contents.
Acknowledgements
The help of Lisa Thorpe with the Boyden chamber assays is gratefully acknowledged
This work was supported by grants from The MRC, Cancer Research UK and the BBSRC to GJG and from INNOCHEM to GJG and AR.
References
- 1.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A. The chemokine system: redundancy for robust outputs. Immunol Today. 1999;20:254–257. doi: 10.1016/s0167-5699(99)01469-3. [DOI] [PubMed] [Google Scholar]
- 3.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 4.Murphy PM, Baggiolini M, Charo IF, Hebert CA, Horuk R, Matsushima K, Miller LH, Oppenheim JJ, Power CA. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–176. [PubMed] [Google Scholar]
- 5.Nibbs R, Graham G, Rot A. Chemokines on the move: control by the chemokine “interceptors” Duffy blood group antigen and D6. Semin Immunol. 2003;15:287–294. doi: 10.1016/j.smim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Mantovani A, Bonecchi R, Locati M. Tuning inflammation and immunity by chemokine sequestration: decoys and more. Nat Rev Immunol. 2006;6:907–918. doi: 10.1038/nri1964. [DOI] [PubMed] [Google Scholar]
- 7.Graham GJ, Locati M. Regulation of the immune and inflammatory responses by the ‘atypical’ chemokine receptor D6. Journal of Pathology. 2013;229:168–175. doi: 10.1002/path.4123. [DOI] [PubMed] [Google Scholar]
- 8.Graham GJ. D6 and the atypical chemokine receptor family: novel regulators of immune and inflammatory processes. Eur J Immunol. 2009;39:342–351. doi: 10.1002/eji.200838858. [DOI] [PubMed] [Google Scholar]
- 9.Ulvmar MH, Hub E, Rot A. Atypical chemokine receptors. Experimental cell research. 317:556–568. doi: 10.1016/j.yexcr.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonecchi R, Locati M, Galliera E, Vulcano M, Sironi M, Fra AM, Gobbi M, Vecchi A, Sozzani S, Haribabu B, Van Damme J, Mantovani A. Differential recognition and scavenging of native and truncated macrophage-derived chemokine (macrophage-derived chemokine/CC chemokine ligand 22) by the D6 decoy receptor. J Immunol. 2004;172:4972–4976. doi: 10.4049/jimmunol.172.8.4972. [DOI] [PubMed] [Google Scholar]
- 11.Fra AM, Locati M, Otero K, Sironi M, Signorelli P, Massardi ML, Gobbi M, Vecchi A, Sozzani S, Mantovani A. Cutting edge: scavenging of inflammatory CC chemokines by the promiscuous putatively silent chemokine receptor D6. J Immunol. 2003;170:2279–2282. doi: 10.4049/jimmunol.170.5.2279. [DOI] [PubMed] [Google Scholar]
- 12.Nibbs RJ, Wylie SM, Yang J, Landau NR, Graham GJ. Cloning and characterization of a novel promiscuous human beta-chemokine receptor D6. J Biol Chem. 1997;272:32078–32083. doi: 10.1074/jbc.272.51.32078. [DOI] [PubMed] [Google Scholar]
- 13.Nibbs RJ, Kriehuber E, Ponath PD, Parent D, Qin S, Campbell JD, Henderson A, Kerjaschki D, Maurer D, Graham GJ, Rot A. The beta-chemokine receptor D6 is expressed by lymphatic endothelium and a subset of vascular tumors. Am J Pathol. 2001;158:867–877. doi: 10.1016/s0002-9440(10)64035-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee KM, McKimmie CS, Gilchrist DS, Pallas KJ, Nibbs RJ, Garside P, McDonald V, Jenkins C, Ransohoff R, Liu L, Milling S, Cerovic V, Graham GJ. D6 facilitates cellular migration and fluid flow to lymph nodes by suppressing lymphatic congestion. Blood. 2011;118:6220–6229. doi: 10.1182/blood-2011-03-344044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee KM, Nibbs RJB, Graham GJ. D6: the ‘crowd controller’ at the immune gateway. Trends in Immunology. 2013;34:7–12. doi: 10.1016/j.it.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 16.Martinez de la Torre Y, Buracchi C, Borroni EM, Dupor J, Bonecchi R, Nebuloni M, Pasqualini F, Doni A, Lauri E, Agostinis C, Bulla R, Cook DN, Haribabu B, Meroni P, Rukavina D, Vago L, Tedesco F, Vecchi A, Lira SA, Locati M, Mantovani A. Protection against inflammation- and autoantibody-caused fetal loss by the chemokine decoy receptor D6. Proc Natl Acad Sci U S A. 2007;104:2319–2324. doi: 10.1073/pnas.0607514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madigan J, Freeman DJ, Menzies F, Forrow S, Nelson SM, Young A, Sharkey A, Moffet A, Graham GJ, Greer IA, Rot A, Nibbs RJB. Chemokine Scavenger D6 Is Expressed by Trophoblasts and Aids the Survival of Mouse Embryos Transferred into Allogeneic Recipients. Journal of Immunology. 2010;184:3202–3212. doi: 10.4049/jimmunol.0902118. [DOI] [PubMed] [Google Scholar]
- 18.McKimmie CS, Fraser AR, Hansell C, Gutierrez L, Philipsen S, Connell L, Rot A, Kurowska-Stolarska M, Carreno P, Pruenster M, Chu CC, Lombardi G, Halsey C, McInnes IB, Liew FY, Nibbs RJ, Graham GJ. Hemopoietic cell expression of the chemokine decoy receptor D6 is dynamic and regulated by GATA1. J Immunol. 2008;181:3353–3363. doi: 10.4049/jimmunol.181.5.3353. [DOI] [PubMed] [Google Scholar]
- 19.Hansell CAH, Schiering C, Kinstrie R, Ford L, Bordon Y, McInnes IB, Goodyear CS, Nibbs RJB. Universal expression and dual function of the atypical chemokine receptor D6 on innate-like B cells in mice. Blood. 2011;117:5413–5424. doi: 10.1182/blood-2010-11-317115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber M, Blair E, Simpson CV, O’Hara M, Blackburn PE, Rot A, Graham GJ, Nibbs RJ. The chemokine receptor D6 constitutively traffics to and from the cell surface to internalize and degrade chemokines. Mol Biol Cell. 2004;15:2492–2508. doi: 10.1091/mbc.E03-09-0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jamieson T, Cook DN, Nibbs RJ, Rot A, Nixon C, McLean P, Alcami A, Lira SA, Wiekowski M, Graham GJ. The chemokine receptor D6 limits the inflammatory response in vivo. Nat Immunol. 2005;6:403–411. doi: 10.1038/ni1182. [DOI] [PubMed] [Google Scholar]
- 22.Martinez de la Torre Y, Locati M, Buracchi C, Dupor J, Cook DN, Bonecchi R, Nebuloni M, Rukavina D, Vago L, Vecchi A, Lira SA, Mantovani A. Increased inflammation in mice deficient for the chemokine decoy receptor D6. Eur J Immunol. 2005;35:1342–1346. doi: 10.1002/eji.200526114. [DOI] [PubMed] [Google Scholar]
- 23.Vetrano S, Borroni EM, Sarukhan A, Savino B, Bonecchi R, Correale C, Arena V, Fantini M, Roncalli M, Malesci A, Mantovani A, Locati M, Danese S. The lymphatic system controls intestinal inflammation and inflammation-associated Colon Cancer through the chemokine decoy receptor D6. Gut. 59:197–206. doi: 10.1136/gut.2009.183772. [DOI] [PubMed] [Google Scholar]
- 24.Whitehead GS, Wang T, DeGraff LM, Card JW, Lira SA, Graham GJ, Cook DN. The chemokine receptor D6 has opposing effects on allergic inflammation and airway reactivity. Am J Respir Crit Care Med. 2007;175:243–249. doi: 10.1164/rccm.200606-839OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Codullo V, Baldwin HM, Singh MD, Fraser AR, Wilson C, Gilmour A, Hueber AJ, Bonino C, McInnes IB, Montecucco C, Graham GJ. An investigation of the inflammatory cytokine and chemokine network in systemic sclerosis. Annals of the rheumatic diseases. 2011;70:1115–1121. doi: 10.1136/ard.2010.137349. [DOI] [PubMed] [Google Scholar]
- 26.Singh MD, King V, Baldwin H, Burden D, Thorrat A, Holmes S, McInnes IB, Nicoll R, Shams K, Pallas K, Jamieson T, Lee KM, Carballido JM, Rot A, Graham GJ. Elevated Expression of the Chemokine-Scavenging Receptor D6 Is Associated with Impaired Lesion Development in Psoriasis. American Journal of Pathology. 2012;181:1158–1164. doi: 10.1016/j.ajpath.2012.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bazzan E, Saetta M, Turato G, Borroni EM, Cancellieri C, Baraldo S, Savino B, Calabrese F, Ballarin A, Balestro E, Mantovani A, Cosio MG, Bonecchi R, Locati M. Expression of the atypical chemokine receptor D6 in human alveolar macrophages in Chronic Obstructive Pulmonary Disease. Chest. 2012 doi: 10.1378/chest.11-3220. [DOI] [PubMed] [Google Scholar]
- 28.Cochain C, Auvynet C, Poupel L, Vilar J, Dumeau E, Richart A, Recalde A, Zouggari Y, Yin KY, Bruneval P, Renault G, Marchiol C, Bonnin P, Levy B, Bonecchi R, Locati M, Combadiere C, Silvestre JS. The Chemokine Decoy Receptor D6 Prevents Excessive Inflammation and Adverse Ventricular Remodeling After Myocardial Infarction. Arteriosclerosis, thrombosis, and vascular biology. 2012 doi: 10.1161/ATVBAHA.112.254409. [DOI] [PubMed] [Google Scholar]
- 29.Nibbs RJ, Gilchrist DS, King V, Ferra A, Forrow S, Hunter KD, Graham GJ. The atypical chemokine receptor D6 suppresses the development of chemically induced skin tumors. J Clin Invest. 2007;117:1884–1892. doi: 10.1172/JCI30068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Liberto D, Locati M, Caccamo N, Vecchi A, Meraviglia S, Salerno A, Sireci G, Nebuloni M, Caceres N, Cardona PJ, Dieli F, Mantovani A. Role of the chemokine decoy receptor D6 in balancing inflammation, immune activation, and antimicrobial resistance in Mycobacterium tuberculosis infection. J Exp Med. 2008;205:2075–2084. doi: 10.1084/jem.20070608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horuk R. BX471: a CCR1 antagonist with anti-inflammatory activity in man. Mini Rev Med Chem. 2005;5:791–804. doi: 10.2174/1389557054867057. [DOI] [PubMed] [Google Scholar]
- 32.Johnston B, Burns AR, Suematsu M, Issekutz TB, Woodman RC, Kubes P. Chronic inflammation upregulates chemokine receptors and induces neutrophil migration to monocyte chemoattractant protein-1. J Clin Invest. 1999;103:1269–1276. doi: 10.1172/JCI5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Speyer CL, Gao H, Rancilio NJ, Neff TA, Huffnagle GB, Sarma JV, Ward PA. Novel chemokine responsiveness and mobilization of neutrophils during sepsis. Am J Pathol. 2004;165:2187–2196. doi: 10.1016/S0002-9440(10)63268-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Callen JP. Neutrophilic dermatoses. Dermatologic clinics. 2002;20:409–419. doi: 10.1016/s0733-8635(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 35.Saavedra AP, Kovacs SC, Moschella SL. Neutrophilic dermatoses. Clinics in dermatology. 2006;24:470–481. doi: 10.1016/j.clindermatol.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 36.Lee SC, Brummet ME, Shahabuddin S, Woodworth TG, Georas SN, Leiferman KM, Gilman SC, Stellato C, Gladue RP, Schleimer RP, Beck LA. Cutaneous injection of human subjects with macrophage inflammatory protein-1 alpha induces significant recruitment of neutrophils and monocytes. J Immunol. 2000;164:3392–3401. doi: 10.4049/jimmunol.164.6.3392. [DOI] [PubMed] [Google Scholar]
- 37.Bonecchi R, Polentarutti N, Luini W, Borsatti A, Bernasconi S, Locati M, Power C, Proudfoot A, Wells TN, Mackay C, Mantovani A, Sozzani S. Up-regulation of CCR1 and CCR3 and induction of chemotaxis to CC chemokines by IFN-gamma in human neutrophils. J Immunol. 1999;162:474–479. [PubMed] [Google Scholar]
- 38.Struyf S, Menten P, Lenaerts JP, Put W, D’Haese A, De Clercq E, Schols D, Proost P, Van Damme J. Diverging binding capacities of natural LD78beta isoforms of macrophage inflammatory protein-1alpha to the CC chemokine receptors 1, 3 and 5 affect their anti-HIV-1 activity and chemotactic potencies for neutrophils and eosinophils. Eur J Immunol. 2001;31:2170–2178. doi: 10.1002/1521-4141(200107)31:7<2170::aid-immu2170>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]


